INTRODUCTION
Stress is present in everyday life and is one of the most prevalent conditions experienced by humans, regardless of age, gender, race, and social status. Stress is defined as an internal perception toward real or perceived external stimuli that threatens homeostasis and triggers physiological responses that involve the endocrine, nervous, and immune systems [1,2]. Two stressors frequently investigated are physiological and psychosocial stresses. In addition to subjective and hormonal responses, both stresses also share common neural substrates [3]. Studies on animals as well as population-based research on humans have demonstrated the impact of chronic stress on metabolic, endocrine, and psychological processes. Chronic stress is linked to a higher risk of biological, psychological, and social issues. Moreover, stress is thought to be a significant risk factor for the aging process, and it can manifest early in life if it is persistently present [4].
Prolonged exposure to psychosocial stress has been linked to an increased incidence of major depressive disorder [5]. According to several animal model studies, several stressors can change the structure and function of the brain, resulting in behaviors linked to depression [6]. Psychosocial stress elicits biological and behavioral responses due to the activation of the hypothalamic-pituitary-adrenal (HPA) axis, particularly stress hormones known as corticosterone [7]. The HPA axis mediates the inhibition of the hypothalamic–pituitary–gonadal (HPG) axis, which is responsible for reproductive function and healthy aging in both men and women [8,9]. Additionally, studies showed that an increasing level of brain pro-inflammatory cytokines contributes to the pathophysiology of stress-induced anxiety and depression, suggesting the critical involvement of inflammation [10,11]. Oxidative stress, an imbalance between the production of radicals and antioxidants, also contributes to pathological conditions related to psychosocial stress [12]. There are several methods to induce psychosocial stress in experimental animals, including overcrowding, social defeat, the restrain stress model, and so on. In this study, psychosocial stress is induced by the overcrowding method, which has been shown to effectively induce psychosocial stress in mice and rats and increase corticosterone [13,14].
To attenuate the negative impact of psychosocial stress, many investigations on the effectiveness and underlying processes of plant-based antidepressant and anti-stress medicines have been carried out recently. It is well documented that herbal medicines might lessen the negative impacts of anxiety and depression [15]. An in vitro study revealed that A. keiskei, a flavonoid-rich traditional plant from Japan, contains chemicals that selectively inhibit monoamine oxidases (MAOs) with an IC50 value of 3.43 μM, which suggests its antidepressant property [16]. Studies in mice with psychosocial stress have reported that flavonoids can prevent HPA axis activation, reduce corticosterone levels, regulate hippocampal glucocorticoid receptors (GRs), and prevent nerve cell damage due to its activity to bind 5-HT1A [17]. In addition, a study reported that flavonoid inhibits corticosterone elevation due to reduced corticotropin-releasing factor by blocking the DNA binding activities of the GR [18]. The preliminary study that has been conducted showed that 20 and 40 mg/kgBW/day of ethanolic extract of Angelica keiskei leaves (AKE) could prevent increased corticosterone levels in overcrowding-subjected male and female rats (data not shown).
Despite the broad effect of stress in human and in vitro studies that suggested the potential role of AKE to ameliorate stress-induced impairment, there has been no research focusing on the effect of AKE on overcrowding-induced stress in animal models, particularly the impact on behavioral, neuroendocrine, antioxidant, and inflammatory responses. Thus, this study aimed to analyze the effects of AKE in overcrowding-subjected rats and focused on the application of AKE to improve several behavioral (anxiety- and depressive-like behaviors), neuroendocrine 5-hydroxytryptamine (5-HT, corticosterone, testosterone, and estrogen), antioxidant superoxide dismutase (SOD) and malondialdehyde (MDA), and inflammatory (TNF-alpha) parameters, explicitly emphasizing the differential effects of AKE on male and female rats.
MATERIALS AND METHODS
Animals and experimental groups
This study used a randomized pretest-posttest control group design, carried out using health rats (Rattus norvegicus), Wistar strain, male (n = 20) and female (n = 20), healthy, aged 6 months, weighing 180–200 g. The rats were purchased from the experimental animal breeding facility of the Medical Faculty, at Udayana University, and were kept under standard conditions [19]. The environmental conditions for animal housing were controlled at stable condition of 12 hours light/dark cycle, reduced light intensity (1–25 lx), temperature of 22°C–24°C, humidity of 40%–60%, and ad libitum sources of food and water throughout the experiment. Male and female rats were each divided randomly into two groups (10 rats per group). The control group was given 2 ml of distilled water orally 2 hours before psychosocial stress induction. The treatment group was given 20 mg/kgBW/day of AKE wo hours before psychosocial stress induction. Psychosocial stress was induced using the previously reported overcrowding method [13]. In brief, rats were placed in 12 × 12 × 18 cm cages, three rats per cage (movement space per rat was 48 cm2), for 4 hours per day, 28 days in a row. After 4 hours, rats were returned to their respective standard 40 × 30 × 15 cm cages (3 rats per cage, movement space per rat was 400 cm2). All procedures and protocols in this study were approved by the Institutional Animal Ethic Committee of the Faculty of Medicine and Health Sciences, Atma Jaya Catholic University of Indonesia, Jakarta, Indonesia (Approval No. 01/08/KEP-FKIKUAJ/2023).
Preparation of the extract
Dried leaves of A. keiskei were purchased from a local market (Denpasar, Bali, Indonesia), ground into a coarse powder, and passed through a 40-mesh sieve (425 µm). AKE powder was mixed with five volumes of 70% ethanol for 48 hours, filtered using filter paper (Whatman No. 1), and the solvent was dissolved with a rotary evaporator. Then, the ethanolic extract of AKE was kept at −20°C until further use.
Behavioral tests
Before and after 28 days of overcrowding and AKE treatment, rats were subjected to behavioral testing, including an open field test and a forced swim test to assess anxiety- and depressive-related behaviors, respectively. All behavior tests were carried out in the light phase. In the open field test, rats were placed individually in the center of an acrylic box (40 × 40 × 40 cm). The base of the box was equipped with 10 × 10 cm square line grids. The movements of each rat were recorded for 6 minutes. The time spent in the 4 squares in the center of grids and 12 peripheral squares was calculated. Next, the rearing behavior, or the number of times the rat completely stood on both legs in a vertical upright position, was measured in the open field test as an indicator of anxiety level. The chamber was wiped with 95% ethanol between trials to remove any scent clues left by the previous subject rat [20]. In the forced swim test, rats were individually placed in transparent cylindrical glass containers (21 cm diameter, 50 cm height) containing 23°C water for 5 minutes. After 5 minutes, rats were removed to a transient drying cage. The forced swim test was recorded, and the amount of time rats remained immobile was quantified as depressive-like behavior [21].
Hormonal analysis
The levels of corticosterone, testosterone, and estrogen were quantified from rats’ serum. Blood was collected before and 28 days after treatment by utilizing sterile 75 µl EDTA-coated capillary tubes to puncture the retro-bulbar sinus from the medial canthus of the eye, and serum was isolated by centrifugation. The levels of serum corticosterone, testosterone, and estrogen were measured by enzyme-linked immunosorbent assay (ELISA) kits purchased from Bioassay Technology Laboratory (Shanghai, China) following the manufacturer’s instructions for corticosterone (Cat no. E0828Ra), testosterone (Cat no. EA0023Ra), and estrogen (Cat no. E1297Ra).
Measurement of brain tissue 5-HT, SOD, MDA, and TNF-alpha levels
The levels of neurotransmitter 5-HT, oxidative stress markers (SOD and MDA), and inflammatory cytokines (TNF-alpha) were measured from the brain tissue (prefrontal cortex) homogenates after behavioral tests and blood collection. To obtain brain tissue homogenates, the brains of the rats were collected and homogenized in Dulbecco’s Modified Eagle Medium F-12 (Wako Pure Chemical, Osaka, Japan) on ice, centrifuged at 4°C for 20 minutes at 10,000 rpm, and the obtained supernatant was collected for further experiments. The levels of 5-HT (Cat no. CSB-E08364r, Cusabio, TX), SOD (Cat no. E1444Ra, Bioassay Technology Laboratory), MDA (Cat no. E0156Ra, Bioassay Technology Laboratory), and TNF-alpha (Cat no. E0764Ra, Bioassay Technology Laboratory) were measured in the brain tissue homogenates using commercially available rat ELISA kits according to the respective manufacturer’s protocol.
Data analysis
The data were presented as mean ± SEM and analyzed with IBM SPSS Statistics for Windows, version 23.0. Statistical comparisons were performed with independent sample t-test or three-way mixed analysis of variance (ANOVA) as described in the figure legends. Differences were significant when p < 0.05 (*), <0.01 (**), or <0.001 (***).
RESULTS
AKE attenuates anxiety- and depressive-like behaviors caused by overcrowding
In the open field test, three-way mixed ANOVA revealed a statistically significant three-way interaction between time, gender, and AKE treatment (F = 8.877, p = 0.004, ηp2 = 0.110) for the time spent in the center and peripheral grids. Chronic overcrowding-treated control rats spent significantly shorter and longer time in the center grids and the peripheral grids, respectively, at day 28 compared to day 0 (p < 0.001 for male and p < 0.001 for female). Examination on the rearing behavior revealed a significant three-way interaction between time, gender, and AKE treatment (F = 38.078, p < 0.001, ηp2 = 0.346). Control rats showed a significantly reduced number of rearing movements in both males and females with a similar extent (p < 0.001 for male and p < 0.001 for female). Together, these results suggest that overcrowding induces anxiety-like behavior to a similar degree in both males and females. Stress also led to a considerable increment in the duration of immobilization in the forced swim test. There were significant three-way interactions between time, gender, and AKE treatment (F = 94.501, p < 0.001, ηp2 = 0.568) on the duration of immobilization in the forced swim test. The elevation of immobilization time was higher in female rats (131.00 ± 10.66 seconds) compared to male rats (111.57 ± 10.16 seconds). These results indicate that female rats show more depressive-like behavior than male rats following overcrowding-induced stress.
Next, the effects of AKE on these anxiety- and depressive-like behaviors were tested. At day 28, group treated with daily oral AKE showed an elevated and reduced time spent in the center grids and peripheral grids, respectively (μd = 47.90, p < 0.001 for male and μd = 72.60, p < 0.001 for female), an increased number of rearing movements (μd = 4.20, p < 0.001 for male and μd = 2.90, p < 0.001 for female), and an elevated time of immobilization (μd = 57.00, p < 0.001 for male and μd = 53.10, p < 0.001 for female) compared to control stress rats. Together, these findings suggest that AKE presents antidepressant activity in animal models of anxiety and depression (Fig. 1). In addition, any adverse effect on the group of rats treated with AKE was not observed at any time point.
Effects of AKE on the hormonal biomarkers in social overcrowding-subjected rats
Serum corticosterone (male and female), testosteron (male only), and estrogen (female only) levels were determined before and at the end of the experiment using the ELISA technique. Three-way mixed ANOVA revealed a statistically significant three-way interaction between time, gender, and AKE treatment (F = 218.685, p < 0.001, ηp2 = 0.752) for the level of corticosterone parameter. Consistent with the anxiety- and depressive-like behavior, the exposure of control rats to a 28-day of social overcrowding stress significantly raised the level of corticosterone in the control male (from 4.32 ± 0.90 to 18.01 ± 2.54 ng/ml, p < 0.001) and female (from 55.23 ± 10.23 to 191.42 ± 22.66 ng/ml, p < 0.001) rats. However, this increment was completely ameliorated by treatment with AKE (μd = 14.29, p < 0.001 for male and μd = 135.07, p < 0.001 for female). Next, the levels of testosterone and estrogen in male and female rats, respectively, as a representative of HPG axis function were measured. As expected, the testosterone level was significantly decreased by overcrowding (F = 43.894, p < 0.001), and this depletion was partially restored by AKE treatment (F = 19.876, p < 0.001). Similarly, estrogen levels were substantially declined by overcrowding (F = 19.573, p < 0.001), and AKE protected rats from this depletion (F = 9.067, p < 0.001). These findings suggest the beneficial effects of AKE on overcrowding-induced stress that may be attributed to blunting the detrimental effect of the stress on fertility (Fig. 2).
Role of AKE on the expression of neurotransmitters in the brain
Psychogenic stressors have been widely established to affect the activity of neurotransmitters, the signaling molecules between neurons, particularly norepinephrine, dopamine, and serotonin (5-HT). Neurotransmitters are important for depression and stress [22]. In the present study, the levels of brain 5-HT as a representative monoamine neurotransmitter were determined at the end of the experiment using the ELISA technique. The levels of 5-HT were comparable in male and female rats. The AKE-treated group significantly elevated the brain 5-HT levels in stressed rats, both male (F = 77.575, p < 0.001) and female (F = 75.599, p < 0.001) (Fig. 3A). These results indicate that AKE restored the social overcrowding-induced depletion of monoamine neurotransmitter levels, which may play a beneficial role in the improvement of depression in rats.
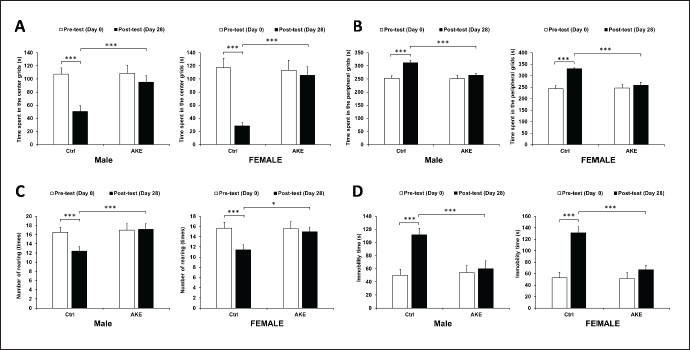 | Figure 1. Effects of overcrowding and AKE on anxiety- and depressive-related behaviors. (A-C) AKE-treated stressed male and female rats exhibited a lower anxiety-like behavior than that of control stressed rats in the open field test, as shown by reduced time spent in the center grids (A), increased time spent in the peripheral grids (B), and a reduced number of forelimbs rearing (C). (D) AKE-treated stressed male and female rats exhibited a lower depressive-like behavior than that of control stressed rats in the forced swim test, as indicated by increased time immobility. Data represent means ± SEM (n = 10), three-way mixed ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001. [Click here to view] |
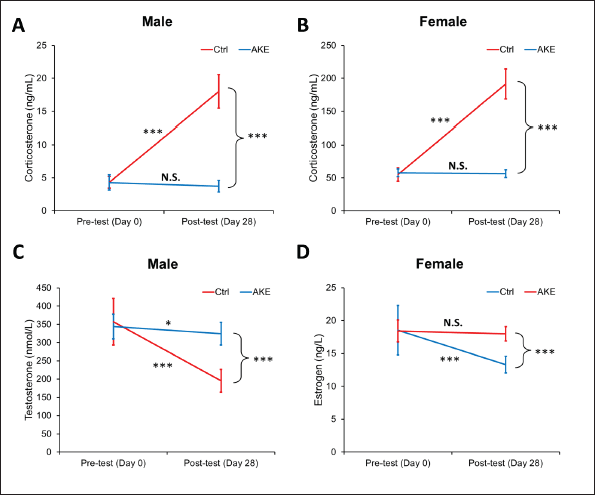 | Figure 2. Changes in plasma corticosterone, testosterone, and estrogen levels in overcrowding- and AKE-treated rats. (A) The plasma concentration of corticosterone in male rats; (B) the plasma concentration of corticosterone in female rats; (C) the plasma concentration of testosterone in male rats; and (D) the plasma concentration of estrogen in female rats before and after overcrowding and AKE treatment for 28 days. Data represent means ± SEM (n = 10), three-way mixed ANOVA for Figures A and B, two-way mixed ANOVA for Figures C and D, *p < 0.05, **p < 0.01, ***p < 0.001. [Click here to view] |
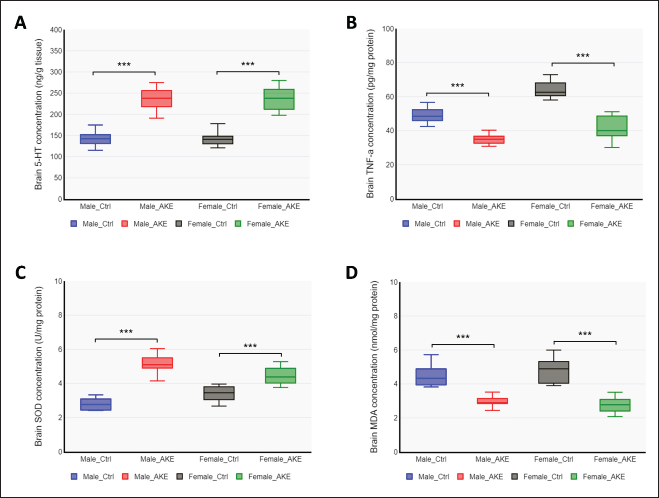 | Figure 3. The effect of AKE on serotonin, TNF-alpha, and redox status biomarkers. Rats were subjected to overcrowding treatment for 4 hours for 28 consecutive days, with or without AKE supplementation. (A) The neurotransmitter 5-HT, (B) the pro-inflammatory cytokine TNF-a, (C) the antioxidant enzyme SOD, and (D) the biomarker for oxidative damage MDA, were measured by ELISA from brain tissue homogenates. Data represent means ± SEM (n = 10), two-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001. [Click here to view] |
AKE modulates the oxido-inflammatory responses of the brain
The depressive disorder caused by psychosocial stress is a combination of brain tissue damage as a result of oxidative stress and chronic inflammation [23]. Oxidative stress is caused by reduced antioxidant levels along with elevated reactive oxygen species (ROS). The proinflammatory cytokine TNF-alpha has been shown to regulate mood and depression [24]. Thus, whether AKE could modulate inflammatory factors and oxidative stress was investigated. To this end, the expression of TNF-alpha, the antioxidant enzyme SOD, and the oxidative damage marker MDA in the brain were measured. These data showed that AKE treatment in male and female rats subjected to overcrowding greatly reduced brain TNF-alpha content (F = 38.213, p < 0.001 for male and F = 102.826, p < 0.001 for female). AKE treatment led to increased SOD content, indicative of improved antioxidant status in the brains of male (F = 128.753, p < 0.001) and female rats (F = 24.668, p < 0.001) (Fig. 3C). The marker of oxidative stress, total MDA content, was significantly lower both in the brains of male (F = 39.805, p < 0.001) and female rats (F = 67.729, p < 0.001) (Fig. 3D). These results revealed that AKE may protect the brain from damage caused by psychosocial stress due to its ability to enhance oxido-inflammatory responses.
DISCUSSION
A study in 2021 reported that the global prevalence of psychological distress amid the COVID-19 pandemic was estimated at 50.0% [25]. There was a trend towards an increase in psychosocial stress in adolescents [26] and adults [27]. Psychosocial stress has been strongly linked to overall health, yet the intricate processes that connect stress to biological dysfunction are still poorly understood [28]. Various studies seem to suggest the involvement of neuroendocrine and autonomic nervous system abnormalities, inflammation, and oxidative stress [29,30]. Research on animal models of chronic psychosocial stress has demonstrated that behavioral alterations in several tests, such as the open field test and the forced swim test, can mimic the symptoms of clinical anxiety and depression, respectively [31].
The results of the present study showed that 28 days of overcrowding treatment in rats induced a stress-like behavioral and biochemical state, which could be reversed by the AKE. The changes in the behavioral and biochemical state of the rats were solely due to overcrowding and AKE treatments, since the environmental conditions for animal housing were controlled at stable conditions. The environments were kept stable throughout the experiments with 12 hours of light/dark cycle, reduced light intensity (1–25 lx), temperature of 22°C–24°C, humidity of 40%–60%, and ad libitum sources of food and water. Many studies have scientifically proven the role of psychosocial stress as a risk factor for depression and depressive symptoms [32]. Indeed, chronic overcrowding treatment in rats in the present study exhibited anxiety- and depressive-related behaviors compared to the pretest. These specific effects were mediated by an alteration in physiological state, including increased corticosterone, decreased testosterone and estrogen, and depleted serotonin (5-HT) levels. Mechanistically, the activation of the HPA axis leads to a surge in corticosterone production [33], resulting in dysregulation of neurotransmitters in the cortical region of the brain, which in turn causes depression-like symptoms [34]. Behavioral changes in stressed rats were also associated with the elevation of MDA, the downregulation of SOD, and the upregulation of TNF-alpha expression in the brain. Co-treatment of overcrowding with AKE attenuated the alterations in behaviors, hormones, neurotransmitters, antioxidants, and cytokine levels elicited by the overcrowding-induced stressful condition.
A proven experimental animal model of psychosocial stress has clearly demonstrated that low levels of brain 5-HT increase stress vulnerability [35]. Hence, most of the current antidepressant medications aim to raise monoamine neurotransmitter concentrations, particularly 5-HT, through selective serotonin reuptake inhibitors. This inhibition is then expected to enhance serotonin signaling [36]. High corticosterone and low 5-HT are the main molecular mechanisms of stress-related depression. A study by Tafet et al. [37] showed that serotonin absorption is increased by heightened cortisol brought on by stress, both at rest and in response to nerve stimulation. In this study, AKE treatment in overcrowding-subjected rats reduced plasma corticosterone levels, which in turn affected serotonergic transmission [31], demonstrating its potent antidepressant activity. The results of the present study are in line with those of previous studies, where antidepressant drugs effectively reduced corticosterone and increased monoamine neurotransmitter levels [38].
In addition to the neuroendocrine systems, there is growing evidence for the involvement of inflammation and oxidative stress in psychosocial stress-related pathology [30,39]. The expression levels of pro-inflammatory cytokines (e.g., IL-1β, TNF-alpha, IL-6, and IL-8) and anti-inflammatory cytokines (e.g., IL-4 and IL-10) in brain tissue were upregulated and downregulated, respectively, by psychosocial stress [40,41]. In the present study, AKE-treated stressed rats showed a lower brain TNF-alpha level, suggesting that AKE prevents brain inflammation induced by overcrowding. The mechanism of the anti-inflammatory property of AKE was revealed by Lee et al. [42] using the RAW 264.7 cell line, which involves the suppression of mitogen-activated protein kinases and nuclear factor-kappaB activation pathways. The anti-inflammatory activity of AKE is mainly attributed to its flavonoid content [43].
Long-term exposure to psychosocial stress can induce enormous ROS and oxidative damage, triggering various cellular responses such as neurodegeneration, tissue damage, and cell death that are responsible for depressive symptoms [12,44]. A study reported that chronic psychosocial stress causes redox perturbations in the brain tissue of rats [45]. In the present study, MDA and SOD were used as oxidative stress markers. MDA is produced as a byproduct of lipid peroxidation, and SOD acts as a radical scavenger by catalyzing the dismutation of the superoxide anion radical into non-reactive molecules [46,47]. Studies showed that the animal model of psychosocial stress exhibits elevated MDA levels and reduced levels of SOD in the brain tissue [48], causing the depletion of 5-HT levels and triggering anxiety- and depressive-related behaviors, as evidenced by the open field and forced swim tests. The AKE treatment in the present study significantly decreased brain MDA levels, accompanied by elevated brain SOD levels, demonstrating the antioxidant properties of AKE. The results of the present study are in line with those of previous studies that showed the beneficial effects of natural antioxidants to attenuate anxiogenic and depressogenic effects [49,50]. A recent meta-analysis study revealed a correlation between the consumption of antioxidant supplements and enhanced states of anxiety and depression, validating their potential as a therapeutic complement to traditional antidepressants [51].
The AKE used in the present study contained 11,523.66 mg QE-Eq/g flavonoids, 3,100.41 mg GAE-Eq/g polyphenols, 10,569.44 mg TAE-Eq/g tannins, an antioxidant capacity of 28,294 mg/l GAEAC, and an IC 50% of 80.16 mg/l. A database of species-metabolites called KNApSAcK (http://www.knapsackfamily.com/KNApSAcK/) identified at least 12 types of bioactive compounds in A. keiskei, including archangelicin, deoxydihydroxanthoangelol H, isobavachalcone, isobavachin, isopimpinellin, osthenol, xanthoangelol, xanthokeismin, xanthotoxin, 4-Hydroxyderricin, and cynaroside. An in vitro study by Kim et al. [16] demonstrated that three of the components of AKE (xanthoangelol, 4-hydroxyderricin, and cynaroside) possess antidepressant activity by inhibiting the activity of MAOs, the enzyme responsible for the degradation of amine neurotransmitters, and dopamine β-hydroxylase (DBH). They found that xanthoangelol acts as a nonselective MAO (MAO-A and MAO-B) and DBH inhibitor, 4-hydroxyderricin is a selective MAO-B inhibitor, and cynaroside is a selective DBH inhibitor [16].
In addition to AKE, many medicinal plant sources have been documented to ameliorate the negative impacts of anxiety and depression [15]. The Caryophyllus aromaticus extract with a total flavonoid content of 9,381.70 mg QE-Eq/g provides antidepressant effects in albino rats [52]. Sargassum horneri and Zataria multiflora extracts have also been shown to reduce depressive-like behaviors in animal models of stress [53,54]. Lychee peel extract with 90.05 ± 0.26 mg QE-Eq/g total flavonoid content reduces depressive-like behavior in chronic restraint stress-subjected rats [48]. In addition, supplementation of Javanese chili extract (Piper longum) with 327.20 mg QE-Eq/g total flavonoid content is also capable of providing antidepressant effects [55]. Rosemary and green tea extracts containing total flavonoid levels of 9,075 QE-Eq/g and 605.48 QE-Eq/g, respectively, have been shown to reduce corticosterone levels in stressed animals [56,57]. These reports highlight the potential of natural products with a wide variety of active compounds to treat neuropsychiatric disorders. With the higher content of active compounds (especially flavonoids), AKE is expected to be more effective in preventing and treating anxiety- and depressive-related behaviors and the corresponding biochemical dysregulation. AKE offers a more compelling prospect than synthetic antidepressants and anxiolytics due to the lower toxicity of natural products. It is widely recognized that herbal medicinal products have a lower risk for toxicity compared to synthetic drugs [58]. In this study, the side effect of AKE was not observed during experiments at any time point. These findings were also supported by several previous studies that report no adverse side effects for AKE treatment in experimental animal models, and toxicity testing in rats demonstrated that the AKE does not cause mortality or organ damage [59,60]. Despite the promising potential of AKE, further investigation is required to establish the mechanisms of action of AKE as an antidepressant and to perform a thorough toxicological evaluation.
In the present study, control non-stressed animals were not included, which may compromise the conclusion drawn from the results. For the behavioral examinations and hormonal assays, the pretest data were obtained and analyzed, thus the observed effects of overcrowding and AKE treatments could be sufficiently concluded. However, the examination of the brain 5-HT, TNF-alpha, SOD, and MDA levels, where pretest data collection is not possible, cannot be adequately compared because the baseline levels of such substances are not available. This fact limits the conclusion drawn from the brain neurotransmitter, pro-inflammatory cytokine, and oxidative stress markers, as it cannot be discerned as to whether overcrowding causes changes to these parameters and whether AKE treatments reversed them. Although several previous studies have reported that overcrowding induces changes in 5-HT [61,62], TNF-a [63], SOD [64], and MDA [65,66] levels, further studies with control non-stressed animals are required to provide more convincing evidence on the role of AKE as an anti-depressant by modulating the brain neurotransmitters, pro-inflammatory cytokines, and antioxidant signaling pathways.
CONCLUSION
In the present study, experimental results show that AKE exhibits antidepressant activities in overcrowding-subjected rats by preventing dysregulation in neuroendocrine, inflammatory, and oxidative stress pathways. The behavioral changes were reversed by AKE, along with lower corticosterone and higher testosterone and estrogen; suggesting that AKE prevents stress-induced infertility and aging-related disorders. In addition, AKE restored the neurotransmitter 5-HT. Furthermore, the SOD was upregulated and MDA and TNF-alpha were downregulated by AKE, demonstrating the potent antioxidant and anti-inflammatory activities of AKE. In conclusion, this study is the first to validate the antidepressant activities of the natural phytomedicine AKE in an animal model, suggesting its potential to be used in clinical settings after clinical trials.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This study was supported by a Grant-in-Aid from Atma Jaya Catholic University of Indonesia (Hibah Kompetitif Dosen Pemula tahun 2024) and a Postdoctoral Grant from WCU UNDIP (Batch IV, 2023).
CONFLICTS OF INTEREST
The author declares that there are no conflicts of interest related to this study.
ETHICAL APPROVALS
The study protocol was approved by the Institutional Animal Ethic Committee of Faculty of Medicine and Health Sciences, Atma Jaya Catholic University of Indonesia, Jakarta Indonesia (Approval No. 01/08/KEP-FKIKUAJ/2023).
DATA AVAILABILITY
All data generated in this study are included in this manuscript.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A. The impact of stress on body function: a review. EXCLI J. 2017;16:1057–72. CrossRef
2. James KA, Stromin JI, Steenkamp N, Combrinck MI. Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Front Endocrinol (Lausanne). 2023;14:1085950. CrossRef
3. Kogler L, Müller VI, Chang A, Eickhoff SB, Fox PT, Gur RC, et al. Psychosocial versus physiological stress—meta-analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage. 2015;119:235–51. CrossRef
4. Yegorov YE, Poznyak AV, Nikiforov NG, Sobenin IA, Orekhov AN. The link between chronic stress and accelerated aging. Biomedicines. 2020;8:198. CrossRef
5. Richter-Levin G, Xu L. How could stress lead to major depressive disorder? IBRO Rep. 2018;4:38–43. CrossRef
6. Planchez B, Surget A, Belzung C. Animal models of major depression: drawbacks and challenges. J Neural Transm. 2019;126:1383–408. CrossRef
7. Chen X, Gianferante D, Hanlin L, Fiksdal A, Breines JG, Thoma MV, et al. HPA-axis and inflammatory reactivity to acute stress is related with basal HPA-axis activity. Psychoneuroendocrinology. 2017;78:168–76. CrossRef
8. Xia F, Wang N, Han B, Li Q, Chen Y, Zhu C, et al. Hypothalamic-pituitary-gonadal axis in aging men and women: increasing total testosterone in aging men. Neuroendocrinology. 2017;104:291–301. CrossRef
9. Bhatta S, Blair JA, Casadesus G. Luteinizing hormone involvement in aging female cognition: not all is estrogen loss. Front Endocrinol (Lausanne). 2018;9:544. CrossRef
10. Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. CrossRef
11. Brymer KJ, Romay-Tallon R, Allen J, Caruncho HJ, Kalynchuk LE. Exploring the potential antidepressant mechanisms of TNFα antagonists. Front Neurosci. 2019;13:98. CrossRef
12. Correia AS, Cardoso A, Vale N. Oxidative stress in depression: the link with the stress response, neuroinflammation, serotonin, neurogenesis and synaptic plasticity. Antioxidants. 2023;12:470. CrossRef
13. Uarquin DG, Meyer JS, Cardenas FP, Rojas MJ. Effect of overcrowding on hair corticosterone concentrations in Juvenile Male Wistar Rats. J Am Assoc Lab Anim Sci. 2016;55:749–55.
14. Pryce CR, Fuchs E. Chronic psychosocial stressors in adulthood: studies in mice, rats and tree shrews. Neurobiol Stress. 2017;6:94–103. CrossRef
15. Yeung KS, Hernandez M, Mao JJ, Haviland I, Gubili J. Herbal medicine for depression and anxiety: a systematic review with assessment of potential psycho-oncologic relevance. Phyther Res. 2018;32:865–91. CrossRef
16. Kim JH, Son YK, Kim GH, Hwang KH. Xanthoangelol and 4-hydroxyderricin are the major active principles of the inhibitory activities against monoamine oxidases on Angelica keiskei K. Biomol Ther. 2013;21:234–40. CrossRef
17. Patil SP, Liu C, Alban J, Yang N, Li XM. Glycyrrhiza uralensis flavonoids inhibit brain microglial cell TNF-α secretion, p-IκB expression, and increase brain-derived neurotropic factor (BDNF) secretion. J Tradit Chinese Med Sci. 2014;1:28–37. CrossRef
18. Kawabata K, Kawai Y, Terao J. Suppressive effect of quercetin on acute stress-induced hypothalamic-pituitary-adrenal axis response in Wistar rats. J Nutr Biochem. 2010;21:374–80. CrossRef
19. Kartiko BHBH, Siswanto FMFM. Overtraining elevates serum protease level, increases renal p16INK4α gene expression and induces apoptosis in rat kidney. Sport Sci Health. 2018;14:1–7. CrossRef
20. Seibenhener ML, Wooten MC. Use of the open field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015;96:e52434. CrossRef
21. Yankelevitch-Yahav R, Franko M, Huly A, Doron R. The forced swim test as a model of depressive-like behavior. J Vis Exp. 2015;97:52587. CrossRef
22. Hayley S, Merali Z, Anisman H. Stress and cytokine-elicited neuroendocrine and neurotransmitter sensitization: implications for depressive illness. Stress. 2003;6:19–32. CrossRef
23. Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BWJH. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–75. CrossRef
24. Liu YN, Peng YL, Liu L, Wu TY, Zhang Y, Lian YJ, et al. TNFα mediates stress-induced depression by upregulating indoleamine 2,3-dioxygenase in a mouse model of unpredictable chronic mild stress. Eur Cytokine Netw. 2015;26:15–25. CrossRef
25. Nochaiwong S, Ruengorn C, Thavorn K, Hutton B, Awiphan R, Phosuya C, et al. Global prevalence of mental health issues among the general population during the coronavirus disease-2019 pandemic: a systematic review and meta-analysis. Sci Rep. 2021;11:10173. CrossRef
26. dos Santos AT, Soares FC, Lima RA, dos Santos SJ, de Silva CR M, Bezerra J, et al. Violence and psychosocial stress: a 10-year time trend analysis. J Affect Disord. 2021;295:116–22. CrossRef
27. Jackson SE, Brown J, Shahab L, McNeill A, Munafò MR, Brose L. Trends in psychological distress among adults in England, 2020-2022. JAMA Netw Open. 2023;6:e2321959. CrossRef
28. Beutel TF, Zwerenz R, Michal M. Psychosocial stress impairs health behavior in patients with mental disorders. BMC Psychiatry. 2018;18:375. CrossRef
29. Won E, Kim YK. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the etiology of depression. Curr Neuropharmacol. 2016;14:665–73. CrossRef
30. Kim E, Zhao Z, Rzasa JR, Glassman M, Bentley WE, Chen S, et al. Association of acute psychosocial stress with oxidative stress: evidence from serum analysis. Redox Biol. 2021;47:102138. CrossRef
31. Belovicova K, Bogi E, Csatlosova K, Dubovicky M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip Toxicol. 2017;10:40–3. CrossRef
32. Duchaine CS, Aubé K, Gilbert-Ouimet M, Bruno Pena Gralle AP, Vezina M, Ndjaboue R, et al. Effect of psychosocial work factors on the risk of depression: a protocol of a systematic review and meta-analysis of prospective studies. BMJ Open. 2019;9:e033093. CrossRef
33. Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6(2):603–21. CrossRef
34. Dean J, Keshavan M. The neurobiology of depression: an integrated view. Asian J Psychiatr. 2017;27:101–11. CrossRef
35. Sachs BD, Ni JR, Caron MG. Brain 5-HT deficiency increases stress vulnerability and impairs antidepressant responses following psychosocial stress. Proc Natl Acad Sci. 2015;112:2557–62. CrossRef
36. Jakobsen JC, Katakam KK, Schou A, Hellmuth SG, Stallknecht SE, Leth-Møller K, et al. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and trial sequential analysis. BMC Psychiatry. 2017;17:58. CrossRef
37. Tafet G, Toister-Achituv M, Shinitzky M. Enhancement of serotonin uptake by cortisol: a possible link between stress and depression. Cogn Affect Behav Neurosci. 2001;1:96–104. CrossRef
38. Fasipe O. Neuropharmacological classification of antidepressant agents based on their mechanisms of action. Arch Med Heal Sci. 2018;6:81. CrossRef
39. Boyle CC, Cole SW, Irwin MR, Eisenberger NI, Bower JE. The role of inflammation in acute psychosocial stress-induced modulation of reward processing in healthy female adults. Brain, Behav Immun – Heal. 2023;28:100588. CrossRef
40. Johnson JD, Barnard DF, Kulp AC, Mehta DM. Neuroendocrine regulation of brain cytokines after psychological stress. J Endocr Soc. 2019;3:1302–20. CrossRef
41. Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl). 2016;233:1637–50. CrossRef
42. Lee HJ, Choi TW, Kim HJ, Nam D, Jung SH, Lee EH, et al. Anti-inflammatory activity of Angelica keiskei through suppression of mitogen-activated protein kinases and nuclear factor- κ B activation pathways. J Med Food. 2010;13:691–9. CrossRef
43. Kil YS, Pham ST, Seo EK, Jafari M. Angelica keiskei, an emerging medicinal herb with various bioactive constituents and biological activities. Arch Pharm Res. 2017;40:655–75. CrossRef
44. Tsuboi H, Tatsumi A, Yamamoto K, Kobayashi F, Shimoi K, Kinae N. Possible connections among job stress, depressive symptoms, lipid modulation and antioxidants. J Affect Disord. 2006;91:63–70. CrossRef
45. Geddie H, Cairns M, Smith L, van Wyk M, Beselaar L, Truter N, et al. The impact of chronic stress on intracellular redox balance: a systems level analysis. Physiol Rep. 2023;11:e15640. CrossRef
46. Pradhany RC, Siswanto FM, Sukoco H, Suarsana IN, Suartini IGAA. L-carnitine prevents hepatic steatosis in deep-frying oil-treated rat. Biomed Pharmacol J. 2022;15:1751–8. CrossRef
47. Wisesa IBGR, Sukoco H, Siswanto FM. The oxidant effect of bisphenol A (BPA) can be decoupled from its endocrine disruptor property. J Phys Conf Ser. 2020;1430:012007. CrossRef
48. Phachonpai W, Preedapirom W, Wuthiyan K, Junkaew A, Tongun T. Lychee peel extract attenuates depression-like behavior in a rat model of chronic restraint stress. J Appl Pharm Sci. 2024;14(02):118–25. CrossRef
49. Kumburovic I, Selakovic D, Juric T, Jovicic N, Mihailovic V, Stankovic JK, et al. Antioxidant effects of Satureja hortensis L. Attenuate the anxiogenic effect of cisplatin in rats. Oxid Med Cell Longev. 2019;2019:1–15. CrossRef
50. Gautam M, Agrawal M, Gautam M, Sharma P, Gautam A, Gautam S. Role of antioxidants in generalised anxiety disorder and depression. Indian J Psychiatry. 2012;54:244. CrossRef
51. Wang H, Jin M, Xie M, Yang Y, Xue F, Li W, et al. Protective role of antioxidant supplementation for depression and anxiety: a meta-analysis of randomized clinical trials. J Affect Disord. 2023;323:264–79. CrossRef
52. Mathiazhagan S, Anand S, Parthiban R, Sankaranarayanan B, Suresh S. Antidepressant-like effect of ethanolic extract from Caryophyllus aromaticus in albino rats. IOSR J Dent Med Sci. 2013;4:37–40.
53. Park I, Kim J, Kim M, Lim DW, Jung J, Kim MJ, et al. Sargassum horneri extract attenuates depressive-like behaviors in mice treated with stress hormone. Antioxidants. 2023;12:1841. CrossRef
54. Arab Z, Hosseini M, Mashayekhi F, Anaeigoudari A. Zataria multiflora extract reverses lipopolysaccharide-induced anxiety and depression behaviors in rats. Avicenna J Phytomed. 2020;10:78–88.
55. Mao QQ, Xian YF, Ip SP, Che CT. Involvement of serotonergic system in the antidepressant-like effect of piperine. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35:1144–7. CrossRef
56. Machado DG, Neis VB, Balen GO, Colla A, Cunha MP, Dalmarco JB, et al. Antidepressant-like effect of ursolic acid isolated from Rosmarinus officinalis L. in mice: evidence for the involvement of the dopaminergic system. Pharmacol Biochem Behav. 2012;103:204–11. CrossRef
57. Chen WQ, Zhao XL, Hou Y, Li ST, Hong Y, Wang DL, et al. Protective effects of green tea polyphenols on cognitive impairments induced by psychological stress in rats. Behav Brain Res. 2009;202:71–6. CrossRef
58. Dai W, Feng K, Sun X, Xu L, Wu S, Rahmand K, et al. Natural products for the treatment of stress-induced depression: pharmacology, mechanism and traditional use. J Ethnopharmacol. 2022;285:114692. CrossRef
59. Maronpot RR. Toxicological assessment of Ashitaba chalcone. Food Chem Toxicol. 2015;77:111–9. CrossRef
60. Son HU, Yoon EK, Cha YS, Kim MA, Shin YK, Kim JM, et al. Comparison of the toxicity of aqueous and ethanol fractions of Angelica keiskei leaf using the eye irritancy test. Exp Ther Med. 2012;4:820–4. CrossRef
61. Daniels WM, Pietersen CY, Carstens ME, Daya S, Stein D. Overcrowding induces anxiety and causes loss of serotonin 5HT-1a receptors in rats. Metab Brain Dis. 2000;15:287–95. CrossRef
62. Abdul Shukkoor MS, Bin Baharuldin MTH, Mat Jais AM, Mohamad Moklas MA, Fakurazi S. Antidepressant-like effect of lipid extract of Channa striatus in chronic unpredictable mild stress model of depression in rats. Evid-Based Complement Altern Med. 2016;2016:1–17. CrossRef
63. Loginova NA, Loseva EV, Sarkisova KY, Kudrin VS. Effects of interferon-α on depressive-like behavior and brain neurochemistry in rats housed in standard and overcrowding conditions. J Evol Biochem Physiol. 2023;59:2005–21. CrossRef
64. Nirmal J, Babu CS, Harisudhan T, Ramanathan M. Evaluation of behavioural and antioxidant activity of Cytisus scoparius link in rats exposed to chronic unpredictable mild stress. BMC Complement Altern Med. 2008;8:15. CrossRef
65. Aparna S, Patri M. Benzo[a]pyrene exposure and overcrowding stress impacts anxiety-like behavior and impairs learning and memory in adult zebrafish, Danio rerio. Environ Toxicol. 2021;36:352–61. CrossRef
66. Bi B, Yuan Y, Zhao Y, He M, Song H, Kong L, et al. Effect of crowding stress on growth performance, the antioxidant system and humoral immunity in hybrid sturgeon. Aquac Rep. 2023;28:101468. CrossRef