INTRODUCTION
Diabetes mellitus has been shown to be associated with several types of cancer as evidenced by epidemiological research. Diabetes mellitus is associated with a heightened vulnerability to certain types of cancer. Conversely, some types of cancer and cancer treatments might potentially result in the development of diabetes mellitus. Genetic factors, obesity, inflammation, oxidative stress, hyperglycemia, hyperinsulinemia, cancer treatments, insulin, and some oral hypoglycemic medications are believed to contribute to the link between diabetes mellitus and cancer [1]. A correlation between diabetes and breast cancer has been established by a combination of articles obtained from the database and keyword searches. Type 2 diabetes is a prevalent medical condition that impacts around 7% of adults in prosperous countries. Moreover, 10% to 20% of patients who are diagnosed with breast cancer also have diabetes. Old age and being overweight are the primary risk factors for type 2 diabetes. These characteristics are likewise associated with an increased likelihood of acquiring breast cancer [2]. A study done with a sample size of 38,000 women revealed that 15% of them had diabetes, which heightened their vulnerability to breast cancer. Subsequent examination indicates that those with diabetes had a 20% higher likelihood of developing breast cancer compared to those without diabetes [3]. Currently, there is increasing focus on the exploration of natural substances that have the potential to reduce blood sugar levels and perhaps act as preventive measures against breast cancer.
Cannabis sativa L. subsp. (hemp) is in the cannabis family (cannabaceae). Hemp is a plant that originated in Central Asia and spread to East Asia, India, and Europe [4]. There have been past research reports studying the chemical composition of hemp. It has been found that hundreds of substances can be separately identified, and some of them have unique structures. Most of the substances found in hemp are in these groups: alkaloids, fatty acids, esters, quinones, flavonoids, stilbenoids, terpenoids, and cannabinoids [5]. The flavonoids can be extracted from hemp flowers, leaves, stems, and pollen. Compounds within the flavonoids group exhibit diverse pharmacological effects, such as inhibiting the production of prostaglandin E2 in cannaflavins A and B, as well as inhibiting rat lens aldose reductase through orientin and quercetin [6]. Stilbenoids are phenolic compounds that are commonly found in many plants. More than 10 types of stilbenoids have been reported to have been isolated from hemp and marijuana, and have the effect of inhibiting bacteria from substances in the stilbenoids group. In addition, compounds within this category exhibit anti-inflammatory properties, hinder cancer cell growth, protect brain cells from blood vessel lining cell death, and act as antioxidants [7]. Terpenoids, also known as isoprenoids, represent another extensive group of metabolites present in plants [8]. There are many terpenoids with interesting pharmacological effects that can be found in cannabis and hemp, especially in flowers. Cannabinoids can be classified into a number of substances that comprise the group. Meroterpenoids are a type of compound that forms when an olivetol-type polyketide (C12 or C9) reacts with a monoterpene unit. An example of a substance in the group of cannabinoids that has been isolated from important hemp plants and is believed to have psychotropic effects is Δ9-tetrahydrocannabinol (Δ9-THC), where THC works by binding to cannabinoid receptors (CB1 and CB2) in the brain.
The identification of cannabinoid receptors suggests that the body can produce its own internal compounds resembling THC, known as endogenous cannabinoids [9]. These are compounds within the cannabinoid group that the body can naturally produce, such as anandamide, along with similar substances reminiscent of opioids, including endorphins, enkephalins, and dynorphins. Besides the well-known substance Δ9-THC, approximately 100 other cannabinoids have been isolated from hemp, categorized into seven main groups: cannabigerol (CBG), cannabicyclol (CBL), cannabidiol (CBD), cannabielsoin (CBE), Δ9-THC, and cannabicoumaronone (CBCON) [10]. The flowers of hemp contain powerful amounts of THC and CBD and have several uses and research applications. However, there have been few phytochemical or biological studies on other parts of this plant. Therefore, this research focused on extracting and separating substances into enriched fractions containing active ingredients from various parts of hemp, including branches (CB), leaves (CL), roots (CR), and stems (CS). These substances have the potential to inhibit diabetes mellitus via α-glucosidase enzyme inhibition and breast cancer cells (MCF-7 and MDA-MB-231 cells).
MATERIALS AND METHODS
Chemicals and reagents
Methanol, hexane, dichloromethane ethyl acetate, ethanol, and acetone were purchased from (RCL Labscan, Bangkok). Ethanol, sodium carbonate, methanol, aluminum chloride, sodium hydroxide, sodium chloride, hydrochloric acid, phosphate buffer, Folin-Ciocalteu reagent, silica gel 7734, sephadex LH-20, thin-layer chromatography (TLC) silica gel 60, dimethyl sulfoxide (DMSO), maltose, and sucrose were purchased from Merck (Darmstadt, Germany). Trypsin, Dulbecco’s Modified Eagle Medium (DMEM) media, fetal bovine serum, 3-(4,5-dimethyl thiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT), annexin Annexin V-Fluorescein Isothiocyanate (V-FITC), propidium iodide (PI), rat intestinal acetone powder, quercetin, and gallic acid were purchased from Sigma-Aldrich (St. Louis, MO). Glucose-kit was purchased from Human (Magdeburg, Germany). Acarbose (Glucobay®, 100 mg) was purchased from Bayer Healthcare (Leverkusen, Germany).
Hemp preparation
Hemp (C. sativa L. subsp.) was obtained courtesy of the Highland Research and Development Institute (Public Organization). A voucher specimen (PCMU0023355) has been deposited at the Faculty of Pharmacy, Chiang Mai University (Chiang Mai, Thailand).
Extraction and fractionation of enriched fractions
Samples of branches (CB), leaves (CL), roots (CR), and stems (CS) were dried at 50°C until the moisture content was reduced to 6%, as determined by an infrared moisture analyzer (Kett, FD610). The dried samples were then ground into a fine powder using a blender to enhance the efficiency of the subsequent extraction process. For each sample, 500 g of the powdered material was weighed and subjected to maceration extraction using 3 l of 99.9% methanol for 7 days, repeated three times for each sample. The filtered solution was evaporated under reduced pressure using a rotary evaporator (RII, Buchi, Germany) to obtain methanol crude extracts of CB, CL, CR, and CS. Each methanol crude extract was suspended in a mixture of methanol (700 ml) and water (300 ml) and partitioned with 99.9% dichloromethane (100 ml) in a separatory funnel. After evaporation under reduced pressure, 70% methanol and dichloromethane extracts were obtained. Preliminary bioassay tests indicated that the dichloromethane extracts from CB, CL, CR, and CS were more effective in inhibiting the growth of MCF-7 and MDA-MB-23 cancer cells compared to the 70% methanol extracts. Therefore, the dichloromethane extracts were further fractionated to obtain enriched fractions using silica gel chromatography (60 G Merck column, 120 mm ID × 7 cm length). The separation was performed with a six-gradient system of EtOAc-n-hexane (0:1, 1:9, 2:8, 1:1, 1:8, and 1:0 v/v). The enriched fractions were monitored and combined using TLC to yield eight enriched fractions, including CB1 (50 mg) and CB2 (70 mg) from branches, CL3 (150 mg) and CL4 (120 mg) from leaves, CR5 (60 mg) and CR6 (20 mg) from roots, and CS7 (30 mg) and CS8 (65 mg) from stems.
Determination of phytochemical constituents
The previously described method was used to determine the total amounts of tetrahydrocannabinol (THC) and CBD [11]. The Folin-Ciocalteu method, which has been reported previously, was used to determine the total phenolic content [12]. The flavonoid content was determined using the aluminum chloride colorimetric technique [12]. The previously described method was used to determine the total terpenoid content [13]. Using bromocresol green (BCG) as a reagent to create a yellow-colored result, the total alkaloid content was determined [13].
α-Glucosidase inhibitory activities
The inhibitory activity of α-glucosidase against rat intestinal maltase and sucrase was determined following our previous investigation. Maltase and sucrase were obtained from a crude enzyme solution derived from rat intestinal acetone powder (Sigma, St. Louis). 30 ml of a 0.9% NaCl solution was used to homogenize one gram of rat intestinal acetone powder. Following a 30-minutes centrifugation at 12,000 rpm, the crude enzyme solution was aliquoted and examined. The isolated fraction was dissolved in 10 µl of DMSO at various concentrations (0.01, 0.05, 0.1, 0.5, and 1.0 mg/ml) and then mixed with 30 µl of 0.1 M phosphate buffer (pH = 6.9) and 20 µl of the substrate solution (10 mM maltose; 100 mM sucrose). Next, 20 µl of the crude enzyme solution was added and a glucose assay kit was used (SU-GLLQ2, human, 80 µl). Maltose was incubated in the reaction mixture for 10 minutes, and sucrose for 40 minutes at 37°C. After five minutes in a water bath at 100°C, the process was stopped. Using a SpectroStar Nano microplate reader, absorbance at 520 nm was used to quantify the enzymatic activity. The formula used to determine the percentage of inhibition was [(A0 – A1)/A0] × 100, where A0 and A1 represent the absorbance levels with and without the sample, respectively. A plot of the % inhibition versus sample concentration was used to get the IC50 value. As the positive control, acarbose was employed. Every experiment was run in triplicate [12].
Kinetic study of α-glucosidase inhibition
The α-glucosidase (rat intestinal maltase and sucrase) inhibitory effect was determined based on our previous report. A 0.1 M phosphate buffer (pH = 6.9, 30 µl) was mixed with increasing concentrations of the substrate solution (maltose: 2–10 mM, 20 µl; sucrose: 10–100 mM, 20 µl) and enriched fractions (1 mg/ml in DMSO, 10 µl) at various concentrations (0, 0.01, 0.1, 0.5, 1.5, 2.0, 2.5, and 3.0 mg/mL). The mixture included a crude enzyme solution (20 µl) and an 80 µl glucose assay kit. The mixture was incubated for 10 (for maltose) and 40 minutes (for sucrose) at 37°C at 520 nm, and then the activity of α-glucosidase was measured. Acarbose was employed as a positive control in the triplicate assay. A Lineweaver-Burk plot was used to determine the different forms of inhibition. The Ki and Ki? values were determined by creating secondary plots based on the Lineweaver-Burk plot’s slope and intercept in relation to the concentration of the active chemical [12].
Cell culture
MDA-MB-231 and MCF-7 breast cancer cells were obtained from the Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University. 10% fetal bovine serum was added to the DMEM medium to maintain the breast cancer cells. Until the culture achieved confluence, cells were stored at 37°C and in a 5% humidified environment. Cells were washed in Phosphate Buffered Saline (PBS) free of calcium and magnesium before cell collection. Trypsinization was carried out using 0.25% trypsin, and the cells were incubated for five minutes at 37°C. After that, cells were collected and washed before being spun down at 1,500 rpm for five minutes at 25°C in a refrigerated centrifuge.
Cell viability assay
The cells were distributed into wells at a concentration of 5 × 103 cells per well, using 100 µl of DMEM medium with 10% fetal bovine serum. The samples were placed in a controlled environment with a temperature of 37°C and a CO2 concentration of 5% for an incubation period of one night. The specimens were subjected to various treatments throughout the incubation process. The amounts of FBS and DMSO in all conditions were consistently 5% and 0.1%, respectively. The control solvent used was a solution containing 0.1% DMSO. At 24, 48, and 72 hours, a concentration of 5 mg/ml of 3-(4,5-dimethyl thiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) was introduced into each well, and then the plate was placed back in the incubator at 50°C for 3 hours to allow color development. The plates were analyzed at a wavelength of 550 nm using an EnSpire™ 2,300 multilabel plate reader manufactured by Perkin Elmer in the United States. The formula used to calculate the percentage of inhibition was (Atreatment/Acontrol cell) × 100, where Atreatment represents the absorbance of the treatment and Acontrol cell represents the absorbance of the control cell [14].
Apoptosis assay
1 × 105 cells were placed in each well of a 6-well plate. The cells were subjected to a sequence of treatments for a period of 24 hours after being incubated overnight. The cells were removed by trypsinization after being reconstituted in a binding buffer. 100 µl of cell suspension was produced and incubated at room temperature in the absence of light with 100 µl of Annexin V-FITC and PI. At the end of the incubation time, 400 l of binding buffer was added to each tube. Subsequently, the cells were analyzed using the Becton Dickinson Fluorescence Activated Cell Sorting (BD FACS) Calibur flow cytometer from Becton Dickinson, USA. The Annexin V-positive cells that had undergone apoptosis were counted and compared to the control group [15].
Statistical analysis
Each experiment was carried out in triplicate. Mean values are displayed with the ± standard deviation (SD). Using the GraphPad prism and one-way analysis of variance (ANOVA) software from IBMSPSS Statistics 22, significant results were identified using a p value of less than 0.05. All values are represented by *.
RESULTS AND DISCUSSIONS
Determination of phytochemical constituents
The most common substances found in C. sativa L. subsp. are alkaloids, fatty acids and esters, terpenoids, quinones, flavonoids, stilbenoids, and cannabinoids [5]. The total quantities of THC, CBD, phenolic, flavonoid, terpenoids, and alkaloid compounds were determined. The results are shown in Table 1. Each enriched fraction contained THC, CBD, phenolic, flavonoids, terpenoids, and alkaloid components. The majority of phytochemicals were detected in CL3 and CL4. CL3 had a concentration of 0.16% ± 0.01% w/w, 2.32% ± 0.41% w/w, 57.25 ± 0.41 µg/g dry mass, 60.24 ± 1.24 mg/g dry mass, 2,578.35 ± 1.56 mg/g dry mass, and 30.12 ± 1.10 mg/g dry mass, while CL4 had a concentration of 0.12% ± 0.01%w/w, 2.37% ± 0.70%w/w, 45.21±0.43 µg/g dry mass, 58.14 ± 0.31 mg/g dry mass, 1,987.78 ± 1.87 mg/g dry mass, and 29.87 ± 1.14 mg/g dry mass. Considering the different parts of the C. sativa L. subsp., a high amount of substances and different nutritional values were found. Meroterpenoids, the most abundant of which are THC and CBD, were found at up to 0.2% and 2%, respectively [16]. In addition, compounds in the flavonoids group were found. These are methylated isoprenoid cannabis compounds that can be separated from the flowers, leaves, branches, and pollen of C. sativa L. subsp. Cannflavins A and B [6].
Evaluation of α-Glucosidase inhibition
An additional strategy for regulating blood sugar levels involves investigating the inhibition of carbohydrate absorption in the intestinal tract, specifically by inhibiting the activity of α-glucosidase. This approach is among the most effective techniques used to reduce postprandial glucose levels [17]. Therefore, the purpose of this investigation was to determine whether a basic enzyme extract containing maltase and sucrase, with a mechanism of action similar to that in rats, could inhibit α-glucosidase derived from the small intestine of rats, thereby simulating the human body [18]. The enriched fraction obtained from CL3 leaves exhibited the highest ability to inhibit maltase and sucrase, as demonstrated by IC50 values of 3.36 ± 0.90 and 2.15 ± 0.29 mg/ml, respectively, which are comparable to the values of drugs. As shown in Table 2, acarbose is statistically significant at a confidence level of p < 0.05, followed by CL4. Studies on active substances from plants and herbs have revealed various groups of compounds with the potential to inhibit α-glucosidase although the effectiveness of these deterrents may vary. These compounds include up to 103 types of flavonoids, such as xanthones, flavanones, flavans, anthocyanins, and chalcones [19]. Studies have reported that THC and CBD are also effective in inhibiting α-glucosidase. At the molecular level, the enzyme is inhibited by binding to enzyme molecules. It has been found that THC binds to the enzyme with hydrogen bonds and Van der Waals interactions, while CBD binds to the enzyme only with Van der Waals interactions. Moreover, it has been observed that inhibition occurs with IC50 values of 3.0±0.37 and 5.5±0.37 µg/mL, respectively, which is better than acarbose (IC50 of 488.6 ± 10.23 µg/ml) [20].
Enzyme kinetic study
An investigation was carried out using Lineweaver-Burk plots to determine the mechanism by which this inhibition occurred [12]. The mechanism of inhibition was studied for CL3 since it was the most effective inhibitor of maltase and sucrase. CL3 demonstrated an order of parallel Lineweaver-Burk plots in relation to maltase (Fig. 1A) in that both the maximal velocity value (Vmax) and the Michaelis constant (Km) exhibited a decline. Therefore, the results of this study suggest that CL3 exhibited noncompetitive inhibition of maltase. The enzyme-substrate-inhibitor (ESI) complex dissociation constant (Ki?) was determined to be 0.77 mg/ml using the secondary plot relating the inhibitor concentration (X axis) and the Y intercept (Y axis). CL3, on the other hand, exhibited a pattern of linear trends that intersected the X axis; maintained the same value of Km; and reduced the value of Vmax (Fig. 1B). The result suggests that CL3 exhibited noncompetitive inhibition of sucrase. The enzyme-inhibitor (EI) complex exhibited a dissociation constant (Ki) of 0.40 mg/ml, which is demonstrated by the secondary plot showing inhibitor concentration (X axis) versus slope (Y axis). The dissociation constants for noncompetitive inhibition of the ESI (Ki?) and EI (Ki) complexes are shown in Table 3. This study delineates solely an enriched fraction, possibly arising from the substances within CL3 exhibiting synergistic effects that induce alterations in the enzyme’s conformation, or impede the active site’s ability to catalyze the chemical reaction. Further inquiry is imperative to isolate and elucidate the structure of the substances present in CL3, and to delve deeper into the inhibition mechanism.
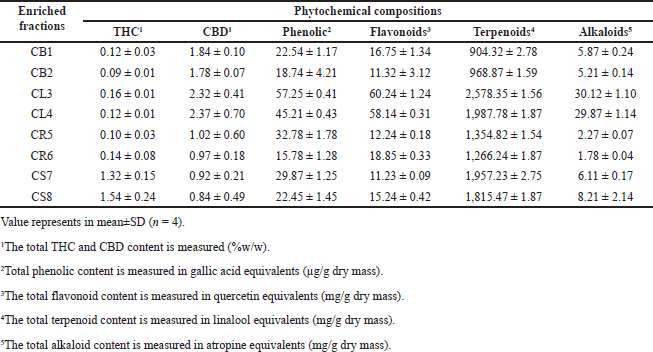 | Table 1. Phytochemical compositions of enriched fractions from C. sativa L. subsp. [Click here to view] |
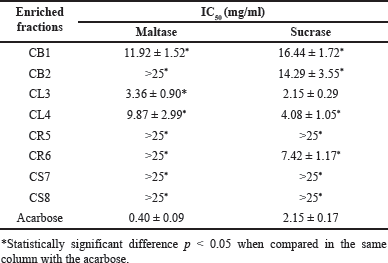 | Table 2. α-Glucosidase inhibitory efficiency of enriched fractions from C. sativa L. subsp. [Click here to view] |
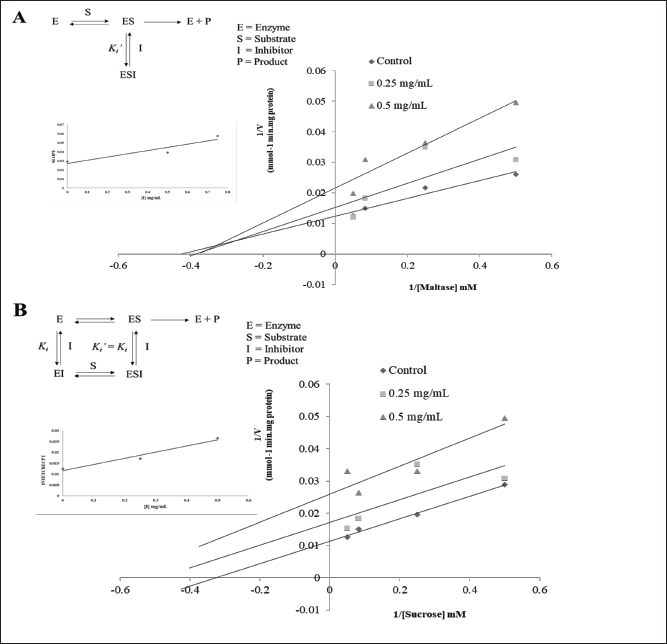 | Figure 1. Lineweaver-Burk, secondary plots, and putative inhibitory mechanisms of the enriched fraction (CL3) against maltase (A) and sucrase (B). [Click here to view] |
Inhibition of enriched fractions against breast cancer cells
The examination of the survival of two types of breast cancer cells, namely MCF-7 cells that respond to estrogen and progesterone, and MDA-MB-23 cells that do not respond to estrogen and progesterone, was conducted. These cells were administered with enriched fractions at concentrations of 25, 50, 100, and 200 ppm, and were incubated for 24 hours. As a result, there was a decrease in the percentage of survival. The reduction exhibited variability depending on the dose of the extract, with MDA-MB-23 cells demonstrating a more favorable reaction compared to MCF-7 cells, as seen in Figure 2. Apoptosis in cultured breast cancer cells (MCF-7 and MDA-MB-231) was evaluated using a flow cytometer. The inducing impact was tested by combining Annexin V-Fluorescein with phosphatidyl serine. Apoptosis occurred. The experiment was performed on both types of cultured breast cancer cells, with analysis of cultured breast cancer cells for changes in shape and structure. Monitoring was done to determine the precise stage at which the extract induced apoptosis in the test cancer cells by employing fluorescent labeling. Afterward, the mortality of mature breast cancer cells was assessed at doses of 125, 250, 500, and 1,000 µg/ml. To assess the capacity of the enriched fractions to trigger programmed cell death (apoptosis) in cultured breast cancer cells (MDA-MB-231 and MCF-7), we employed PI and Annexin staining. The level of apoptosis in cultured MDA-MB-231 and MCF-7 breast cancer cells was measured using V-FITC. The test results show that all extracts, when given at a concentration of 1,000 μg/ml, had the ability to trigger apoptosis in both kinds of cultured breast cancer cells. The study shows that the CS7 and CS8 extracts were much more efficient than DMSO in causing programmed cell death in cultured breast cancer cells, with a confidence level of p < 0.05. As seen in Figure 3, the stronger responses of MDA-MB-231 breast cancer cells to the extract compared with MCF-7 breast cancer cells may be due to the former being a triple-negative breast cancer cell type (TNBC), characterized by the absence of three receptors: estrogen receptor (ER-), progesterone receptor, and HER2 receptor. MCF-7 breast cancer cells express estrogen receptors (ER+) and progesterone receptors (PR+), but do not create the human epidermal growth factor receptor-2 (Her2-) protein [21]. The effectiveness of the concentrated fraction derived from the roots and stems in suppressing cancer cells in various forms is linked to the cannabinoids THC and CBD. Research findings suggest that a concentration as low as 14 µM of THC can effectively impede the growth of breast cancer cells [22]. Furthermore, studies have shown that THC can hinder the activation of ERs produced by estradiol in MCF-7 breast cancer cells. Other research has shown that THC demonstrates resistance to the growth of certain types of cancer cells. It operates by inhibiting angiogenesis and reducing metastasis in certain types of cancer by triggering programmed cell death (apoptosis) and preventing the growth of cancer cells. Other possible methods include stopping the cell cycle and limiting the formation of new blood vessels (antiangiogenic effects) [23].
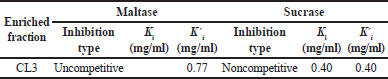 | Table 3. Kinetic parameters of the enriched fraction (CL3) against α-glucosidase. [Click here to view] |
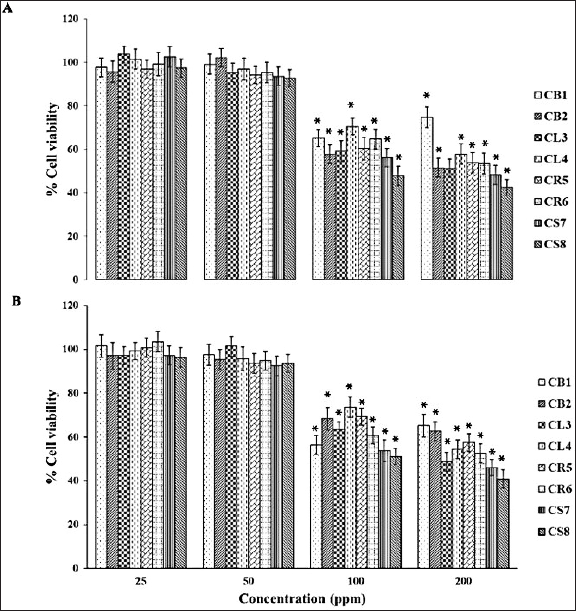 | Figure 2. (A) MCF-7 and (B) MDA-MB-231 cells after treatment with an enriched fraction for 24 hours. Data are presented as mean ± SD (n = 3 independent experiments). One-way ANOVA analysis with a *p value < 0.05 is considered significant, compared to the DMSO. [Click here to view] |
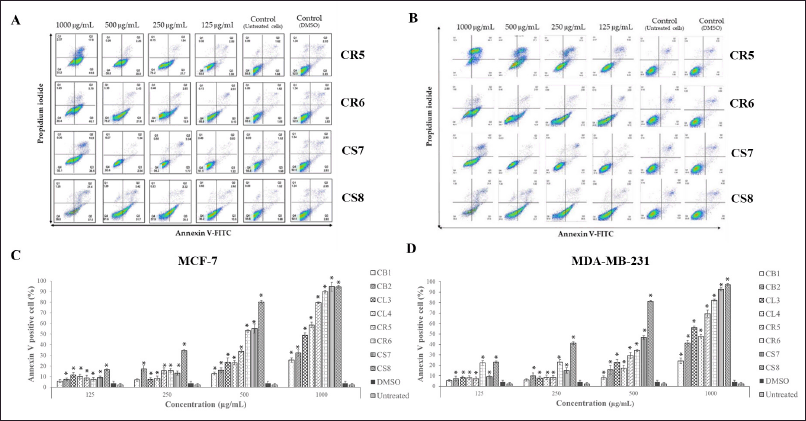 | Figure 3. The MCF-7 and MDA-MB-231 Annexin V-positive cells had enriched fractions. (A) and (B) are the results from the flow cytometry after 24 hours. (C) and (D) population of apoptotic cells. The results are presented as mean ± SD (n = 3 independent experiments). One-way ANOVA analysis with a *p value < 0.05 was considered significant compared to the DMSO. [Click here to view] |
This study reveals an abundance of phytochemicals in enriched fractions produced from different components of hemp. Compounds that efficiently inhibit both maltase and sucrase of glucosidase from rat intestines, as well as breast cancer cells, could originate either as major compounds or through synergistic actions. Hence, it is important to conduct further study of the structure of a pure chemical to confirm its composition. Furthermore, the research should further explore complex information, ultimately leading to the development of pharmaceuticals designed for medical treatment for individuals diagnosed with diabetes and breast cancer.
CONCLUSION
The hemp (C. sativa L. subsp.) extract was found to contain THC, CBD, phenolics, flavonoids, terpenoids, and alkaloids. Their ability to inhibit α-glucosidase and their potential in combating breast cancer were investigated. The presence of phytochemicals in these plant extracts might contribute to the inhibitory effect observed, particularly in the case of CL3, which exhibited higher activity against α-glucosidase. Moreover, it can be inferred that the concentrated extract of C. sativa L. subsp. induced cytotoxicity and apoptosis in MCF-7 and MDA-MB-231 cells under laboratory conditions. Therefore, C. sativa L. subsp. indicates potential as an effective therapy for breast cancer in the field of alternative and complementary medicine. Further investigation will be necessary to isolate and identify the specific chemical components of C. sativa L. subsp. that indicate acute and chronic toxicity, and which could be responsible for these effects.
ACKNOWLEDGMENTS
The financial support for the research was provided by the Thai traditional medical knowledge fund, which is administered by the Department of Thai Traditional and Alternative Medicine under the Ministry of Public Health. Additionally, the work has been supported by the fundamental fund (FF 2023) from Rajamanjala University of Technology Srivijaya (Nakhon Si Thammarat Campus).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Zhu B, Qu S. The relationship between diabetes mellitus and cancers and its underlying mechanisms. Front Endocrinol. 2022;13:800995. CrossRef
2. Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes mellitus and breast cancer. Lancet Oncol. 2005;6(2):103–11. CrossRef
3. Jordt N, Kjærgaard KA, Thomsen RW, Borgquist S, Cronin-Fenton D. Breast cancer and incidence of type 2 diabetes mellitus: a systematic review and meta-analysis. Breast Cancer Res Treat. 2023;202(1):11–22. CrossRef
4. Chaachouaya N, Azerouala A, Bencharkia B, Douirab A, Zidaneb L. Cannabis sativa L.: a review on traditional uses, botany, phytochemistry, and pharmacological aspects. Trad Integrative Med. 2023;8(1):97–116. CrossRef
5. Malabadi RB, Kolkar KP, Chalannavar RK. Cannabis sativa: ethnobotany and phytochemistry. Int J Innov Sci Res Rev. 2023;5(2):3990–8.
6. Bautista JL, Yu S, Tian L. Flavonoids in cannabis sativa: biosynthesis, bioactivities, and biotechnology. ACS Omega. 2021;6(8):5119–23. CrossRef
7. Kundan M, Gani U, Fayaz M, Angmo T, Kesari R, Rahul VP, et al. Two R2R3-MYB transcription factors, CsMYB33 and CsMYB78 are involved in the regulation of anthocyanin biosynthesis in Cannabis sativa L. Ind Crops Prod. 2022;188:115546. CrossRef
8. Watanakul P, Phattaralerphong J, Pitija K, Nakmee PS. Terpenoid profiling of Thai strain cannabis leaves (Cannabis sativa L. subsp. sativa) by Headspace (HS) couple with GC/MS. JoP. 2024;4(1):3–11.
9. Gary R, Trina H, Joshua AH, Joshua E, Brian H, Alexandros M. Cannabis sativa: an overview. Nutraceuticals. 2021;2:603–624. CrossRef
10. van Klinken BJ-W, Stewart ML, Kalgaonkar S, Chae L. Health-promoting opportunities of hemp hull: the potential of bioactive compounds. J Diet Suppl. 2024;1–15. CrossRef
11. Grafinger KE, Krönert S, Broillet A, Weinmann W. Cannabidiol and tetrahydrocannabinol concentrations in commercially available CBD E-liquids in Switzerland. Forensic Sci Int. 2020;310:110261. CrossRef
12. Thanakorn D, Pennapa R, Suntaree A, Chalermpong S, Mary AL. Effects of in vitro simulated gastrointestinal digestion on the α-glucosidase inhibitory activities of Thai folk remedies: synergistic effects with Acarbose. Trop J Nat Prod Res. 2023;7(12):5548–57. CrossRef
13. Rachpirom M, Ovatlarnporn C, Thengyai S, Sontimuang C, Puttarak P. Dipeptidyl peptidase-IV (DPP-IV) inhibitory activity, antioxidant property and phytochemical composition studies of herbal constituents of Thai folk anti-diabetes remedy. Walailak J Sci Technol. 2016;13(10):803–14.
14. Rangsinth P, Sharika R, Pattarachotanant N, Duangjan C, Wongwan C, Sillapachaiyaporn C, et al. Potential beneficial effects and pharmacological properties of ergosterol, a common bioactive compound in edible mushrooms. Foods. 2023;12(13):2529. CrossRef
15. Nilkhet S. Anti-tumor activities of auricularia polytricha extracts in hepatocellular carcinoma cell line (HepG2) and breast cancer cell lines (MCF-7 AND MDA-MB-231). Bangkok, Thailand: Chulalongkorn University; 2019.
16. Nagy DU, Cianfaglione K, Maggi F, Sut S, Dall’Acqua S. Chemical characterization of leaves, male and female flowers from spontaneous Cannabis (Cannabis sativa L.) growing in Hungary. Chem Biodiv. 2019;16(3):e1800562. CrossRef
17. Damsud T, Tedphum T, Sukkrong C. Alternative protein-enriched food matrices with concentrated ripe mango (Mangifera indica L.) bioflavonoids for topical applications. Mater Today. 2021;43:2525–30. CrossRef
18. Doungwichitrkul T, Damsud T, Phuwapraisirisan P. α-glucosidase inhibitors from cold-pressed black sesame (Sesamum indicum) meal: characterization of new furofuran lignans, kinetic study, and in vitro gastrointestinal digestion. J Agric Food Chem. 2024;72(2):1044–54. CrossRef
19. Yin Z, Zhang W, Feng F, Zhang Y, Kang W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci Hum Wellness. 2014;3(3–4):136–74. CrossRef
20. Suttithumsatid W, Shah MA, Bibi S, Panichayupakaranant P. α-Glucosidase inhibitory activity of cannabidiol, tetrahydrocannabinol and standardized cannabinoid extracts from Cannabis sativa. Curr Res Food Sci. 2022;5:1091–7. CrossRef
21. Jia M, Li Y, Xin H-L, Hou T-T, Zhang N-D, Xu H-T, et al. Estrogenic activity of osthole and imperatorin in MCF-7 cells and their osteoblastic effects in Saos-2 cells. Chin J Nat Med. 2016;14(6):413–20. CrossRef
22. Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318(3):1375–87. CrossRef
23. Hermanson DJ, Marnett LJ. Cannabinoids, endocannabinoids, and cancer. Cancer Metastasis Rev. 2011;30(3–4):599–612. CrossRef