INTRODUCTION
Sepsis, including severe sepsis and septic shock, is a health problem that affects millions of people worldwide each year and can kill one in four. There are 48.9 million cases and 11 million deaths related to sepsis worldwide, which accounts for nearly 20% of all global deaths [1]. In Asia, the prevalence of sepsis is 22.4%, with a higher mortality rate in lower-income countries and lower-middle-income countries than in high-income countries, and costly [2]. An observational study conducted from 2013 to 2016 in Indonesia identified 14,076 sepsis patients across four medical centers. The study found that more than half of the patients (58.3%) died, while only 41.7% survived [3]. It is worth noting that the mortality rate was 60% for sepsis patients with multifocal infections and higher for those with surgical site infections, at 74.2% [3]. The burden of sepsis is much higher in pediatric patients, with a mortality rate of 76.1%. Among these, 41.8% died within the first 24 hours due to septic shock [4].
The economic burden of sepsis is also a significant global issue. Data from the United States (US) in 2013 showed that sepsis costs more than US$24 billion in hospital costs, representing 13% of total hospital costs [5]. A 2018 study in the US showed that sepsis is a disease with a high economic burden where hospitalization costs increase as the severity of sepsis [5]. The findings from a systematic review encompassing 26 studies on sepsis costs highlighted the substantial economic burden, with the total hospital cost per patient varying from €1,101 to €91,951 [6].
In Indonesia, the second major economic burden is sepsis patients with multifocal infections and patients with single focal lower respiratory tract infections, costing (US$48 million and US$33 million, respectively, within 100,000 sepsis patients [3]. Sepsis is a devastating systemic response that is typically caused by bacterial infection but could also be the result of other infections such as viruses, parasites, or fungi. Its treatment requires medical care, including the use of antimicrobials, and intravenous fluids. Common signs of sepsis include fever, fast heart rate, rapid breathing, confusion, and body pain. When the blood pressure drops significantly, it can damage the lungs, kidneys, liver, and other organs. Severe damage can lead to severe sepsis, septic shock, multiple organ failure, and death [7,8], where the speed and suitability of therapy given in the early hours after developing severe sepsis can affect the outcome [9]. Therefore, prompt and appropriate management of treatment is needed. One of the therapies for sepsis is antibiotics, which should be given as early as possible, namely in the first hour after a patient is diagnosed with sepsis [10], where delay in giving antibiotics can increase the risk of death for sepsis patients. Empirical antibiotics should be given as early as possible while waiting for blood culture results, then if the culture results are available, definitive antibiotics can be given to patients according to the results of bacterial culture. That can reduce the incidence of antibiotic resistance and excessive use of antibiotics. However, routine examination of bacterial cultures in sepsis patients has not yet been carried out, and there are still hospitals that do not have a germ map. This has the potential to cause antibiotic resistance, which can result in increasing length of stay and costs of patient care at the hospital [11–13]. Furthermore, bacterial culture examination using patient blood samples can increase patient care costs, but the administration of appropriate definitive antibiotics according to culture results can reduce the length of stay. On the other hand, without carrying out bacterial culture examinations, patient care costs can be cheaper because there is no need for bacterial culture examinations. Still, it can increase the length of stay, inappropriate use of antibiotics, and subsequent antibiotic resistance. In addition, with the implementation of the national health insurance system in Indonesia, namely JKN, the basis for reimbursement and health financing should include the results of the economic evaluation study.
Several economic evaluation studies have explored the cost-effectiveness of using culture-based antibiotic strategies and empirical antibiotic approaches in various countries [14–16]. The findings from these studies consistently indicated that applying antibiotic prescriptions based on culture test results was a more cost-effective approach compared to empirical antibiotics [14–16]. However, the amount of evidence is still limited and no study has been conducted in sepsis patients.
In Indonesia, an economic evaluation study compared the use of empirical versus definitive antibiotics for community-acquired pneumonia using patient data from hospitals in Surabaya [17]. However, no economic evaluation study has compared the use of empirical versus definitive antibiotics for sepsis patients using real-world data of hospitalized patients yet to be practical hospitalized. Recognizing the necessity for such an economic evaluation and addressing the aforementioned research gap, this study was conducted.
Hence, based on several observations we can deduce that sepsis patients treated with definitive antibiotics showed a much better response compared to patients treated with empirical antibiotics hence lowering patient’s length of stay and medical cost, and the primary objective was to assess the cost-effectiveness of using definitive antibiotics compared to empiric antibiotics for sepsis in hospitals in Indonesia. The outcomes of this study will serve as valuable input for policymakers, including those within hospitals, and as a practical guideline for the strategic selection of antibiotics for patients with sepsis.
METHOD
Model structure
This cost-effectiveness analysis used a decision tree analysis model to evaluate the cost-effectiveness of definitive antibiotics compared to empiric antibiotics in a simulated cohort of 1,000 sepsis patients. Economic analysis was employed from the healthcare payer’s perspective with a 1-year time horizon. The decision tree compared two options: a) patients on empiric antibiotic treatment (ET) during hospitalization and b) patients on culture-based treatment (CBT) whose treatment was adjusted based on the results of a bacterial culture that had been performed on the patient’s blood sample. The CBT group was treated with empiric antibiotics per Indonesian guidelines 24 hours post-hospitalisation. After that, the culture results were given, and the patient was treated with a specific therapy. Definitive antibiotics given after the culture results come out can be different from the empirical antibiotics previously received by the patient (antibiotic change or AC), or there is no change in the use of antibiotics (fixed antibiotics or No AC). We assumed that the laboratory staff’s competency level was similar in performing and reporting blood culture results from septic patients. In the ET group, we assumed that antibiotic treatment was empiric during hospitalization. Decision tree termination statuses for CBT and ET are presented as recovery or death (Fig. 1).
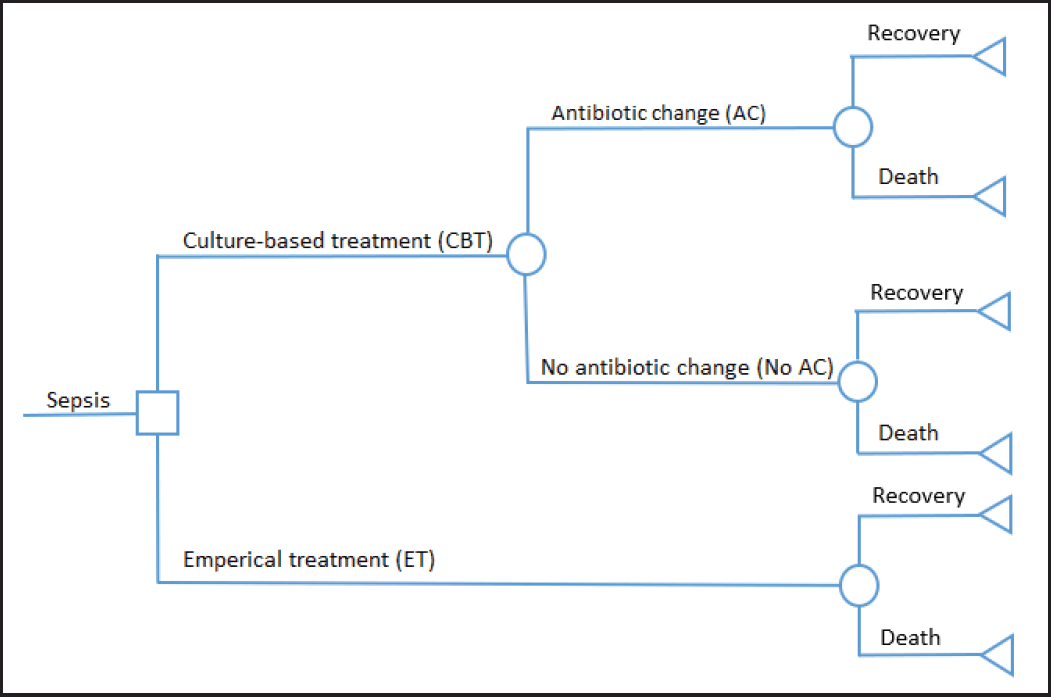 | Figure 1. Decision tree model. [Click here to view] |
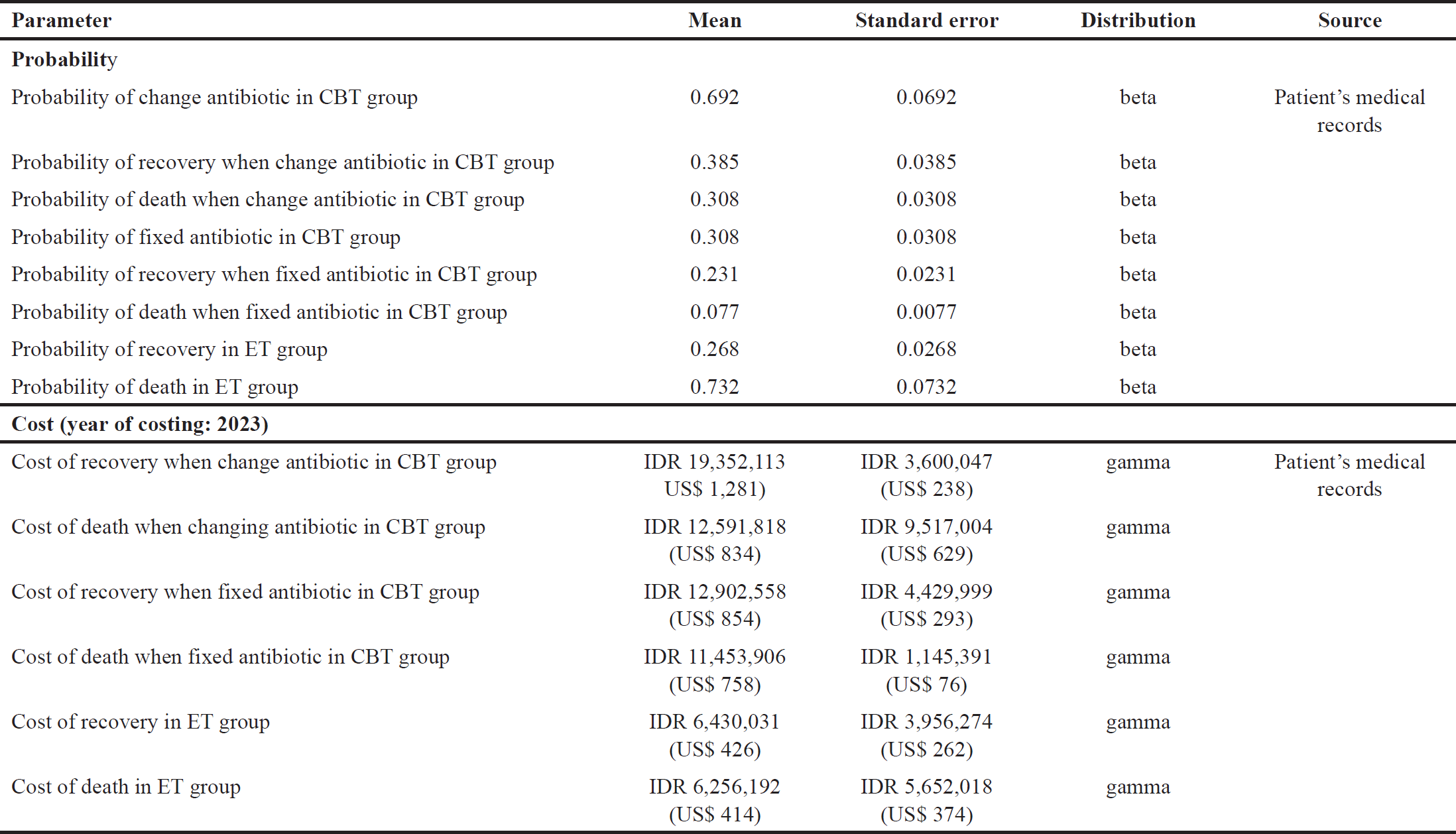 | Table 1. Input parameters used in decision-tree model. [Click here to view] |
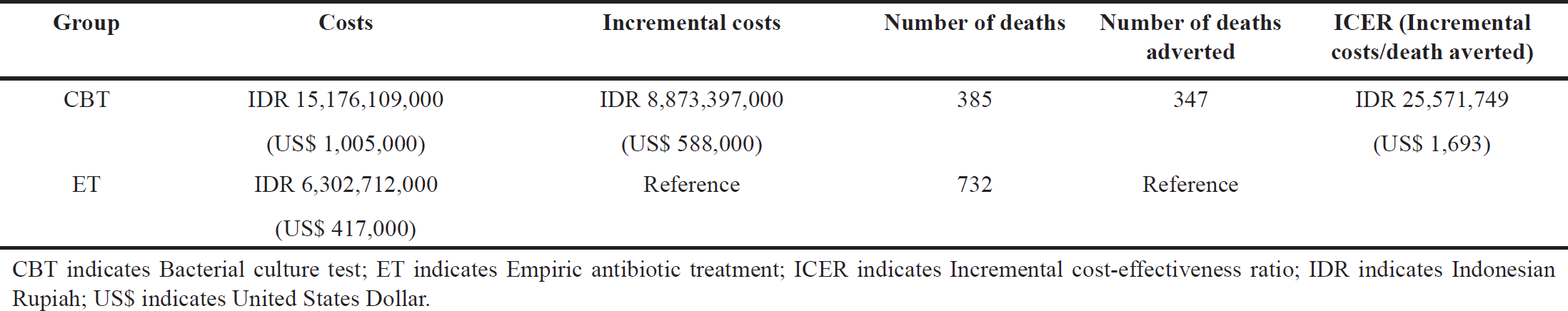 | Table 2. Base-case results of cost-effectiveness analysis (A hypothetical cohort of 1,000 patients entering the model). [Click here to view] |
Data input
The parameters used in the model are presented in Table 1. Individual data on adult sepsis patients who underwent hospitalization at the Tabanan Hospital, in Bali, Indonesia, for 2 years, from 2020 to 2022, were taken from medical records. This hospital is a teaching hospital owned by the local government and is a referral hospital for other areas in Tabanan, Bali. We used data from adult patients who were over 18 years old. Patients diagnosed with sepsis were determined by a doctor with the same competence to treat sepsis patients. The clinical data and costs used in this study were obtained from the medical record, pharmacy, and hospital finance departments, where the data were anonymous and confidential. This study received approval from the ethical commission at Tabanan Hospital with number 445/220/TIMKORDIK/RSUD/2023, and it was decided not to require a review in terms of patient consent because the study was conducted retrospectively. The study met the agreement with Indonesian research conduct and the Declaration of Helsinki [18].
The clinical outcome of this study was a number of deaths averted. Using the payer perspective, only direct medical costs were estimated, including hospitalization, drug, laboratory tests, and medical staff costs. The costs were expressed in US$ with conversion on July 27, 2023, amounting to US$ 1 = IDR 15,107 based on data from Bank Indonesia [19]. The results were presented as incremental cost-effectiveness ratio (ICER), which presents incremental costs per number of deaths adverted.
Sensitivity analysis
We conducted a one-way sensitivity analysis to test the robustness of the model on ICER by the change in the lower and upper limits of the parameters. We also conducted probabilistic sensitivity analysis (PSA) using a Monte Carlo simulation with 1,000 random cohorts on the corresponding distributions. A gamma distribution was used for costs, whereas a beta distribution was used for probabilities. We constructed the cost-effectiveness acceptability curve to draw the relationship between the ICER and Indonesian threshold willingness to pay (WTP). Based on the World Health Organization—Choosing Interventions for Cost Effectiveness criterion (WHO-CHOICE), a particular intervention is highly cost-effective when the ICER is less than the WTP, defined as the Indonesian cost-effectiveness threshold of gross domestic product (GDP) per capita. In this study, we applied the threshold WTP was the Indonesian GDP per capita in 2022 at US$ 4,788 [20], equal to IDR 72,333,083 with the conversion rate of US$ 1 = IDR 15,107 based on data from Bank Indonesia [19].
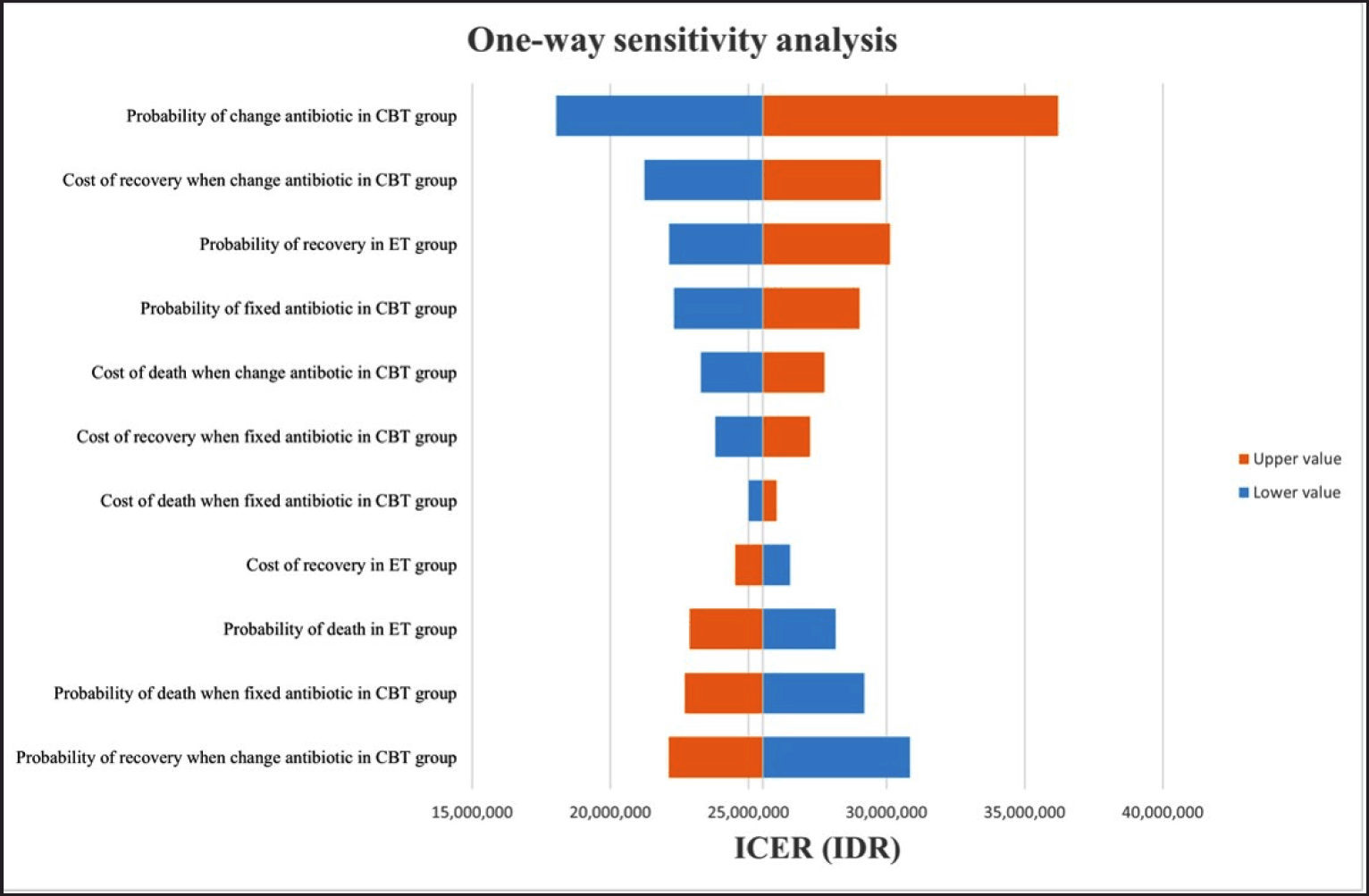 | Figure 2. Tornado diagram illustrating results from sensitivity analysis for CBT versus ET. CBT indicates Culture-based treatment; ET indicates Empiric antibiotic treatment; ICER indicates Incremental cost-effectiveness ratio; IDR indicates Indonesian Rupiah. [Click here to view] |
The results are presented as a Tornado diagram for one-way sensitivity analysis, cost-effective (CE) plane, and cost-effectiveness acceptability curve (CEAC) for PSA. All analyses were conducted using Microsoft Office Excel.
RESULTS
Characteristics of patients
A total of 84 medical records from adult patients were collected. Among these patients, 40 (47.6%) were aged between 61 and 80, with 48 (57.1%) being male and 49 (58.3%) having multiple comorbidities. Regarding the treatment methods, 13 (15.5%) patients were treated with CBT, and 71 (84.5%) received ET. At the end of treatment, a total of 57 patients died, which accounted for 67.9% in both CBT and ET groups. The details of patient characteristics are presented in Supplementary 1.
Cost-effectiveness analysis
The results of cost-effectiveness analyses are shown in Table 2. In terms of treatment efficacy, patients who were treated with CBT demonstrated a considerably reduced mortality rate compared to those treated with ET, with 385 deaths in the CBT group versus 732 in the ET group. CBT resulted in the prevention of 347 patient deaths. In terms of treatment costs, CBT increased costs by IDR 8,873,397,000 compared to ET (IDR 15,176,109,000 [US$ 1,005] versus IDR 6,302,712,000 [US$ 417,000]), resulting in an ICER of IDR 25,571,749 (US$ 1,693) per death averted. This ICER suggested that implementing CBT for sepsis patients was considered cost-effective compared to ET, given the WTP threshold of one GDP per capita (IDR 72,333,083 [US$ 4,788]).
Sensitivity analysis
One-way sensitivity analysis was conducted for probabilities and costs to determine influential variables with the most impact on the decision tree results and is presented as the Tornado diagram (Fig. 2). The most significant factors affecting the ICER were probabilities for antibiotic change on the CBT, followed by costs of recovery with antibiotic change in the CBT group and probability of recovery in the ET group. Varying each variable independently within a given range had minimal impact on the ICER, and Indonesia’s value remained below one GDP.
The results of PSA are shown in Figures 3 and 4. The results indicated that using WTP thresholds of one and three GDP per capita, 72% and 80% probability of CBT was a cost-effective option compared to ET, respectively (Fig. 4).
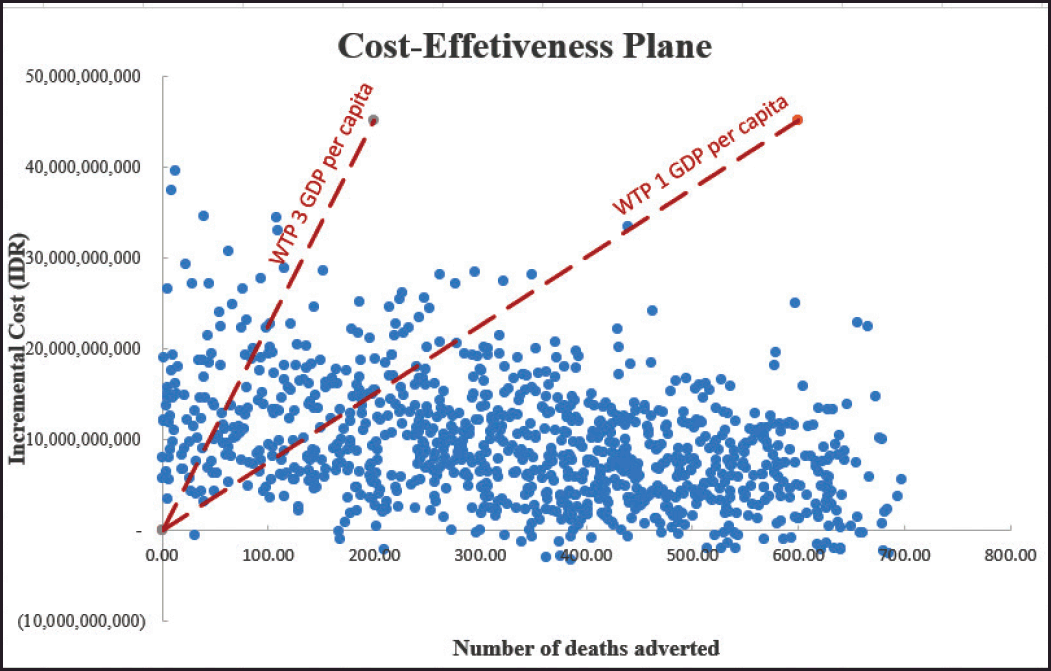 | Figure 3. Incremental cost-effectiveness plane for CBT versus ET. The red line depicts ICER threshold equal to one and three times the Indonesian GDP. (A hypothetical cohort of 1,000 patients entering the model). Each point represents incremental number of deaths adverted and incremental cost of using culture-based treatment versus empiric antibiotic treatment in sepsis patients from 1,000 simulations. CBT indicates Culture-based treatment; ET indicates Empiric antibiotic treatment; GDP indicates Gross domestic products; ICER indicates Incremental cost-effectiveness ratio; IDR indicates Indonesian Rupiah; WTP indicates Willingness-to-pay. [Click here to view] |
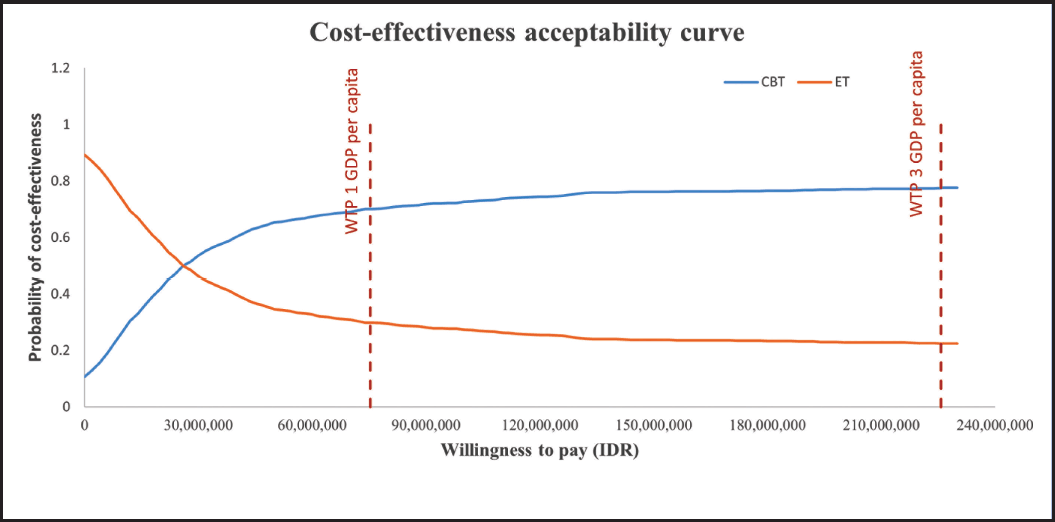 | Figure 4. The cost-effectiveness acceptability curve for antibiotics treatment with CBT and ET in sepsis patients. CBT indicates Culture-based treatment; ET indicates Empiric antibiotic treatment; GDP indicates Gross domestic products; IDR indicates Indonesian Rupiah; WTP indicates Willingness-to-pay. [Click here to view] |
DISCUSSION
This study aimed to estimate the cost-effectiveness of antibiotics with CBT versus ET for sepsis patients in Bali, Indonesia, from a payer perspective. The data used were real-world data retrieved from Tabanan Hospital. The results showed that antibiotic treatment based on the evaluation of cultures was more likely cost-effective compared to empirical treatment, with the ICER being IDR 25,571,749 (US$ 1,693) per death averted, less than Indonesia’s GDP per capita. The finding aligned with the results of the PSA analysis, underscoring the robustness of the analytic decision model. The results of the one-way sensitivity analysis showed that the probability of changing antibiotics in the CBT group had the most effect on the ICER. However, the range of ICER values remained within the WTP threshold as per the WHO guidelines.
The results of a previous study using the same model as the current study yielded similar findings. Applying CBT in treating adult Indonesian patients with community-acquired pneumonia was considered cost-saving with a total savings of US$ 1,066,885 and an increase of 247 years in improved outcomes [17]. Currently in Indonesia, culture test costs are partially covered by national health insurance. These results can inform policymakers about the feasibility of including these tests, especially lab expenses, in national health insurance in the treatment of sepsis patients, potentially contributing to improved healthcare practices. Additionally, it also provides evidence to physicians regarding the effectiveness of CBT in sepsis treatment for future clinical practice.
Some economic evaluations came up with the same conclusion that CBT therapy was cost-effective compared to ET. A recent study in Taiwan conducted in young patients who had Helicobacter pylori infection, came up with a similar conclusion, finding that CBT was more cost-effective than ET. With each 10% increase in the number of patients successfully treated, the cost decreased by US$ 24,058 for CBT, which was higher than the US$ 20,241 reduction seen with ET [14]. Proper antibiotic use is critical in preventing antibiotic resistance, particularly in sepsis patients. The use of broad-spectrum antibiotics contributes to the increase of antimicrobial resistance and increases the cost of treatment [21]. Compared to empirical therapy, the adjustment of antibiotic therapy, according to the results of blood cultures, reduced € 19,800 for 7 days of treatment, and the cost of adjustment of antibiotics was 23% less than empirical therapy [16].
Regarding the probability of being cost-effective, our study shows that using the WTP threshold of one and three GDP per capita, a 72%–80% probability of CBT was a cost-effective option compared to ET, respectively. The results from a trial-based economic evaluation in patients with urinary tract infections showed a similar probability. Using antibiotics followed by culture results was cost-effective compared to immediate antibiotics when the value for a day of avoiding symptoms was over £10, with 70% certainty of cost-effective [15]. However, the data about the utility loss of this study was not derived directly from the patient. Therefore, there is a need for further economic evaluation using real-world data to evaluate the cost-effectiveness of CBT compared to ET in various diseases.
There is a fact that the number of economic evaluation studies that compare the cost-effectiveness of CBT and ET still needs to be improved. To inform evidence-based practices and mitigate the risk of antimicrobial resistance, there is a crucial need for more comprehensive economic evaluations, not only in Indonesia but also in other countries, to compare countries. Future studies should extend their scope to various bacterial infections, enabling a thorough comparison of the cost-effectiveness of CBT and ET. Additionally, conducting research with data from multiple hospitals within the country will enhance the relevance and applicability of findings, providing a more accurate reflection of the country’s healthcare context.
The strength of this study was that it used real-world data in our model. We collected data from Tabanan Hospital, encompassing direct medical costs and probability of death, changing antibiotics, and recovery in both CBT and ET among sepsis patients. For our sensitivity analysis, we performed both one-way sensitivity and probability sensitivity analyses. These analyses consistently supported the cost-effectiveness of CBT when compared to ET. This study has some limitations that need to be considered. Firstly, the data utilized in the model was derived from a single hospital in Indonesia with a limited number of patients as such, the findings may not reflect the situation across the entirety of Indonesia. Additionally, while constrained by the availability of patients during the study period and the capacity of the hospital, we ensured that the sample was representative of the adult sepsis patient population in the Tabanan region. Secondly, our study adhered to the WHO’s recommendation for a cost-effectiveness threshold of one to three times the GDP per capita [22]. This threshold helps determine if an intervention is very cost-effective (ICER less than one GDP per capita) or cost-effective (ICER between one and three GDP per capita). However, the decision to include an intervention in the benefit package should not rely solely on the ICER and cost-effectiveness threshold [23]. Additional evidence regarding the budget impact and feasibility of implementation should also be considered. Future research should focus on estimating the budget impact and analyzing the feasibility of CBT in sepsis patients in Indonesia.
CONCLUSION
In conclusion, CBT was estimated to be better in terms of cost-effectiveness with an increased number of deaths averted for sepsis patients during their hospitalization. To note, CBT was way better to apply in adult sepsis patients and for guiding to select the appropriate antibiotics during the hospitalizations.
ACKNOWLEDGMENTS
The authors thank Dr. David for his clinical insights into data interpretation.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Health Research Ethic Committee of Tabanan Hospital, Bali, Indonesia with approval number 445/220/TIMKORDIK/RSUD/2023. We do not to require a review in terms of patient consent because the study was conducted retrospectively. The study met the agreement with Indonesian research conduct and the Declaration of Helsinki.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–11. CrossRef
2. Tirupakuzhi Vijayaraghavan BK, Adhikari NKJ. Sepsis epidemiology and outcomes in Asia: advancing the needle. Am J Respir Crit Care Med. 2022;206(9):1059–60. CrossRef
3. Purba AKR, Mariana N, Aliska G, Wijaya SH, Wulandari RR, Hadi U, et al. The burden and costs of sepsis and reimbursement of its treatment in a developing country: an observational study on focal infections in Indonesia. Int J Infect Dis. 2020;96:211–8. CrossRef
4. Pudjiadi AH, Putri ND, Wijaya S, Alatas FS. Pediatric sepsis profile in a tertiary-care hospital in Indonesia: a 4-year retrospective study. J Trop Pediatr. 2023;69(5):fmad029. CrossRef
5. Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E. Epidemiology and costs of Sepsis in the United States-an analysis based on timing of diagnosis and severity level. Crit Care Med. 2018;46(12):1889–97. CrossRef
6. van den Berg M, van Beuningen FE, Ter Maaten JC, Bouma HR. Hospital-related costs of sepsis around the world: a systematic review exploring the economic burden of sepsis. J Crit Care. 2022;71:154096. CrossRef
7. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–10. CrossRef
8. Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent J-L. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2(1):16045. CrossRef
9. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228. CrossRef
10. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–247. CrossRef
11. Martin M, Moore L, Quilici S, Decramer M, Simoens S. A cost-effectiveness analysis of antimicrobial treatment of community-acquired pneumonia taking into account resistance in Belgium. Curr Med Res Opin. 2008;24(3):737–51. CrossRef
12. Martin M, Quilici S, File T, Garau J, Kureishi A, Kubin M. Cost-effectiveness of empirical prescribing of antimicrobials in community-acquired pneumonia in three countries in the presence of resistance. J Antimicrob Chemother. 2007;59(5):977–89. CrossRef
13. Kuti JL, Capitano B, Nicolau DP. Cost-effective approaches to the treatment of community-acquired pneumonia in the era of resistance. Pharmacoeconomics. 2002;20(8):513–28. CrossRef
14. Hung CW, Chen SC, Ku LE, Sheu BS, Yang YJ. A culture-based strategy is more cost effective than an empiric therapy strategy in managing pediatric Helicobacter pylori infection. Front Pediatr. 2022;10:860960. CrossRef
15. Turner D, Little P, Raftery J, Turner S, Smith H, Rumsby K, et al. Cost effectiveness of management strategies for urinary tract infections: results from randomised controlled trial. BMJ. 2010;340:c346. CrossRef
16. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2005;57(2):326–30. CrossRef
17. Purba AKR, Ascobat P, Muchtar A, Wulandari L, Dik JW, d’Arqom A, et al. Cost-effectiveness of culture-based versus empirical antibiotic treatment for hospitalized adults with community-acquired Pneumonia In Indonesia: a real-world patient-database study. Clin Outcomes Res. 2019;11:729–39. CrossRef
18. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. CrossRef
19. Bank Indonesia. Exchange rates on Transaction 2023 [cited 2023 6 November]. Available from: https://www.bi.go.id/en/statistik/informasi-kurs/transaksi-bi/default.aspx
20. World Bank. GDP per capita (current US$)—Indonesia 2023 [cited 2023 July 27]. Available from: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=ID
21. Mariita KM, Chirima HA, Maina CK. Broad spectrum antibiotic use among in-patients at a hospital in Nairobi, Kenya. Int J Basic Clin Pharmacol. 2018;8(1):1–7. CrossRef
22. Hutubessy R, Chisholm D, Edejer TT. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003;1(1):8. CrossRef
23. Bertram MY, Lauer JA, De Joncheere K, Edejer T, Hutubessy R, Kieny MP, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94(12):925–30. CrossRef
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the journal's website: Link here [https://japsonline.com/admin/php/uploadss/4349_pdf.pdf].