INTRODUCTION
Psoriasis is an incredibly prevalent, long-lasting, immune-related, inflammatory, papulosquamous, non-contagious, uncomfortable, and debilitating hyperproliferative dermatological disorder. This is identifiable through erratically excessive keratinocyte formation along with distinction, and also the buildup of leukocytes with polymorphonuclear structures in the epidermis, as well as the activation of T cells. Typically, erythematous papules or lesions surrounding silvery scales are the most prevalent method to identify this [1–3] In the study by Nabawy Mohamed et al. [4], the most common clinical indicators of psoriasis include skin scaling in around 92% of cases, itchiness in 72%, redness in 69%, lethargy in 27% of cases, inflammation in 23% of cases, blistering in 20% of cases, and oozing of blood in 20% of cases. Psoriasis falls within the National Organization for Rare Disorders criteria of a rare inflammatory, persistent, recurring dermatological disorder [5]. In accordance with Egeberg et al. (2019) and Sewerin et al. (2019), the global incidence of psoriasis is estimated to be between 2% and 3% worldwide and between 8% and 11% in several Northern European nations [6,7]. Despite the fact that this disease can appear at any point in life, it frequently appears in people between the age of 50 and 60 years [8]. The extent of the illness ranges from a fingernail pit to superficial lesions (on a small area of skin to the whole body which can be linked to arthritis).
The long-term use of synthetic drugs to treat psoriasis is strongly linked to serious side effects, such as the development of stomach ulcers, skin and bone thinning, and early cataracts. Other side effects include dermatitis from topical vitamin D use, alopecia from long-term use of salicylic acid on the scalp, and xeroderma from coal tar products. Therefore, safety monitoring is crucial during psoriasis treatment [9,10].
?Aloe emodin, also known as 1, 8-dihydroxy-3-(hydroxymethyl)-9, 10-anthraquinone, is a naturally occurring anthraquinone derivative, found in the latex of Aloe vera [11,12]. It has numerous therapeutic advantages and is a non-carcinogenic medication. Aloe emodin exhibits an extensive spectrum of pharmacological characteristics, including anti-proliferative, anticancer, wound healing, analgesic, anti-inflammatory, antibacterial, and antiviral properties, which have proven to be beneficial and effective for treating psoriasis, as reported by the most recent research [13,14]. Aloe emodin serves as one of the active ingredients of the liquid solution ProZ92, which is sold as a natural psoriasis treatment [15]. A number of proteins, including Protein Kinase C (PKC), B-cell lymphoma 2 (bcl-2), caspases (linked to apoptosis and antiproliferative effects), and other members of the Mitogen-activated Protein Kinase family (linked to wound healing activity), have been shown to be regulated by aloe emodin, which has been demonstrated to possess therapeutic benefits [16]. Aloe emodin is quickly distributed in the blood, rapidly metabolized into aloe emodin glucuronides and rhein sulfates along with other unidentified metabolites with a terminal half-life of 50 hours and is eliminated via urine and feces, following first-order kinetics and two-compartment model [17,18]. High levels of aloe emodin have been reported in the liver, spleen, and renal tissues of healthy rats compared with those in experimental rats. Given its poor intestinal absorption, short elimination half-life, and low bioavailability, traditional oral administration of aloe emodin is not the most effective method of application [19].
To the best of our knowledge, there has been no documented work for aloe emodin alone in the form of topical hydrogel using polymers such as carbopol 940, chitosan, and hydroxyl propyl methyl cellulose K15M (HPMC K15M). The objective of the present study was to investigate the pharmacological potential of aloe emodin-loaded topical hydrogel in the management of imiquimod (IMQ)-induced human plaque-type psoriasis in BALB/c mice.
MATERIALS AND METHODS
Materials
Aloe emodin 95% was procured from Avra Synthesis Pvt. Ltd. Ananthpuram, Andhra Pradesh. Polymers Carbopol 940, Chitosan, and HPMCK15M were procured from Himedia Pvt. Ltd, Triethanolamine from Loba Chemie Pvt. Ltd., analytical-grade Methanol, Rankem Chemicals.
Methods
Preformulation study
A preformulation study of aloe emodin included melting point by a capillary method using digital melting point apparatus, solubility, and partition coefficient determination by quantitative shake flask method [20–22]. The compatibility of aloe emodin with selected polymers was evaluated using differential scanning calorimetry (DSC). An isothermal stress testing method was used in the stability testing device (T26/HAO-L, Technico) at 50°C for 4 weeks. The samples were scanned from 30°C to 300°C at a rate of 30°C per minute temperature change with a hold time of 30°C for 1 minute using DSC [23–25].
Optimization of polymeric concentrations and preparation of topical hydrogels
Blank polymeric hydrogels were prepared using a series of concentrations of carbopol 940, chitosan, and HPMC K15M from 0.5%–4.0% w/v by soaking them in respective gelling solvents for 6 hours and mixing them to form a homogeneous mixture. Physicochemical properties such as physical appearance, pH, viscosity, and spreadability were evaluated as optimizing parameters [26–28]. Formulations showing the desired physicochemical properties were selected for aloe emodin loading. The hydrogels were prepared by adding 1.0 g each of carbopol 940, chitosan, and HPMC K15M in 100 ml of 20:80 mixture of propylene glycol and purified water (in case of carbopol 940 and HPMC K15M) to aid solubility of aloe emodin and 100 ml of 1% solution of glacial acetic acid in purified water (in case of chitosan) as chitosan exhibits its gelling in acidic media. The mixture was soaked for 6 hours and then stirred constantly using a magnetic stirrer (100 rpm) for a further 6 hours until complete gelling (care should be taken to avoid air entrapment). To this was added 1.0 g of aloe emodin dissolved in analytical-grade methanol, Rankem Chemicals while stirring the content continuously. The dispersion was neutralized and cross linked by drop wise addition of triethanolamine (in case of carbopol 940). Thereafter, the hydrogel was sonicated to remove entrapped air bubbles. The formulation was further allowed to stabilize at room temperature for 24 hours. The prepared hydrogels (20 g) were filled in clean wide-mouthed tightly capped bottles [26–28]. The Formula for the optimized batches of hydrogel formulations is depicted in Table 1.
Characterization of aloe emodin-loaded hydrogel formulations
Physical appearances of the developed hydrogels were evaluated by visual perception and with the help of a simple microscope [25,28,29]. The pH of the prepared formulations was determined using a pre-calibrated digital pH meter (Microanalytica, SD Fine Chem LLP) [25,30,31]. The viscosities and rheological behavior of the prepared hydrogels were assessed at 25°C ± 0.5°C using a Brookfield viscometer RVDV-E with Spindle no. 6 (spindle factor 1,000) at 50 rpm [25,30]. A self-assembled system with two slides (an upper moving slide and a lower moving slide) was used to measure the spreadability and the amount of time consumed for the upper slide to separate from the fixed bottom slide was measured after spreading 2.0 g of gel over the pan. Spreadability was then calculated using the following formula
 | Table 1. Formulation of topical hydrogels. [Click here to view] |
where S is spreadability; W is the weight (g) of the tide to the upper slide; L is the length (cm) of glass slide; T is the time (s) taken to separate the slide completely [24,25,30] The extrudability test measured the weight required to extrude a topical hydrogel ribbon measuring 0.5 cm in length in 10 s from a coated aluminum tube. The following formula was then used to calculate the extrudability: [30,31].
For drug loading, an accurately weighed 50 mg quantities of prepared topical hydro gel were dissolved in 50 ml methanol and shaken for 2 hours on a mechanical stirrer to promote the solubility of the incorporated aloe emodin. These solutions were quantitatively transferred to volumetric flasks, and appropriate dilutions were made. The solution was then filtered using 0.45-mm Millipore filter paper and measured spectrophotometrically at 428.5 nm against methanol as the blank. The following equation was used to calculate the amount of drug loaded: [25,32,33].
where Ic is the initial concentration and Fc is the final concentration.
Franz diffusion cell with 25 ml receiver volume capacity and 0.77 cm2 diffusion area was used to evaluate the in vitro drug permeation of prepared hydrogels in pH 6.8 phosphate buffer solution using presaturated dialysis membrane (MWCO 12–14 k Da) at 37°C ± 0.5ºC with continuous stirring of the receiver contents. During the process, equal volumes of samples (1.0 ml) were withdrawn at hourly intervals and replenished with the same volume of phosphate buffer (pH 6.8). All samples were estimated spectrophotometrically for aloe emodin at 428.5 nm. The cumulative percentage of drug permeated was calculated for each time (in hour) interval. In vitro drug release data were interpreted using zero-order, first-order, and Higuchi and Korsmeyer-Peppas kinetic models [24,25]. The abdomen skin of mice with an area of 0.77 cm2 was clamped between the donor and receptor compartments of the diffusion cell in such a way that the stratum corneum side of the skin was toward the donor compartment. The skin was harvested and washed with isopropyl alcohol to remove fat. Permeation studies in triplicate were conducted to quantify the permeated drug per unit area and time [34,35].
Accelerated stability study
To determine the durability of topical hydrogel compositions containing 1% w/w aloe emodin, both hot and cold temperature cycling were considered. In airtight glass containers, each topical hydrogel formulation was alternately maintained at 4°C for 24 hours and 45°C for 24 hours with 75% ± 2% relative humidity (RH) for six cycles. Furthermore, each gel was divided into three samples, which were kept under different storage conditions of 4°C ± 1ºC, 25°C ± 1ºC, and 45°C ± 1ºC with 75% ± 2% RH and observed for the same period as that of the temperature cycling test. Before and after the stability test, the physical properties, including color, homogeneity was determined by visual observation, pH, using a pre-calibrated digital pH meter (Microanalytica, SD Fine Chem LLP), the viscosities and rheological behavior of the prepared hydrogels were assessed at 25°C ± 0.5°C using a Brookfield viscometer RVDV-E with Spindle no. 6 (spindle factor 1,000) at 50 rpm, and spreadability by a self-assembled system with two slides (an upper moving slide and a lower moving slide) as per the procedure described earlier in formulation characterization [36,37].
In vivo skin irritation, antipsoriatic, and acute dermal toxicity study
The animal studies were carried out at the College of Pharmacy, Sir Satya Sai University of Technology and Medical Sciences, Sehore, India and the study protocol was approved by IAEC of Sir Satya Sai University with the approval no: 1587/PO/Re/S/11/CPCSEA. Draize patch test on mice was used for the skin irritation test. Animals in good health were divided into four groups, each with five animals. Hairs on the dorsal surface skin were removed 24 hours before the start of the experiment. No formulation was utilized for group 1 (the control group); whereas, a single dose of 0.8% formalin solution, 0.1% tacrolimus cream, and aloe emodin-loaded topical hydrogel were applied to group 2 (the negative control), group 3 (standard drug treated group) and group 4 (aloe emodin hydrogel treated group), respectively. At 24, 48, and 72 hours later, the skin was rinsed with saline water. The extent of erythema and edema were considered while calculating the mean score using the previously published grading system for skin reactions. Each rabbit received a 0–4 score of primary irritation at 24, 48, and 72 hours after therapy, depending on the degree of erythema shown. Based on the presence of edema, a second 0–4 score was considered. The irritation reaction in each group was categorized using the primary irritation index [15,34,38,39].
?PII = Σ SPI (Test) Σ SPI (Blank)/Number of animals (v)
The antipsoriatic potential of the selected aloe emodin topical hydrogel was evaluated using an IMQ-induced human plaque-type psoriasis model in BALB/c mice [40,41]. Female Swiss albino mice (8–10 weeks old) weighing approximately 20–25 g were procured from the animal facility center. Throughout the entire period of acclimatization and experimentation, mice (n = 6) were kept in cages with a 12-hour light/dark cycle, controlled temperature (23°C), and humidity (50%) along with free access to regular food pellet diet and water [42]. The animals were divided randomly into four experimental groups, each with six animals, after 7 days of acclimatization. Gp1: Naive group/untreated animals (Healthy animals receiving no induction and treatment); Gp2: Negative control (Animals with the disease only); Gp3: Standard control (subjects receiving standard tacrolimus ointment); and Gp4: Animals treated with the chosen aloe emodin topical hydrogel. Following the next day of hair removal, psoriasis-like effects were induced in all animal groups except the normal control group by careful application (avoiding direct contact to IMQ cream) of marketed IMQ cream 5% Imiquad® at a dose of 62.5 mg cream (containing 3.125 mg of the active compound daily) for 11 consecutive days [40,41]. The standard control group animals received a dose of 10 mg/kg/day of tacrolimus cream 0.1% daily from 12th to 24th days of the treatment period, whereas the treatment group received the prepared topical hydrogel formulation of aloe emodin at a dose of 50 mg/kg/day for the same study period as that of the standard control group of animals. PASI scoring was employed to determine the severity of redness, skin thickness, and scaling on 1st, 3rd, 7th, 11th, 14th, 17th, 21st, and 25th days. Ear thickness was measured using a calibrated digital screw gauge [43]. On the 25th day, the animals were sacrificed, and skin sections of representative animals from each group were isolated and stored in 10% v/v formalin in normal saline and stained with hematoxylin and eosin for histopathological evaluation to determine pathological changes such as acanthosis, inflammatory infiltrates, hyperkeratosis, and parakeratosis, which are landmarks of psoriasis induction. Images were recorded using a microscope (Nikon, USA) at 10 magnification. Blood samples (1.0 ml) were collected from the tail veins on the 25th day for tissue necrosis factor (TNF-α) assay employing enzyme-linked immunosorbent assay (ELISA) kits.
For the acute dermal toxicity, both male and female rats were divided into two groups for the investigation. The dorsal skin surface’s hair was shaved. Animals in the test group had their dorsal skin surface treated with the prepared hydrogel. Body weight, scoring for acute dermal toxicity, and hematological parameters were assessed during the experiment, which lasted for 14 days and were then compared with the animals in the control group [44].
 | Figure 1. DSC curve for drug polymer interaction study; a) aloe emodin b) carbopol 940 c) physical mixture of aloe emodin and carbopol (1:1) Note ‘larger images are available in supplementary materials’. [Click here to view] |
 | Table 2. Optimization of polymer concentration for blank hydrogels. [Click here to view] |
 | Table 3. Characterization of hydrogel formulations. [Click here to view] |
 | Figure 2. a) Cumulative % drug permeated curve for PHGL1, 2, and 3 aloe emodin hydrogel containing carbopol 940, chitosan, and HPMC K15M, b) Ex vivo drug permeation curve for PHGL1 aloe emodin hydrogel containing carbopol 940 as gelling agent. [Click here to view] |
 | Table 4. Kinetic study of in vitro and ex vivo drug permeation. [Click here to view] |
Statistical analysis of data
Data obtained from the results of the antipsoriatic study were expressed as Mean ± SD and analyzed using one-way analysis of variance (ANOVA) at a 95% confidence level followed by Dunnett’s test. p values <0.05 were considered significant.
RESULTS
In the current research, an attempt was made to formulate and assess aloe emodin topical hydrogel containing aloe emodin as the therapeutic component and chitosan, HPMC K15M, and carbopol 940 as gel-forming agents (0.25%–4.0% w/v) for managing plaque-like psoriasis. This study involved skin sensitivity evaluation, acute dermal toxicity test, in vivo anti-psoriatic investigation, in vitro and ex vivo drug penetration studies, and rapid stability evaluations.
Preformulation study
During the preformulation studies, the melting point of aloe emodin was found to be 224°C, whereas its solubility and partition coefficient was reported as 0.0123 ± 0.00152 µg/ml in phosphate buffer pH 6.8 and 2.885 ± 0.686 in n-octanol and water (1:1), respectively. Experimental investigations established the label-compliant melting point of aloe emodin at 224ºC, confirming the compound’s purity. The results of DSC were juxtaposed (Fig. 1) with the individual thermograms to determine whether the distinctive troughs in the DSC curve associated with the drug-polymer mixture had appeared or disappeared. Aloe emodin and carbopol 940 had indicative troughs at 224°C and 116ºC, respectively, whereas the mixture›s DSC demonstrated melting points that were on the higher end of the range, at 232°C and 124°C, respectively (Fig. 1). Results of different physicochemical properties of prepared blank polymeric hydrogels prepared with different concentrations (0.5%–4.0%) of polymers are depicted in table 2.
 | Table 5. Accelerated stability study of the selected topical hydrogel formulation. [Click here to view] |
 | Figure 3. i) Phenotypical observations of dorsal skin, ii) Phenotypical observations of change in ear pinna, and iii) Histopathological images of skin sections in a) Untreated mice group with no treatment, b) Disease control group initially treated with IMQ 5% cream for 11 consecutive days showing psoriasis like inflammation and erythema lesions, c) Standard drug treated group with topical applications of tacrolimus 0.1% cream, and d) aloe emodin hydrogel treated group with topical application 1% aloe emodin hydrogel from day 12 onward; and iv) Data representing one way ANOVA analysis of in vivo antipsoriatic study of the prepared topical aloe emodin hydrogel formulation for a) change in ear thickness during antipsoriatic study with ****p < 0.0001 vs. Normal control, disease induced and standard drug treated group, b) PASI score analysis during disease induction period (1–11 days) with *p < 0.01 vs. normal control group, and c) PASI score analysis during treatment phase (12–24 days) with **p < 0.01 vs. normal control group. [Click here to view] |
 | Figure 4. Results of the ELISA experiment performed for (TNF-α) with skin homogenate using ELISA kits as per the manufacturer’s protocol. Values are expressed as mean ± SD, n = 6/group with *p < 0.01 versus normal control group and disease-induced group.**p < 0.001 versus normal control and disease induce group. [Click here to view] |
Characterization of optimized hydrogel formulations
Near the required pH range of 5.4–6.8, the produced topical hydrogels’ pH was found to range from 6.867 ± 0.015 to 6.91 ± 0.254 when studied in triplicate (Table 3) [45]. The measured viscosity of the formulation was found to be 31402.33 ± 149.0783 mPaS (Table 3). According to rheological findings from the study, a pseudoplastic (non-Newtonian) system exhibited shear thinning along with thixotropic behavior at 25°C as viscosity decreased with increasing shear rate [46]. The prepared 1% topical hydrogel had an excellent spreadability of 16.257 ± 0.946 g.cm/s (spreadability was measured as the diameter of the circle) (Table 3) compared with a previously reported range of 2.5–3.4 g.cm/s [47]. When 100 g was used, the total amount of hydrogel formulation of 1% aloe emodin was 72.283% ± 0.481% (Table 3). When aloe emodin gel was prepared with carbopol 940, chitosan, and HPMC K15M, the difference in percentages was quite significant, with carbopol at 85.773% (±0.621), chitosan at 66.383% (±0.284), and HPMC K15M at 77.48% (±1.061), which indicated that employing carbopol for drug loading was more effective than chitosan and HPMC K15M (Table 3).
 | Table 6. Scoring for acute dermal toxicity studies. [Click here to view] |
 | Table 7. Hematological and blood parameters. [Click here to view] |
In vitro and ex vivo drug permeation studies:
When calculating the cumulative percentage of drug released for aloe emodin topical hydrogels 1%, the hydrogel with carbopol 940 released the maximum concentration of drug (78%) of all three preparations (Fig. 2a). The hydrogel exhibiting the highest release was subjected to an ex vivo drug permeation assay, which revealed almost 84% of drug release (Fig. 2b) during a prolonged 8 hours investigation. When studied for zero, first, Higuchi’s, and Korsemeyer-Peppa’s release kinetic models, R2 value was found to be highest (0.993) in the Korsemeyer-Peppa’s kinetic model (Table 4).
Accelerated stability studies
The prepared formulation was observed to be a clear translucent viscous gel with no grittiness or homogeneity throughout the stability experiments. Variation in pH, viscosity, spreadability, and percentage drug assay was found to vary from 6.747 ± 0.025 to 6.937 ± 0.051, 31423 ± 114.896 to 31803 ± 168.250, 17.202 ± 0.567 to 17.884 ± 0.565, and 86.317 ± 0.489 to 94.286 ± 0.343, respectively (Table 5).
In vivo skin irritation, antipsoriatic, and acute dermal toxicity studies
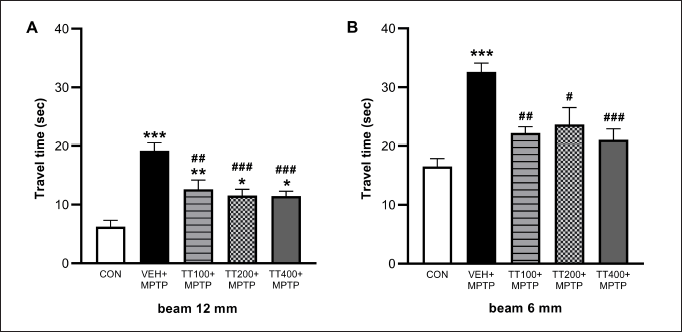
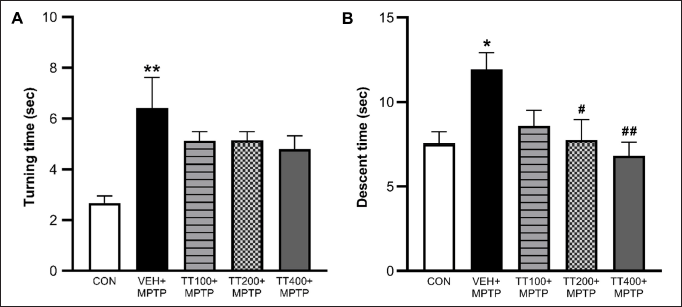
There were no signs of erythema and edema when scoring was performed in comparison with the normal controlled and the irritation-induced groups of animals. Local application of IMQ cream (5%) resulted in pathological alterations resembling psoriasis. Further evidence of psoriasis-like development in Gp2 was provided by high PASI scores (8.55 ± 0.49), increased ear thickness (245.17 ± 4.36 μm), and increased levels of TNF-α (126.11 ± 1.45pg/ml) compared with Gp1 (0.0 ± 0.0, 242.33 ± 0.96 µm and 23.14 ± 0.45pg/ml for PASI score, ear thickness and TNF-α level, respectively). By day 11, the Gp2 mice exhibited typical psoriasis-like dermatitis with mildly intrusive erythema (Fig. 3. i.). On days 1, 3, 7, and 11, the PASI score in Gp2 increased steadily (0.54 ± 0.15 to 8.55 ± 0.49) (Fig. 3.iv.b, 3.iv.c.). The characteristic psoriasis symptoms of parakeratosis, Munro’s microabscesses, prominent acanthosis with prolonged rete ridges, and severe inflammatory cell infiltration were observed upon histopathological examination (Fig. 3.iii.b.). TNF-α levels were substantially higher in the Gp2 (126.11 ± 1.45 pg/ml) than in Gp1 (23.14 ± 0.45) (Fig. 4). These observations indicate that IMQ-induced human plaque-type psoriasis in BALB/c mice has been successfully established.
The synthesized 1% aloe emodin hydrogel demonstrated stronger antipsoriatic action when measured by PASI scores (0.55 ± 0.122) as well as ear thickness (228 ± 5.56 µm). TNF-α level was also significantly reduced (45.15 ± 0.25 pg/ml). The results were diminished compared with 1% tacrolimus cream, which is commercially available as the gold standard. Aloe emodin hydrogel therapy had superior pharmacological effects to tacrolimus cream (0.1%), which was commercially available, and the score obtained from the PASI was significantly reduced (from 8.25 ± 0.37 to 0.65 ± 0.23 for tacrolimus and from 9.4 ± 2.01 to 0.55 ± 0.12 for aloe emodin hydrogel) (Fig. 3ivb-3ivc). Gp2 exhibited a noteworthy disparity in the thickness (245.17 ± 4.36 µm) of the left and right ears among the treatment cohorts, namely Gp3 and Gp4 (240.83 ± 6.05 and 228 ± 5.59, respectively), as evidenced by Figure 3iia. TNF-α level in Gp2 was noticeably elevated (126.11 ± 1.45 pg/ml) compared with that in Gp1 (23.14 ± 0.45 pg/ml), whereas in Gp4 and Gp3, TNF-α levels declined significantly (62.87 ± 0.55 and 45.15 ± 0.25, respectively) (Fig. 4).
As shown in Table 6, for acute dermal toxicity with an initial dose of 200 mg of aloe emodin scoring the body weights of animals were observed to increase (from 194.366 ± 3.46 to 211.32 ± 2.99 in males and from 162.88 ± 2.39 to 176.32 ± 5.61 in females in control groups while from 201.11 ± 4.13 to 222.88 ± 3.69 in males and from 183.78 ± 2.03 to 200.11 ± 1.66 in females for the aloe emodin topical hydrogel treated animal group) while scoring for the erythema, eschar formation, was found nil for both the treatment normal control and aloe emodin hydrogel treated groups. Hematological and blood parameters were also unchanged in both groups (Table: 7).
DISCUSSION
During preformulation studies, the experimental investigation established the label-compliant melting point of aloe emodin at 224°C, confirming the compound’s purity. When compared with the previously reported solubility in methanol and partition coefficient in n-octanol and water (1:1) of aloe emodin, which were <0.025 µg/m\l in methanol [48] and 3.25in n-octanol and water (1:1) [49], respectively. Preformulation study findings suggest that the candidate drug is suitable for transdermal delivery. Aloe emodin and carbopol 940 had indicative troughs at 224°C and 116ºC, respectively, whereas the mixture›s DSC demonstrated melting points that were on the higher end of the range, at 232°C and 124°C, respectively (Fig. 1). DSC analysis was used to analyze the drug-polymer compatibility assessment, and the results showed that there were no interactions between the drug and the excipient throughout the entire duration of the investigation.
For the optimization of hydrogel formulations, blank polymeric hydrogels were prepared using polymeric concentrations (0.5%–4.0%) and checked for parameters such as physical appearance, pH, spreadability, and viscosity. Formulations containing carbopol (1%), chitosan (2%), and HPMC K15M (2%), which were all created as blank hydrogel formulations, were chosen as optimal hydrogels because they had superior physical characteristics. Optimized formulations were prepared as per the formula depicted in formula depicted in Table 1, in which carbopol 940, chitosan, and HPMC K15M were used as gelling agents, mixes of water and propylene glycol as gelling solvent, methyl paraben and propyl paraben as preservatives. The formula for the optimized batches of hydrogel formulations is depicted in Table 1.
The prepared optimized hydrogel formulations were done for pH, viscosity and rheological behavior, spreadability, extrudability, drug loading, drug content analysis, and so on. Human skin normally has a pH between 4.5 and 7.4, with a pH of 6.8 being considered as the average. The pH of the compositions meant for application on the skin should remain within this range. In addition, the permeability and stability of drugs are directly impacted by the pH value of topical preparations. The measured viscosity of the formulation was within the intended range of 15–25 cps [50] According to the rheological findings from the study, a pseudoplastic (non-Newtonian) system exhibited shear thinning along with thixotropic behavior at 25°C, as viscosity decreased with increasing shear rate in terms of rotation per minute (RPM). Spreadability is a crucial factor because it indicates the area of the hydrogel coverage region. Additionally, it shows that the formulations viscosity is suitable and appropriate. The spreadability of the prepared formulations was found to be within the desired range. Given that gel packing is critical in delivering the right amount of gel out of jars or extrusion out of collapsible tubes, its extrudability is a significant empirical test to determine the force necessary for releasing the product out of the tube. The extrudability of the prepared formulations was appreciably good. To determine the percentage of the total medications that could be loaded inside the formulation, a drug loading study was conducted. Drug loading for carbopol was found to be more effective than that for chitosan and HPMC K15M.
The hydrogel with carbopol 940 released the maximum concentration of drug (78%), of all three preparations (Fig. 2a). The hydrogel exhibiting the highest release was put through an ex vivo drug permeation assay, which revealed almost 84% of drug release (Fig. 2b) during a prolonged 8 hours investigation. Additional ex vivo and in vivo investigations were conducted using carbopol hydrogel containing 1% aloe emodin. To explore the release sequence, information pertaining to drug permeation from in vitro and ex vivo experiments was also analyzed for various release kinetic models, including Zero, First, Higuchi’s and Korsemeyer-Peppas model. The R2 value was found to be highest (0.993) in case of Korsemeyer peppas kinetic model. Therefore, it was concluded that drug release from hydrogel was following a sustained release pattern.
According to the ICH specifications, the produced formulations’ accelerated stability investigations were conducted. Spreadability, viscosity, and physical appearance were considered as factors for analyzing stability. The developed hydrogel formulation’s appearance was clear throughout the stability experiments, and no appreciable variations were found. Results showed no discernible variations in pH, viscosity, spreadability, and drug content.
The findings of the skin irritation study demonstrated that utilizing carbopol 940 loaded with aloe emodin did not cause any dermal irritation or inflammation, proving that it was non-irritant when applied to the skin. Moreover, viscosity enhancement brought on by the incorporation of carbopol® 940 created a three-dimensional framework of networks that minimized the possibility of aloe emodin coming into direct contact with the dermal layers, which was believed to reduce any negative effects [51].
The antipsoriatic impact of aloe emodin has been documented in the literature [16,42]. In the present study, we looked at whether an aloe emodin hydrogel based on carbopol might alleviate the immune system’s dysfunction in IMQ-induced plaque psoriasis in BALB/c mice. To investigate the effectiveness of 1% aloe emodin hydrogel, a murine model of human psoriasis was created using IMQ. Psoriasis is characterized by prominent skin thickening, erythema, and scaling.
During the antipsoriatic study, results revealed that IMQ application triggered and aggravated psoriasis in mice, which was similar to a prior study [52]. By day 11, the mice from Gp2 exhibited typical psoriasis-like dermatitis that looked to have mildly intrusive erythema (Fig. 3ib). The characteristic psoriasis symptoms of parakeratosis, Munro’s microabscesses, prominent acanthosis with prolonged rete ridges, and severe inflammatory cell infiltration were all evident upon histopathological examination (Fig. 3iiib). The aforementioned observations indicated that IMQ-induced human plaque-type psoriasis in BALB/c mice was successfully established. Figure 3id illustrates the phenotypical alterations in the mouse’s skin after the sixth day of therapy in relation to aloe emodin hydrogel. The normal control, negative (detrimental) control, positive (advantageous) control, and the 1% aloe emodin hydrogel were all used as comparison points. The synthesized 1% aloe emodin hydrogel demonstrated stronger antipsoriatic action when measured by PASI scores as well as ear thickness. TNF-α level was also significantly reduced. The results were diminished compared with those of 0.1% tacrolimus cream, which is commercially available as the gold standard. Aloe emodin hydrogel therapy had superior pharmacological effects to tacrolimus cream (0.1%), which was commercially available, and the score obtained from the PASI was significantly reduced (Fig. 3ivb-3ivc). To examine the impact of prepared hydrogel on the recovery of psoriatic skin back to normal and to spot psoriatic characteristics, histopathology of skin samples was conducted. Figure 3iii displays skin samples from various groups in both phenotypic and H&E-stained forms. While Gp2 depicts psoriatic skin, which includes hyperkeratosis, parakeratosis, acanthosis, and subtle chronic inflammatory infiltrates in the dermis, Gp1 depicts normal skin histology, which is characterized by normal and healthy dermis and epidermis. Gp3 demonstrated a decrease in epidermal thickness compared with Gp2. Gp4’s histological findings, in which the epidermis was normalized, and fewer infiltrates were noticed, were strikingly comparable to those of Gp1’s. TNF-α, which is synthesized by T cells and triggered macrophages and is expressed in plasma membranes, facilitates the immune system’s reaction along with inflammation [53]. The level of TNF-α in the blood collected on the 25th day of the protocol was utilized in current research to assess the effectiveness of topically administered 1% aloe emodin hydrogel since this kind of cytokine plays an essential role in psoriasis. TNF-α level in Gp2 was noticeably elevated in comparison to Gp1, while in Gp4 and Gp3, TNF-α levels declined significantly (Fig. 4). The combined results indicated that the hydrogel formulation of aloe emodin 1 % containing carbopol 1% as gelling agent exhibited appreciable antipsoriatic efficacy when applied topically. A similar in vivo anti-psoriatic activity study using Perry’s mouse tail model and skin safety studies has been reported by Divya et al. [15] for the more potential benefit of the system for topical delivery of acitretin and aloe emodin in psoriasis.
CONCLUSION
Numerous studies and reviews have noted the antipsoriatic properties of aloe emodin. In Ayurvedic medications, aloe emodin has been combined with various other phytochemicals in marketed products, but none of them include aloe emodin alone. Thus, an endeavor was undertaken to fabricate a topical hydrogel formulation of aloe emodin. After assessing the prepared hydrogel formulations that were optimized, a hydrogel containing 1% carbopol 940 and aloe emodin (1%) was found to be promising for the effective management of psoriasis. The prepared formulation also passed the acute dermal toxicity study. Exploring nano-delivery systems, using a combination of synthetic and other phytochemicals along with aloe emodin for achieving the synergistic effect, or trying other delivery approaches for the effective management of psoriasis can help in handling current challenges provided preclinical and clinical research should be undertaken for the generation and establishment of more effective and safe data. It would also help in catering to the individual needs of patients with psoriasis and making their lives easier. Further studies involving human subjects are essential to validate the effectiveness and safety of the aloe emodin topical hydrogel in clinical settings, beyond preclinical models.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
The protocol for in vivo animal studies was approved by the Institutional Animal Ethical Committee of the College of Pharmacy, Sri Satya Sai University of Technology and Medical Sciences, Madhya Pradesh, India, with approval number. 1587/PO/Re/S/11/CPCSEA.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
ACKNOWLEDGMENT
The authors are thankful to the Amity Institute of Pharmacy, Amity University, Lucknow Campus, and Gurukula Kangri Deemed to be University, Haridwar, Uttarakhand, India, for providing the laboratory facilities for the study. The authors are thankful to Dr. Chandra Kishore Tyagi, Professor, College of Pharmacy, Sri Satya Sai University of Technology and Medical Sciences for monitoring the animal study.
REFERENCES
1. Zhou X, Chen Y, Cui L, Shi Y, Guo C, Advances in the pathogenesis of psoriasis: from keratinocyte perspective. Cell Death Dis. 2022;24;13(1):81. CrossRef
2. Pandey A, Shukla AK, Dubey RC,A review on the important phytochemicals and their role in psoriasis. J Appl and Nat Sci. 2021;13(3):880–96. CrossRef
3. Parisi R, Iskandar IY, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;28:369. CrossRef
4. Nabawy Mohamed E, Mohamed Abd Al-Aal E, Abdallah Abdel-Mordy M. Knowledge and self-care practices among Psoriatic patients in Benha City. J Nurs Sci Benha Uni. 2021;2(2):261–72. CrossRef
5. Aghmiuni AI, Khiavi AA. Medicinal plants to calm and treat psoriasis disease. Aromat Med Plants. 2017;2016:1–28. CrossRef
6. Egeberg A, Andersen YM, Thyssen JP. Prevalence and characteristics of psoriasis in Denmark: findings from the Danish skin cohort. BMJ Open. 2019;9(3):e028116. CrossRef
7. Sewerin P, Brinks R, Schneider M, Haase I, Vordenbäumen S. Prevalence and incidence of psoriasis and psoriatic arthritis. Ann Rheum Dis. 2019;78(2):286–7. CrossRef
8. Osuna CG, García SR, Martín JC, Jiménez VG, López FV, Santos-Juanes J. Use of biological treatments in elderly patients with skin psoriasis in the real world. Life. 2021;11(12):1348. CrossRef
9. Sakthi PS, Vani PB, Kumar PR. A comparative review on conventional and traditional medicine in the treatment of psoriasis. Res J. Pharm Tech. 2020;13(11):5642–6. CrossRef
10. Daniyal M, Akram M, Zainab R, Munir N, Shah SMA, Liu B, et al. Progress and prospects in the management of psoriasis and developments in phyto-hytoress and prospects iDermatol Ther. 2019;2(3):e12866. CrossRef
11. Españo E, Kim J, Kim JK. Utilization of aloe compounds in combatting viral diseases. Pharmaceuticals. 2022;15(5):599. CrossRef
12. Salehi B, Albayrak S, Antolak H, Kr?giel D, Pawlikowska E, Sharifi-Rad M, et al. Aloe genus plants: from farm to food applications and phytopharmacotherapy. Int J Mol Sci. 2018;19(9):2843. CrossRef
13. ?eker KG, Küpeli AE, Yücel Ç, Bahad?r Ac?kara Ö, Sobarzo-Sánchez E. Advances in understanding the role of aloe emodin and targeted drug delivery systems in cancer. Oxid Med Cell Longev. 2022;2022:7928200. CrossRef
14. Sánchez M, González-Burgos E, Iglesias I, Gómez-Serranillos MP. Pharmacological update properties of Aloe vera and its major active constituents. Molecules. 2020;25(6):1324. CrossRef
15. Divya G, Panonnummal R, Gupta S, Jayakumar R, Sabitha M. Acitretin and aloe-emodin loaded chitin nanogel for the treatment of psoriasis. Eur J Pharm Biopharm. 2016;107:97–109. CrossRef
16. Lin HJ, Chao PD, Huang SY, Wan L, Wu CJ, Tsai FJ. Aloelemodin suppressed NMDA-induced apoptosis of retinal ganglion cells through regulation of ERK phosphorylation. Phytother Res. 2007;21(11):1007–14. CrossRef
17. Zaffaroni A. Degrees of efficiency and degrees of minimality. SIAM J Control Optim. 2003;42(3):1071–86. CrossRef
18. Yu CP, Shia CS, Lin HJ, Hsieh YW, Lin SP, Hou YC. Analysis of the pharmacokinetics and metabolism of aloe-emodin following intravenous and oral administrations in rats. Biomed Chromatogr. 2016;30(10):1641–7. CrossRef
19. Fang F, Wang JB, Zhao YL, Jin C, Kong WJ, Zhao HP, et al. A comparative study on the tissue distributions of rhubarb anthraquinones in normal and CCl4-injured rats orally administered rhubarb extract. J Ethnopharmacol. 2011;137(3):1492–7. CrossRef
20. Yalkowsky SH, Alantary D. Estimation of melting points of organics. J Pharm Sci. 2018;107(5):1211–27. CrossRef
21. Veseli A, Kristl A, Akelj S. Proof-of-concept for a miniaturized shake-flask biopharmaceutical solubility determination by sonic mixing. Pharmazie. 2020;75(12):626–31. CrossRef
22. Baluja S, Kulshrestha A, Movalia J. 1-Octanol-water partition coefficient of some cyanopyridine and chalcone compounds. Rev Colomb Cienc Quim Farm. 2017;46(3):342–56. CrossRef
23. Srivastava N, Fatima Z, Kaur CD, Rizvi DA. Berberine chloride dihydrate enthused nanovesicles for the management of dermatitis nanovesicles for dermatitis. Nanosci Nanotechnol. 2021;11(3):300–13. CrossRef
24. Ali KM, Sharif MB, Pooranian M, Rezai A. Preparation and optimization of silibinin-loaded chitosan–fucoidan hydrogel: an in vivo evaluation of skin protection against UVB. Pharm Dev Technol. 2021;26(2):209–19. CrossRef
25. Singh N, Goyal K, Sondhi S, Jindal S. Development and characterization of barbaloin gel for the safe and effective treatment of Psoriasis. J Drug Deliv Ther. 2020;10(5):188–97. CrossRef
26. Dantas MG, Reis SA, Damasceno CM, Rolim LA, Rolim-Neto PJ, Carvalho FO, et al. Development and evaluation of stability of a gel formulation containing the monoterpene borneol. Scientific World J. 2016;2016:7394685. CrossRef
27. Saher T, Manzoor R, Abbas K, Mudassir J, Wazir MA, Ali E, et al. Analgesic and anti-inflammatory properties of two hydrogel formulations comprising polyherbal extract. J Pain Res. 2022;26:1203–19. CrossRef
28. Dejeu IL, Vica? LG, Vlaia LL, Jurca T, Mure?an ME, Pallag A, et al. Study for evaluation of hydrogels after the incorporation of liposomes embedded with caffeic acid. Pharmaceuticals. 2022;15(2):175. CrossRef
29. Woodruff CM, Botto N. The role of patch testing in evaluating delayed hypersensitivity reactions to medications. Clin Rev Allergy Immunol. 2022;62(3):548–61. CrossRef
30. Khanna K, Sharma D, Khar RK, Karwasra R, Sharma N, Nishad DK, et al. A comparative study of chitosan gel and soframycin in the management of wounds. Int JLow Extrem Wounds. 2020;19(2):148–57. CrossRef
31. Ghedini E, Pizzolitto C, Albore G, Menegazzo F, Signoretto M, Operti L, et al. Sulfadiazine-based drug delivery systems prepared by an effective sol–gel process. J Solgel Sci Technol. 2017;83:618–26. CrossRef
32. Mackiewicz M, Romanski J, Drozd E, Gruber-Bzura B, Fiedor P, Stojek Z, et al. Nanohydrogel with N, N′-bis (acryloyl) cystine crosslinker for high drug loading. Int J Pharm. 2017;523(1):336–42. CrossRef
33. Rizvi DA, Fatima Z, Kaur CD. Antipsoriatic and anti-inflammatory studies of Berberis aristata extract loaded nanovesicular gels. Pharmacon Mag. 2017;13(Suppl 3):S587. CrossRef
34. Asad MI, Khan D, ehman AU, Elaissari A, Ahmed N. Development and in vitro/in vivo evaluation of pH-sensitive polymeric nanoparticles loaded hydrogel for the management of psoriasis. Nanomaterials. 2021;11(12):3433. CrossRef
35. Najafi-Taher R, Ghaemi B, Amani A. Delivery of adapalene using a novel topical gel based on tea tree oil nano-emulsion: permeation, antibacterial and safety assessments. Eur J Pharm Sci. 2018;120:142–51. CrossRef
36. Maske PP, Lokapure SG, Nimbalkar D, Malavi S, D’souza JI. In vitro determination of sun protection factor and chemical stability of Rosa kordesii extract gel. J Pharm Res. 2013;1;7(6):520–4. CrossRef
37. Guideline IH. Pharmaceutical development, Q8 (2R). U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER); 2009.
38. Gokhale JP, Mahajan HS, Surana SJ. Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: in vivo and in vitro studies. Biomed Pharmacother. 2019;112:108622. CrossRef
39. Salem HF, Nafady MM, Kharshoum RM, Abd El-Ghafar OA, Farouk HO. Novel enhanced therapeutic efficacy of dapoxetine HCl by nano-vesicle transdermal gel for treatment of carrageenan-induced rat paw edema. AAPS PharmSci Tech. 2020;21:1–3. CrossRef
40. Saka R, Jain H, Kommineni N, Chella N, Khan W. Enhanced penetration and improved therapeutic efficacy of bexarotene via topical liposomal gel in imiquimod induced psoriatic plaque model in BALB/c mice. J Drug Deliv Sci Tech. 2020;58:101691. CrossRef
41. Srivastava N, Fatima Z, Kaur CD, Rizvi D. Berberine chloride dihydrate enthused nanovesicles for the management of dermatitis nanovesicles for dermatitis. Nanosci Nanotechnol. 2021;11(3):300–13. CrossRef
42. Rapalli VK, Sharma S, Roy A, Alexander A, Singhvi G. Solid lipid nanocarriers embedded hydrogel for topical delivery of apremilast: In-vitro, ex-vivo, dermatopharmacokinetic and anti-psoriatic evaluation. J Drug Deliv Sci Tech. 2021;63:102442. CrossRef
43. Chen H, Lu C, Liu H, Wang M, Zhao H, Yan Y, et al. Quercetin ameliorates imiquimod-induced psoriasis-like skin inflammation in mice via the NF-κB pathway. Int Immunopharmacol. 2017;48:110–7. CrossRef
44. OECD. Test No. 402: acute dermal toxicity, OECD guidelines for the testing of chemicals, section 4. Paris, France: OECD Publishing; 2017. CrossRef
45. Han YG, Aoyagi M, Asakawa M, Shimizu T. Facile fabrication and magnetic properties of a one-dimensional magnetite peapod in a lipid nanotube. ACS Appl Mater Interfaces. 2012;4(5):2439–44. CrossRef
46. Aiyalu R, Govindarjan A, Ramasamy A. Formulation and evaluation of topical herbal gel for the treatment of arthritis in animal model. Braz J Pharm Sci. 2016;52:493–507. CrossRef
47. El-Kased RF, Amer RI, Attia D, Elmazar MM. Honey-based hydrogel: in vitro and comparative in vivo evaluation for burn wound healing. Sci Rep. 2017;7(1):9692. CrossRef
48. Jabeen M, Boisgard AS, Danoy A, El Kholti N, Salvi JP, Boulieu R, et al. Advanced characterization of imiquimod-induced psoriasis-like mouse model. Pharmaceutics. 2020;12(9):789. CrossRef
49. Özenver N, Saeed M, Demirezer LÖ, Efferth T. Aloe-emodin as drug candidate for cancer therapy. Oncotarget. 2018;9(25):17770. CrossRef
50. Berillo D, Kozhahmetova M, Lebedeva L. Overview of the biological activity of anthraquinons and flavanoids of the plant rumex species. Molecules. 2022;27(4):1204. CrossRef
51. Patel HK, Barot BS, Parejiya PB, Shelat PK, Shukla A. Topical delivery of clobetasol propionate loaded microemulsion based gel for effective treatment of vitiligo–part II: rheological characterization and in vivo assessment through dermatopharmacokinetic and pilot clinical studies. Colloids and Surfaces B: Biointerfaces. 2014;119:145–53. CrossRef
52. Ahmad S, Minhas MU, Ahmad M, Sohail M, Abdullah O, Badshah SF. Preparation and evaluation of skin wound healing chitosan-based hydrogel membranes. AAPS PharmSci Tech. 2018;19:3199–209. CrossRef
53. Sathe P, Saka R, Kommineni N, Raza K, Khan W. Dithranol-loaded nanostructured lipid carrier-based gel ameliorate psoriasis in imiquimod-induced mice psoriatic plaque model. Drug dev Ind pharm. 2019;45(5):826–38. CrossRef