INTRODUCTION
The bitter taste of medications dramatically restricts the development of oral formulations, hinders clinical applicability, and decreases patient compliance, especially for pediatric and geriatric applications. Based on a survey by the American Association of Pediatricians, the unpleasant taste was reported as one of the most significant barriers to pediatric compliance to treatment for acute (a rate of 90.8%) and chronic (a rate of 83.9%) illnesses in Pediatrics [1]. The bitter taste of drugs can be masked by either reducing the bitter ingredient solubility in saliva or reducing the ability of these ingredients to interact with the taste buds [2]. Both the choice of the taste masking method and the amount of excipients used depend on the severity of the bitter taste, the dose of active pharmaceuticals, the shape and particle size distribution of a drug, the desired dosage form, drug solubility, and the ionic characteristics of the drug [2].
Polymeric microencapsulation is intended for various pharmaceutical applications, including drug protection, targeting, controlling release, avoiding gastric irritation, and taste masking [3,4]. Mechanical microencapsulation was suggested to be the most preferable method for drug taste-masking compared to complexation and hot melting [5]. Microencapsulation is a process by which micron-sized particles or droplets of a liquid or solid drug are coated with a film of polymeric materials that control the dissolution of the loaded drug. The most commonly used pH-sensitive polymer is Eudragit®. This polymer is widely used for pharmaceutical applications such as taste masking, enteric film coating, and controlled drug release [6]. Eudragit E is a polyacrylate cationic polymer based on dimethyl aminoethyl methacrylate, methyl methacrylate, and butyl methacrylate. Eudragit E is soluble in the acidic gastric fluid (pH 1–5) but forms permeable, swellable, and insoluble films at pH ≥ 5. Thus, it can be used as a coating polymer in microencapsulation to delay or prevent the drug release in saliva [7]. Eudragit EPO is known to be used in pharmaceutical formulations of several solid dosage forms [6], and it was reported to be used for taste masking of clindamycin prepared as oral disintegrated tablets [8].
On the other hand, Eudragit S is soluble at pH > 7 because of more esterified groups than carboxylic groups. Thus, it is usually investigated for use in colon-targeting applications [6]. Eudragit S100 microspheres were reported to successfully mask the bitter taste of chlorpheniramine using the double emulsion solvent diffusion technique [9].
Several methods can be employed for microencapsulation of the drug inside the polymer. Some conventional methods, like solvent evaporation (SE), rely on newer technologies like supercritical fluid technology. Solvent evaporation or extraction is performed by dissolving the drug and polymer in a suitable organic solvent, emulsifying them with a continuous phase, and finally evaporating the solvent to encapsulate the drug inside the polymer [10]. When using the polymeric microencapsulation method to mask the bitter taste, the coating polymer has to be insoluble at the salivary pH (pH 6–8).
Supercritical fluid technology has been efficiently used for the bitter taste masking of drugs. The process employed in supercritical fluid technology relies on the criteria of the drug and polymer used. It can be employed as a solvent or as an anti-solvent technique. It can be used for drug loading or microencapsulation and to dry the sample and remove organic solvent residues from the prepared samples[11,12].
For the loading of drugs into polymeric materials, SFT-CO2-assisted impregnation is a promising alternative to conventional methods that usually involve the use of suitable solvents. In the SFT-CO2 method, the solvent is replaced by CO2 in its supercritical phase, which serves multiple functions simultaneously. First, it dissolves the drug and transfers it to the polymer surface. Second, it acts as a diffusion enhancer by temporarily modifying some polymer properties. Finally, it induces drug precipitation because of its desaturation in CO2 when the system is depressurized [13–16]. Co-solvents can be added to adjust polarity and enhancement of the supercritical CO2 impregnations when required.
Azithromycin has broad-spectrum antibacterial activity against various respiratory, skin, urogenital, and other infections [17]. Although practically water-insoluble, Azithromycin is freely soluble in organic solvents (e.g., anhydrous ethanol and methylene chloride) [18]. Different dosage forms of Azithromycin are available on the market, such as capsules, film-coated tablets, granules for suspension, and microspheres in oral extended-release formulations [18,19]. Nevertheless, the extreme bitterness of this drug is considered one of its limitations that should be overcome [20]. The most widely used method to mask the taste of Azithromycin is tablet film coating. Unfortunately, this method cannot be used in pediatrics due to difficulty swallowing tablets. Therefore, a taste-masked Azithromycin suspension for oral administration is commercially available for pediatrics. However, this suspension suffered from physical instability [21]. Several attempts were reported to develop Azithromycin taste-masked formulations, including titanium nanohybrid preparations [22], Eudragit L100 microspheres produced by SE and spray drying methods [21], microspherical particles of ethylcellulose combined with ethyl acetate prepared using solvent diffusion technique [23], Azithromycin incorporated pellets that composted of Eudragit RL and Eudragit L30D were also reported to mask the taste of Azithromycin [24], Azithromycin complexation with ion exchange resin was also investigated for masking its bitter taste [25], we have recently reported a polymeric foam that was formulated using of supercritical fluid technology in a single-step, the produced foaming matrix can mask the bitter taste of Azithromycin without the need of an organic solvent use [26].
In this work, we prepare blank polymeric microparticles using the SE method and investigate the loading of Azithromycin drug into these particles employing supercritical fluid technology as an attempt to increase loading efficiency and minimize drug loss was performed using supercritical fluid technology. Besides that, this allows these microparticles to incorporate different active ingredients due to the diffusional and solvation properties of the supercritical fluid. This study primarily aimed to compare the widely studied nonaqueous emulsification SE method with the supercritical fluid technology method for drug loading into Eudragit polymeric microparticles.
MATERIALS AND METHODS
Material
Azithromycin dihydrate was kindly supplied by Hikma Company (Amman, Jordan). Eudragit Epo and Eudragit S100 were purchased from Röhm GmbH (Germany). Liquid paraffin was purchased from AZ Chem (India). Potassium dihydrogen phosphate was obtained from AZ Chem for Chemicals (Thunder Bay, ON, Canada). Sodium chloride and calcium chloride anhydrous were supplied by BBC chemicals for the lab (Holland). Sodium hydroxide was provided by Scharlau Chemie (Spain). Carbon dioxide (CO2) was supplied by the Jordanian Gas Company (Amman, Jordan). Water high-performance liquid chromatography (HPLC) grade was obtained from LabChem (USA). All of these substances were used in their form provided without any alteration.
Preparation of Azithromycin microparticles by SE method
To prepare the microparticles by emulsification and SE method (SE formulation), 2 g of different weight ratios (Table 1) of Azithromycin and polymer were dissolved in 7 ml acetone. Also, 0.35 g of magnesium stearate were dissolved in 3 ml acetone and mixed with the drug and polymer dispersion. This acetone dispersion was emulsified into liquid paraffin (in a ratio of 1 to 10 of acetone to oil) at a fixed room temperature. The system was mixed at 700 rpm for one hour. For the first 20 minutes, the container was covered with parafilm. Then, the cover was removed to allow SE. The microparticles were then filtered and washed with n-hexane. Finally, the microspheres were permitted to dry overnight at ambient temperature.
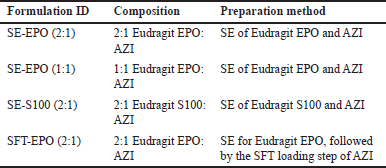 | Table 1. The component of the prepared Azithromycin (AZI) formulations prepared by SE (SE, into Eudragit EPO or Eudragit S100) or SE followed by supercritical fluid technology (SFT, using Eudragit EPO) for AZI loading. [Click here to view] |
Preparation of Azithromycin microparticles by supercritical fluid method (SFT-CO2)
The formulation preparation by supercritical fluid technology (SFT formulation) was initiated by preparing drug-free microparticles and loading the drug using the SFT inside the prepared microparticles. The working supercritical conditions were just above the carbon dioxide transitions to the supercritical phase at critical points of 7.38 MPa and 31.1°C [27].
The sample was prepared in two steps: first, the microparticles were papered using the SE method with a procedure similar to the SE formulation without the addition of Azithromycin drug. The second step was loading Azithromycin inside the prepared microparticles as follows: the prepared microparticles were transferred to a preheated 200 ml cylindrical vessel made of stainless steel (Fig. 1). The CO2 was purged constantly from the top of the vessel at a 6 kg/hour rate. The pressure and temperature were controlled and maintained at 10 MPa and 40°C, respectively, for two hours. The temperature was controlled using a heating jacket, and the pressure was kept constant using a back pressure regulator. The second cylindrical vessel, known as the separator, receives CO2 and maintains CO2 above its critical pressure and temperature, it is kept at 6 MPa and 40°C, respectively. After that, CO2 is recycled into the high-pressure vessel, and as soon as the sample is incubated there, this supercritical fluid (SCF) cycle is repeated. At the end of the experiment, the SCF technology vessel was depressurized gradually to reach atmospheric pressure, and the collected samples (SFT formulation) were evacuated and stored in dry conditions at room temperature.
The supercritical method is described in depth in our earlier publication [28].
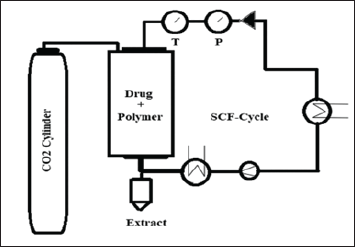 | Figure 1. Supercritical Fluid Technology (SFT) unit was designed by Eurotechnica®. [Click here to view] |
Characterization of Azithromycin microparticles
The prepared formulations, raw drugs, and their physical mixtures were evaluated using Fourier transform infrared (FTIR), PXRD, DSC, in vitro dissolution, and scanning electron microscopy (SEM) studies as described below.
FTIR spectroscopy
Sample particles were ground and mixed with potassium bromide powder using a mortar and pestle until well dispersed. The FTIR analysis was done at a resolution of 0.04 cm−1 over a 4750–250 cm−1 frequency range.
PXRD analysis
The PXRD analysis used an X-ray powder diffractometer with cobalt radiation (40 Kilovolts (KV), 30 mass attenuation coefficient (MA). Scanning was performed over a 2-theta range of 5–70.
DSC analysis
DSC thermograms of samples (5 mg each) were generated using a Shimadzu DSC-50. Samples were heated under nitrogen in sealed aluminum pans at 10°C/minutes within 30–300°C. The samples’ DSC thermograms were compared to an empty sealed aluminum pan.
In vitro drug dissolution studies
The exposure of the mouth to the release of Azithromycin was evaluated by testing Azithromycin (100 mg Azithromycin or equivalent from formulation) dissolution (in triplicate) in 50 ml of simulated salivary fluid (SSF, pH 7.4) over 3 minutes at 37°C ± 0.5°C and 50 rpm. SSF is made by dissolving sodium chloride (40 millimolar (mM)), potassium dihydrogen phosphate (12 mM), and calcium chloride (1.5 mM) in demineralized water up to 1 l [29]. The addition of sodium hydroxide resulted in a final pH of 7.4. Samples (2 ml each) were collected at 30, 60, 90, 120, 150, 180, and 300 seconds. The last sample was collected at 300 seconds (5 minutes) to evaluate the effect of longer residence time in the mouth. A nylon syringe filter (0.22 µm) was used to filter samples immediately before being assayed (at 210 nm) for Azithromycin. Filtered samples (100 µl) were injected into the HPLC [30].
The HPLC (Merck Hitachi) system consisted of an L-7200 autosampler, L-7150 pump, L-7455 diode array detector, L-7612 solvent degasser, and D-7000 interface. The mobile phase comprised phosphate buffer solution (pH 7.5 ± 0.05) and methanol (80:2, v/v). The isocratic separation was done at a 1.2 ml/min flow rate, where Azithromycin had a retention time of 8 minutes [30].
SEM
The Jeol-JSM-5300 SEM, Tokyo, Japan, was used to evaluate the best formulation (SE-EPO (2:1)) morphological properties at a magnification power of 2400x.
In vivo evaluation of taste masking
The protocol for the in vivo experiments used herein was approved by the Institutional Review Board of King Abdullah University Hospital (Irbid, Jordan) (Approval No. 15/147/2022, date: 24/2/2022). Ten healthy volunteers (five females and five males) participated in this study. In brief, the study protocol was explained to the volunteers, and each volunteer signed the required consent form. During the experiment, each volunteer received either 50 mg of raw Azithromycin or the best formulation (i.e., SE-EPO (2:1)) containing an equivalent Azithromycin dose. The volunteer was instructed to keep it in his/her mouth for 60 seconds. The degree of bitterness was recorded on a scale from 0 to 3 (0: no bitter taste; 1: slightly bitter; 2: moderately bitter; and 3: highly bitter). The bitterness assessment for each volunteer was performed after 20, 20, and 30 seconds. The volunteers were instructed to spit out the samples immediately following the evaluation.
RESULTS AND DISCUSSION
The resulting encapsulation efficiencies of the prepared formulations were 52.0 ± 0.03, 91.2 ± 6.2, 68.8 ± 1.8, and 64.9 ± 1.6 for SE-EPO (2:1), SFT-EPO (2:1), and SE-S100 (2:1), respectively. Using supercritical technology to load drugs (SFT-EPO (2:1)) resulted in higher encapsulation efficiency and lower drug loss compared to formulations (SE-EPO (2:1) and SE-S100 (2:1)) where drug-loading was done using the SE method. The lower encapsulation of Azithromycin when loaded using the SE method can be attributed to drug partitioning into the solvent before microparticle solidification [31]. The differences in encapsulation efficiency between Eudragit EPO and Eudragit S100 might be attributed to differences in the chemical functional groups of each polymer [31].
FTIR SPECTROSCOPY
The FTIR spectrum of raw Azithromycin dihydrate (Fig. 2-A) shows the characteristic peaks of Azithromycin at 1,049 cm−1 (C–O–C symmetrical stretching), 1166 cm−1 (C–O–C asymmetrical stretching), and 1720 cm−1 (C = O stretch). Similar peaks were reported in the literature [32]. No significant changes in the FTIR peaks (Fig. 2-B) were induced upon loading Azithromycin into Eudragit EPO polymer using the two methods (SE-EPO (2:1) and SFT-EPO (2:1)). Indicating the compatibility of the drug with the used ingredients without the alteration of Azithromycin therapeutic efficacy. The FTIR spectrum of SE-S100 (2:1) shows a deviation in the IR peaks (Fig. 2-C), which can be due to Azithromycin-polymer interaction. Similar intermolecular interactions between the Azithromycin amine and the Eudragit L100 carbonyl group were reported before [21].
PXRD analysis
The prominent characteristic peak of Azithromycin was found at two theta of 9.90°, in agreement with the characteristic peak of crystalline Azithromycin dihydrate [33]. In the current work, Azithromycin was amorphous (Fig. 3) in the formulation prepared using the SE method (SE-EPO (2:1)). Therefore, it can be deduced that the drug was successfully loaded inside the Eudragit EPO polymer. In contrast, the formulation where Azithromycin was loaded by SFT (SFT-EPO (2:1)) showed a decrease in the crystallinity of Azithromycin. The presence of sharp crystalline peaks in the supercritical sample can indicate lower incorporation of the drug inside the polymeric microparticles (Fig. 3); this can be explained by the drug precipitation on the surface of the polymer when supercritical impregnation used for drug loading [34–36].
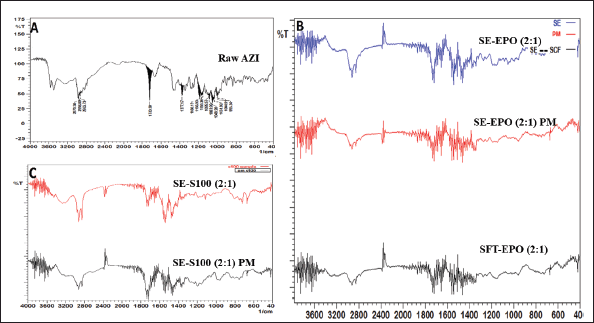 | Figure 2. FTIR spectrum of (A) Raw Azithromycin (AZI) drug; (B) Azithromycin-containing Eudragit EPO (polymer: drug ratio of 2:1) where AZI was loaded using SE method (SE-EPO (2:1)) or supercritical fluid technology (SFT-EPO (2:1)) as compared to their corresponding physical mixture (SE-EPO (2:1) PM); and (C) Azithromycin-containing Eudragit S100 (polymer: drug ratio of 2:1) where AZI was loaded using SE (SE-S100 (2:1)) as compared to the corresponding physical mixture (SE-S100 (2:1) PM). [Click here to view] |
It can be concluded that the drug loading methodology can have an impact on drug localization, as the use of the SE method resulted in the incorporation of the drug inside the polymeric matrix; meanwhile, the use of the SFT-CO2 method resulted in drug precipitation on the surface of the polymer as suggested by the PXRD results.
DSC analysis
Different products containing Azithromycin on the market exhibited different thermal behaviors ranging from single to multiple (two) DSC endotherms as compared to a single DSC endotherm for the reference standard as reported by the United States Pharmacopoeia (USP) [37]. Figure 4-D shows the endothermic peak of Azithromycin at 102°C as recorded in the current work, which includes multiple endotherms. This DSC endotherm (Fig. 4-D) might correspond to the dehydration or recrystallization steps involving the fusion and recrystallization of the anhydrous phase into a new crystalline lattice for peritectic transition [37]. However, the recrystallization exotherm was not detected between the two endotherms.
The DSC thermograms of SE-EPO (2:1) suggested the inclusion of Azithromycin inside the Eudragit EPO polymer (Fig. 4-A). Meanwhile, a sharp endothermic peak of Azithromycin was still observed (Fig. 4-C) in the DSC of SFT-EPO (2:1). Thus, it can be concluded that Azithromycin is loaded near the microparticle’s surface or its dispersion in the crystalline form which agrees with PXRD results [38].
In vitro dissolution studies
The in vitro dissolution studies in SSF were pursued to evaluate the taste masking efficiency of the prepared formulations (SE-EPO (2:1) and SFT-EPO (2:1)) as compared to raw Azithromycin and a commercially available reference product (Fig. 5). Both SE-EPO (2:1) and SFT-EPO (2:1) decreased Azithromycin release in SSF compared to raw Azithromycin and a commercially available reference product. The sample prepared using the SE method (SE-EPO (2:1)) was associated with the minimum percent of cumulative drug release in SSF. Therefore, SE-EPO (2:1) can be considered the most promising formulation for Azithromycin bitter taste masking. Similar observations were reported in the literature where the minimal release of Azithromycin in SSF (i.e., maximal taste masking efficacy) was from using formulations (made from Eudragit S100 and Eudragit L100) as prepared using the SE technique [39]. The faster release rate of Azithromycin from SFT-EPO compared to SE-EPO can be explained by the existence of the drug near the surface of polymeric microparticles, as suggested by the DSC and XRD results.
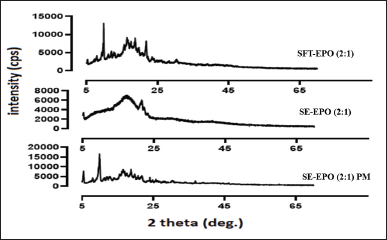 | Figure 3. PXRD pattern of Azithromycin loaded using SE method (SE-EPO (2:1)) or supercritical fluid technology (SFT-EPO (2:1)) as compared to their corresponding physical mixture (SE-EPO (2:1) PM). [Click here to view] |
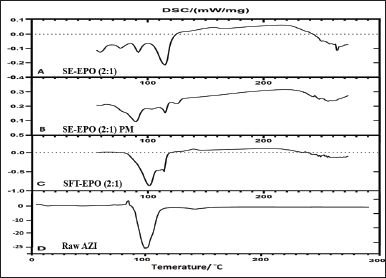 | Figure 4. DSC thermograms of (A) Azithromycin (AZI)-containing Eudragit EPO (polymer: drug ratio of 2:1) where AZI was loaded using SE method (SE-EPO (2:1)); (B) Corresponding physical mixture (SE-EPO (2:1) PM); (C) Azithromycin-containing Eudragit EPO (polymer: drug ratio of 2:1) where AZI was loaded using supercritical fluid technology (SFT-EPO (2:1)); and D- Raw AZI. [Click here to view] |
When comparing the two studied methods for drug loading, it can be concluded that the drug loading methodology can have an impact on drug release properties, as the use of the SFT-CO2 method is associated with a faster drug release rate from the prepared formulation.
Morphological properties
The SEM image (Fig. 6) was taken for the best formulation (SE-EPO (2:1)) based on the results of PXRD (Fig. 3) and relevant in vitro dissolution studies (Fig. 5). The sample prepared by SE (SE-EPO (2:1)) showed superior properties in terms of precipitation of the Azithromycin in the amorphous form as indicated by the efficient microencapsulation and efficiency in decreasing the in vitro release of the drug in SSF. The SEM image (Fig. 6) of SE-EPO (2:1) shows spherical microparticles (78.3 µm) with a relatively rough surface.
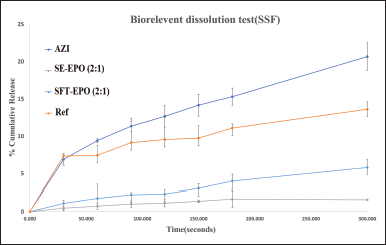 | Figure 5. In vitro dissolution results in SSF (pH 7.4, 37 ± 0.5°C, 50 rpm) of raw Azithromycin (AZI); Azithromycin-containing Eudragit EPO (polymer: drug ratio of 2:1) where AZI was loaded using SE method (SE-EPO (2:1)) or supercritical fluid technology (SFT-EPO (2:1)) as compared to a reference commercial preparation available in the Jordanian market(Zithromax®200mg/5ml, Pfizer, US) (Ref). [Click here to view] |
In vivo taste masking evaluation
The in vivo taste masking test was performed for the most successful formulation (SE-EPO (2:1)) as prepared by SE. The success of the taste masking ability of the developed microparticles was evaluated in vivo in ten healthy volunteers in a two-period study as compared to raw Azithromycin (Table 2). Most volunteers reported an improved taste masking capacity over 60 seconds for the prepared microparticles compared to the raw Azithromycin. These in vivo results of taste-masking ability can be directly correlated with the decrease in the in vitro drug release in SSF (Section “In vitro dissolution studies,” Fig. 5).
Previous studies investigating Eudragit L100 microparticles prepared using SE indicated a promising taste masking efficacy. The bitter taste masking of Azithromycin can be explained by the intermolecular interaction between the amine group of Azithromycin and the carbonyl group in the carrier [21]. In the current work, the cationic Eudragit EPO polymer was used for the molecular dispersion of Azithromycin within the polymeric matrix. Therefore, Azithromycin’s access to taste buds was hindered, and thus, the Azithromycin’s bitter taste was masked; this was confirmed by the in vivo taste-making study and the decreased Azithromycin release in vitro in SSF by the cationic polymer Eudragit EPO-based formulation as prepared and loaded with drug using the SE technique (SE-EPO (2:1)).
From the study results, it can be concluded that the compared drug loading methodologies can impact drug localization, encapsulation efficiency, and release properties. The selection of the suitable method should be directly linked to the intended application. In this study, the SE method resulted in slower release rates in a salivary medium, confirmed by the in vivo taste-making evaluation.
 | Figure 6. SEM image (magnification power = 2400×) of Azithromycin (AZI)-containing Eudragit EPO (polymer: drug ratio of 2:1) where AZI was loaded using SE method (SE-EPO (2:1). [Click here to view] |
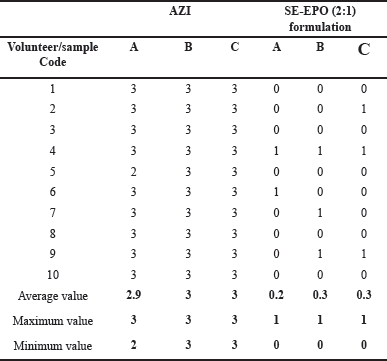 | Table 2. Taste panel scores for the in vivo clinical taste study of raw Azithromycin (AZI) as compared to the best formulation prepared herein (i.e., SE-EPO (2:1)). SE-EPO (2:1) was prepared and loaded with AZI in Eudragit EPO (polymer: drug ratio of 2:1) using the SE method. Each subject was evaluated at three time points: (A) 10 seconds, (B) 30 seconds, and (C) 60 seconds. [Click here to view] |
CONCLUSION
The bitter taste masking of Azithromycin dehydrate was successfully applied in the current work using a nonaqueous emulsion SE method. Using the Eudragit EPO polymer, the Azithromycin’s in vitro release was decreased, and the crystalline nature was lost. The FTIR results indicated no Azithromycin-Eudragit EPO interaction as opposed to loading Azithromycin in Eudragit S100. Using the supercritical fluid technology to load Azithromycin into Eudragit EPO resulted in higher loading efficiency (91% as compared to 52%) but faster in vitro release (6% as compared to <2%) of Azithromycin as compared to using the SE method. Thus, despite supercritical fluid technology and SE being promising methods in vitro, SE resulted in the best performance. This performance was confirmed in the in vivo clinical study in healthy subjects.
ACKNOWLEDGMENTS
The authors acknowledge Yarmouk University, the University of Jordan, and Jordan University of Science and Technology for all their administrative and facility support. The authors also thank Al-Hikma Company (Amman, Jordan) for providing Azithromycin.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research was funded by the Deanship of Research at Jordan University of Science and Technology (Irbid, Jordan) through Grant number 164/2022.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The protocol for the in vivo experiments used herein was approved by the Institutional Review Board of King Abdullah University Hospital (Irbid, Jordan) (Approval No. 15/147/2022, date: 24/2/2022). Ten healthy volunteers (five females and five males) participated in this study. In brief, the study protocol was explained to the volunteers, and each volunteer signed the required consent form.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Pediatrics AAO. Periodic Survey# 44: patient compliance with prescription regimens. 2015.
2. Chirag JP, Tyagi S, Dhruv M, Ishita M, Gupta A, Mohammed Usman M, et al. Pharmaceutical taste masking technologies of bitter drugs: a concise review. J Drug Discov Ther. 2013;1(5):39–46.
3. Patil V, Tambe V, Pathare B, Dhole S. Modern taste concealing techniques in pharmaceuticals: a review. WJPPS. 2014;3:293–316.
4. Jyothi NVN, Prasanna PM, Sakarkar SN, Prabha KS, Ramaiah PS, Srawan G. Microencapsulation techniques, factors influencing encapsulation efficiency. J Microencapsul. 2010;27(3):187–97. CrossRef
5. Al-kasmi B, Alsirawan MB, Bashimam M, El-zein H. Mechanical microencapsulation: the best technique in taste masking for the manufacturing scale-effect of polymer encapsulation on drug targeting. J Control Release. 2017;260:134–41. CrossRef
6. Patra CN, Priya R, Swain S, Jena GK, Panigrahi KC, Ghose D. Pharmaceutical significance of Eudragit: a review. Future J Pharm Sci. 2017;3(1):33–45. CrossRef
7. Thakral S, Thakral NK, Majumdar DK. Eudragit®: a technology evaluation. Expert Opin Drug Deliv. 2013;10(1):131–49. CrossRef
8. Cantor SL, Khan MA, Gupta A. Development and optimization of taste-masked orally disintegrating tablets (ODTs) of clindamycin hydrochloride. Drug Dev Ind Pharm. 2015;41(7):1156–64. CrossRef
9. Jelvehgari M, Valizadeh H, Kiafarab F, Afandipourc L. Taste masking and characterization of chlorpheniramine maleate by using enteric polymers carrier system. J Rep Pharm Sci. 2013;2(1):45–58. CrossRef
10. Ghorab MM, Zia H, Luzzi LA. Preparation of controlled release anticancer agents I: 5-fluorouracil-ethyl cellulose microspheres. J Microencapsul. 1990;7(4):447–54. CrossRef
11. Lee C-W, Kim S-J, Youn Y-S, Widjojokusumo E, Lee Y-H, Kim J, et al. Preparation of bitter taste masked cetirizine dihydrochloride/β-cyclodextrin inclusion complex by supercritical antisolvent (SAS) process. J Supercrit Fluids. 2010;55(1):348–57. CrossRef
12. Obaidat R, Aleih H, Mashaqbeh H, Altaani B, Alsmadi MtM, Alnaief M. Development and evaluation of cocoa butter taste masked ibuprofen using supercritical carbon dioxide. AAPS PharmSciTech. 2021;22:1–13. CrossRef
13. Machado ND, Mosquera JE, Martini RE, Goñi ML, Gañán NA. Supercritical CO2-assisted impregnation/deposition of polymeric materials with pharmaceutical, nutraceutical, and biomedical applications: a review (2015–2021). J Supercrit Fluids. 2022;191:105763. CrossRef
14. AlSheyyab RY, Obaidat RM, Altall YR, Abuhuwaij RT, Ghanma RR, Ailabouni AS, et al. Solubility enhancement of nimodipine through preparation of Soluplus® dispersions. J Appl Pharm Sci. 2019;9(9):030–7. CrossRef
15. Obaidat RM, Alnaief M, Mashaqbeh H. Investigation of carrageenan aerogel microparticles as a potential drug carrier. Aaps Pharm. 2018;19:2226–36. CrossRef
16. Bagheri H, Notej B, Shahsavari S, Hashemipour H. Supercritical carbon dioxide utilization in drug delivery: experimental study and modeling of paracetamol solubility. Eur J Pharm Sci. 2022;177:106273. CrossRef
17. Joshi J. Azithromycin drug profile. Indian J Otolaryngol Head Neck Surg. 1995;47(4):331–3. CrossRef
18. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles of drug substances, excipients and related methodology. Elsevier, Amsterdam, The Netherlands, pp 1–40, 2014. CrossRef
19. Harrison TS, Keam SJ. Azithromycin extended release. Drugs. 2007;67(5):773–92. CrossRef
20. Fiese E, Steffen S. Comparison of the acid stability of azithromycin and erythromycin A. J Antimicrob Chemother. 1990;25(suppl_A):39–47. CrossRef
21. Tung N-T, Tran C-S, Nguyen T-L, Hoang T, Trinh T-D, Nguyen T-N. Formulation and biopharmaceutical evaluation of bitter taste masking microparticles containing azithromycin loaded in dispersible tablets. Eur J Pharm Biopharm. 2018;126:187–200. CrossRef
22. Amin F, Khan S, Shah SMH, Rahim H, Hussain Z, Sohail M, et al. A new strategy for taste masking of azithromycin antibiotic: development, characterization, and evaluation of azithromycin titanium nanohybrid for masking of bitter taste using physisorption and panel testing studies. Drug Design Dev Ther. 2018:3855–66. CrossRef
23. Hu L, Pan J, Liu C, Xu H, Luo L. Preparation, characterization and taste-masking properties of microspheres containing azithromycin. J Pharm Pharm. 2009;61(12):1631–5. CrossRef
24. Chen Y, Liu Y, Wu C, Pan X, Peng T. Dry suspension containing coated pellets with pH-dependent drug release behavior for the taste-masking of Azithromycin. AAPS PharmSciTech. 2022;24(1):21. CrossRef
25. Siddiqui F, Shoaib MH, Ahmed FR, Qazi F, Yousuf RI, Usmani MT, et al. Formulation development and optimization of taste-masked azithromycin oral suspension with ion exchange resins: Bioanalytical method development and validation, in vivo bioequivalence study, and in-silico PBPK modeling for the paediatric population. J Drug Deliv Sci Technol. 2023;79:104048. CrossRef
26. Mashaqbeh H, Obaidat RM, Alsmadi MtM. Solvent-free method for masking the bitter taste of azithromycin dihydrate using supercritical fluid technology. Drug Dev Indust Pharm. 2024;50:1–10. CrossRef
27. Budisa N, Schulze-Makuch D. Supercritical carbon dioxide and its potential as a life-sustaining solvent in a planetary environment. Life. 2014;4(3):331–40. CrossRef
28. Alnaief M, Obaidat R, Mashaqbeh H. Effect of processing parameters on preparation of carrageenan aerogel microparticles. Carbohydr Polym. 2018;180:264–75. CrossRef
29. Guhmann M, Preis M, Gerber F, Pöllinger N, Breitkreutz J, Weitschies W. Development of oral taste masked diclofenac formulations using a taste sensing system. Int J Pharm. 2012;438(1–2):81–90. CrossRef
30. Waghule SN, Jain NP, Patani CJ, Patani AC. Method development and validation of HPLC method for determination of azithromycin. Der Pharma Chemica. 2013;5(4):166–72.
31. Ali M, Walboomers XF, Jansen JA, Yang F. Influence of formulation parameters on encapsulation of doxycycline in PLGA microspheres prepared by double emulsion technique for the treatment of periodontitis. J Drug Deliv Sci Technol. 2019;52:263–71. CrossRef
32. Adeli E. Preparation and evaluation of azithromycin binary solid dispersions using various polyethylene glycols for the improvement of the drug solubility and dissolution rate. Braz J Pharm Sci. 2016;52(1):1–13. CrossRef
33. Montejo-Bernardo J, García-Granda S, Bayod-Jasanada M, Llorente I, Llavonab L. An easy and general method for quantifying Azithromycin dihydrate in a matrix of amorphous Azithromycin. Arkivoc. 2005;9:321–31. CrossRef
34. Braga ME, Pato MTV, Silva HSC, Ferreira EI, Gil MH, Duarte CM, et al. Supercritical solvent impregnation of ophthalmic drugs on chitosan derivatives. Jf Supercr Fluids. 2008;44(2):245–57. CrossRef
35. Champeau M, Thomassin JM, Tassaing T, Jerome C. Drug loading of sutures by supercritical CO2 impregnation: effect of polymer/drug interactions and thermal transitions. Macromole Mater Eng. 2015;300(6):596–610. CrossRef
36. Champeau M, Thomassin J-M, Tassaing T, Jérôme C. Drug loading of polymer implants by supercritical CO2 assisted impregnation: a review. J Control Release. 2015;209:248–59. CrossRef
37. Timoumi S, Mangin D, Peczalski R, Zagrouba F, Andrieu J. Stability and thermophysical properties of azithromycin dihydrate. Arabian J Chem. 2014;7(2):189–95. CrossRef
38. Alnaief M, Obaidat R, Mashaqbeh H. Loading and evaluation of meloxicam and atorvastatin in carrageenan microspherical aerogels particles. J Appl Pharm Sci. 2019;9(1):083–8. CrossRef
39. Alsayad R, Laham A. Investigation of the effects of some process variables on the azithromycin microencapsulation by the quasi-emulsion solvent evaporation method. Res J Pharm Technol. 2023;16(8):3909–14. CrossRef