INTRODUCTION
The Ginger family Zingiberaceae is among the most sizeable plant families, with approximately 50 genera and over 1,300 species thriving in tropical and subtropical forests [1,2]. Alpinia is an important and widespread genus, mainly found in South and Southeast Asia as part of the highly diverse Zingiberaceae family which contains approximately 250 species globally and 35 native to Vietnam [3,4]. These Alpinia plants are considered folk medicinal plants and spices in some countries including China, Japan, India, and Vietnam. Parts of the plant are commonly used to treat digestive ailments, upset stomachs, vomiting, and intestinal infections. Many species of Alpinia have many different medicinal properties and are used as medicine in Vietnamese traditional medicine [5,6]. Most varieties of Alpinia contain essential oils and have many potential applications based on their biological activity. Regarding the chemistry of the genus Alpinia, studies have centered on analyzing the essential oil components and connected biological functions—antibacterial, anticancer, antioxidant, and so on, across species such as A. galanga, A. zerumbet, and A. cancarata [6–9].
Recently, we have described a new species in Alpinia genus, Alpinia nelumboides Nob.Tanaka, T.T.K. Van & V. Hoang in Lam Dong, Vietnam, and Laos. This species has the Vietnamese name “Ri?ng sen” [10] (https://www.ipni.org/n/77315593-1). Thus far, the only studies on this recently documented species have examined the pseudo-stem and rhizome essential oils, analyzing their chemical profile and antioxidant potential [11]. However, other biological activities have not been reported. Therefore, this investigation targets elucidating the constituent compounds within the extracted essential oils of A. nelumboides rhizome and leaves samples, followed by an assessment of unreported biological properties. We further evaluated their antimicrobial activities against common pathogenic microbes, cytotoxic effects on cancerous and normal cell lines, as well as α-glucosidase inhibitory properties. Results from the phytochemical profiling and bioassays will enhance the current understanding of the chemicals and bioactive potentials of the rhizome, leaves, and pseudo-stem components of A. nelumboides. The findings can provide insight into the medicinal values of A. nelumboides to support its potential pharmaceutical applications.
MATERIALS AND METHODS
Materials
Alpinia nelumboides (Fig. 1) were collected in Di Linh District and cultivated in Da Lat City, Lam Dong Province (Vietnam). Taxonomic authentication utilized morphological assessment for the plant was conducted [10], https://www.ipni.org/n/77315593-1), and type specimen (PHH1004920, PHH1004921) was deposited at the PHH herbarium of the University of Science, Ho Chi Minh city. Before experimental use, the materials underwent washing with water, cleaning, and storage in clean air.
The Center for Research and Application in Bioscience in Ho Chi Minh City, Vietnam, provided clinically isolated antibiotic-resistant bacterial strains, including Enterococcus faecium, Staphylococcus aureus, Acinetobacter baumannii, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Candida albicans. The Department of Genetics at VNUHCM-University of Science provided NCI H460, HepG2, and fibroblast cell lines. A procurement of apramycin was made from GoldBio (USA). The following items were purchased from Sigma (USA): dimethyl sulfoxide, ethanol, trichloroacetic acid, sulforhodamine B, PBS, DPPH, 4-nitrophenyl-α-D-glucopyranoside, trolox, and α-glucosidase.
Extraction of essential oils
The freshly chopped rhizomes and leaves (400 g each) underwent a 6-hour hydrodistillation process using a 2,000 ml capacity Clevenger-esque apparatus loaded with 1,000 ml of water, according to the Vietnamese Pharmacopoeia (The Committee of Vietnamese Pharmacopoeia 2017). Following separation from the hydrodistillation, the hydrophobic essential oil layers were dehydrated using anhydrous sodium sulfate and then kept refrigerated for preservation before impending analysis. The essential oil yields were averaged over three repeated experiments and calculated based on dividing the weight (g) of the essential oil by the weight (g) of the fresh weight of plant materials.
Analysis of the essential oils by GC-FID/MS
Gas chromatography of the essential oils utilized an Agilent 8,860 GC with an Agilent 122-5532 DB-5MS column (30 m × 0.25 mm × 0.25 μm). The GC oven program ramped from 60°C to −240°C at 3°C/minute with the injector and detector maintained at 250°C. The essential oil sample was diluted with hexane solvent at a ratio of 0.1 mg/ml. Samples were injected in splitless mode with 1.00 ml/minute nitrogen carrier gas flow at 11.724 psi. For GC/MS, an Agilent 8,890 GC coupled to a 5977B MSD was equipped with a 19091S-433UI HP-5MS column (30 m × 0.25 mm × 0.25 μm). Helium carrier gas flow was 1.0509 ml/minute at 8.8085 psi. The injection volume was 1.0 μl with a 15:1 split ratio and 70.007 eV ionization voltage [12].
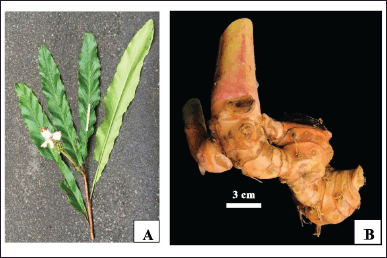 | Figure 1. Alpinia nelumboides Nob. Tanaka, T.T.K. Van & V. Hoang (A. Leaves, B. Rhizomes). [Click here to view] |
Constituent identification initially utilized comparisons of acquired mass spectra against the NIST 2020 compound library. Supplementary confirmation involved matching retention indices for each constituent with indices reported in the literature, specifically the compilation published by Adams in 2007 [13]. Peak area normalization was used to determine the relative percentage of each component in the oil. Response factors were not computed.
Antimicrobial activity
An agar well diffusion assay assessed essential oil bioactivity against select microbial pathogens [14]. The pathogenic cultures were incubated in nutrient broth at 37°C for 18 hours to reach the exponential growth phase. The cultures were diluted with sterile 0.9% NaCl to produce 1.5 × 108 CFU/ml microbial solutions for each pathogen. On Mueller–Hinton agar (MHA) plates, 100 µl of every microbial solution was distributed. On the MHA plates, 8 mm-diameter wells were created by inserting sterile points. In each well, 25 µl of each essential oil was added. Following 37°C, 16–18 hour incubation of inoculated MHA plates, the potency of test essential oils was assessed by measurements of inhibition zone diameters surrounding wells, compared to apramycin and DMSO as positive and negative controls, respectively.
Each essential oil was added to MHA at a concentration spanning from 0.5 mg/ml to 32 mg/ml at a two-fold dilution to determine the minimum inhibitory concentration (MIC) [15]. On the MHA plates containing a serial dilution of the essential oil, 5 × 105 CFU of each microbial pathogen were deposited. After an 18-hour incubation at 37°C, the MIC was determined as the lowest dilution of oil on MHA that completely inhibited the development of the pathogen. The complete inhibition of microbial growth was defined as the absence of any visible colonies on the agar plate at the respective essential oil concentration. This conclusion was based on comparing the appearance of colonies to the reference wells containing apramycin and DMSO.
Anticancer activity
The cytotoxicity of essential oils was assessed on three animal cell lines utilizing the Sulforhodamine B (SRB) method [16]. Cells were seeded in 96-well plates at a density of 104 cells per well for Hep G2 and fibroblast cell lines and at 7.5×103 cells per well for NCI-H460. After 24 hours of culture, the cells were exposed to different concentrations of each essential oil and incubated for an additional 48 hours. The cells were fixed for 1–3 hours with a cold 50% (w/v) trichloroacetic acid solution, rinsed, and stained for 20 minutes with 0.2% (w/v) SRB. Following five treatments with a 1% acetic acid solution, the solubilization of the protein-bound dye was carried out using a 10 mM Tris base solution. At 492 nm and 620 nm, optical density values were determined using a 96-well microtiter plate reader. The growth inhibition percentage (I%) was determined using the formula: I% = (1 - [ODt/ODc]) × 100%, where ODt and ODc denote the optical density values of the test sample and the control sample, respectively. Camptothecin served as the positive control, while DMSO was utilized as the negative control.
Alpha-glucosidase inhibition activity
In a 96-well plate, 120 μl of each essential oil and 20 μl of α-glucosidase at a concentration of 1 unit/ml were dispensed into each well. The mixture was then incubated for 15 minutes at 37°C. Subsequently, 20 μl of a 5 mM p-Nitrophenyl-α-D-glucopyranoside solution was introduced into each well, and the incubation continued for an additional 15 minutes at 37°C. The reaction was terminated by adding 80 μl of a 0.2 M Na2CO3 solution to each well. The quantitative measurement of enzyme activity was performed at an absorbance of 405 nm. All samples were evaluated at five concentrations around the IC50 values. The following equation was used to determine the percentage of inhibition: I (%) = [1—(Asample/Acontrol)] × 100% [17]. The assay used acarbose as the standard control.
Statistical analyses
Statistical analyses were conducted using GraphPad Prism 9. Each experiment was independently replicated at least three times. The data were presented as mean values accompanied by standard deviations and error bars.
RESULTS AND DISCUSSION
Chemical composition of essential oils
Essential oils from the fresh rhizomes and fresh leaves of A. nelumboides, growing in Vietnam were yellow and obtained a yield of 0.20% and 0.22% ± 0.02%, respectively. Table 1 and Table 2 (Supplementary Figures 1 and 3) indicate the chemical constituents and their percentages present in the rhizomes and leaves of A. nelumboides essential oils. Results revealed 99.75% and 88.19% of detectable substances in rhizomes and leaves essential oils, respectively. There were 14 compounds detected in rhizomes essential oils, while this number was 74 in leaves essential oils.
Notes: The percentage values were determined through FID peak area normalization, employing the response factor exclusively for main components (≥ 10%). LRIs (linear retention indices) on HP-5MS Ultra Inert [Lit: literature [13], Obs: observed].
Compared with rhizome essential oil, leaves essential oil contains a chemical composition with more abundant components. Eucalyptol is known as one main components in the oil with the highest percentage in both rhizome and leaves essential oil (71.23% in rhizome essential oil and 32.82% in leaves essential oil). A. nelumboides was morphologically most similar to A. kwangsiensis, but the essential oils of their rhizomes were different which was the main constituent of rhizome A. kwangsiensis essential oil was terpinene-4-ol (14.3%) [9]. Differences in the number of compounds detected in the rhizome and leaves essential oils were similar to previous research on Alpinia vietnamica with 45 compounds detected from leaves and 36 compounds detected from the rhizome [18]. The essential oil from leaves and rhizomes of Kaempferia galanga Linn also showed that 108 and 81 compounds were detected, respectively [19]. Interestingly, the essential oil from this species in the publication of Nguyen Hoang Tuan et al. [11] shows that rhizome essential oil contains the main components of 8-cineole, linalool, (E)-citral, (Z)-citral,α-pinene, limonene, and β-pinene which was different from our study. Also in the study of Nguyen Hoang Tuan et al. [11], the essential oils of rhizome and pseudo-stem had a large amount of polyphenols, which had high antioxidant capacity, while the essential oils in our study showed weak antioxidant activity (IC50 for DPPH assay was over 35 mg/ml). These differences could be explained by the influence of environmental factors. As reported in previous studies, seasonal variations, geographical locations, and weather conditions can affect the chemical compositions and biological activities of essential oils extracted from medicinal plants. Variations in soil properties, sunlight exposure, rainfall patterns, and so on, contribute to changes in the biosynthesis and accumulation of secondary metabolites in plants grown under different environmental conditions [20]. However, it should be noted that the quantitative data reported in this study were obtained through peak area normalization, which provides only semi-quantitative results without considering potential response factor variations.
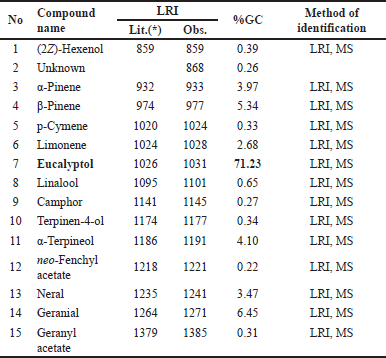 | Table 1. Chemical composition of rhizomes A. nelumboides essential oil. [Click here to view] |
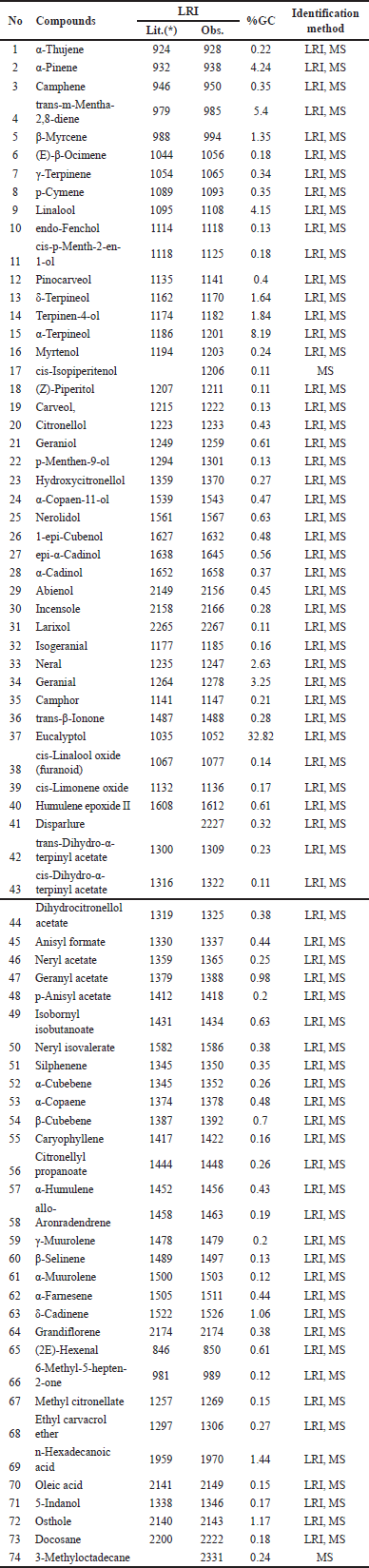 | Table 2. Chemical composition leaves A. nelumboides essential oil. [Click here to view] |
Antimicrobial activity
The antimicrobial activity of essential oil from A. nelumboides was performed by agar well diffusion method, as shown in Table 3. The essential oil extracted from the rhizomes and leaves of A. nelumboides were effective against Gram-negative bacteria, Gram-positive bacteria, and fungi. Rhizome essential oil was more active than leaves essential oil which was 6/8 test microorganisms sensitive to the leaves essential oil, 8/8 test microorganisms sensitive to the rhizomes essential oil. On E. faecium, S. aureus, and P. aeruginosa, the inhibition zone diameters of the rhizomes essential oil (38, 26, 25 mm) were greater than those of apramycin at a concentration of 1 mg/ml (18, 21, 21 mm).
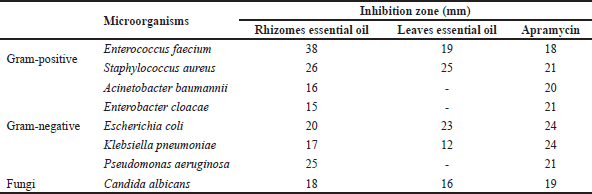 | Table 3. Antimicrobial activity of the rhizomes and leaves essential oil from A. nelumboides. [Click here to view] |
In general, the essential oil extracted from the rhizomes and leaves exhibited a range of antibacterial and antifungal properties against a variety of bacterial and fungal species. A similar result was found in the study of E. fimbriobracteata essential oil [21] and A. fragrans essential oil from Iran [22]. According to a study on the antibacterial activity of Z. spectabile Griff, the essential oil from the leaves had no antibacterial activity, but the essential oil from the rhizomes did, which was due to a large variation in the chemical composition of essential oil [23]. The results of our analysis could be explained by the difference in concentrations of antibacterial compounds, such as eucalyptol [24], that accumulate in the rhizomes (71.23%) versus the leaves (32.82%). Moreover, eucalyptol is the primary antibacterial component of many antimicrobial-active plants [24,25]. Therefore, the essential oil of A. nelumboides rhizomes, whose primary constituent was eucalyptol, is a potential alternative for the control of multiple microorganisms. Following this, we determined the antibacterial activity of essential oil from A. nelumboides by determining the MIC of essential oil against susceptible microorganism strains. The MIC values of essential oil against susceptible bacteria strains were obtained in Table 4.
The rhizomes essential oil exhibited antibacterial activity against all 8 microbiological strains, with MIC values ranging from 8 to 16 mg/ml. The antibacterial activity of leaves essential oil ranged from 4 mg/ml to 16 mg/ml against E. faecium, S. aureus, E. coli, K. pneumoniae, and C. albicans. The anti-C. albicans activity of the leaves essential oil was determined to be more impressive than that of the microorganisms examined in this study. The MIC values of rhizomes essential oil from A. nelumboides were stronger than S. abrotanoides essential oil on E. coli (MIC = 64 mg/ml), P. aeruginosa (MIC = 64 mg/ml), but its value was identical on S. aureus (MIC = 8 mg/ml), and C. albicans (MIC = 8 mg/ml) [26].
ANTICANCER ACTIVITY
The anticancer activity of the essential oil of A. nelumboides was determined using the SRB method, and their results are shown in Table 5.
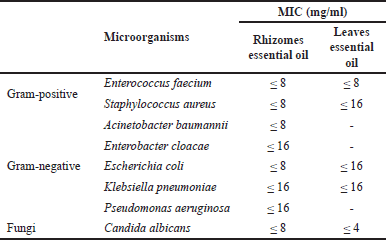 | Table 4. MIC results of the rhizomes and leaves essential oils from A. nelumboides. [Click here to view] |
 | Table 5. Cytotoxic activity of the rhizomes and leaves essential oils from A. nelumboides. [Click here to view] |
The IC50 value of essential oil greater than 300 µg/ml was considered non-toxic, whereas the IC50 value was between 10 and 50 µg/ml; 50–100 µg/ml and 100–200 µg/ml were evaluated for strong, moderate, and weak toxic, respectively [27]. The IC50 values indicated that the rhizomes essential oil had a moderate cytotoxic effect on the NCI-H460 cell line and a weak cytotoxic effect on the HepG2 cell line. This essential oil was not fibroblast-toxic. The IC50 values of the leaves essential oil for all three human cell lines were greater than 300 µg/ml, indicating no cytotoxicity for these human cell lines.
Similar to many previous investigations, the rhizomes and leaves of A. nelumboides essential oil displayed varying degrees of cytotoxicity [26, 28,29]. Eucalyptol is an anti-cancer and metastasis-preventing agent [27]. Variations in the quantities of compounds with cytotoxic activity may account for differences in the toxicity of various essential oils [26]. Therefore, the increased concentration of eucalyptol in the essential oil of the rhizome may account for its enhanced anticancer activity. According to a previous study, selective index (SI) = IC50 for normal cell line/IC50 for respective cancerous cell line, and a selective index (SI) > 1 denotes a drug with greater efficacy against tumor cells than toxicity against normal cells [30]. Because the IC50 of the rhizomes essential oil was greater than 300 µg/ml on fibroblasts compared with the IC50 on cancer cell lines (NCI-H460 and HepG2), this essential oil could have a selective effect on these cancer cell lines. Thus, the rhizomes essential oil of A. nelumboides had a potential alternative for further research on treatment for cancer diseases.
While the results demonstrate promising anticancer effects, testing on additional cancer cell lines from various tissue origins would provide a broader understanding of the essential oil of anticancer potential. Moreover, in vivo studies are warranted to validate the anticancer effects observed in vitro and investigate any potential toxicity or off-target effects in animal models.
Alpha-glucosidase inhibition activity
Table 6 displayed the α-glucosidase inhibitory activity results of A. nelumboides essential oils. A. nelumboides essential oil exhibited the α-glucosidase inhibition activity with the IC50 of leaves essential oil was 2.42 ± 0.22 mg/ml while the values of rhizomes essential oil were higher than 3 mg/ml. The IC50 values of A. nelumboides essential oil extracted from leaves were lower than those of Centaurea calcitrapa essential oil from Iraq (IC50 = 4.38mg/ml) [31]. The hydrolysis of nonreducing α-1,4-linked glucose molecules from disaccharides or oligosaccharides is catalyzed by α-glucosidase. α-glucosidase enzymes complete carbohydrate breakdown into monosaccharides until units are taken into the body [32]. Glucosidase inhibitors have the potential to be utilized to treat diabetes. Therefore, our data preliminary find out the potential of A. nelumboides essential oil in diabetes treatment.
The findings of this study demonstrate the potential of Alpinia nelumboides essential oils, particularly from the rhizomes, as promising natural sources for pharmaceutical applications. The observed anticancer, antibacterial, and antidiabetic properties present opportunities for developing novel therapeutic agents or functional ingredients. Additionally, this research enhances our understanding of the applicational capabilities of these essential oils compared to the preliminary findings reported earlier since just focus on antioxidation activity [11]. However, further research is required to isolate and characterize the bioactive components, optimize extraction processes, and conduct comprehensive preclinical and clinical evaluations before practical applications can be realized in the pharmaceutical industry.
 | Table 6. The α-glucosidase inhibition of the rhizomes and leaves essential oil from A. nelumboides. [Click here to view] |
CONCLUSION
A bearing oil plant, Alpinia nelumboides Nob. Tanaka, T.T.K. Van & V. Hoang, named as “Ri?ng sen” by Vietnamese was found and studied on taxonomy, essential oils, and their bioactivities. The chemical composition of the oils identified by GC-FID/MS and LRI showed that this species was rich in eucalyptol essential oils. By the rich in eucalyptol in particular and rich in oxygenated compounds in general, the essential oils showed high potential for bioactivities. Rhizomes and leaves essential oils exhibited different levels of antibacterial activity, anticancer activity, and α-glucosidase enzyme inhibition to varying degrees.
Authorship
VTTK and TTL carried out experiments, performed statistical analysis, and wrote the manuscript. TTNT conducted experiments chemical analysis experiments. VH and CHN designed experiments, supervised experiments, and reviewed the manuscript. The manuscript underwent review by the full author list before obtaining consent for submission from each party for journal publication.
ACKNOWLEDGMENTS
The authors thank the Center for Research and Application in Bioscience for providing the bacterial strains for this study, and the Department of Genetics at VNUHCM-University of Science for supplying the NCI H460, HepG2, and fibroblast cell lines.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This study was supported by the University of Science, VNU-HCM, under grant number SH-CNSH 2022-10, Vietnam.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed have been included in this research article.
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the journal’s website: Link Here [THIS IS A DUMMY LINK THAT WILL BE CHANGED AFTER PUBLICATION].
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Springer-Verlag Berlin Heidelberg, Germany. The families and genera of vascular plants. Springer Available from: https://www.springer.com/series/1306
2. Holttum RE, Holttum RE. The Zingiberaceae of the Malay Peninsula. Gard Bull Singap. 1950 Jun 30;13:1–249.
3. P.H. Ho. An illustrated flora of Vietnam. Ho Chi Minh City, Vietnam: Youth Publish House; 2023. Vol. 3, pp 432–61.
4. Smith RM. Alpinia (Zingiberaceae): a proposed new infrageneric classification. Edinb J Bot. 1990 Mar;47(1):1–75. CrossRef
5. Victório CP. Therapeutic value of the genus Alpinia, Zingiberaceae. Rev Bras Farmacogn. 2011 Feb;21:194–201. CrossRef
6. Van HT, Thang TD, Luu TN, Doan VD. An overview of the chemical composition and biological activities of essential oils from Alpinia genus (Zingiberaceae). RSC Adv. 2021 Nov 23;11(60):37767–83. CrossRef
7. Thi Huong L, Dai D, Van Chung M, Dung D, Ogunwande I. Constituents of essential oils from the leaf, stem, root, fruit and flower of Alpinia macroura K. Schum. 2017 Jan 1;16:26–33.
8. Adhikari B. Distribution of Alpinia (Zingiberaceae) and their use pattern in Vietnam. J Biodivers Endanger Species. 2014 Jan 1;02. CrossRef
9. Nhan NT, Lan CT, Linh LD, Huong LT, Dai DN, Ogunwande IA. Chemical compositions of essential oils and antimicrobial activity of Alpinia kwangsiensis from Vietnam. J Essent Oil Bear Plants. 2021 Jul 4;24(4):714–23. CrossRef
10. Tanaka N, Hoang V, Kieu Van T, Tram N, Tagane S, Funakoshi H, et al. A new species of alpinia (Zingiberaceae: subgenus Alpinia subsect. Catimbium) from Laos and Vietnam. 2023 Feb 23;49:25–32.
11. Nguyen Hoang T, Tran-Trung H, Giang LD, Triet NT, Tran Van C, C. Vu D, et al. Alpinia nelumboides Nob.Tanaka, T.T.K.Van & V.Hoang: phytochemical analysis and antioxidant activities of pseudo-stem and rhizome essential oils. Nat Prod Res. 2023;0(0):1–8. CrossRef
12. van Den Dool H, Dec. Kratz P. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J Chromatogr A. 1963 Jan 1;11:463–71. CrossRef
13. Adams R. Identification of essential oil components by gas chromatography / mass spectroscopy. Soc Mass Spectrometry. 1995;Vol. 16(11):65–120.
14. Do TH, Duong TH, Nguyen HT, Nguyen TH, Sichaem J, Nguyen CH, et al. Biological activities of lichen-derived monoaromatic compounds. Molecules. 2022 Jan;27(9):2871. CrossRef
15. Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163–75. CrossRef
16. Selective cytotoxicity of a Vietnamese traditional formula, Nam Dia long, against MCF-7 cells by synergistic effects—PubMed [Internet]. Available from: https://pubmed.ncbi.nlm.nih.gov/27421261/
17. Phongpaichit S, Nikom J, Rungjindamai N, Sakayaroj J, Hutadilok-Towatana N, Rukachaisirikul V, et al. Biological activities of extracts from endophytic fungi isolated from Garcinia plants. FEMS Immunol Med Microbiol. 2007 Dec;51(3):517–25. CrossRef
18. Tra NT, Ha NX, Tuyen NV, Thuy Linh NT, Thu Ha NT, Cham BT, et al. Essential oils of Alpinia vietnamica rhizomes and leaves: chemical composition, cytotoxicity, α-glucosidase inhibition, and molecular docking approach. Nat Prod Commun. 2023 Oct;18(10):1934578X231206280—[Internet]. Available from: https://journals.sagepub.com/doi/10.1177/1934578X231206280
19. Bhuiyan MNI, Begum J, Anwar MN. Essential oils of leaves and rhizomes of Kaempferia galanga Linn. Chittagong Univ J Biol Sci [Internet]. 2008 Jan 1; Available from: https://paperity.org/p/254070735/essential-oils-of-leaves-and-rhizomes-of-kaempferia-galanga-linn
20. Masotti V, Juteau F, Bessière JM, Viano J. Seasonal and phenological variations of the essential oil from the narrow endemic species Artemisia molinieri and its biological activities. J Agric Food Chem. 2003 Nov 19;51(24):7115–21. CrossRef
21. Ud-Daula AFMS, Demirci F, Abu Salim K, Demirci B, Lim LBL, Baser KHC, et al. Chemical composition, antioxidant and antimicrobial activities of essential oils from leaves, aerial stems, basal stems, and rhizomes of Etlingera fimbriobracteata (K.Schum.) R.M.Sm. Ind Crops Prod. 2016 Jun 1;84:189–98. CrossRef
22. Composition and antibacterial activity of essential oils of Artemisia Fragrans Willd. Leaves and roots from Iran [Internet]. Available from: https://journals.sagepub.com/doi/epdf/10.1177/1934578X0900400223
23. Sivasothy Y, Awang K, Ibrahim H, Thong KL, Fitrah N, Koh XP, et al. Chemical composition and antibacterial activities of essential oils from Zingiber spectabile Griff. J Essent Oil Res. 2012 Jun;24(3):305–13. CrossRef
24. Nguyen DD, Nguyen-Ngoc H, Tran-Trung H, Nguyen DK, Nguyen LTT. Limonene and eucalyptol rich essential oils with their antimicrobial activity from the leaves and rhizomes of Conamomum vietnamense N.S. Lý & T.S. Hoang (Zingiberaceae). Pharmacia. 2023 Jan 26;70(1):91–6. CrossRef
25. M?czka W, Duda-Madej A, Górny A, Grabarczyk M, Wi?ska K. Can eucalyptol replace antibiotics? Mol Basel Switz. 2021 Aug 14;26(16):4933. CrossRef
26. Investigation of the composition, antimicrobial, antioxidant, and cytotoxicity properties of Salvia abrotanoides essential oil [Internet]. Available from: https://www.hindawi.com/journals/ecam/2022/9304977/ CrossRef
27. Döll-Boscardin PM, Sartoratto A, Sales Maia BHL de N, Padilha de Paula J, Nakashima T, Farago PV, et al. In vitro cytotoxic potential of essential oils of Eucalyptus benthamii and its related terpenes on tumor cell lines. Evid-Based Complement Altern Med ECAM. 2012;2012:342652. CrossRef
28. Minyi T, Hong Y, Wu X, Zhang M, Lin B, Zhou Y. Chemical constituents and cytotoxic activities of essential oils from the flowers, leaves and stems of zingiber striolatum Diels. Rec Nat Prod. 2019 Nov 26;14:144–9. CrossRef
29. Donadu MG, Trong Le N, Viet Ho D, Quoc Doan T, Tuan Le A, Raal A, et al. Phytochemical compositions and biological activities of essential oils from the leaves, rhizomes and whole plant of hornstedtia bella Škorni?k. Antibiotics. 2020 Jun 18;9(6):334. CrossRef
30. Synthesis, antiproliferative activity and molecular docking studies of novel doubly modified colchicine amides and sulfonamides as anticancer agents—PMC [Internet]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7221574/
31. Kaskoos R. In-vitro α-glucosidase inhibition and antioxidant activity of methanolic extract of Centaurea calcitrapa from Iraq. Am J Essent Oils Nat Prod [Internet]. 2013 Jul 1; Available from: https://www.semanticscholar.org/paper/In-vitro-%CE%B1-glucosidase-inhibition-and-antioxidant-Kaskoos/23ab8e90ebec1495530715d77115dd645d1d3f2a
32. ?orkovi? I, Gašo-Soka? D, Pichler A, Šimunovi? J, Kopjar M. Dietary polyphenols as natural inhibitors of α-amylase and α-glucosidase. Life. 2022 Nov;12(11):1692. CrossRef