INTRODUCTION
Endophytic fungi are microorganisms that asymptomatically live in the plant tissues during the entire or certain part of their life cycle. Many studies have shown the capability of these microorganisms to produce structurally diverse secondary metabolites with various pharmacological activities, including antimicrobial and anticancer [1]. Endophytic fungi also attracted attention due to their potential to produce valuable bioactive compounds originally derived from medicinal plants, as exemplified by the isolation of anticancer agents, paclitaxel and camptothecin, among others from endophytic fungi [2,3]. It is estimated that only around 1–2% of 300,000 known plant species have been studied for their fungal endophytes [4], indicating that exploration of endophytic fungi from plants offers great opportunities in the search for bioactive molecules.
In particular, medicinal plants have been repeatedly reported in many previous studies as one of the promising sources of talented endophytic fungal strains capable of producing bioactive compounds. For instance, an investigation on endophytic Fusarium sp. BZCB-CA isolated from Chinese medicinal plants Bothriospermum chinense led to the isolation of lateropyrone, which exhibited antibacterial activity toward several bacterial strains with MICs ranging from 3.1 to 25 µM. In addition, rubrofusarin isolated from this fungal strain displayed cytotoxicity against various cancer cells with IC50 values ranging from 6.2 to 7.7 µM [5]. Our recent investigation on methanolic extracts of endophytic fungi Penicillium steckii AAB-01 and Daldinia eschscholtzii AAB-05, associated with medicinal plants Antidesma bunius, revealed their cytotoxicity against breast cancer cells MCF-7 and 4T1 [6].
Continuing our endeavor to explore the antimicrobial and cytotoxic metabolites from endophytic fungi, in the present study we investigated fungal endophytes from Zingiber officinale var. rubrum, which is commonly known as red ginger. Red ginger is renowned for its ethnopharmacological uses and diverse therapeutic properties, such as antifungal [7], antihyperglycemic [8], antihypertensive [9], analgesia [10], and antibacterial [11]. Rhizomes of red ginger are rich in essential oil which mainly consists of monoterpenes and sesquiterpenes, and showed anti-biofilm activity [12]. Moreover, gingerol and shogaol derivatives, along with cinnamic acids, ethyl cinnamate, and ethyl p-methoxycinnamate were reported from the red ginger extract in a previous study [13]. Secondary metabolites produced by medicinal plants might be influenced by the interaction between the host plant and their associated fungal endophytes [14,15]; therefore, investigating the endophytic fungi associated with red ginger holds the potential to discover bioactive compounds.
A previous study on endophytic fungi isolated from red ginger, collected in Bogor, Indonesia, led to the isolation of endophytic Curvularia affinis, Fusarium solani, and Glomerella cingulata which could inhibit the growth of plant pathogenic fungus Fusarium oxysporum [16]. More recently, ethyl acetate extract of fungal endophyte Aspergillus terreus isolated from the rhizome of red ginger growing in Bengkulu, Indonesia, was reported to possess antimicrobial properties against Candida albicans, Staphylococcus aureus, and Escherichia coli with the inhibition zone of 8.3, 14.4, and 16.9 mm, respectively [17]. Herein, we reported eight fungal endophytes isolated from stems, rhizomes, and roots of red ginger, collected from Bali, Indonesia. Following the fermentation procedure, the resulting fungal extract was tested for antimicrobial, toxicity, and cytotoxicity against breast cancer cells 4T1 and MCF-7, as well as noncancer cells Vero.
MATERIALS ND METHODS
Fungal material
The endophytic fungi were isolated from the healthy and fresh stems, rhizomes, and roots of the red ginger plant growing in Tabanan Regency, Bali, Indonesia. The host plant was collected in December 2021. Authentication of the host plant, red ginger (Zingiber officinale var. rubrum), was done in the Herbarium Biologi Udayana, Udayana University, and was deposited under voucher specimen no. PY-ZOR09 (HBU)top of form. The isolation of fungal endophytes was carried out as previously described [6]. Each of the collected plant parts was thoroughly washed under running tap water for 3 minutes. Surface sterilization was then performed by immersing the samples in 70% EtOH for 2 minutes and allowed to dry. As the negative control, sterilized samples were pressed onto a petri dish containing an isolation medium, consisting of malt extract (Merck), Bacto agar (Difco BD), and chloramphenicol (Nalgene) in demineralized water. The same sample was then aseptically cut into smaller pieces before being placed into the second isolation medium. The inoculated samples were incubated in ambient conditions for several days to allow the growth of fungal mycelia. Once the fungal mycelia were observed, they were transferred into a new agar plate to obtain the pure fungal isolate. For long-term preservation, pure fungal isolates were cultured on media containing malt extract, Bacto agar, yeast extract, and glycerol (Vivantis) in demineralized water.
Identification of endophytic fungi
The pure fungal isolates were then identified through the comparison of the sequence of the internal transcribed spacer (ITS) region. The fungal genomic DNA was extracted using Quick-DNA Fungal/Bacterial Miniprep Kit (Zymo Research) following the manufacturer’s instructions. PCR procedure was done to amplify the region of ITS1-5.8S-ITS2 rDNA from the extracted fungal DNA utilizing forward primer, ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) (IDT), and reverse primer, ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (IDT) according to the procedure described by Putra et al. [18]. Meanwhile, for those that were unable to be identified through ITS region analysis, their D1/D2 domain of the large subunit (LSU) region was analyzed by amplifying it using forward NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3’′) (IDT) and reverse NL4 (IDT) (5′-GGTCCGTGTTTCAAGACGG-3′) primers. Sequencing analysis of the PCR products was done by 1st BASE. The resulting sequence was compared to the deposited sequence in the database of NCBI GenBank through the Basic Local Alignment Search Tool (BLAST) for nucleotides program, to search its closest homologous taxon. The sequence of the samples and their related homologous taxon were aligned using the MUSCLE algorithm and the phylogenetic tree was reconstructed using MEGA version 11.0.11 by a neighbor-joining algorithm with replication of 1,000 bootstraps.
Fermentation and extraction
To produce a sufficient amount of extract, each fungal isolate was subjected to fermentation on rice media, following the procedure described before [6]. Briefly, each pure fungal isolate in Petri dish agar was excised into small blocks and seeded onto two conical flasks (each 1,000 ml) containing a medium of autoclaved 100 g rice in 110 ml distilled water. Each flask was incubated at ambient temperature for 3–4 weeks until the rice media were completely covered by the fungal mycelia. Fermentation was terminated by pouring 500 ml ethyl acetate into each fermentation flask, then agitated on a shaker at 150 rpm for 6–8 hours. The mixture was filtered under reduced pressure and the filtrate was concentrated in vacuo in a rotary evaporator to obtain the crude extract. The crude extract was partitioned between methanol consisting of 10% water and n-hexane. The aqueous phase was concentrated in vacuo to dryness and the resulting methanolic extract was subjected to antimicrobial, toxicity, and cytotoxic assays, in addition to phytochemical analysis.
Preliminary identification of phytochemicals
Identification of phytochemicals was done to detect the presence of alkaloids, terpenoids, polyphenols, flavonoids, and saponins in fungal methanolic extracts using the procedure described earlier [18].
Antimicrobial assay
Evaluation of the antimicrobial properties of the fungal extracts was carried out utilizing the broth microdilution method under the protocol described by the Clinical and Laboratory Standards Institute (CLSI) [19]. Briefly, each fungal methanolic extract was pre-dissolved in 20 µl DMSO (Merck) before the assay. Next, in the 96-microwell plates (Iwaki), each methanolic extract solution was serially diluted to afford a final concentration from 1,000 to 1.95 µg/ml. Four bacterial strains, i.e., S. aureus ATCC 6538, methicillin-resistant S. aureus (MRSA) ATCC 3351, Staphylococcus epidermidis ATCC 12228, Pseudomonas aeruginosa ATCC 9027, along with a fungal strain C. albicans ATCC 10231 were selected for the antimicrobial assay. Two-fold dilution of chloramphenicol with concentrations ranging from 0.0625 to 32 µg/ml and ketoconazole with concentrations ranging from 0.5 to 256 µg/ml were included as positive controls in the assay against the tested bacteria and fungus, respectively. All the experiment was conducted in triplicates. The minimum inhibition concentration (MIC) value was determined from the lowest concentration of extract that can inhibit the growth of the tested microorganism.
Toxicity
Toxicity screening was performed employing the brine shrimp lethality test (BSLT) as described earlier [6,20]. Each fungal methanolic extract was dissolved in DMSO and then diluted in artificial seawater resulting in a series of concentrations ranging from 1 to 1,000 μg/ml. To prepare the brine shrimp larvae, 20 g of Artemia salina eggs (Supreme Plus) were incubated in a brine incubator loaded with 300 ml artificial seawater [9.5 g artificial sea salt (Himedia) in 300 ml distilled water]. The eggs were maintained under a continuous light regime and aeration for 24 hours until they hatched to be mature nauplii. Active nauplii free from eggshells in the illuminated side of the incubator were drawn for toxicity assay. Toxicity screening was conducted by transferring 10 nauplii using a Pasteur pipette to the vials supplemented with a serial concentration of fungal extract. Nauplii were also placed in vials containing artificial seawater consisting of 0.5% DMSO as a negative control. Each test was carried out in triplicates, and the vials were kept at room temperature under constant light for 24 hours. Afterward, the number of dead nauplii in each vial was counted, and the percentage of mortality was calculated with the following equation [21]:
With SPSS v.26., the value of median lethal concentration (LC50) of each methanolic extract was estimated using probit analysis of concentration versus mortality.
Cytotoxicity assay
The 4T1, MCF-7, and Vero cell lines were maintained by the Cancer Chemoprevention Research Center, Faculty of Pharmacy UGM, Indonesia. Sub-confluent cultures of 4T1, MCF-7, and Vero were harvested and re-suspended in a Dulbecco’s Modified Eagle Medium (Gibco) at a 1.0 × 105 cells/ml density. A 100 μl aliquot of this cell suspension was seeded in 96-well cell culture plates (Iwaki) at a final density of 3.0 × 103, 2.5 × 103, and 1.0 × 104 cells/well, respectively. Cells were incubated for 24 hours to allow adherence and further incubated with fresh culture medium as the control or treated at increasing concentrations (5–500 μg/ml) of the fungal extracts for 24 hours. The cell viability of the extracts was assessed using the CCK-8 kit assay (Dojindo, Japan). The absorbance of each well was measured using a Micro-titer Plate Reader (BioRad) at 490 nm wavelength. The percent viability of cells was calculated using the following equation:
where A is the absorbance.
RESULTS
Isolation and identification of endophytic fungi
Eight endophytic fungal strains were isolated from various parts of red ginger. Fungal isolates designated as ZOR-S1-1, ZOR-S1-3, ZOR-S1-4, and ZOR-S1-4.1 were isolated from the stems. Two fungal isolates ZOR-Rh1-1 and ZOR-Rh1-3 were obtained from the rhizomes, while isolates ZOR-Br1-1 and ZOR-Br1-2 were isolated from the roots of red ginger. The macroscopic appearance of each fungal isolate is shown in Figure 1.
Species determination of the endophytic fungi was primarily done by the sequence comparison of ITS1-5.8S-ITS2 rDNA. For the isolate ZOR-Rh1-1, since no amplified DNA band was observed in the gel electrophoresis after PCR on its ITS region, this fungal isolate was further identified through the analysis of the D1/D2 domain of its LSU region. However, fungal isolates ZOR-S1-4 and ZOR-Br1-2 remained unidentified as we were unable to amplify this fungal DNA either using amplification of the ITS region or the D1/D2 domain of the LSU region.
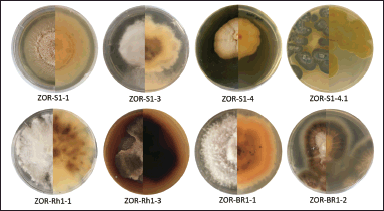 | Figure 1. The front (half left) and reverse (half right) views of each endophytic fungal colony isolated from various parts of red ginger grown on an agar dish containing malt extract, yeast extract, glycerol, and Bacto agar in demineralized water. [Click here to view] |
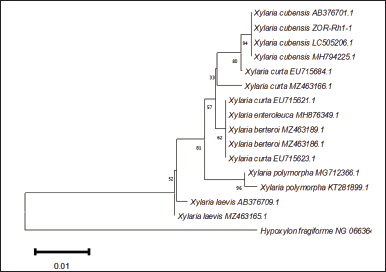
Amplification of the analyzed region employing PCR protocol yielded a single band of DNA segment with a size ranging from 500 to 750 base pairs, visualized on gel agarose electrophoresis as depicted in Figure 2. To predict the taxon of each endophytic fungus, the obtained sequences were compared to the fungal sequences deposited in the GenBank database utilizing the BLAST nucleotide algorithm of NCBI. As a result, the fungal sequences comparison indicated high sequence homology to six distinct species with good similarity percentage (>99%) and E values (Table 1). In addition, the phylogenetic tree construction showed that each isolate lineage differentiated into six primary clades with good bootstrap support, as shown in Figures 3 and 4.
Alignment analysis of the sequences suggested that isolate ZOR-S1-1 belonged to the species of Microdochium colombiense with 99.8% similarity to this species (OP855525.1), which was in line with its phylogenetic analysis with 99% bootstrap support. Whereas, isolate ZOR-S1-3 showed 99.8% similarity to Phlebiopsis flavidoalba (MZ087901.1), supported with high bootstrap support (100%). In addition, isolate ZOR-S1-4.1 was identified as P. citrinum proven by its similarity of 99.8% with this species (MN249873.1) and bootstrap support of 99% on phylogenetic tree. Moreover, sequence alignment showed that the ZOR-Rh1-3 isolate possessed high similarity (100%) with Dactylonectria anthuriicola (KP942924.1), confirmed with bootstrap support of 95% on the phylogenetic study. Isolate ZOR-Br1-1 had high sequence homology (99.8%) with the species of Setophoma terrestris (MN522036.1) which was supported by 99% bootstrap support on phylogenetic analysis. Finally, isolate ZOR-Rh1-3 displayed significant sequence similarity (99.8%) to the Xylaria cubensis (AB376701.1) species, which was confirmed by a 95% bootstrap support in the phylogenetic analysis.
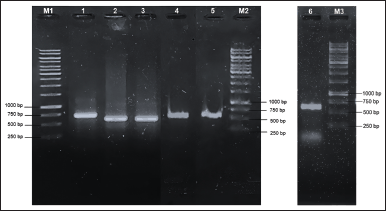 | Figure 2. Electrophoregram of amplified ITS rDNA from (1) ZOR-S1-3; (2) ZOR-S1-4.1; (3) ZOR-S1-1; (4) ZOR-BR1-1; (5) ZOR-Rh1-3; and amplified LSU region of (6) ZOR-Rh1-1. M1: 1 kb DNA marker (lane 1-3); M2: 1 kb DNA marker (lane 4-5); M3: 1 kb DNA marker (lane 6). [Click here to view] |
Preliminary identification of phytochemicals
Phytochemical screening revealed the presence of alkaloids and terpenoids in all fungal extracts, except for X. cubensis ZOR-Rh1-1 extract which showed positive results only for alkaloids (Table 2). Polyphenols were detected in four fungal extracts produced by P. citrinum ZOR-S1-4.1, D. anthuriicola ZOR-Rh1-3, S. terrestris ZOR-Br1-1, and isolate ZOR-Br1-2.
Antimicrobial activity
The antimicrobial activity of each methanolic extract produced by the isolated fungal endophytes from red ginger was evaluated. The MIC values of each fungal methanolic extract toward the tested microbial strains are displayed in Table 3. Among the tested extracts, methanolic extract from S. terrestris ZOR-Br1-1 was found to show pronounced antimicrobial activity against S. aureus ATCC 6538, S. epidermidis ATCC 12228, and C. albicans ATCC 10231 with MIC values of 31.3, 125, and 15.6 µg/ml, respectively. Meanwhile, the methanolic extract of D. anthuriicola ZOR-Rh1-3 along with isolate ZOR-Br1-2 showed weaker inhibition against the tested S. aureus and S. epidermidis with MIC values of 62.5 and 500 µg/ml. In addition, weak inhibition was found in the assay of methanolic extract of P. citrinum ZOR-S1-4.1 against S. aureus ATCC 6538, MRSA ATCC 3351, S. epidermidis ATCC 12228, and P. aeruginosa ATCC 9027 with MIC values of 500 µg/ml. Likewise, P. flavidoalba ZOR-S1-3 possessed weak inhibition against S. aureus ATCC 6538 with a MIC value of 500 µg/ml. Extracts of M. colombiense ZOR-S1-1, isolate ZOR-S1-4, and X. cubensis ZOR-Rh1-1 were found inactive toward all the tested microbial strains up to the tested concentration of 1,000 µg/ml.
Brine Shrimp Lethality Test
The result of toxicity screening employing BSLT as displayed in Figure 5, showed that extracts of D. anthuriicola ZOR-Rh1-3, X. cubensis ZOR-Rh1-1, isolate ZOR-Br1-2, and M. colombiense ZOR-S1-1 were found to have strong toxicity against A. salina nauplii with LC50 values ranging from 6.8 to 70.2 µg/ml. The remaining methanolic extracts exhibited moderate toxicity with LC50 values starting from 114 to 770 µg/ml.
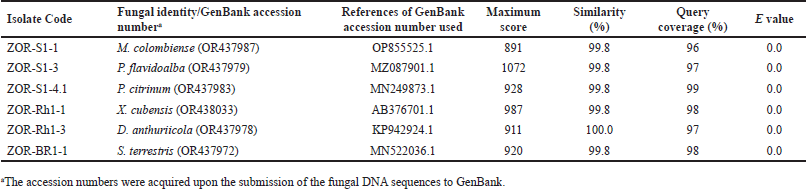 | Table 1. BLAST-N result of sequenced PCR products from endophytic fungi associated with red ginger. [Click here to view] |
 | Figure 3. Phylogenetic analysis of M. colombiense ZOR-S1-1, P. flavidoalba ZOR-S1-3, P. citrinum ZOR-S1-4.1, D. anthuriicola ZOR-Rh1-3, and S. terrestris ZOR-BR1-1 through the ITS region comparison, using the neighbor-joining algorithm, employing 1,000 bootstraps for support. [Click here to view] |
Cytotoxicity potency against breast cancer cells and selectivity of tested extracts
We further explored the cytotoxicity of the fungal extracts against breast cancer cells. We used 4T1 and MCF-7 cell lines which represent triple-negative breast cancer (TNBC) and non-TNBC subtype breast cancer cells, respectively. The selectivity of samples was evaluated by employing a normal cell line, Vero. We treated the cells with the fungal extracts in a series of 5–500 µg/ml concentrations. In the TNBC cell lines, 4T1, all the treatments with the fungal extracts decreased cell viability (Fig. 6A) in a dose-dependent manner, where isolate ZOR-Br1-2 showed the highest toxicity with IC50 values of 38 ± 5.6 µg/ml. Extracts with IC50 values of 2–89 µg/ml are categorized as a moderate cytotoxic agent [22]. Based on this category, in addition to isolate ZOR-Br1-2, endophytic isolate ZOR-S1-4, D. anthuriicola ZOR-Rh1-3, and P. citrinum ZOR-S1-4.1 also have moderate cytotoxicity (Table 4). We observed similar results when we treated the non-TNBC cells, MCF-7 cells, with the fungal extracts at the same series of concentrations. Samples treatment showed a dose-dependent manner toward cell viability (Fig. 6B), and isolate ZOR-Br1-2 was also the most potent sample with an IC50 value of 14 ± 3.9 µg/ml. It was followed by the activity of isolate ZOR-S1-4, D. anthuriicola ZOR-Rh1-3, S. terrestris ZOR-Br1-1, and P. citrinum ZOR-S1-4.1 as shown in Table 4. However, the MCF-7 cells were more sensitive than 4T1 cells as indicated by the lower IC50 value of samples on those tested cells. Other samples showed lower cytotoxicity with IC50 values of more than 100 µg/ml. Moreover, we also checked the samples’ effect on normal epithelial cells using the Vero cell lines, a normal kidney cell, to evaluate the selectivity of the samples. The top four potent samples, fungal isolates ZOR-Br1-2 and ZOR-S1-4, D. anthuriicola ZOR-Rh1-3, and P. citrinum ZOR-S1-4.1, have a selectivity index (SI) of 12, 8, 3, and 3, respectively (Fig. 6C), indicating that they are selective to cancer cells. A SI of more than three indicates high selectivity in cancer cells compared to noncancerous cells [23].
 | Figure 4. Phylogenetic analysis of X. cubensis ZOR-Rh1-1 through the region of LSU comparison, using the neighbor-joining algorithm with 1,000 bootstraps. [Click here to view] |
DISCUSSION
Medicinal plants serve as a valuable reservoir of endophytic fungi with the ability to produce a wide variety of bioactive substances. Diversity and species richness of endophytic fungi among the same host plant species are primarily shaped by the geographical site and environmental factors of the plant’s habitat, such as humidity, temperature, and sunlight intensity [2]. This implies that distinct endophytic fungi can be isolated even from the same medicinal plants that have been investigated before. In our study, we identified different endophytic fungal species in contrast to earlier studies that also isolated endophytic fungi from red ginger. Several endophytic fungi across various genera were reported to be associated with red ginger, for instance: Acremonium, Cochliobolus, Colletotrichum, Curvularia, Fusarium, Glomerella [16], and Aspergillus [17].
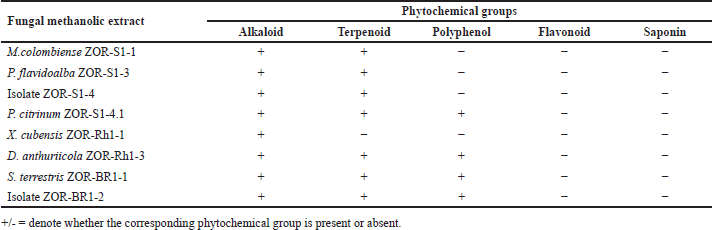 | Table 2. Preliminary detection of phytochemical groups from methanolic extracts of endophytic fungi isolated from red ginger. [Click here to view] |
 | Table 3. Antimicrobial activity of methanolic extracts of red ginger-derived endophytic fungi. [Click here to view] |
In the present study, we isolated eight fungal strains, six of which were identified as M. colombiense, P. flavidoalba, P. citrinum, D. anthuriicola, S. terrestris, and X. cubensis. Xylaria cubensis was identified through the analysis of domain D1/D2 of the LSU region. While the ITS region is regarded as the standard barcode in fungal identification [24], the level of variability of this region is not uniform across all fungal species [25]. Apart from the ITS, the D1/D2 domain is also useful in the determination of fungal species based on DNA sequences [26]. It is proven in this study that fungal identification could be achieved by analyzing the D1/D2 region, particularly for the molecular identification of isolate ZOR-Rh1-1.
Endophytic fungi isolated from red ginger in the present study have been also previously reported from different host plants. Microdochium colombiense was formerly investigated for being an endophyte in Musa sapientum [27]. Phlebiopsis flavidoalba was reported as an endophyte associated with Gastrodia elata (Orchidaceae) [28]. Endophytic P. citrinum was repeatedly isolated from a wide range of hosts, such as Triticum aestivum [29], Jatropha heynei [30], Cephalotaxus mannii [31], and Rhodomyrtus tomentosa [32]. Dactylonectria anthuriicola was previously isolated from Anthurium sp. [33]. Setophoma terrestris was found to be associated with the inner tissue of Brassica oleracea var. acephala [34], Psidium guajava [35], and Armoracia rusticana [36]. Meanwhile, X. cubensis was reported as an endophytic fungus found in Litsea akoensis [37], Lychnophora ericoides [38], and Asimina triloba [39]. It is the first record of these endophytic fungi being isolated from red ginger.
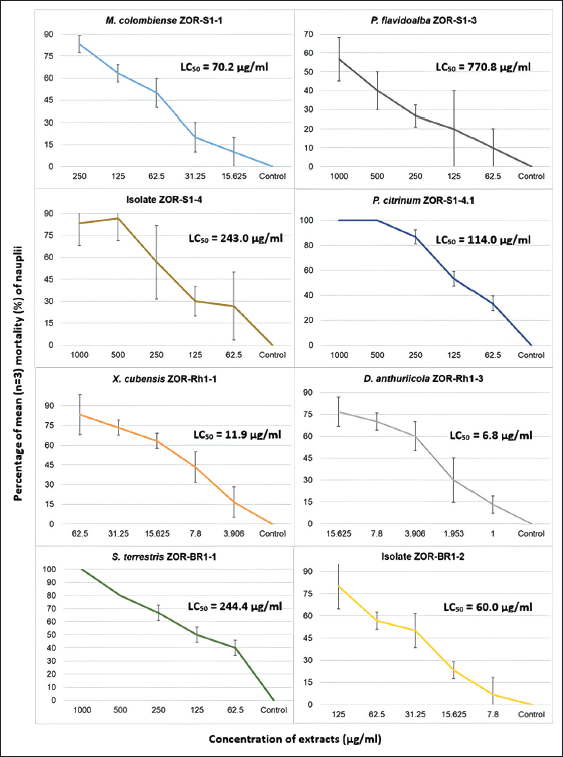 | Figure 5. The result toxicity assay through brine shrimp lethality test, showing the relationship between mean mortality percentage (n = 3) of nauplii (y axis) at each tested concentration of fungal methanolic extracts (µg/ml) (x axis). LC50 (µg/ml) of each methanolic extract is included in each graph. [Click here to view] |
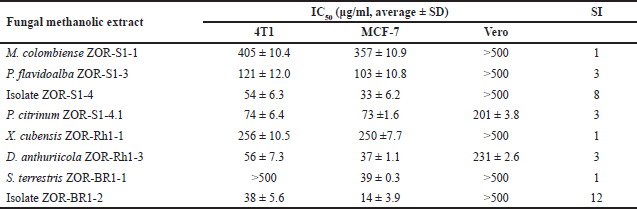 | Table 4. IC50 values (µg/ml) of the tested fungal extracts against 4T1, MCF-7, and Vero cell lines along with their SI. [Click here to view] |
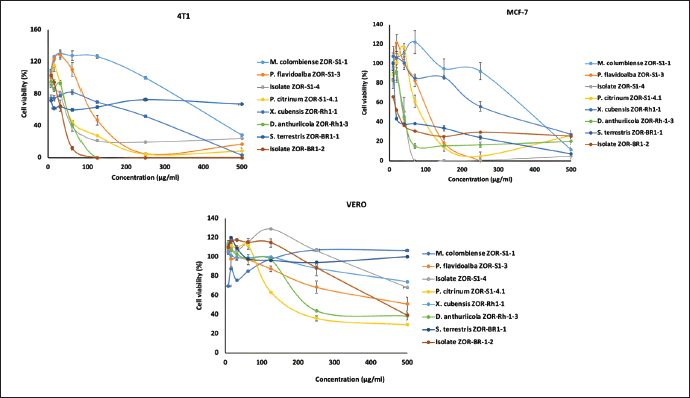 | Figure 6. Cytotoxicity of the metabolites against 4T1, MCF-7, and Vero cells. Cells were grown in a 96-well plate as described in the Materials and Methods. Cells were treated with metabolites at 5–500 μg/ml and incubated for 24 hours. The availability of the cells was analyzed using CCK-8 kit assay and then calculated based on their absorption at 490 nm. [Click here to view] |
Some isolated fungi in this study were previously shown to have antimicrobial and cytotoxic potential. Setophoma terrestris obtained from the leaves litter in the mangrove ecosystem produced secalonic acid A, penicillixanthone A, and hypothemycin, which displayed remarkable cytotoxic activities against colon and melanoma cell lines with IC50 values starting from 0.2 to 2.1 μM. In addition, secalonic acid G and blennolide J were also isolated from this fungal strain and exhibited antimicrobial activity against S. aureus with MIC values of 39 and 43 μg/ml, respectively [40]. Moreover, co-cultivation of S. terrestris with Bacillus amyloliquifaciens led to the isolation of a novel compound, blennolide K, which was found to be active against MCF-7 cells with an IC50 value of 4.8 μM [41]. Penicillium citrinum isolated from a hydrothermal vent was revealed to produce citrinin together with its derivatives, which served as promising leads in the development of α-glucosidase and ATP-citrate lyase inhibitory agents [42]. New citrinin derivatives, peniciriols A and B, were isolated from P. citrinum of Rhodomyrtus tomentosa, but did not show any cytotoxic and antibacterial activity [32]. Penicitrinols C and E from P. citrinum found in a sediment estuary in China, showed weak cytotoxic activities toward HL-60 cancer cells [43]. Secalonic acid A from P. citrinum of Tapiscia sinensis, was revealed to have cytotoxicity against an array of cancer cell lines with IC50 values ranging from 11–25 µg/ml [44].
Several investigations on Microdochium revealed that fungal strains from this genus could produce brocaeloids, cillifuranone, leptosphaeric acid, 15β-hydroxyl-(22E,24R)-ergosta-3,5,8,22-tetraen-one, (22E,24R)-6β-methoxyergosta-7,22-diene-3β,5α-diol [45], isocoumarins [46], and sesquiterpenes [47], with broad-ranging bioactivities, including anticancer, antimycobacterial [45], antifungal, antibacterial, and antialgal [46]. Furthermore, some bioactive compounds were formerly reported for being isolated from the species of Phlebiopsis, including hydroxystrepantibin D, p-terphenyls derivatives [48], phlebiopsin A–C, methyl-terfestatin A, and o-orsellinaldehyde [49], which showed antioxidant [48] and antifungal [49] activities.
Dactylonectria was reported to produce brefeldins A and C, 7-dehydrobrefeldin A, methyl tetradecanoate, anthraquinone ZSU-H85, and (3β,5α,6β,22E)-ergosta-7,22-diene-3,5,6-triol [50]. Moreover, X. cubensis was previously reported to produce polyhydroxyanthraquinones [39], succinic acid derivatives, mellein derivatives, cytochalasin D, 2-chloro-5-methoxy-3-methylcyclohexa-2,5-diene-1,4-dione, isosclerone [51], sesquiterpenoids, aliphatic derivative, alkaloids, and isocoumarins [37]. Cytochalasin D showed cytotoxicity toward KB cells with an IC50 value of 3.99 µg/ml. Meanwhile, compound 2-chloro-5-methoxy-3-methylcyclohexa-2,5-diene1,4-dione displayed mild growth inhibition against S. aureus and MRSA with MIC values of 128 µg/ml [51].
Based on the cytotoxicity assay of the fungal extracts in our study, we observed that although 4T1 cells and MCF-7 cells showed similar responses toward samples, the non-TNBC cells (MCF-7) cells were more sensitive than the TNBC cells (4T1 cells). In cancer therapy, TNBC and non-TNBC, which express estrogen receptors (ERs) and progesterone receptors (PRs), have gained much attention. Recent clinical studies have demonstrated the effectiveness of anti-endocrine drugs in treating HR-positive breast cancer. However, TNBC lacks an appropriate therapeutic target, and chemotherapy is the primary treatment option [52]. Conventional approaches to combating those problems have proven inadequate. Chemotherapy has drawbacks, especially when it comes to side effects that may reduce a patient’s quality of life. Lack of appetite, nausea, vomiting, constipation, and hair loss are a few of the side effects that chemotherapy patients frequently encounter [53].
It is well-known that the TNBC cells were characterized by lacking expression or no amplification of estrogen receptor, progesterone receptor, and HER-2. Therefore, TNBC also lacks targeted therapy. In the clinic, TNBC exhibits more aggressive cells, often relapses, short progression-free survival, and overall survival (OS) compared to non-TNBC. In addition, certain mutations including p53 and PIK3CA, increased expression of HIF1-α and MYC, amplification of cyclin E1, and loss of PTEN and RB1 have been associated with chemo-resistance of the TNBC patients [54]. On the other hand, MCF-7 is a model of ER+/PR+ cells that can be targeted via those receptors. The different sensitivities of 4T1 and MCF-7 cells (Table 4) in our study may indicate that the samples’ mechanism of action may involve more of the ER signaling. However, the IC50 values of the top four of the samples (unidentified fungal isolates ZOR-Br1-2 and ZOR-S1-4, D. anthuriicola ZOR-Rh1-3, and P. citrinum ZOR-S1-4.1) against 4T1 cells are relatively low (less than 100 µg/ml), giving a promising indication to be developed further. This includes the isolation of the main compound and characterization of the compound to seek a new candidate for breast cancer therapy, particularly the TNBC type.
CONCLUSION
Eight endophytic fungal strains, identified as M. colombiense ZOR-S1-1, P. flavidoalba ZOR-S1-3, P. citrinum ZOR-S1-4.1, Dactylonectria anthuriicola ZOR-Rh1-3, S. terrestris ZOR-Br1-1, X. cubensis ZOR-Rh1-1, including two unidentified isolates ZOR-S1-4 and ZOR-Br1-2, were isolated from red ginger. Extract of S. terrestris ZOR-Br1-1 was found to have the most substantial inhibition toward the growth of S. aureus and C. albicans with MIC of 31.3 and 15.6 µg/ml. Meanwhile, in the toxicity screening, D. anthuriicola ZOR-Rh1-3 extract revealed the most potent toxicity with the LC50 value of 6.8 µg/ml. When subjected to cytotoxicity testing on breast cancer cells MCF-7 and 4T1, extracts of D. anthuriicola ZOR-Rh1-3, P. citrinum ZOR-S1-4.1, unidentified isolates ZOR-S1-4 and ZOR-Br1-2, showed strong to moderate activity with IC50 values starting from 14 to 74 µg/ml. Given their bioactivity potentials, further studies of S. terrestris ZOR-Br1-1, D. anthuriicola ZOR-Rh1-3, and P. citrinum ZOR-S1-4.1 extracts on their antimicrobial and cytotoxic metabolites hold significance in the bioprospecting of endophytic fungi.
ACKNOWLEDGMENT
The authors thank Dr. Junita Hardini for her assistance in the authentication of the host plant red ginger, used in this study.
AUTHOR CONTRIBUTIONS
Conceptualization and research design are done by NPA. Data acquisition, analysis, and interpretation are done by NPA, IPYAP, NPEK, NN, RIJ, and EM. Drafting and revision of the manuscript, as well as a review of the final version of the manuscript, are done by NPA, IPYAP, NPEK, NN, RIJ, and EM. Funding acquisition is done by NPA, NPEK, and RIJ. All authors have read and agreed to the published version of the manuscript.
FINANCIAL SUPPORT
Research funding from Universitas Udayana, Bali, Indonesia, grant no. B/78.842/UN14.4.A/PT.01.03/2022 is gratefully acknowledged. Research on cytotoxicity assay was supported by the Post-doctoral Program Batch II 2022 from the Directorate of Research, Universitas Gadjah Mada, Yogyakarta, Indonesia, grant no. 13602/UN1.P.II/Dit-Lit/PT.01.04/2022.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Wen J, Okyere SK, Wang S, Wang J, Xie L, Ran Y, et al. Endophytic fungi: an effective alternative source of plant-derived bioactive compounds for pharmacological studies. JoF. 2022;8:205. CrossRef
2. Rai N, Kumari Keshri P, Verma A, Kamble SC, Mishra P, Barik S, et al. Plant Associated fungal endophytes as a source of natural bioactive compounds. Mycology. 2021;12:139–59. CrossRef
3. Gupta A, Meshram V, Gupta M, Goyal S, Qureshi KA, Jaremko M, et al. Fungal endophytes: microfactories of novel bioactive compounds with therapeutic interventions; a comprehensive review on the biotechnological developments in the field of fungal endophytic biology over the last decade. Biomolecules. 2023;13:1038. CrossRef
4. Strobel G. The emergence of endophytic microbes and their biological promise. J F. 2018;4:57. CrossRef
5. Ariantari NP, Frank M, Gao Y, Stuhldreier F, Kiffe-Delf A-L, Hartmann R, et al. Fusaristatins D–F and (7S,8R)-(−)-Chlamydospordiol from Fusarium sp. BZCB-CA, an Endophyte of Bothriospermum chinense. Tetrahedron. 2021;85:132065. CrossRef
6. Ariantari NP, Putra IPYA, Leliqia NPE, Yustiantara PS, Proborini MW, Nugraheni N, et al. Antibacterial and cytotoxic secondary metabolites from endophytic fungi Associated with Antidesma bunius Leaves. J Appl Pharm Sci. 2023;13:132–43. CrossRef
7. Marwan H, Hayati I, Mulyati S. Effectiveness of biofungicide formula on rhizome rot disease of red ginger and its plant growth. Biodiversitas. 2023;24:2143–8. CrossRef
8. Khafyah N, Dewi ST, Jumain. The effectiveness of red ginger extract (Zingiber officinale var. rubrum) on decreased blood glucose levels in mice (Mus musculus). Ihj. 2023;2:16–21. CrossRef
9. Razali N, Dewa A, Asmawi MZ, Mohamed N, Manshor NM. Mechanisms underlying the vascular relaxation activities of Zingiber officinale var. rubrum in thoracic aorta of spontaneously hypertensive ats. J Integr Med. 2020;18:46–58. CrossRef
10. Fajrin FA, Imandasari N, Barki T, Sulistyaningrum G, Afifah, Kristiningrum N, et al. The antioxidant activity of red ginger oil in aloxan-induced painful diabetic neuropathy in mice model. Thai J Pharm Sci. 2019;43:69–75
11. Rialita T, Nurhadi B, Puteri RD. Characteristics of microcapsule of red ginger (Zingiber officinale var. rubrum) essential oil produced from different arabic gum ratios on antimicrobial activity toward Escherichia coli and Staphylococcus aureus. Int J Food Prop. 2018;21:2500–8. CrossRef
12. Rinanda T, Isnanda RP, Zulfitri. Chemical analysis of red ginger (Zingiber officinale Roscoe var rubrum) essential oil and its anti-biofilm activity against Candida albicans. Nat Prod Commun. 2018;13:1587–90. CrossRef
13. Yamauchi K, Natsume M, Yamaguchi K, Batubara I, Mitsunaga T. Structure-activity relationship for vanilloid compounds from extract of Zingiber officinale var rubrum Rhizomes: effect on extracellular melanogenesis inhibitory activity. Med Chem Res. 2019;28:1402–12. CrossRef
14. Strobel G, Daisy B, Castillo U, Harper J. Natural products from endophytic microorganisms. J Nat Prod. 2004;67:257–68. CrossRef
15. Caruso G, Abdelhamid MT, Kalisz A, Sekara A. Linking endophytic fungi to medicinal plants therapeutic activity. A case study on Asteraceae. Agriculture. 2020;10:286. CrossRef
16. Ginting RCB, Sukarno N, Widyastuti U, Darusman LK, Kanaya S. Diversity of endophytic fungi from red ginger (Zingiber officinale Rosc.) plant and their inhibitory effect to Fusarium oxysporum plant pathogenic fungi. HAYATI J Biosci. 2013;20:127–37. CrossRef
17. Handayani D, Sari HC, Julianti E, Artasasta MA. Endophytic fungus isolated from Zingiber officinale Linn. var. rubrum as a source of antimicrobial compounds. J App Pharm Sci. 2023;13:115–20. CrossRef
18. Putra IPYA, Utami KS, Hardini J, Wirasuta IMAG, Ujam NT, Ariantari NP. Fermentation, bioactivity and molecular identification of endophytic fungi isolated from mangrove Ceriops tagal. Biodiversitas. 2023;24:3091–8. CrossRef
19. CLSI. M100—Performance standards for antimicrobial susceptibility testing. 32nd ed. Wayne, PA: CLSI; 2022
20. Niksic H, Becic F, Koric E, Gusic I, Omeragic E, Muratovic S, et al. Cytotoxicity screening of Thymus vulgaris L. Essential oil in brine shrimp nauplii and cancer cell lines. Sci Rep. 2021;11:13178. CrossRef
21. Waghulde S, Kale MK, Patil V. Brine shrimp lethality assay of the aqueous and ethanolic extracts of the selected species of medicinal plants. Proceedings. 2019;41:47. CrossRef
22. Nordin ML, Abdul Kadir A, Zakaria ZA, Abdullah R, Abdullah MNH. In vitro investigation of cytotoxic and antioxidative activities of Ardisia crispr against breast cancer cell lines, MCF-7 and MDA-MB-231. BMC Complement Altern Med. 2018 Mar 12;18(1):87. CrossRef
23. Prayong P, Barusrux S, Weerapreeyakul N. Cytotoxic activity screening of some indigenous thai plants. Fitoterapia. 2008;79:598–601. CrossRef
24. Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a Universal DNA barcode marker for fungi. Proc Natl Acad Sci U. S. A. 2012;109:6241–6. CrossRef
25. Nilsson RH, Kristiansson E, Ryberg M, Hallenberg N, Larsson K-H. Intraspecific ITS variability in the kingdom fungi as expressed in the international sequence databases and its implications for molecular species identification. Evol Bioinform Online. 2008;4:193–201. CrossRef
26. Kang M-J, Choi Y-S, Kim S. A comparison of the ability of fungal internal transcribed spacers and D1/D2 domain regions to accurately identify Candida glabrata clinical isolates using sequence analysis. Biomed Sci Lett. 2018;24:430–4. CrossRef
27. Hernández-Restrepo M, Groenewald JZ, Crous PW. Taxonomic and phylogenetic re-evaluation of Microdochium, Monographella and Idriella. Persoonia. 2016;36:57–82. CrossRef
28. Li T, Gao JL, Huang JH, Gu L, Zou J, Wu XJ. Phlebiopsis xuefengensis sp. Nov. from Gastrodia elata (Orchidaceae) in Hunan Province, Southern China. S Afr J Bot. 2021;142:299–304. CrossRef
29. Kaur R, Saxena S. Penicillium citrinum, a Drought-tolerant endophytic fungus isolated from Wheat (Triticum aestivum L.) leaves with plant growth-promoting abilities. Curr Microbiol. 2023;80:184. CrossRef
30. Ashoka GB, Shivanna MB. Antibacterial, antioxidant, and anticancer activities of Penicillium citrinum Thom. Endophytic in Jatropha heynei. J App Pharm Sci. 2023;13:196–207. CrossRef
31. Vu THN, Quach NT, Le PC, Pham QA, Do TT, Chu HH, et al. Bioprospecting endophytic fungi isolated from Cephalotaxus mannii Hook f. as prolific sources of antibacterial, anticancer, and antioxidant agents. Microbiology. 2023;92:284–92. CrossRef
32. Wei S, Sang Z, Zhang Y, Wang H, Chen Y, Liu H, et al. Peniciriols A and B, two new citrinin derivatives from an endophytic fungus Penicillum citrinum TJNZ-27. Fitoterapia. 2023;169:105572. CrossRef
33. Cabral A, Groenewald JZ, Rego C, Oliveira H, Crous PW. Cylindrocarpon root rot: multi-gene analysis reveals novel species within the Ilyonectria radicicola species complex. Mycol Progress. 2012;11:1–34. CrossRef
34. Poveda J, Zabalgogeazcoa I, Soengas P, Rodríguez VM, Cartea ME, Abilleira R, et al. Brassica oleracea var. acephala (Kale) improvement by biological activity of root endophytic fungi. Sci Rep. 2020;10:20224. CrossRef
35. de Medeiros LS, Abreu LM, Nielsen A, Ingmer H, Larsen TO, Nielsen KF, et al. Dereplication-guided isolation of depsides Thielavins S–T and lecanorins D–F from the endophytic fungus Setophoma sp. Phytochemistry. 2015;111:154–62. CrossRef
36. Szucs Z, Plaszkó T, Cziáky Z, Kiss-Szikszai A, Emri T, Bertóti R, et al. Endophytic fungi from the roots of Horseradish (Armoracia rusticana) and their interactions with the defensive metabolites of the glucosinolate–myrosinase–isothiocyanate system. BMC Plant Biol. 2018;18:85. CrossRef
37. Fan N-W, Chang H-S, Cheng M-J, Hsieh S-Y, Liu T-W, Yuan G-F, et al. Secondary metabolites from the endophytic fungus Xylaria cubensis. Helv Chim Acta. 2014;97:1689–99. CrossRef
38. Caraballo-Rodríguez AM, Mayor CA, Chagas FO, Pupo MT. Amphotericin B as an inducer of griseofulvin-containing guttate in the endophytic fungus Xylaria Cubensis FLe9. Chemoecology. 2017;27:177–85. CrossRef
39. Sica VP, Rees ER, Tchegnon E, Bardsley RH, Raja HA, Oberlies NH. Spatial and temporal profiling of griseofulvin production in Xylaria cubensis using mass spectrometry mapping. Front Microbiol. 2016;7:544. CrossRef
40. El-Elimat T, Figueroa M, Raja HA, Graf TN, Swanson SM, Falkinham III JO, et al. Biosynthetically distinct cytotoxic polyketides from Setophoma terrestris. EurJOC. 2015;2015:109–21. CrossRef
41. Arora D, Chashoo G, Singamaneni V, Sharma N, Gupta P, Jaglan S. Bacillus amyloliquefaciens induces production of a novel blennolide K in coculture of Setophoma terrestris. J Appl Microbiol. 2018;124:730–9. CrossRef
42. Chen S, Tian D, Wei J, Li C, Ma Y, Gou X, et al. Citrinin derivatives from Penicillium citrinum Y34 that inhibit α-glucosidase and ATP-citrate lyase. Front Mar Sci. 2022;9:961356. CrossRef
43. Chen L, Liu W, Hu X, Huang K, Wu J-L, Zhang Q-Q. Citrinin derivatives from the marine-derived fungus Penicillium citrinum. Chem Pharm Bull. 2011;59:515–7. CrossRef
44. Li X, Zhang L, Liu Y, Guo Z, Deng Z, Chen J, et al. A New metabolite from the endophytic fungus Penicillium citrinum. Nat Prod Commun. 2013;8:1934578X1300800. CrossRef
45. Zhang J, Wang Z, Song Z, Karthik L, Hou C, Zhu G, et al. Brocaeloid D, a novel compound isolated from a wheat pathogenic fungus, Microdochium majus 99049. Synth Syst Biotechnol. 2019;4:173–9. CrossRef
46. Zhang W, Krohn K, Draeger S, Schulz B. Bioactive isocoumarins isolated from the endophytic fungus Microdochium bolleyi. J Nat Prod. 2008;71:1078–81. CrossRef
47. Gavrilova OP, Orina AS, Kessenikh ED, Gustyleva LK, Savelieva EI, Gogina NN, et al. Diversity of physiological and biochemical characters of Microdochium fungi. C B. 2020;17:e2000294. CrossRef
48. Choi D-C, Ki D-W, Kim J-Y, Lee I-K, Yun B-S. P-Terphenyl glucosides from the culture broth of Phlebiopsis castanea. J Antibiot. 2023;76:52–5. CrossRef
49. Kälvö D, Menkis A, Broberg A. Secondary metabolites from the root rot biocontrol fungus Phlebiopsis gigantea. Molecules. 2018;23:1417. CrossRef
50. Ming Q, Huang X, He Y, Qin L, Tang Y, Liu Y, et al. Genome mining and screening for secondary metabolite production in the endophytic fungus Dactylonectria alcacerensis CT-6. Microorganisms. 2023;11:968. CrossRef
51. Klaiklay S, Rukachaisirikul V, Sukpondma Y, Phongpaichit S, Buatong J, Bussaban B. Metabolites from the mangrove-derived fungus Xylaria cubensis PSU-MA34. Arch Pharm Res. 2012;35:1127–31. CrossRef
52. Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Primers. 2019;5:66. CrossRef
53. Altun I, Sonkaya A. The most common side effects experienced by patients were receiving first cycle of chemotherapy. Iran J Public Health. 2018;47:1218–9
54. Lee K-L, Kuo Y-C, Ho Y-S, Huang Y-H. Triple-negative breast cancer: current understanding and future therapeutic breakthrough targeting cancer stemness. Cancers. 2019;11:1334. CrossRef