INTRODUCTION
The liver has a powerful role in the metabolism as it battles with diverse noxious chemicals, and environmental pollutants that prompt reactive oxygen and nitrogen species production, causing several ailments [1]. Hepatic fibrogenesis, overconsumption of alcohol, and obesity (considered as the key factor coupled with non-alcoholic fatty liver disease, simple steatosis, and cirrhosis) are associated with liver disease [2].
Carbon tetrachloride (CCl4) can induce intoxication in animals and hence serve as an experimental model to replicate oxidative stress. Based on its molecular characteristics, it could be inferred that CCl4 can induce hepatotoxicity in animals. The oxidative stress triggered by CCl4 produces radicals, leading to the impairment of deoxyribonucleic acid (DNA), proteins, and lipid peroxidation in liver tissues [2]. Animals use antioxidant systems to reduce oxidative stress. Antioxidant administration guards the tissues against the destructive consequences of free radicals, e.g., reactive oxygen species, plus lipid peroxidation, thus it may obstruct the progress of chronic diseases related to the liver [3].
The scientific evidence supporting the protective properties of certain natural products from medicinal plants employed for treating disorders remains insufficient and requires authentication. One such plant is Musa balbisiana Colla (Musaceae), which is rich in nutritional value and medicinal properties. It grows up in Southeast and Central Asia. Its fruit is full of vitamins, minerals, and dietary fibers, bioactive compounds, for instance, polyphenols, flavonoids, and carotenoids, which emphasize its dietary and remedial worth [4]. It is an affluent resource of high potassium, chloride, calcium, and Carbonate. C16, C18 fatty acid, and ferulic acid are reported to be present in the seeds of M. balbisiana Colla [5]. Also, sinapic acid, p-hydroxybenzoic, salicylic acid, vanillic acid, gentisic acid, gallic acid, and p-coumaric acid, other phenolic compounds are reported to be present in the fruit of M. balbisiana Colla [6]. These phyto-constituents may be responsible for several health benefits and the therapeutic properties of the M. balbisiana Colla fruit.
However, the protective behaviors of fruits of M. balbisiana Colla against hepatotoxicity have not been investigated yet.
The present study aims to investigate the hepatoprotective potential of the methanolic extract of unripe fruit pulp of M. balbisiana Colla (MBME) against CCl4-induced toxicity in Swiss albino mice.
MATERIALS AND METHODS
Chemicals
Folin’s Ciocalteu Reagent, α,α-Diphenyl-β-picrylhydrazyl (DPPH), 2,2′-azinobis (3-ethylbenzthiazoline-6-sulfonic acid) or ABTS, Trolox were bought from SRLPvt. Ltd. (SRL)—Mumbai, India. Alanine transaminase (ALT), aspartate aminotransferase (AST), total protein (TP), alkaline phosphatase (ALP), total bilirubin (TBIL), malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), and glutathione (GSH) detection kits were bought from Sigma Aldrich (Saint Louis, MO). Every chemical was certified as an analytical grade.
Collection of sample and fruit pulp extract preparation
The collection of fresh fruits of M. balbisiana Colla (Musaceae) from the Kamrup Metropolitan district of Assam, India, was performed in November 2021. The collection of fresh fruits of M. balbisiana Colla (Musaceae) from the Kamrup Metropolitan district of Assam, India, was performed in November 2021. These were cleaned, cut, air dried, powdered, and used in the present study. Fine powder (10 g) of the same and 100 ml of methanol were mixed (1:10 ratio) and extracted by cold maceration extraction. The sample mixture was then filtered and allowed to evaporate to achieve the methanolic extract (MBME) and collected plus stored at 4oC for further use [7]. An authenticated voucher specimen of the plant species was submitted to the Herbarium, Department of Botany, Gauhati University, Assam, with the accession number GUBH20010 for identification.
Phytochemical investigation of methanolic extract of M. balbisiana Colla fruit pulp
Qualitative tests of the methanolic extract of M. balbisiana Colla fruit pulp were conducted to explore carbohydrates, proteins, fats, alkaloids, flavonoids, and phenols [8]. Quantitative analysis of flavonoids, phenols, and evaluation of the antioxidant activity of methanolic extract of M. balbisiana Colla fruit pulp was conducted according to the standard procedure [9].
Total phenolic content
Determination of total phenolic contents in the methanolic extract of M. balbisiana Colla fruit pulp was performed by the Folin–Ciocalteu colorimetric technique [10]. Various concentrations of Gallic acid and the methanolic extract of M. balbisiana Colla fruit pulp, i.e., 50, 100, 150, 250, 350, and 500 μg/ml were prepared from the stock solution of Gallic acid and the methanolic extract of M. balbisiana Colla fruit pulp in the same way. Then, 5 ml 10% Folin–Ciocalteu reagent followed by 4 ml 7% Na2CO3 was put to each concentration of the Gallic acid and the methanolic extract of M. balbisiana Colla fruit pulp in sequence, followed by shaking and incubation at 40°C in a water bath for 30 minutes. After this, the absorbance for each concentration was recorded at 760 nm compared to the blank. The experiments were performed in triplicates, and the calibration curve was plotted using average absorbance values of several concentrations.
Total flavonoid contents
The total flavonoid content in the methanolic extract of M. balbisiana Colla fruit pulp was detected by an already-established method [11]. Various concentrations of quercetin and the methanolic extract of M. balbisiana Colla fruit pulp (50, 100, 150, 200, and 250 μg/ml) were prepared from their stock solutions. To 1 ml quercetin from each concentration, 4 ml distilled water, 0.3 ml 5% NaNO2, 0.3 ml 10% AlCl3 followed by 2 ml 1M NaOH were added to the solutions, respectively. The volume of the solutions was then made up to 10 ml by adding 4.4 ml of distilled water, followed by incubation for 15 minutes under room temperature and measurement of absorbance at 510 nm. Total flavonoid content was articulated as quercetin equivalent (mg QE/g) via the linear equation attained from the standard calibration curve.
Free radical scavenging activity of the methanolic extract of M. balbisiana Colla fruit pulp
The free radical scavenging activity of the methanolic extract of M. balbisiana Colla fruit pulp was measured by DPPH and ABTS radical scavenging assay.
DPPH assay
The DPPH assay was conducted by procedures followed by Ravi et al. [12] with minor modifications. From the stock solution of both the standard and the methanolic extract of M. balbisiana Colla, different diluted concentrations of 25, 50, 75, 100, 125, and 150 µg/ml were made. Methanol and 3 ml of 1M DPPH were the blank and the negative control, in that order. Now, to each concentration of ascorbic acid and the methanolic extract of M. balbisiana Colla, 4 ml of 1M DPPH was added. Then the volume of all the solutions was made up to 10 ml by adding 99% methanol. These solutions were mixed and incubated for approximately 30 minutes in the dark at room temperature. Absorbance was noted against the blank at 517 nm.
ABTS assay
ABTS assay was performed by preparing 7 mM ABTS stock solution and 2.45 mM potassium persulphate stock solution from which the working solution of ABTS + reagent was prepared. The control in this assay was ABTS + solution without antioxidants and the blank was 100% methanol without ABTS +. The working standard solutions of Trolox (0.2, 0.4, 0.6, 0.8, 1, and 1.2 mM) were prepared from the stock solution of the standard 2 mM Trolox, from which 10 µl of each dilution and different dilutions of the methanolic extract of M. balbisiana Colla fruit pulp (5, 10, 15, 20, 25, and 30 µl) were mixed with 200 µl of ABTS + reagent followed by incubation for 30 minutes and absorbance measurement was executed at 734 nm [13].
In vivo hepatoprotective activity
Assignment of animals and administration of CCl4 and drugs
All the experiments were performed with healthy young Swiss albino mice (adult and male, 20 to 30 g). Five groups of mice were arbitrarily assembled, each including six male Swiss albino mice (n = 6). Group I (normal control) was given distilled water (1 ml/kg) orally for 28 days and olive oil (1 ml/kg) twice a week and group II (toxic CCl4 group), III, IV, and V were given a single dose of 1 ml/kg CCl4 in the olive oil 1:1 v/v, intraperitoneally once in a week, group III (CCl4+100mg/kg silymarin) received silymarin 100mg/kg orally for 28 days. Group IV (CCl4+200mg/kg MBME) received 200mg/kg MBME orally for 28 days and group V (CCl4+400mg/kg MBME) received 400 mg/kg MBME orally for 28 days. The doses of CCl4 were administered compliant with the suggested chronic oral exposure reference dose for CCl4 (CASRN 56-23-5) [14–16].
All the experiments were performed with healthy young adult male Swiss albino mice (20 to 30 g) bought from the animal house, Department of Zoology, Gauhati University, Guwahati, Assam, India. All the investigational procedures were scrutinized and permitted by the Institutional Ethical Committee for Animal Welfare having reference number GUIEC/2021/038 and were performed according to current guidelines for the care of laboratory animals of the Institutional Animal Ethics Committee (IAEC) bearing Ref. No. IAEC/Per/2022/PP-IAEC/ 2022-4/01.
Blood and tissue samples collection and body weight measurement
The experiments were concluded on the day 28th and the mice were put on fasting during the night on day 29th. Collection of blood samples in sterilized centrifuge tubes using 2 ml syringes via cardiac puncture followed by liver removal was performed under the control of ketamine. The blood samples were centrifuged after clot formation at 5,000 rpm for ten minutes. The clear serum was then stocked at −20ºC for further use. The body weight of all the experimental animals before and following the experiments was recorded. Right after the sacrifice of all the mice, the liver weight was measured to calculate the liver index. The calculation of the liver index was conducted by applying the formula (liver weight/body weight) × 100 [3].
Investigation of serum biochemical parameters
The clear serums attained from blood samples were used for the measurement of the various liver biochemical markers, such as aspartate transaminase (AST), ALT, ALP, TBIL, and TP by using standard commercial kits, and the manufacturer’s instructions were strictly followed.
Estimation of antioxidant markers and liver peroxidation
One gram of the removed liver was homogenized in 10 ml 50 mM phosphate buffer (ice cold, pH 7.4) and centrifuged for 10 minutes at 2,500 rpm in 4°C the clear serum was exploited to calculate antioxidant markers such as SOD, CAT, GSH, and production of MDA. The assays were performed as per the instructions of the standard commercial kits.
Estimation of serum cytokines
Serum levels of a multifunctional cytokine transforming growth factor-beta (TGF-β) and inflammatory cytokines, Interleukin-6 (IL-6), as well as tumor necrosis factor-alpha (TNF-α), were evaluated in mice with CCl4-induced hepatotoxicity with commercial particular cytokine specific enzyme-linked immunosorbent assay kits as per the manufacturer’s guidelines. The absorbance of the product was measured by using the micro-plate reader at 450 nm.
Immunohistochemistry analysis
The liver tissues of all experimental groups were scrutinized for the expression of TGF-β antibodies by immunohistochemical labeling technique [17]. After deparaffinization and rehydration in various grades of alcohol, antigens were retrieved. The response color was developed by staining and incubation was carried out with anti-TGF-β (MA1-21595 Invitrogen, diluted in the ratio 1:1500) at 4°C for the entire night. These tissues were provided treatment with goat anti-rabbit secondary antibody followed by dehydration and mounting. Slide visualization was performed by a microscope (Leica DM3000, China) at 40× magnification and the percent area of TGF-β was measured by using ImageJ software (National Institutes of Health, USA).
Histopathological examination
Dissection of a portion of the liver was promptly performed and fixed using 10% formalin (neutral buffered) before being dehydrated in a different series of concentrations of alcohol and xylene followed by embedding, block making, trimming, and sectioning of blocks to pieces of 4–5 μm thickness followed by staining in hematoxylin plus eosin (HE) for histopathological study [18].
Statistical analysis
GraphPad Prism 10.0.0 was operated to conduct statistical analysis, and the results were articulated as mean ± SD. One-way analysis of variance (ANOVA) and Welch’s t-tests were operated to analyze the results, and the significance was set at p < 0.05.
RESULT AND DISCUSSION
Phytochemical investigation
Qualitative analysis
A disparity between the free radicals and the antioxidant defense mechanism in animals leads to oxidative stress generation. Free radical production in higher quantities often causes hepatocellular damage by affecting its DNA, protein, and lipid [19,20]. The antioxidants in plants reduce oxidative stress very well. Phytochemical investigation of M. balbisiana Colla methanolic fruit pulp extract included the qualitative analysis of protein, carbohydrate, oils, fats, alkaloid, phenol, and flavonoids, and positive results affirmed its potential nutritive value. The qualitative analysis of the methanolic extract of M. balbisiana Colla fruit pulp shows the existence of carbohydrates, protein, alkaloids, phenols, flavonoids, fats, and oils.
Total phenolic and flavonoid content and free radical scavenging activity
The calibration curve for the total phenolic and flavonoid content is shown in Figure 1. The methanolic extract of M. balbisiana Colla fruit pulp revealed a very high total phenolic content of 654.88 ± 1.75 mg GAE/g and total flavonoid content was 65.04 ± 1.25 quercetin equivalent (mg QE/g). The IC50 of the methanolic extract of M. balbisiana Colla fruit pulp achieved through DPPH and ABTS assay was 86.20 and 15.33 µg/ml, respectively. In contrast, the standard ascorbic acid in the DPPH assay revealed an IC50 value of 18.39 µg/ml, and the standard of ABTS assay, trolox, possessed an IC50 value of 0.73 mM/ml (Fig. 2). The quantitative investigation of phenols unveiled better total phenol content than M. balbisiana Colla ethanolic unripe fruit pulp extract (625.64 ± 0.36 mg GAE/g) [21]. The total flavonoid content of M. balbisiana Colla methanolic fruit pulp extract was estimated as 65.04 ± 1.25 mg QE/g, which, together establishes a correlation with the soaring antioxidant efficacy of M. balbisiana Colla fruit pulp. Our study revealed a better content of phenols and flavonoids in the methanolic fruit extract of M. balbisiana Colla. Phenols and flavonoids are the secondary metabolites produced in plants which mostly contribute to antioxidant properties to mitigate oxidative stress induced by free radicals produced through several metabolic processes. Such antioxidant compounds combat oxidative stress through free radical scavenging activity [20]. In our study, CCl4-induced hepatotoxicity through oxidative stress was significantly reduced by treatment with the methanolic fruit extract of M. balbisiana Colla. The antioxidant activity of the methanolic fruit extract of M. balbisiana Colla may be responsible for this action, as the methanolic fruit extract of M. balbisiana Colla administration reduced the MDA content in the liver tissue produced through lipid peroxidation, a consequence of oxidative stress. Free radical scavenging activities of the natural products and plant extracts are normally assessed by the DPPH plus ABTS free radical scavenging assay [21]. Lower IC50 (50% inhibition) achieved through DPPH along with ABTS free radical scavenging assay reveals a high antioxidant effect of a test compound or extract and vice versa [10]. The antioxidant effect of the methanolic fruit extract of M. balbisiana Colla determined by DPPH and ABTS free radical scavenging assay revealed IC50 of 109.75 mg/ml and 15.33 µg/ml in order which is better than IC50 of 150.24 ± 0.03 mg/ml of ethanolic fruit extract of M. balbisiana Colla [20]. The other species of the genus Musa or the members of the family Musaceae are of medicinal importance, as many of them have been reported to possess antioxidant activity, anti-ulcerogenic, and hepatoprotective activity. The fruit peel and pulp of Musa acuminata exhibit hepatoprotective activity, but the total phenolic content in the methanolic fruit extract of M. balbisiana Colla is better than that of Musa acuminata [22]. The whole plant extract of Musa paradisiaca was also reported to exhibit hepatoprotective activity [23]. The total phenolic content and the antioxidant activity (IC50) through the DPPH assay of the methanolic fruit extract of M. balbisiana Colla were found better than both the methanolic and ethanolic extract of Musa paradisiaca fruit pulp and peel [24]. The stem of Musa sapientum L. was also reported to exhibit hepatoprotective activity in vivo [25]. However, total phenolic content and the DPPH free radical scavenging activity assay of the methanolic fruit extract of M. balbisiana Colla are comparatively found better than the stem and inflorescence extract of Musa sapientum L. [26]. Based on this comparative analysis, it is corroborated that the fruit pulp of M. balbisiana Colla is a better selection than the other species of the genus Musa in terms of the total phenolic content and antioxidant activity.
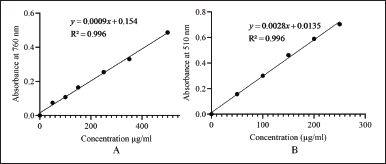 | Figure 1. Calibration curves for the total phenolic content (A) and total flavonoid content (B) of methanolic fruit extract of M. Balbisiana Colla. [Click here to view] |
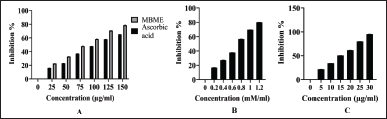 | Figure 2. DPPH assay of the standard ascorbic acid and the methanolic fruit extract of M. balbisiana Colla (A), ABTS assay of the standard Trolox (B), and M. Balbisiana Colla methanolic fruit pulp extract (C). [Click here to view] |
In vivo hepatoprotective activity
Effect of the methanolic extract of M. balbisiana Colla fruit pulp on body and liver weight of mice with liver damage
The changes in body and liver weights, plus liver index, are tabulated in Table 1. In contrast to normal control, a reasonable reduction of the body weights of mice was detected in the CCl4 group (p < 0.05). Conversely, body weight reduction by the CCl4 dosage was compensated very well by a minimum dose (200 mg/kg) plus a high dose (400 mg/kg) of the methanolic extract of M. balbisiana Colla fruit pulp. The CCl4 treatment induced a spike in the liver index, which confirmed hepatomegaly in all groups except normal control. Different doses of the methanolic extract of M. balbisiana Colla fruit pulp lowered the increased liver weight caused by the CCl4 treatment. The CCl4 group recorded the highest loss of body weight among all the treatment groups. However, despite the toxic effect of CCl4, different dosages of the methanolic extract of M. balbisiana Colla fruit pulp indemnified the loss (p < 0.05). The CCl4 treatment also reduced the liver weight. Subsequently, it increased the liver index, indicative of hepatomegaly. Hepatomegaly is an anomalous enlargement of the normal liver that generates serious clinical symptoms [27]. Similar reports focusing on the declined body weight together with elevated liver weight in the hepatotoxic animal groups have been published in various studies [3,28]. This condition was reverted by a different dosage of the methanolic extract of M. balbisiana Colla fruit pulp.
Outcome of the methanolic extract of M. balbisiana Colla fruit pulp treatment on liver enzyme markers in hepatic damage
CCl4 administration elevated the aspartate transaminase, ALP, ALT, and TBIL levels and reduced TP levels in all treatment groups, excluding normal control (Table 2). But the treatment with a low dose (200 mg/kg) along with the high dose (400 mg/kg) of the methanolic extract of M. balbisiana Colla fruit pulp lowered the aspartate transaminase, ALP, ALT, and TBIL levels and raised the TP levels in contrast to the CCl4 group (p < 0.05). Free radicals produced by CCl4 attach to proteins, and DNA along with lipids of hepatocytes leading to oxidative stress injury, peroxidation of lipids, and hepatocellular disruption leading to the elevation in the serum aspartate transaminase, ALP, ALT, and TBIL levels and reduction in TP level [8,16]. In our study, serum aspartate transaminase, ALP, ALT, and TBIL levels were the highest and the TP level was the lowest in the CCl4 group when compared to the other groups. This suggested hepatotoxicity in the toxic group. Administration of the methanolic extract of M. balbisiana Colla fruit pulp brought these levels close to near normal. Similar explanatory results have been reported by a few studies [27,29,30].
Effect of the methanolic extract of M. balbisiana Colla fruit pulp on liver enzyme markers and production of MDA in hepatic damage
The consequences of the methanolic extract of M. balbisiana Colla fruit pulp treatment on levels of the hepatic antioxidant enzymes and MDA contents generated via lipid peroxidation are presented in Table 3. The toxic CCl4 reduced the SOD, CAT, and GSH levels, and a noticeable elevation in the MDA generation through lipid production (p < 0.05) against normal control. But the administration of the methanolic extract of M. balbisiana Colla fruit pulp raised the SOD, CAT, and GSH levels and a decline in the MDA contents dose-wise versus the CCl4 group (p < 0.05). As a defense mechanism against hepatic injury, liver cells employ antioxidant enzymes such as SOD and CAT, and a non-enzymatic antioxidant GSH. Oxidative stress induces SOD, CAT, and GSH activity which serves as a quantitative assessment of oxidative stress in hepatocytes. Free radicals trigger MDA formation via lipid peroxidation in hepatocytes, which specify hepatocellular damage [3,27]. In our study, lower levels of SOD, CAT, and GSH and higher MDA levels have been observed in the CCl4 group in contrast with the normal control (p < 0.05). Conversely, high and low doses of the methanolic extract of M. balbisiana Colla fruit pulp raised the SOD, CAT, and GSH levels and reduced the levels in contrast to the CCl4 group (p < 0.05). Thus, the methanolic extract of M. balbisiana Colla fruit pulp protected the liver tissue from free radicals by raising these enzyme concentrations along with the GSH in the hepatic tissue. CCl4 also increased the MDA concentration by promoting lipid peroxidation. Lipid peroxidation attacks the antioxidant system in the tissue. The methanolic extract of M. balbisiana Colla fruit pulp lowered this concentration in contrast to the CCl4 group (p<0.05). Similar findings have been reported by earlier studies [27,29,31].
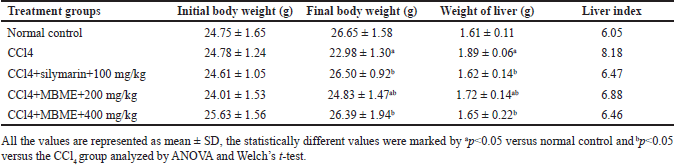 | Table 1. Effect of the methanolic fruit extract of M. balbisiana Colla on the body and liver weight. [Click here to view] |
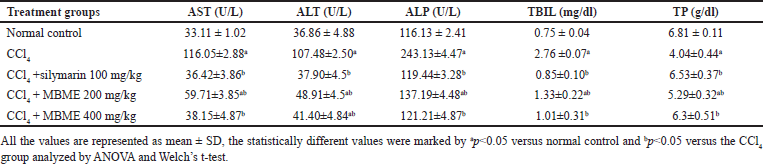 | Table 2. Effect of the methanolic fruit extract of M. balbisiana Colla on liver antioxidant enzymes. [Click here to view] |
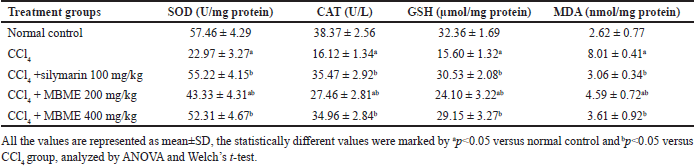 | Table 3. Effect of the methanolic fruit extract of M. balbisiana Colla on liver antioxidant enzymes. [Click here to view] |
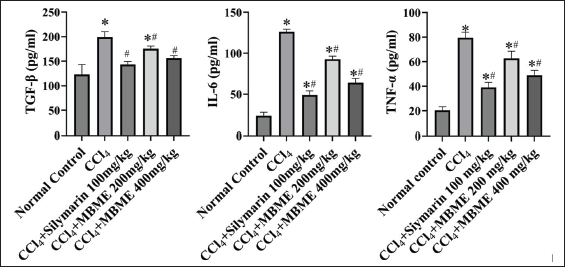 | Figure 3. Effect of the methanolic fruit extract of M. balbisiana Colla on serum proinflammatory cytokines TGF-β and IL-6. All the values are represented as mean±SD, the statistically different values were marked by *p < 0.05 versus normal control and #p < 0.05 versus the CCl4 group, analyzed by ANOVA and Welch’s t-test. [Click here to view] |
Effect of the methanolic extract of M. balbisiana Colla fruit pulp on serum cytokines
The TGF-β, IL-6, and TNF-α serum concentrations evaluated from each treatment group have been depicted in Figure 3. Among all the groups, the toxic CCl4 group displayed the highest spike in these cytokines levels (p < 0.05). Both doses of the methanolic extract of M. balbisiana Colla fruit pulp declined these cytokines levels in a dose-wise manner (p < 0.05). Tissue injury produces IL-6 while TGF-β drives pathophysiological conditions of hepatic disease [32,33]. Acute inflammation results in TNF-α production [34]. Treatment with CCl4 resulted in extensive tissue damage, inflammation, and higher production of TGF-β. The methanolic extract of M. balbisiana Colla fruit pulp reduced the serum concentration of pro-inflammatory cytokines IL-6 and TNF-α and multifunctional cytokine TGF-β levels dose wise (p < 0.05, contrast to the CCl4 group) despite the influence of CCl4. Comparable reports are made available on several related studies [35–37].
Immunohistochemistry analysis
TGF-β expression level in hepatic tissues by immunohistochemical staining has been presented in Figure 4. The expression level of TGF-β was maximum in the CCl4 group and minimum in normal control. Though dose wise, the methanolic extract of M. balbisiana Colla fruit pulp treatment attenuated the TGF-β expression levels (p < 0.05, contrast to the CCl4 group). TGF-β (TGF-β) superfamily (three isoforms, i.e., TGF-β1, TGF-β2, and TGF-β3) augments hepatic fibrogenesis by acting on hepatic satellite cells [38,39]. A study has reported that CCl4 treatment up-regulates TGF-β by production of excess reactive oxidative species [39]. The TGF-β level was elevated in the toxic CCl4 group. The TGF-β level was suppressed by the methanolic extract of M. balbisiana Colla fruit pulp administration (p < 0.05 contrast to the CCl4 group). Similar results of different extracts of medicinal plants on the modulation of TGF-β were reported [40,41].
Histopathological analysis
The histopathological results of the liver of all mice from all treatment groups are documented in Figure 5. The liver tissues of the normal control exhibit standard architecture of hepatic tissue, such as well-arranged hepatocytes and no central vein alterations (Fig. 5A). In contrast to the normal control, extensive fatty changes, marked alteration in central veins, spotty hepatocytic necrosis, and swelling have been observed in the toxic CCl4 group (Fig. 5B). Liver tissues of high dosage of the methanolic extract of M. balbisiana Colla fruit pulp administered group V revealed diminished fatty acid changes and central vein alterations and minimal necrosis (Fig 5E). The histopathological result of the silymarin (reference) administered group III features near standard structural features of the hepatic tissues (Fig. 5C). The histopathological findings of hepatic tissues from different test groups confirm the hepatoprotective potential of the methanolic extract of M. balbisiana Colla fruit pulp. Major histological alterations such as fat deposition, cellular necrosis, and changes in central vein architecture were observed within the liver tissues of animals treated with CCl4 in contrast to normal control. However, these changes were restored by the methanolic extract of M. balbisiana Colla fruit pulp in a dose-wise manner.
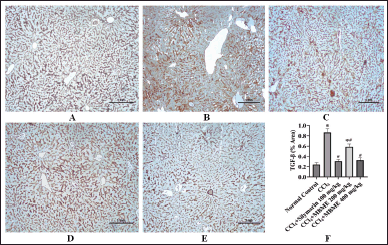 | Figure 4. Microphotographs of immune-stained hepatic tissues against TGF-β (A–E) and comparison of the percent area of TGF-β in the experimental groups (F). (A) normal control, (B) CCl4 group, (C) CCl4+silymarin 100mg/kg) group, (D) low dose (CCl4+MBME 200 mg/kg) MBME group, and (E) high dose (CCl4+MBME 400 mg/kg) MBME group (scalar bar: 1 mm). [Click here to view] |
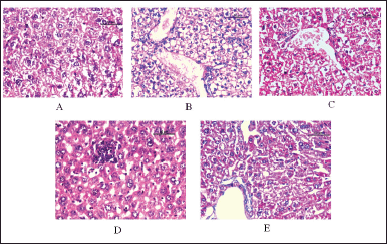 | Figure 5. Effect of the methanolic fruit extract of M. balbisiana Colla on histopathological changes in liver tissues of treatment groups: (A) normal control, (B) CCl4 group, (C) CCl4+silymarin 100 mg/kg) group, (D) low dose (CCl4+MBME 200 mg/kg) MBME group, and (E) high dose (CCl4+MBME 400 mg/kg) MBME group (scalar bar: 50 µm). [Click here to view] |
CONCLUSION
The results of our current study revealed that the methanolic fruit extract of M. balbisiana Colla possesses hepatoprotective activity through its high antioxidant activity. These findings deduced the reduction of hepatic biomarkers, and liver index, as well as the restoration of normal body weight, enzymatic and non-enzymatic antioxidant components in the liver tissue, and normal architecture of liver tissues through the treatment with methanolic fruit extract of M. balbisiana Colla (MBME). Our investigation also demonstrated that administering methanolic extract derived from unripe fruit pulp of MBME effectively decreased both the serum concentration of TGF-β and its expression within hepatic tissue in mice afflicted with significant liver damage induced by CCl4 hepatotoxicity. Notably, TGF-β serves as a pivotal regulator of liver fibrosis and other liver-related ailments, making it a prime target for assessing the hepatoprotective potential of natural products or plant extracts. Our findings indicate promising efficiency against TGF-β, contributing to substantial protection against hepatic injury. Further exploration of bioactive compounds accountable for such activity is desirable. Conclusively, as a future prospect, the findings of our present study can contribute to the discovery, synthesis, and development of novel and promising hepatoprotective drugs.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
ETHICAL APPROVALS
All the experiments were performed with healthy young adult male Swiss albino mice (20 to 30 g) bought from the animal house, Department of Zoology, Gauhati University, Guwahati, Assam, India. All the investigational procedures were scrutinized and permitted by the Institutional Ethical Committee for Animal Welfare having reference no. GUIEC/2021/038 and were performed according to current guidelines for the care of laboratory animals of Institutional Animal Ethics Committee (IAEC) bearing Ref. No. IAEC/Per/2022/PP-IAEC/ 2022-4/01.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Marques TR, Caetano AA, Henrique S, Cesar P, Braga MA, Henrique A et al. Antioxidant activity and hepatoprotective potential of lyophilized extract of Acerola bagasse against CCl4-induced hepatotoxicity in Wistar rats. J Food Biochem. 2018;42(6):e12670. CrossRef
2. Ramos-Lopez O, Martinez-Lopez E, Roman S, Fierro NA, Panduro A. Genetic, metabolic and environmental factors involved in the development of liver cirrhosis in Mexico. World J Gastroenterol. 2015;21(41):11552–66. CrossRef
3. Li L, Zhou YF, Li YL, Wang LL, Arai H, Xu Y. In vitro and in vivo antioxidative and hepatoprotective activity of aqueous extract of Cortex Dictamni. World J Gastroenterol. 2017;23(16):2912–27. CrossRef
4. Borborah K, Borthakur S, Tanti B. Musa balbisiana Colla-taxonomy, traditional knowledge and economic potentialities of the plant in Assam, India. Indian J Tradit Knowl. 2016;15:116–20. Available from: https://www.researchgate.net/publication/290202562_Musa_balbisiana_colla-taxonomy_traditional_knowledge_and_economic_potentialities_of_the_plant_in_Assam_India
5. Deka P, Kashyap A, Sharma D, Baruah C. A review on Musa balbisiana Colla. Int J Pharm Sci Invention. 2019;7(7):14–7.
6. Russell WR, Labat A, Scobbie L, Duncan GJ, Duthie GG. Phenolic acid content of fruits commonly consumed and locally produced in Scotland. Food Chem. 2019;115(1):100–4
7. Dharajiya D, Patel P, Patel M, Moitra N. In vitro antimicrobial activity and qualitative phytochemical analysis of Withania somnifera (L.) Dunal extracts. Int J Pharmaceutical Sci Rev Res. 2014;27(2):349–54. Available from: https://www.researchgate.net/publication/279769610_In_vitro_Antimicrobial_Activity_and_Qualitative_Phytochemical_Analysis_of_Withania_somnifera_L_Dunal_Extracts
8. Pandey A, Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn Phytochem. 2014;2(5):115–9.
9. Chandra S, Khan S, Avula B, Lata H, Yang MH, Elsohly MA et al. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: a comparative study. Evid Based Complement Alternat Med. 2014;2014:253875. CrossRef
10. Phuyal N, Jha PK, Raturi PP, Rajbhandary S. Total phenolic, flavonoid contents, and antioxidant activities of fruit, seed, and bark extracts of Zanthoxylum armatum DC. Sci World J. 2020;2020:8780704. CrossRef
11. Carmona-Hernandez JC, Le M, Idárraga-Mejía AM, González-Correa CH. Flavonoid/polyphenol ratio in Mauritia flexuosa and Theobroma grandiflorum as an indicator of effective antioxidant action. Molecules. 2021;26(21):6431. CrossRef
12. Ravi L, Manasvi V, Praveena BL. Antibacterial and antioxidant activity of saponin from Abutilon indicum leaves. Asian J Pharm Clin Res. 2016;9(9):344–7. CrossRef
13. Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Hawkins BD. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal. 2006;19(6–7):669–75. CrossRef
14. Makni M, Chtourou Y, Garoui E, Boudawara T, Fetoui H. Carbon tetrachloride-induced nephrotoxicity and DNA damage in rats. Hum Amp Exp Toxicol. 2012;31(8):844–52. CrossRef
15. Ebaid H, Al-Tamimi J, Habila M, Hassan I, Rady A, Alhazza IM. Potential therapeutic effect of synthesized AgNP using curcumin extract on CCl4-induced nephrotoxicity in male mice. J King Saud Univ Sci. 2021;33(2):101356. CrossRef
16. Nbete RZ, Lekpa KD. Hepatomegaly with steatosis affects the normal liver physiology of young adults in port-harcourt metropolis: a sonographic assessment. Asian Res Rep Hepatol. 2021;3:31–6. Available from: https://www.researchgate.net/publication/352363148_Hepatomegaly_with_Steatosis_Affects_the_Normal_Liver_Physiology_of_Young_Adults_in_Port-Harcourt_Metropolis_A_Sonographic_Assessment
17. Kieswich JE, Chen J, Alliouachene S, Caton PW, McCafferty K, Thiemermann C, et al. Immunohistochemistry of kidney a-SMA, Collagen 1, and Collagen 3, in a novel mouse model of reno-cardiac syndrome. Bio Protoc. 2020;10(18):e3751. CrossRef
18. Copper JE, Budgeon LR, Foutz CA, van Rossum DB, Vanselow DJ, Hubley MJ et al. Comparative analysis of fixation and embedding techniques for optimized histological preparation of zebrafish. Comp Biochem Physiol. 2018;208:38–46. CrossRef
19. Betteridge DJ. What is oxidative stress? Metabolism. 2000;49(2 Suppl 1):3–8. CrossRef
20. Cichoz-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20(25):8082–91. CrossRef
21. Irawan C, Utami A, Styani E, Putri ID, Putri RK, Dewanta A et al. Potential of ethanolic extract from Ripe Musa balbisiana Colla Fruit using ultrasound-assisted extraction as an antioxidant and anti-Gout. Pharmacogn J. 2021;13(6):1332–40. CrossRef
22. Abdullah FC, Rahimi L, Zakaria ZA, Ibrahim AL. Hepatoprotective, antiulcerogenic, cytotoxic and antioxidant activities of Musa acuminata peel and pulp. Novel Plant Bioresour Appl Food Med Cosmet. 2014:371–82.
23. Verma P, Paswan SK, Verma S, Singh SP, Rao CV, Shrivastva S, et al. Assessment of hepatoprotective activity of Musa paradisica Linn. Whole plant extract against carbon tetrachloride induced hepatotoxicity in wistar rats. Int J Pharm Sci Res. 2017;8(1):126.
24. Yusof N, Gani H, Sedik SN. Antioxidant and antimicrobial activities of pisang berangan (Musa paradisiaca) pulp and peel extracts. J Agrobiotech. 2023 Oct 26;14(2):71–82.
25. Dikshit P, Tyagi MK, Shukla K, Sharma S, Gambhir JK, Shukla R. Hepatoprotective effect of stem of Musa sapientum Linn in rats intoxicated with carbon tetrachloride. Annals Hepatol. 2016;10(3):333–9.
26. Jayamurthy P, Aparna B, Gayathri G, Nisha P. Evaluation of antioxidant potential of inflorescence and stalk of plantain (Musa sapientum). J Food Biochem. 2013 Feb;37(1):2–7.
27. Childs JT, Esterman AJ, Thoirs KA, Turner RC. Ultrasound in the assessment of hepatomegaly: a simple technique to determine an enlarged liver using reliable and valid measurements. Sonography. 2016;3(2):47–52. CrossRef
28. Khan MA, Ahmad W, Ahmad M, Nisar M. Hepatoprotective effect of the solvent extracts of Viola canescens Wall. ex. Roxb. against CCl4 induced toxicity through antioxidant and membrane stabilizing activity. BMC Complement Altern Med. 2017;17(1):10. CrossRef
29. Almatroodi SA, Anwar S, Almatroudi A, Khan AA, Alrumaihi F, Alsahli MA et al. Hepatoprotective effects of garlic extract against carbon tetrachloride (CCl4)-Induced liver injury via modulation of antioxidant, anti-inflammatory activities and hepatocyte architecture. Appl Sci. 2020;10(18):6200. CrossRef
30. Devaraj E, Roy A, Royapuram VG, Magesh A, Varikalam SA, Arivarasu L et al. β-Sitosterol attenuates carbon tetrachloride–induced oxidative stress and chronic liver injury in rats. Naunyn Schmiedeb Arch Pharmacol. 2020;393(6):1067–75. CrossRef
31. Elsawy H, Badr GM, Sedky A, Abdallah BM, Alzahrani AM, Abdel-Moneim AM. Rutin ameliorates carbon tetrachloride (CCl4)-induced hepatorenal toxicity and hypogonadism in male rats. Peer J. 2019;7:e7011. CrossRef
32. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. CrossRef
33. Aly O, Abouelfadl DM, Shaker OG, Hegazy GA, Fayez AM, Zaki HH. Hepatoprotective effect of Moringa oleifera extract on TNF-α and TGF-β expression in acetaminophen-induced liver fibrosis in rats. Egypt J Med Hum Genet. 2020;21(1):1–9. CrossRef
34. Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s). Microsc Res Tech. 2000;50(3):184–95. CrossRef
35. Ma Z, Sheng L, Li J, Qian J, Wu G, Wang Z, et al. Resveratrol alleviates hepatic fibrosis in associated with decreased endoplasmic reticulum stress-mediated apoptosis and inflammation. Inflammation. 2022;45:812–23. CrossRef
36. Hassan AA, Thabet NM, Abdel-Rafei MK. Hyaluronan as a mediator for the hepatoprotective effect of Diosmin/Hesperidin complex. Pak J Pharm Sci. 2018;31:1191–1201. Available from: https://www.researchgate.net/publication/326413752_Hyaluronan_as_a_mediator_for_the_hepatoprotective_effect_of_DiosminHesperidin_complex
37. Xu Z, Chen Z, Lan T, Han Y, Yang N, Wang C et al. Protective effects of phytic acid on CCl4-induced liver fibrosis in mice. J Food Biochem. 2023;2023:1–11. CrossRef
38. Chávez E, Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P. et al. Resveratrol prevents fibrosis, NF-κB activation and TGF-β increases induced by chronic CCl4 treatment in rats. J Appl Toxicol. 2007;28(1):35–43. CrossRef
39. Liu L, Li XM, Chen L, Feng Q, Xu LL, Hu YY. The effect of gypenosides on TGF-β1/smad pathway in liver Fibrosis Induced by carbon tetrachloride in rats. Intern J Integr Med. 2013;1:1–6. Available from: https://api.semanticscholar.org/CorpusID:51860978
40. Abdel-Hamid NM, Nazmy MH, Wahid A, Abdel-Moniem EM. Jerusalem artichoke attenuates experimental hepatic fibrosis via modulation of apoptotic signaling and fibrogenic activity. Biochem Biotech Res. 2015;3(3)43–50. Available from: https://www.researchgate.net/publication/288920385_Jerusalem_artichoke_attenuates_experimental_hepatic_fibrosis_via_modulation_of_apoptotic_signaling_and_fibrogenic_activity
41. Chale-Dzul J, Pérez-Cabeza de VR, Quintal-Novelo C, Olivera-Castillo L, Moo-Puc R. Hepatoprotective effect of a fucoidan extract from Sargassum fluitans Borgesen against CCl4-induced toxicity in rats. Int J Biol Macromol. 2020;145:500–9. CrossRef