INTRODUCTION
Alzheimer’s disease (AD) is the predominant form of neurodegenerative ailment impacting elderly individuals [1,2]. It is recognized by the deposition of neuritic plaques, which are abnormal and neurofibrillary tangles in the cerebral cortex and subcortical grey matter [3]. According to a recent report by the World Health Organization, it has been estimated that dementia afflicts 55 million individuals worldwide, with more than 60% residing in nations with lower to moderate income levels. With the aging population increasing in every country, there is a chance of an increase in number to 78 million in 2030 and around 139 million in 2050. The report by the Alzheimer’s Association in the U.S. indicates that around 5.8 million Americans 65 years of age and older are suffering from AD or other dementias in 2020, which could reach up to 13.8 million by 2050 [4]. There are many reports on the pathogenesis of AD, and one of them is the dysfunction of the cholinergic system. Furthermore, a hypothesis showed the involvement of the cholinergic system in the advancement of AD by phenocopying cognitive impairments in rodent models along with acetylcholine receptors (AChRs) antagonism [5–7].
One of the AChRs is the alpha-7-nicotinic acetylcholine receptor (α7nAChRs). The α7 subunit is mostly located in the hippocampus (HIP) and hypothalamus [8]. It has a pentameric structure with a ligand-gated ion channel. Thus, they regulate neuronal excitability as well as neurotransmitter release by regulating the flux of different cations, for example, Na+, K+, and Ca2+, across cell membranes that affect various physiological responses, including memory and behavior [9,10]. However, when compared to other ligand-gated ion channels, these receptors exhibit elevated permeability to Ca2+ ions. This calcium ion, acting as a second messenger, leads to the activation of numerous signaling pathways [11].
Furthermore, because of cholinergic neurons’ increased sensitivity (abundant in α7nAChRs) and the substantial interaction among α7nAChRs and Amyloid Beta (Aβ), coupled with the notable calcium ion selectivity of such receptors, there is considerable interest in targeting them for AD treatment [9]. Therefore, the modulation of α7nAChRs with different agonists can be considered as a future target for the treatment of cognitive disorders such as AD [12]. Moreover, 3’,5’-cyclic nucleotides [Cyclic Adenosine Monophosphate (cAMP) and Cyclic Guanosine Monophosphate (cGMP)] are the “second messengers” that are involved in connecting the extracellular environment to the intracellular environment as well as in transducing the signal of the “first messenger” which can be hormones and neurotransmitters [13]. They are involved in various physiological responses such as neurogenesis, apoptosis, synaptic plasticity, learning and memory function, and other cognitive functions [14]. In the brain of AD patients, the level of cyclic nucleotides seems to be reduced. Moreover, the synaptic function is modulated by the activation of cyclic nucleotide activity [15]. The altered regulation of cyclic nucleotides in AD has prompted novel considerations for targeting these nucleotides in the therapeutic approach to AD [16]. cAMP and cGMP pathways affect each other through mechanisms where cAMP can inhibit guanylate cyclase, lowering cGMP levels, and cGMP can inhibit adenylate cyclase, lowering cAMP levels. This interaction modulates cellular responses and maintains homeostasis. Both pathways play roles in neurotransmitter release and synaptic plasticity, and their dysregulation can disrupt neurotransmission, causing cognitive deficits in AD. Maintaining a balance between these cyclic nucleotides is crucial for proper neuronal function [17]. Hence, it is a recent subject in the advancement of new therapies for AD that focuses on the cGMP and cAMP signaling pathways [18].
It has been demonstrated that enhancing levels of cyclic AMP and GMP by inhibiting the enzyme phosphodiesterase had a positive impact on improving cognitive function in AD. Observations indicate that cyclic nucleotide analogs replicate the effect of stimulating nicotinic cholinergic receptors, inducing a sustained hyperpolarization of the membrane potential in Schwann cells. Furthermore, studies showed that the nicotinic receptor that is present in squid Schwann cells mediates its action via activation of adenylate cyclase [19]. Also, a report suggested that nicotine-induced currents are coupled to the cGMP/ Protein kinasse G pathway [20]. Moreover, STZ is a derivative of glucosamine-nitrosourea, originating from soil bacteria and formulated originally as an anticancer medication. In 1963, it was discovered to trigger diabetes in laboratory animals [21]. Research has explored that Intracerebroventricular (ICV) injection of STZ leads to changes in behavioral functions, which includes memory impairment and neurochemical functions, which is the same as that observed in sporadic AD condition and thus considered as a major experimental model of the early pathophysiological changes in AD [22–24]. In addition, ICV-STZ leads to a deficiency of cholinergic activity and causes mitochondrial dysfunction [25,26].
Moreover, it has been documented by our laboratory that a notable decrease has been observed in the brain’s expression level of α7nAChRswith impairment in mitochondria function and cholinergic activity within the regions of the brain that are sensitive to memory [27,28]. However, there is no documentation regarding the levels of cyclic nucleotides in memory-sensitive regions of the rat brain after the downregulation of α7nAChR via the STZ-induced AD model. Therefore, the present study was undertaken to explore the amounts of cAMP and cGMP levels in the STZ-induced AD model.
MATERIALS AND METHODS
Animals
Swiss albino Wistar rats of body weight 220–250 g were procured from the animal house of the Institute of Pharmaceutical Research, GLA University, Mathura. The rats were grouped into three equal-sized groups, each group containing six rats (the expected disease incidence is 70%, so six animals per group were chosen for the study) and maintained at standard conditions (temperature is 23°C–26°C, relative humidity is 48%–55% as well as light/dark cycle of 12 hour in a cage made up of polyacrylic lined with husk). Rats were provided standard soya-free pellet feed and water ad libitum. The study protocol was approved by the Institutional Animal Ethics Committee of the GLA University, Mathura, India (Approval No. GLAIPR/IAEC/09/22/PhD/02). All research procedures were carried out in strict accordance with the guidelines set forth by CPCSEA (Committee for the Purpose of Control And Supervision of Experiments on Animals).
Reagents and chemicals
STZ, cAMP, and cGMP ELISA kits were obtained from KRISHGEN BioSystem, Ashley Ct, Whittier, California. All remaining chemicals and other reagents were of analytical quality and procured from nearby vendors.
Experimental protocol
The full study regimen was followed for 18 days. Experimental rodents (male albino Wistar rats) were acclimated and divided into three groups, each consisting of six animals labeled Control, Sham, and STZ. Except for the control group, all other groups received anesthesia with a 45 mg/kg dose of pentobarbitone (i.p.). STZ was injected into rats of the STZ group via ICV injection on Day 1 as well as Day 3 of the research protocol. The Sham group received an ICV injection of artificial cerebrospinal fluid (aCSF). According to the experimental timetable, the rats underwent the Morris Water Maze (MWM) test protocol for five consecutive days, i.e., Day 14 to Day 18. Additionally, on Day 18, the animals underwent the Y-maze test. Any-maze™ (Version-4.96, USA) software for video-tracking was used to capture and measure all behavioral activity. Following the successful completion of the behavioral tasks, all rodents were sacrificed via decapitation. Each animal’s brain was removed and microdissected into HIP, PFC, and Amygdale (AMY) for biochemical analysis [29,30].
ICV injection of STZ
Rats were fasted overnight and then anesthetized with sodium pentobarbital (45 mg/kg) intraperitoneally. Afterward, the scalp incision was made, and the rat’s head was placed on a stereotaxic device. Two holes (1.5 mm depth) were drilled on each side of the lateral ventricles [31]. Two perforations were made on either side of the lateral ventricle, each with a depth of 1.5 mm, located 0.8 mm posterior, ±1.5 mm mediolateral to the bregma, and 3.8 mm dorsoventral under the dura [30,31]. The STZ solution dissolved in aCSF was administered into both sides of the lateral ventricle within a 5-minute interval on Day 1 and repeated on Day 3 (at a dose of 3 mg/kg; 5 µl each site, with a 5-minute delay between injections) [32]. Furthermore, 5 µl aCSF was injected into either side of the lateral ventricles of rats of the Sham group.
Assessment of STZ-induced cognitive deficits in various behavioural models
MWM test model for evaluating learning as well as memory
The MWM is commonly employed in animal models to assess learning and memory abilities [33,34]. Briefly, it consists of a circular black pool filled with water. The water container was segmented into four identical quadrants, and a concealed platform was positioned beneath the water surface. The behavior of each animal was recorded by using a video tracking system (ANY-maze video tracking system, Stoelting Co., Version 4.96, USA). The animals’ ability to locate the hidden platform and their escape latency (the time taken to find the platform as a goal) were documented using a video camera and a tracking system. Randomly, four consecutive trials for each animal were taken with a gap of 5 minutes days of practice for 120 seconds each day for 4 days, starting from Day 14 and ending on Day 17 of the research plan. On Day 18, during the experimental procedure, each animal was permitted to explore the pool for 120 seconds to search the hidden platform. The duration of the animal spent in the target quadrant and each quadrant while searching for the platform was noted. Animals’ speed of swimming, as well as the percentage of the overall distance traversed within the specific quadrant, were documented. The duration consumed in the specific quadrant was regarded as an indicator of memory recall and memory development. The average time spent searching for the hidden platform in all quadrants was noted. The average time the animal consumed in the targeted quadrant was used as the measure for memory assessment.
Y-maze test for assessing spontaneous alteration behaviour (SAB)
The Y-maze test paradigm is utilized to evaluate the working memory of rodents. Standard protocol was followed to evaluate working memory in the form of SAB [35,36] on Day 18. The Y-maze apparatus was black painted and consisted of three wooden arms, which were labeled A, B, and C. On the final day of the experiment, i.e., Day 18, each animal was placed randomly in the center of the Y-maze and permitted to move unrestrictedly in the maze for 8 minutes. The count of alterations (defined as consecutive sequences of entering ABC, CAB, or BCA, except ABA), as well as the total count of entries into the arms, were documented.
Biochemical analysis
Cyclic GMP (cGMP) levels and cyclic AMP (cAMP)
cGMP (Ref: KLR0297; Lot: RCGMP0224) and cAMP (Ref: KLR0298; Lot: RCAMP0224) enzyme immunoassay kits (KRISHGEN BioSystem, Ashley Ct, Whittier, California) were used for the estimation of cyclic nucleotides intracellularly in tissue. The procedure adhered to the manufacturer’s guidelines. Results were given as pmol/ml [37].
Analysis of data
The data analysis results were presented as mean ± standard error of the mean. Statistical analysis of the escape latency of the rats from Day 14 to Day 17 in the MWM paradigm was conducted using repeated assessments using two-way analysis of variance (ANOVA), subsequently employing the Bonferroni Post hoc test. Statistical evaluation of extra data was done by employing one-way ANOVA, which was followed by the Student Newman-Keuls post-hoc test to determine the significance between the experimental groups, with a significance level set at p < 0.05.
RESULTS
STZ reduced the rats’ learning and memory development during the MWM test
STZ’s impact on escape latency changes from Day 14 to Day 17 is represented in Figure 1, (A) the time consumed in the targeted quadrant by the rats (B), % of the overall distance traversed in the target quadrant (C) along with the animals’ swimming speed (D) throughout the MWM evaluation. Statistical analysis by two-way ANOVA revealed significant variances in escape latency across the groups of rats [F (2, 60) = 42.17, p < 0.05] and day [F (3, 60) = 57.37, p < 0.05]. Moreover, a notable connection between the group and the day was observed in the escape latency of rats [F (6, 60) = 2.77, p < 0.05]. The Bonferroni post-hoc assessment suggested that the escape latency did not significantly change among rat groups on MWM’s Day 14. Furthermore, on MWM’s Day 15, rodents from the STZ group showcased prolonged escape latency when compared to rats from the Control as well as Sham groups, suggesting a decline in the animals’ learning abilities due to STZ. Furthermore, a similar observation was noted in the rats on Days 16 and 17. One-way ANOVA indicated that notable variances were observed in the duration consumed in the specific quadrant [F (2, 15) = 56.35, p < 0.05] among rats groups and the percentage of the overall distance traversed by the rats in the target quadrant [F (2, 15) =89.08, p < 0.05], on Day 18 of MWM test than other group rats. However, no remarkable variations were noted in the speed of swimming [F (2, 15) = 2.27, p < 0.05] among rat groups. The post-hoc evaluation indicated that on Day 18, rats treated with STZ exhibited a significant decline in both the duration consumed as well as the % of overall distance covered in the specific quadrant when compared to Control and Sham group rats. It suggests that STZ led to a notable impairment in memory formation among the tested animals.
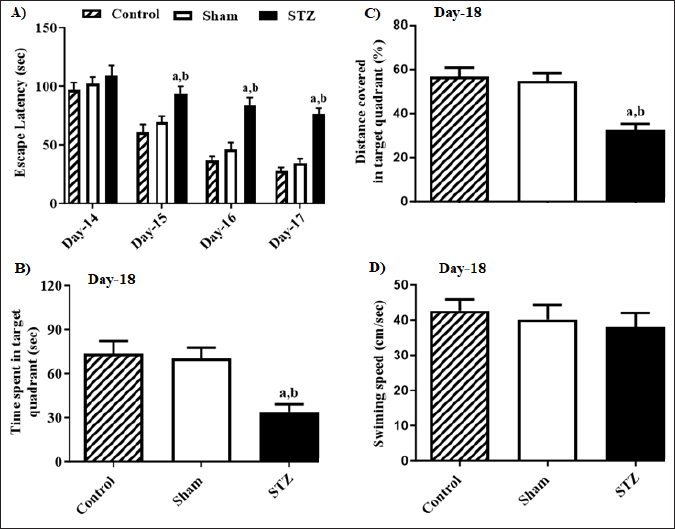 | Figure 1. Effect of STZ-induced changes in the learning and memory of rats in Morris Water Maze test (MWM). (A) STZ-induced changes in escape latency from Day-14 to Day-17 (B) time spent in target quadrant (C) percentage of total distance traveled in the target quadrant and (D) swimming speed on Day-18 during the MWM test. Values are expressed as mean ± SEM (n = 6). ap < 0.05 as compared to Control, bp < 0.05 as compared to Sham (two-way analysis of variance followed by Bonferroni post-hoc test for escape latency from D-14 to D-17 and one-way analysis of variance followed by Student-Newman-Keuls multiple comparison post-hoc test for other parameters). [Click here to view] |
STZ reduced spatial working memory formation of rats in the Y-maze test
Figure 2 displays the impact of STZ on alterations in spatial learning along with memory, as indicated by the percentage of SAB in animals. There were notable variances in the spontaneous behavioral changes [F (2, 15) = 41.10, p < 0.05] among the groups of rats. The post-hoc analysis revealed that rodents subjected to STZ exhibited a substantial decline in SAB during the Y-maze test compared to animals in the Control and Sham groups. It suggests that STZ significantly impaired spatial memory formation, as indicated by SAB performance, in rodents during the Y-maze paradigm.
STZ reduced the concentrations of cyclic nucleotides in distinct brain regions
Figure 3 depicts STZ’s impact on cGMP and cAMP concentrations in the HIP, PFC, and AMY of rats. Statistical variances were observed in cGMP and cGMP levels in rats’ HIP ([F (2, 15) = 93.35, p < 0.05] and [F (2, 15) = 98.82], respectively), PFC ([F (2, 15) = 97.28, p < 0.05] and [F (2, 15) = 48.11, p < 0.05], respectively), and AMY ([F (2, 15 = 87.46, p < 0.05], [F (2, 15) = 31.75, p < 0.05, respectively) among rat groups. The post-hoc analysis reveals a notable reduction in both cGMP and cAMP levels across all brain areas in rats from the STZ group compared to those in the control and sham groups.
DISCUSSION
A previous report from our laboratory showed a decline in the α7nAChR concentration levels in distinct areas of the brain of animals challenged with STZ. Additionally, the ICV administration of STZ adversely affected the functionality, integrity, and bioenergetics of mitochondria in various areas of the rat brain. This downregulation of α7nAChRs observed in memory-related brain regions of rats, occurred alongside other key pathological factors, including cholinergic system dysfunction, accumulation of Aβ, and mitochondrial dysfunction. These findings indicated a potential relationship between the expression levels of α7nAChR and the mitochondrial functioning in the brain regions of rats subjected to STZ. The α7nAChRs may, therefore, serve as a viable alternative target for potential Alzheimer’s therapy [27].
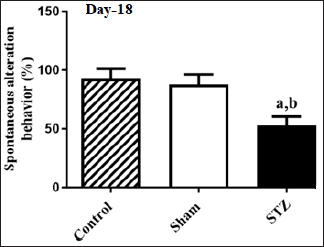 | Figure 2. Effect of STZ on changes in spatial learning and memory in terms of percentage SAB of animals. Values are expressed as mean ± SEM (n = 6). ap < 0.05 as compared to Control, bp < 0.05 as compared to Sham (one-way analysis of variance and Student-Newman-Keuls multiple comparison post-hoc test). [Click here to view] |
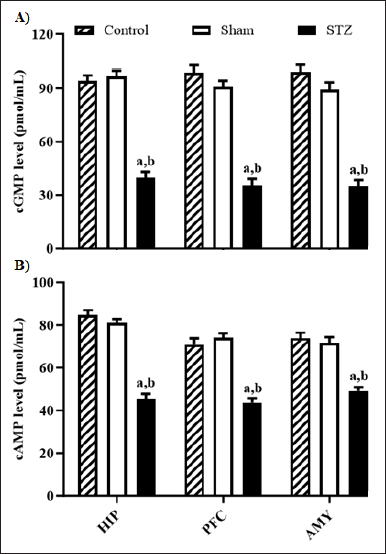 | Figure 3. Effect of STZ-induced changes in the level of (A) cGMP and (B) cAMP in HIP, PFC, and AMY of rat brain. Values are expressed as mean ± SEM (n = 6). ap < 0.05 as compared to Control, bp < 0.05 as compared to Sham (one-way analysis of variance and Student-Newman-Keuls multiple comparison post-hoc test). [Click here to view] |
Since, the amygdala, prefrontal cortex (PFC), and HIP facilitate the development, storage, and retrieval of memories. The HIP is responsible for declarative and spatial memory, the PFC handles working memory and executive functions, and the amygdala deals with emotional aspects. Their interaction is essential for a comprehensive memory system [38]. Cyclic nucleotides, such as cAMP and cGMP, play an important role in the functioning of synapses and the release of neurotransmitters in the brain. When these nucleotides become dysregulated, it can disrupt neurotransmission and lead to cognitive problems in AD [18,39]. Therefore, in this present investigation, we demonstrated that STZ administration (ICV) in experimental rodents led to a notable decrease in the concentrations of cyclic nucleotides within the hippocampal, PFC as well as amygdala regions of these animals’ brains. These results suggest a potential link between the function of α7nAChR and cyclic nucleotide concentration in memory-responsive areas of rats’ brains. Our behavioral studies indicate that ICV injection of STZ led to a significant reduction in learning as well as memory throughout the study (Day 14–Day 17) in the MWM test paradigm and also caused a decrease in spatial memory performance in the Y-maze assessment as reported previously [26]. It is widely acknowledged that AD results in a deterioration of performance in rodents on the MWM test. The learning and memory status assessed through MWM serves as a valuable method for evaluating agents with potential anti-AD properties [40,41]. In this context, STZ induced a significant decline in both the % of overall distance covered as well as the duration consumed in the targeted quadrant, which indicated the STZ attenuated memory formation. Notably, STZ caused a decrease in the SAB percentage in the Y-maze assessment.
Furthermore, in terms of cognition, cyclic nucleotides (cAMP and cGMP) play a critical role in diverse stages of memory, including synaptic plasticity, gene transcription, neurogenesis, neuronal circuitry, and neuronal survival. Hence, the degradation of cyclic nucleotides has been closely linked to cognitive deficits. As a result, targeting cyclic nucleotides as an intracellular messenger emerges as a viable strategy for various biological processes in the brain, exerting significant regulatory influence. Notably, any disruptions in the foundational processes that underlie cognition in AD suggest a crucial involvement of cAMP/cGMP signaling in AD populations [17,42]. In this regard, the current study’s results indicate a significant decrease in the amounts of cyclic nucleotides, including cGMP as well as cAMP, in brain areas of experimental rodents that are memory-responsive. It suggests a potential connection between the reduced cyclic nucleotide levels and the downregulation of α7nAChRs.
CONCLUSION
In summary, STZ results in a decline in the amounts of cyclic nucleotides in the brain regions responsible for memory after STZ administration. The findings suggest a possible direct correlation between α7nAChR activity and cyclic nucleotide levels in memory-sensitive brain regions of STZ-treated animals. Consequently, it is plausible to consider cyclic nucleotides as a potential alternative target to enhance memory dysfunction in AD.
ACKNOWLEDGMENTS
The authors thank the management of GLA University, Mathura, for continuous support and providing a platform to carry out research work.
LIST OF ABBREVIATIONS
AD Alzheimer’s disease
Aβ Amyloid beta
aCSF Artificial cerebrospinal fluid
AMY Amygdale
ANOVA Analysis of variance
cAMP Cyclic adenosine monophosphate
cGMP Cyclic guanosine monophosphate
HIP Hippocampus
ICV Intracerebroventricular
MWM Morris water maze
NAChRs Nicotinic acetylcholine receptors
PFC Prefrontal cortex
PKG Protein kinasse G
SAB Spontaneous alteration behavior
AUTHOR CONTRIBUTIONS
All authors contributed equally to this work. Ahsas Goyal and Neetu Agrawal developed the concept and designed the experiment. Sushma Singh performed material preparation, experimentation, and data collection. Sushma Singh analyzed the results. Sushma Singh wrote the first draft of the manuscript. All authors read and approved the final manuscript.
FINANCIAL SUPPORT
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Institutional Animal Ethics Committee of the GLA University, Mathura, India (Approval No. GLAIPR/IAEC/09/22/PhD/02). All research procedures were carried out in strict accordance with the guidelines set forth by CPCSEA (Committee for the Purpose of Control And Supervision of Experiments on Animals).
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Ren JM, Zhang SL, Wang XL, Guan ZZ, Qi XL. Expression levels of the α7 nicotinic acetylcholine receptor in the brains of patients with Alzheimer’s disease and their effect on synaptic proteins in SH-SY5Y cells. Mol Med Rep. 2020;22(3):2063–75. CrossRef
2. Goyal A, Verma A, Dubey N, Raghav J, Agrawal A, Naringenin. A prospective therapeutic agent for Alzheimer’s and Parkinson’s disease. J Food Biochem. 2022;46(12):e14415. CrossRef
3. Kumar A, Sharma V, Singh V, Kaundal M, Gupta MK, Bariwal J, et al. Herbs to curb cyclic nucleotide phosphodiesterase and their potential role in Alzheimer’s disease. Mech Ageing Dev. 2015;149:75–87. CrossRef
4. Bekdash RA. The cholinergic system, the adrenergic system and the neuropathology of Alzheimer’s disease. Int J Mol Sci. 2021;22(3):1273. CrossRef
5. Varshney V, Garabadu D. Ang(1-7) exerts Nrf2-mediated neuroprotection against amyloid beta-induced cognitive deficits in rodents. Mol Biol Rep. 2021;48(5):4319–31. CrossRef
6. Singh NK, Garabadu D. Quercetin exhibits α7nAChR/Nrf2/HO-1-mediated neuroprotection against STZ-induced mitochondrial toxicity and cognitive impairments in experimental rodents. Neurotox Res. 2021;39(6):1859–79. CrossRef
7. Hoskin JL, Al-Hasan Y, Sabbagh MN. Nicotinic acetylcholine receptor agonists for the treatment of Alzheimer’s dementia: an update. Nicotine Tob Res. 2019;21(3):370–76. CrossRef
8. Papke RL, Horenstein NA. Therapeutic targeting of α7 nicotinic acetylcholine receptors. Pharmacol Rev. 2021;73(3):1118–49. CrossRef
9. Ahmed T, Zahid S, Mahboob A, Farhat SM. Cholinergic system and post-translational modifications: an insight on the role in Alzheimer’s disease. Curr Neuropharmacol. 2017;15(4):480–94. CrossRef
10. Terry AV, Callahan PM. Nicotinic acetylcholine receptor ligands, cognitive function, and preclinical approaches to drug discovery. Nicotine Tob Res. 2019;21(3):383–94. CrossRef
11. Shen JX, Yakel JL. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol Sin. 2009;30(6):673–80. CrossRef
12. Pohanka M. Alpha7 nicotinic acetylcholine receptor is a target in pharmacology and toxicology. Int J Mol Sci. 2012;13(2):2219–38. CrossRef
13. Argyrousi EK, Heckman PRA, Prickaerts J. Role of cyclic nucleotides and their downstream signaling cascades in memory function: being at the right time at the right spot. Neurosci Biobehav Rev. 2020;113:12–38. CrossRef
14. Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev Neurosci. 2007;18(2):93–114. CrossRef
15. Hesse R, Lausser L, Gummert P, Schmid F, Wahler A, Schnack C, et al. Reduced cGMP levels in CSF of AD patients correlate with severity of dementia and current depression. Alzheimers Res Ther. 2017;9(1):17. CrossRef
16. Sharma K, Pradhan S, Duffy LK, Yeasmin S, Bhattarai N, Schulte MK. Role of receptors in relation to plaques and tangles in Alzheimer’s disease pathology. Int J Mol Sci. 2021;22(23):12987. CrossRef
17. Sharma VK, Singh TG, Singh S. Cyclic nucleotides signaling and phosphodiesterase inhibition: Defying Alzheimer’s disease. Curr Drug Targets. 2020;21(13):1371–84. CrossRef
18. Jehle A, Garaschuk O. The interplay between cGMP and calcium signaling in Alzheimer’s disease. Int J Mol Sci. 2022;23(13):7048. CrossRef
19. Elferink JG, VanUffelen BE. The role of cyclic nucleotides in neutrophil migration. Gen Pharmacol. 1996;27(2):387–93. CrossRef
20. Mannai S, Bitri L, Thany SH. cGMP/cGMP-dependent protein kinase pathway modulates nicotine-induced currents through the activation of α-bungarotoxin-insensitive nicotinic acetylcholine receptors from insect neurosecretory cells. J Neurochem. 2016;137(6):931–38. CrossRef
21. Grieb P. Intracerebroventricular streptozotocin injections as a model of Alzheimer’s disease: in search of a relevant mechanism. Mol Neurobiol. 2016;53(3):1741–52. CrossRef
22. Sharma M, Gupta YK. Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci. 2001;68(9):1021–29. CrossRef
23. Grünblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S. Brain insulin system dysfunction in streptozotocinintracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem. 2007;101(3):757–70. CrossRef
24. Gáspár A, Hutka B, Ernyey AJ, Tajti BT, Varga BT, Zádori ZS, et al. Intracerebroventricularly injected streptozotocin exerts subtle effects on the cognitive performance of long-evans rats. Front Pharmacol. 2021;12:662173. CrossRef
25. Esteves IM, Lopes-Aguiar C, Rossignoli MT, Ruggiero RN, Broggini ACS, Bueno-Junior LS, et al. Chronic nicotine attenuates behavioral and synaptic plasticity impairments in a streptozotocin model of Alzheimer’s disease. Neuroscience. 2017;353:87–97. CrossRef
26. Saxena G, Patro IK, Nath C. ICV STZ induced impairment in memory and neuronal mitochondrial function: a protective role of nicotinic receptor. Behav Brain Res. 2011;224(1):50–7. CrossRef
27. Singh NK, Garabadu D. Alpha7 nicotinic acetylcholine receptor down regulation impairs mitochondrial function in streptozotocin-induced sporadic alzheimer’s disease model in rats. Inter J Phar Edu Res. 2021;55(1):153–63. CrossRef
28. Tota S, Kamat PK, Shukla R, Nath C. Improvement of brain energy metabolism and cholinergic functions contributes to the beneficial effects of silibinin against streptozotocin induced memory impairment. Behav Brain Res. 2011;221(1):207–15. CrossRef
29. Palkovits M. Stereotaxic map, cytoarchitectonic and neurochemical summary of the hypothalamic nuclei, rat. In: Jones TC, Mohr U, Hunt RD, Capen CC, editors. Endocrine system. Monographs on pathology of laboratory animals. Berlin, Heidelberg, Germany: Springer; 1983. CrossRef
30. Ng HJ, Whittemore ER, Tran MB, Hogenkamp DJ, Broide RS, Johnstone TB, et al. Nootropic alpha7 nicotinic receptor allosteric modulator derived from GABAA receptor modulators. Proc Natl Acad Sci USA. 2007;104(19):8059–64. CrossRef
31. Herman JP, Watson SJ. The rat brain in stereotaxic coordinates. 2nd ed. In: Paxinos G, Watson C, editors. Cambridge, MA: Academic Press; 1986.
32. Agrawal R, Mishra B, Tyagi E, Nath C, Shukla R. Effect of curcumin on brain insulin receptors and memory functions in STZ (ICV) induced dementia model of rat. Pharmacol Res. 2010;61(3):247–52. CrossRef
33. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. CrossRef
34. Sharma B, Singh N. Defensive effect of natriumdiethyldithiocarbamatetrihydrate (NDDCT) and lisinopril in DOCA-salt hypertension-induced vascular dementia in rats. Psychopharmacology (Berl). 2012;23(3):307–17. CrossRef
35. Mouri A, Noda Y, Hara H, Mizoguchi H, Tabira T, Nabeshima T. Oral vaccination with a viral vector containing Abeta cDNA attenuates age-related Abeta accumulation and memory deficits without causing inflammation in a mouse Alzheimer model. FASEB J. 2007;21(9):2135–48. CrossRef
36. Goyal A, Garabadu D. Bilateral ovariectomy decreases the levels of cyclic nucleotides and nuclear phosphorylated estrogen receptor-alpha in memory-sensitive rat brain regions. J Pharm Edu Res. 2020;54(1):125–34. CrossRef
37. Kaundal M, Zameer S, Najmi AK, Parvez S, Akhtar M. Betulinic acid, a natural PDE inhibitor restores hippocampal cAMP/cGMP and BDNF, improve cerebral blood flow and recover memory deficits in permanent BCCAO induced vascular dementia in rats. Eur. J. Pharmacol. 2018;5(832):56–66. CrossRef
38. Yavas E, Gonzalez S, Fanselow MS. Interactions between the hippocampus, prefrontal cortex, and amygdala support complex learning and memory. F1000Res. 2019 Jul 31;8:F1000 Faculty Rev-1292. CrossRef
39. Donders Z, Skorupska IJ, Willems E, Mussen F, Broeckhoven JV, Carlier A, et al. Beyond PDE4 inhibition: a comprehensive review on downstream cAMP signaling in the central nervous system. Biomed Pharmacother. 2024;(177):117009. CrossRef
40. Bromley-Brits K, Deng Y, Song W. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J Vis Exp. 2011;(53):2920. CrossRef
41. Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–58. CrossRef
42. Giorgi M, Modica A, Pompili A, Pacitti C, Gasbarri A. The induction of cyclic nucleotide phosphodiesterase 4 gene (PDE4D) impairs memory in a water maze task. Behav Brain Res. 2004;154(1):99–106. CrossRef