INTRODUCTION
Neonatal sepsis remains one of the major public health concerns in developing nations despite the enormous advancements in healthcare facilities and infrastructure [1]. The ambiguous signs and symptoms of the disease are one of the primary reasons for its delayed diagnosis and treatment, subsequently resulting in high mortality [2]. Moreover, the lack of reliable biomarkers pertaining to disease identification also adjoins the problem [3]. Thus, the prominent solution to this problem remains to be the blood culture assessment, which precisely takes forty-eight hours to generate any conclusive results, thus leading to the inevitable empirical application of antibiotics, subsequently paving towards antimicrobial resistance [4,5]. Moreover, the outcomes generated through these culture reports can be subjected to false negative (FN) or positive output based on exposure to antenatal antibiotics or sample contamination [6]. Hence, a legitimate and prompt disease diagnosis is crucial for combating the situation.
Biomarkers in neonatal sepsis can play a substantial part in the timely and accurate diagnosis of the disease [7]. A highly sensitive and precisely specific biomarker can ensure a proper differential diagnosis by gathering valid cases and eliminating unsure cases [3]. Moreover, rapid disease diagnosis for swift treatment and management should be one of the primary criteria for an ideal biomarker [8]. However, the root, characteristics, and moment of expression/elevation of a biomarker are also majorly essential in identifying any disease or condition [9]. Therefore, identifying individual biomarkers for recognizing any condition becomes an integral part of healthcare management.
Presepsin, commonly known as the soluble CD14 subtype, is a glycoprotein receptor that interacts with a pathogen’s lipopolysaccharide-binding protein, thereby inducing varied innate immune responses [10,11]. Finally, it is broadly growing as a potential biomarker in the field of neonatal sepsis [12,13]. Extensive literature suggests that it is known to be an accurate diagnostic test for neonatal sepsis when compared to reliable and conventional biomarkers like C-reactive protein (CRP) and procalcitonin (PCT) [13–15]. Moreover, it is also considered a valuable biomarker in distinguishing neonates with suspected sepsis compared to that of PCT [16]. Additionally, it alone has equivalent diagnostic accuracy for neonatal sepsis compared to CRP and PCT combined [6]. Similarly, interleukin-6 (IL-6) is also indicated to be a precise and definite biomarker in recognizing neonatal sepsis, chiefly in cases with premature rupture of membrane (PROM) [17,18]. Furthermore, the upsurge in the levels of IL-6 during the first few hours of infection, before that of acute phase reactants like CRP, makes it an even more desirable biomarker for neonatal sepsis. Additionally, the development of signs and symptoms by the infected neonates, along with standard positive reports, is followed by the uprise in IL-6 levels [19].
Thus, the aim of the review study was to compare the accuracy of biomarkers IL-6 and presepsin in the diagnosis of neonatal sepsis. Our understanding is that this would be the first of its kind to compare these biomarkers, for to date, no systematic review and meta-analysis exists comparing the diagnostic accuracy of these biomarkers. This present systematic review and meta-analysis will assist the health care professionals in building firm and improved decisions pertaining to disease management and supervision, meanwhile encouraging the youthful researchers to investigate numerous plots to try out their groundwork in the field of neonatal sepsis.
METHODS
Prospero registration
The review study has been registered with PROSPERO (CRD42024565507).
Search databases
PubMed, Scopus, excerpta medica database (EMBASE), and CINHAL databases were extensively explored from 1st January 2012 till 31st August 2023 to identify relevant studies. “Presepsin,” “IL-6,” “neonatal sepsis,” and “diagnosis” were a few of the keywords employed to conduct the search. Furthermore, the bibliography of the relevant articles and grey literature from our institutional library were also inspected manually to identify desired studies. A meticulous description of the search history is provided in the supplementary file.
Eligibility criteria
The following criteria were contemplated for the included articles: (1) Functional characterization of neonatal sepsis: Neonates were termed as early onset sepsis; if with positive blood culture within 72 hours of life while late onset sepsis; if with positive blood culture after 72 hours of life. Similarly, neonates who had a positive blood culture report with positive blood culture report within first 28days of life were further termed sepsis patient [20]; (2) The functional definition of neonatal sepsis should have been mentioned in the article; (3) Neonatal sepsis with positive culture report in the experimental group, while culture sterile or healthy neonates in the control group; (4) Case control-prospective, cross-sectional, cohort- prospective studies executed for the diagnosis of neonatal sepsis; (5) Experimental group: estimation of blood serum presepsin and IL-6 performed at the time of suspected clinician appearance along with, before antibiotic therapy was started; (6) Adequate information to be provided in the article to calculate the desired output, i.e., true positive (TP), true negative (TN), false positive (FP), and FN; (7) Research published in the English language were considered.
Meanwhile, exclusion criteria constituted: (1) preclinical, randomized, retrospective, narrative review, systematic review metanalysis studies; (2) standard functional definition of sepsis not mentioned in the study; (3) estimation of presepsin and IL-6 performed in urine or cerebrospinal fluid or in maternal or umbilical cord blood; (4) antibiotics started before estimation of presepsin and IL-6; (5) insufficient data to calculate the desired output.
Study selection
Preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines were pursued for comprehended articles screening [21]. The entire search outcome was checked out by the first two reviewers [P.S] and [E.A.R.S]. However, the full-text article, preceded by title and abstract screening, was examined by two other reviewers [V.K] and [K.A]. The exclusion of all the titles, abstracts, and full text of the articles was based on study design/disease/intervention/outcome and irrelevancy. Meanwhile, the metanalysis of all the included articles was performed by [P.S] and [E.A.R.S] and was supervised by [V.K]. However, any disagreement corresponding to the article incorporation and meta-analysis was cleared through deliberation.
Data extraction and quality assessment
PRISMA of diagnostic test accuracy studies (PRISMA-DTA) checklist was followed by all the reviewers for the data extraction method [22]. Study objective, design, duration, patient characteristics, sample size, biomarker tested, and outcome measures such as the specificity, sensitivity, the positive and negative predictive value-(PPV, NPV) were excerpted from the included articles. Meantime, the quality assessment of the comprehended articles was regulated based on the quality assessment tool of diagnostic accuracy studies-2 (QUADAS-2), which encompasses four realms, i.e., patient selection, reference standard, index test, and flow and timing. Each realm, except the last, was appraised for both risk of bias and applicability purposes. Furthermore, signaling questions were incorporated into the tool to evaluate the risk of bias and the applicability of the articles. Articles included were classified as low grade/high grade/unclear grade of risk of bias based on the following criteria: (1) low-grade risk of bias: if answers to all the questions in all the domains were “yes,” (2) high-grade risk of bias: if the answer to any of the question in any of the domain is “no,” (3) unclear grade risk of bias: any unclear answer to any of the questions in any domain, due to insufficient data. Similarly, the applicability of the included articles was also categorized in an identical manner based on the above criteria [23].
Data synthesis and analysis
Required output data such as TN, TP, FN, and FP were calculated from the formula provided by Molinaro et al. [24]. Spearmen correlation test was employed by Meta-disc (version 1.4) software to analyze the heterogeneity due to the threshold effect of the included articles, where p < 0.05 value signifies a considerable amount of threshold effects [25]. The visual representation of the heterogeneity of the included articles was illustrated by a forest plot. However, heterogeneity caused by the nonthreshold effects was assessed through Higgins I2 statistics value, where value >50% with p < 0.05 implying heterogeneity due to nonthreshold effect. Thus, a bivariate hierarchical model was employed to estimate the summary sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and their corresponding 95% confidence interval using Meta-DTA software [26]. Additionally, the hierarchical summary receiver characteristics (HSROCs) curve was plotted for the diagnosis of neonatal sepsis.
RESULTS
PRISMA guidelines were followed for the data screening process from all embodied articles. An absolute of 1475 articles were retrieved from several distinct databases such as PubMed, cumulative index to nursing and allied health literature (CINAHL), EMBASE, and Scopus. After examining the eligibility criteria, a cumulative of 4 articles were incorporated into the final analysis. The process of screening is illustrated in Figure 1.
Study characteristics
Two of the included studies were conducted in Egypt [28,29], while the remaining two were conducted in Turkey and India, respectively [27,30]. All the aggregated articles were of single-center origin, with a minimal sample size of 55 and a maximum of 168 [27,28]. Three of the studies were of prospective design while one of the studies adopted the cross-sectional study design [28]. Neonatal sepsis was diagnosed as general in two of the identified studies [28,30], while diagnosed as specific cases of late and early-onset neonatal sepsis in the other two studies [27,29]. Healthy neonates: age, gender, and weight-matched were taken as controls in more than half of the studies, i.e., three studies, while a mere single study took culture sterile neonates as the control group [28]. However, the length of life of all the newborns during the diagnosis remains the same in all the studies, i.e., less than 30 days of life. The primary feature of the research articles included is outlined in Table 1.
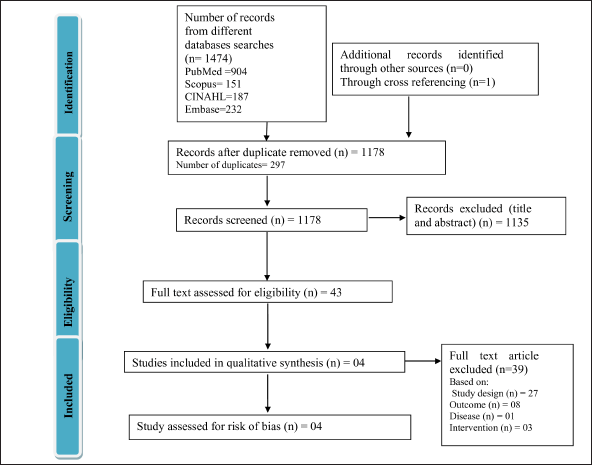 | Figure 1. Preferred reporting item for systematic review and meta-analyses (PRISMA) flow diagram of the comprehended articles using databases PubMed, CINAHL, EMBASE and SCOPUS. [Click here to view] |
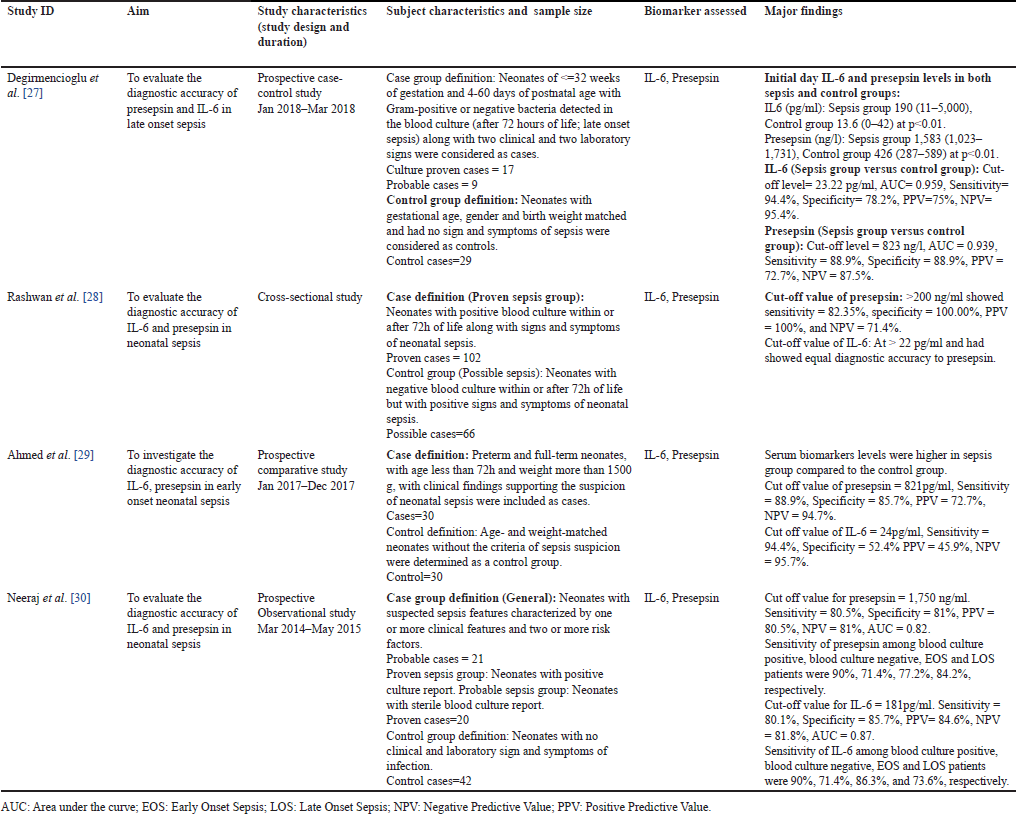 | Table 1. Main features of the comprehended articles, stating the comparison of interleukin 6 and presepsin biomarkers for the early detection of neonatal sepsis. [Click here to view] |
Risk of bias assessment
QUADAS-2 was the tool employed to gauge the risk level of bias and applicability concerns of the research articles included. Three articles had low risk in the “patient selection” realm, while one had high risk. Meanwhile, in the “index and reference standard” section, half of the articles incorporated (two articles) showed low risk while the remaining half were of unclear risk of bias. Furthermore, in the “flow and timing” segment, three of the articles had a low risk of bias, while the remaining one had an unclear risk of bias. In terms of applicability context, in the “patient selection” and “reference standard” division, barely one study had a low risk of bias, and the three had a high risk of bias, while in the “index test” section, the opposite is observed, i.e., three studies have a low risk of bias while exclusively one article had a high risk of bias. The tabular representation of the QUADAS-2 tool for all the included articles is shown in Table 2, while the pictorial representation, in terms of percentage, is shown in Figure 2.
Threshold effect and heterogeneity
There was no significant heterogeneity resulting from the observed threshold effect because the spearmen correlation p-value for presepsin and IL-6 was greater than p = 0.05. For IL-6 and presepsin, the individual spearmen correlation coefficient and p-value were 0.600, p = 0.400, and −0.44, p = 0.600, respectively. Thus, we combined the sensitivity, specificity, PLR, NLR, DOR, and AUC of the incorporated articles. To calculate the heterogeneity resulting from nonthreshold effects, Higgins I2 statistics were used. It was found that, in terms of sensitivity and specificity, presepsin had I2 values of 0% and 75.7%, respectively, whereas IL-6 had 72.2% and 92%, pointing towards heterogeneity caused by nonthreshold effects. Thus, considering the I2 values, a bivariate hierarchical model was employed.
Pooled result and forest plot
The combined sensitivity and the specificity, with the confidence interval of presepsin, were 0.86 (0.81–0.90), 0.89(0.84–0.92), and that for IL-6 were 0.88 (0.82–0.93), 0.78 (0.70–0.84), which surely indicates that presepsin had a better specificity and nearly equivalent sensitivity to that of IL-6. Analogous kinds of output were seen in the case of DOR and positive and NLR. Thus, the pooled result of both presepsin and IL-6 for both sensitivity and specificity are depicted as a forest plot in Figure 3, and the corresponding PLR, NLR, and DOR, along with their respective CI, are summarized in the supplementary file; Table 1.
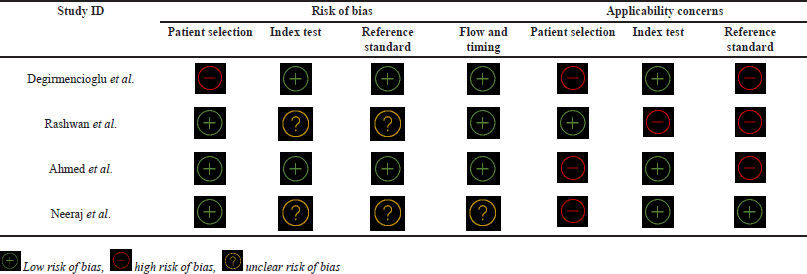 | Table 2. Risk of bias assessment of the included articles by applying QUADAS-2 tool. [Click here to view] |
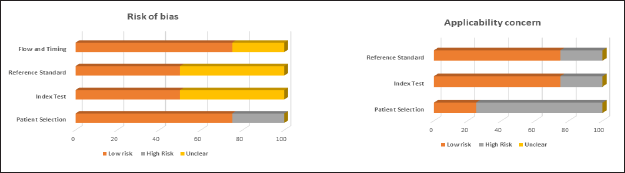 | Figure 2. Risk of bias and applicability concerns of the embodied articles by quality assessment of diagnostic accuracy studies-2 (QUADAS-2) tool. [Click here to view] |
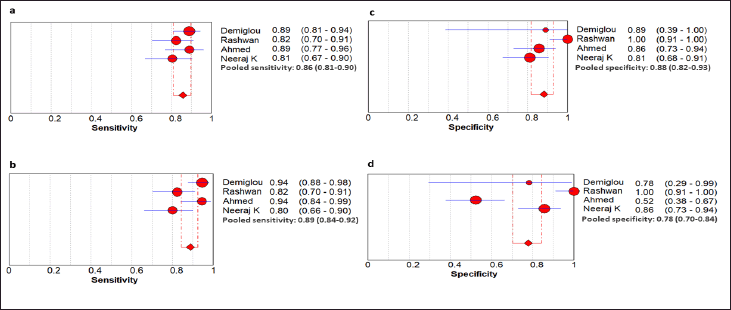 | Figure 3. Pooled sensitivity and specificity of both the biomarkers by forest plot (a) Sensitivity of presepsin, (b) Sensitivity of IL-6, (c) Specificity of presepsin, and (d) Specificity of IL-6. [Click here to view] |
Bivariate hierarchical model and HSROC curve
The bivariate/HSROC model was deduced to assess the diagnostic accuracy of both biomarkers. Furthermore, the HSROC curve was laid out to gauge the same. The AUC of HSROC for presepsin was higher than that of IL-6, i.e., 0.9305 and 0.9190, respectively. The summary operating point of presepsin in terms of sensitivity and specificity was 0.859 (0.808–0.898) and 0.902 (0.731–0.969), while that of IL-6 was 0.893 (0.806–0.944) and 0.852 (0.508–0.970) correspondingly implying higher specificity for presepsin and roughly equivalent sensitivity to that of IL-6. Subsequently, positive and NLRs had also showed akin kind of results where the values of presepsin were higher than that of IL-6, i.e., 8.701 (2.941–25.749) versus 6.050 (1.457–25.126) and 0.125 (0.075–0.211) versus 0.157 (0.114–0.125). Meanwhile, the summary DOR for both presepsin and IL-6 were 55.529 (16.789–183.654) and 48.286 (11.494–202.854) simultaneously, attributing towards better diagnostic capacity of presepsin than IL-6. Furthermore, the random effect correlation of presepsin had a value of −1 with random effect accuracy λ (lambda) of 5.996, random effect threshold θ (theta) of 2.235, and random effect shape effect β (beta) of 2.129. However, the random effect correlation value of IL-6, along with its accuracy, threshold, and shape effect, is largely lower than that of presepsin values. Thus, the detailed summary point statistics of both the biomarkers (presepsin and IL-6) are briefed in Table 3, while the pictorial depiction of the HSROC curve is illustrated in Figure 4.
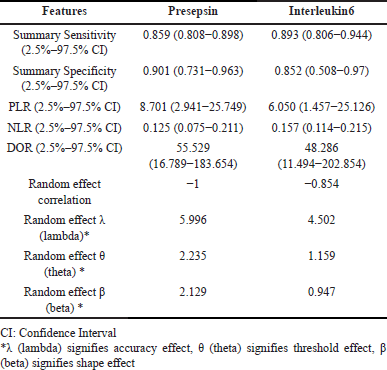 | Table 3. Summary point statistics of presepsin and Interleukin 6 using bivariate hierarchical model. [Click here to view] |
DISCUSSION
Late identification of neonatal sepsis, owing to its incomprehensible signs and symptoms, is one of the elementary bases for its significant mortality rates. Prevailing approaches such like blood culture authentication, and laboratory and clinical investigation are insufficient in providing a rapid and factual diagnosis of the disease. Therefore, an eminently perceptive and profoundly precise biomarker is the need of the hour. Thus, this systematic review and meta-analysis were planned to highlight the efficiency of presepsin and IL-6 in neonatal sepsis diagnosis. To the best of our knowledge, this is the first review to comprehend these two biomarkers for neonatal sepsis diagnosis, as there is a lack of literature evaluating these biomarkers. Thus, the currently studied systematic review and meta-analysis have been characterized based on the three segments, i.e., key findings of the article, comparing the major findings in terms of conventional and latest biomarkers available in neonatal sepsis diagnosis, and advantages of the statistical method adopted to carry out the meta-analysis.
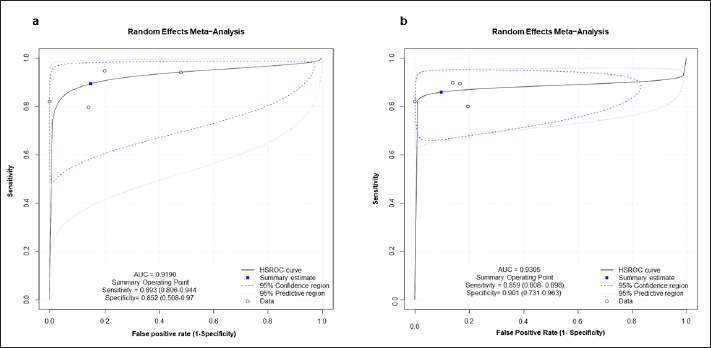 | Figure 4. HSROC curve of both the biomarkers (a) IL-6. (b) Presepsin. [Click here to view] |
Key findings
The fundamental finding of the present meta-analysis is that presepsin showed to have improved diagnostic efficiency compared to that of IL-6. The pooled specificity of presepsin was found to be higher than that of IL-6, indicating better performance in “ruling out” the disease. However, the pooled sensitivity parameter was relatively comparable for both biomarkers, thus suggesting an equal possibility of “ruling in” disease [31]. Nonetheless, these parameters are dependent on disease prevalence and population characteristics and do not translate to individual patients. Thus, instead, the likelihood ratio parameter is used, which is independent of population characteristics and can be translated to individual patients. A high positive and low NLR propound towards good diagnostic performance [32]. Our study showed a similar kind of result, where presepsin had a higher positive and lower NLR as compared to IL-6. Likewise, the value of the area under the curve for the preferable diagnostic ability of presepsin is substantially higher than IL-6. DOR is one of the central factors in evaluating the diagnostic scope of any biomarker, accounting for the threshold effects that sensitivity and specificity do not provide. A higher value of DOR for any biomarker is suggestive of better diagnostic accuracy when compared to that of the other [33]. Our study finding indicates that presepsin had higher DOR than IL-6. However, a minute amount of heterogeneity was noticed in some of the statistical analysis of our study, which may influence the analysis of our findings. Inconsistency in terms of disease severity among the covered articles and involvement of preterm neonates for meta-analysis can be a possible reason for the upsurge in heterogeneity in the output analysis [27–29]. Likewise, the age of newborns can be a potential source of heterogeneity, thus ensuring we to eliminate articles with newborns having sepsis at an age of more than 28 days. However, we have sidelined the “premature newborn” factor pertaining to the diagnosis of neonatal sepsis by presepsin and IL-6, as the availability of data related to this was not reported in the included articles. Similarly, the correlation between gestational age and levels of presepsin could have been considered an important factor for determining the performance of the biomarker [34]. However, we could not evaluate this factor due to the inadequacy of data in the embodied articles. Furthermore, the surge in the levels of presepsin can also be influenced by the microbiological trait of the culture report and thus can play a pivotal role in diagnosing neonatal sepsis [35].. However, we could not assess this prospect, as the requisite data for the same was unavailable in the embodied articles.
Thus, considering the minuscule heterogeneity observed in the study, presepsin could still serve as a potent biomarker for diagnosing neonatal sepsis.
Comparing the major findings of our study based on conventional and latest biomarkers available for diagnosing neonatal sepsis
Presepsin was discovered to have exceedingly high diagnostic efficiency when compared to that of IL-6 was the notable finding of our study. Since there is a lack of research comparing presepsin with IL-6, we examined our findings with the conventional biomarker available in neonatal sepsis diagnosis. A meta-analysis conducted by Ruan 2018 et al. [6], had evidently depicted that presepsin alone had proportionate diagnostic veracity when compared to that of conventional biomarkers such as CRP and PCT, where the pooled sensitivity of CRP, PCT, and presepsin was 0.71 (0.63–0.78), 0.85 (0.79–0.89), and 0.94 (0.80–0.99) correspondingly. Findings from our study displayed the summary sensitivity of presepsin to be 0.860 (0.80–0.89), which was greater than that of conventional markers CRP and PCT mentioned above. Similarly, the HSROC; AUC of presepsin in our study (0.93) was higher than that of CRP and PCT found in Ruan et al. [6]. However, another research by Bellos et al. [12] revealed a similar kind of result where presepsin had superlative sensitivity than CRP and PCT. AUC obtained from this study pertaining to presepsin (0.959) had shown a higher value than that of PCT (0.783) and CRP (0.858) [12]. Analogous findings were discovered in our study where the HSROC; AUC of presepsin was 0.930, which was proportionately identical to that of the previously mentioned study and was greater than that of PCT and CRP. Further, another distinct study had identified presepsin to be an ideal biomarker for early onset sepsis with pooled specificity of 0.91 (0.85–0.95), which is commensurate with our study output, i.e., 0.90 (0.73–0.96) (2.5–97.5CI). However, the pooled sensitivity and DOR were higher than that of our study finding which can be attributed to the diverse model employed for conducting the meta-analysis [36]. In addition, a study conducted by Parri et al. [16], had showcased presepsin to be a reliable biomarker for detecting neonatal sepsis [19]. The output obtained from this study is almost complementary to that of our study findings, where the pooled sensitivity and specificity and AUC of presepsin at a threshold of >600 ng/l were comparable to that of ours. Recently identified biomarkers such as neutrophil CD64 (nCD64) and soluble triggering receptor expressed on myeloid cell-1 (sTREM-1) were also explored for their diagnostic capacity in neonatal sepsis. However, no meta-analysis was available, comparing the diagnosing efficacy of presepsin and the recent biomarkers. Therefore, we compared our study findings with the individual research pertaining to presepsin and the latest biomarkers evaluated together.
Experimentation conducted by Hashem et al. [37] revealed that presepsin and CD64 both were beneficial in detecting neonatal sepsis, with higher specificity (95.5%) detected by presepsin over CD64. The AUC was estimated to be 0.887. Findings from our analysis revealed the summary specificity of 0.901 (0.731–0.963) with HSROC and AUC of 0.930 for presepsin, which indicates acceptable diagnostic accuracy. Similarly, yet another study conducted by Madbouly et al. [38] ascertains presepsin to be of better diagnostic ability, followed by sTREM and CD64. The individual sensitivity and specificity of presepsin were 100% and 86.7%, which was in accordance with the current study results [38].
Thus, the findings gathered suggest that presepsin can be considered a reliable biomarker in detecting neonatal sepsis, especially when compared to conventional ones. Combining presepsin with the latest biomarker can have an augmented effect on the diagnosis. However, a voluminous amount of research is still recommended to consider it to be a lone marker for neonatal sepsis.
Statistical method adopted to conduct the meta-analysis
A hierarchical bivariate model (summary point) and hierarchical summary receiver operating characteristic (HSROC) (summary line) were adopted to carry out the meta-analysis, thereby ensuring improved robustness of the data generated as it accounts for the threshold effect. Threshold effects are usually the criteria considered for conducting any diagnostic study, which largely differs from study to study and thus affects the sensitivity and specificity of any biomarker. The hierarchical bivariate model considers this aspect which is usually overlooked in the case of separate pooling method (Higgins I2 statistics) and thereby provides us with summary specificity and summary sensitivity. Additionally, the correlation between sensitivity and specificity could be analyzed through this model which eventually provides meticulous results as in real case scenarios, sensitivity, and specificity are always endlessly related. Data generated through the separate pooling method disregards this condition. Similarly, plotting the HSROC curve assists in finding out the inter and intra-study heterogeneity, which is impossible with Mosses Littenberg Model, i.e., summary receiver operating characteristic (SROC) curve. Furthermore, optimal weightage to individual study and correlation between sensitivity and specificity are few of the visible features assessed through the HSROC curve, which is beyond the possibility of SROC, thus ensuring robust and definite data output [39]. Additionally, publication bias is a universal concern in any meta-analysis, thus questioning the validity of the outcome. Therefore, Egger’s test or Begg’s test can be employed to analyze the publication bias, ensuring an improved, validated, and reliable result [40]. However, a lesser number of studies may influence the power of the test [41]. Considering only four articles are incorporated into our study, it would be hard to anticipate any kind of potential publication biases by these tests.
Hence, to the best of our knowledge, the data generated through our review can be valuable evidence, thus ensuring high applicability in other clinical settings.
Shortcomings
The inadequacy of the articles related to interleukin 6 and presepsin evaluation collectively is one of the primary shortcomings of the article. Pervasive experimentation, both observational and interventional, is necessary to bring out substantial and concrete evidence related to this. Categorization of neonates into early onset and late-onset sepsis may be considered as a plausible important factor in determining the performance of both the biomarkers as both presepsin and IL-6 could have responded differently in both the categories [28,42]. However, the limitation in the number of included articles restricted us from considering this prospect and, thereby, can be regarded as a sizable drawback. Furthermore, the exclusion of articles in other languages except English can also be contemplated as a limitation.
Hence, results bred through this meta-analysis certainly articulate that presepsin can be considered a superior diagnostic marker than IL-6 in the rapid diagnosis of neonatal sepsis. Evidence generated through this article might help healthcare professionals and clinicians in making improved and judicious decisions related to disease management and treatment.
CONCLUSION
Presepsin can be considered a better diagnostic marker than of IL-6 in rapid diagnosis of neonatal sepsis. Evidence generated through this meta-analysis can guide healthcare professionals in fabricating rationale and thoughtful choices related to disease management and treatment. However, thorough research and experimentation are essential to confirm and validate these findings, thereby ensuring their applicability and reproducibility in real-world settings.
ACKNOWLEDGEMENTS
The authors acknowledge Sawan Mukund for the assimilation and visualization of figures.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The fellowship of the research participant was sponsored by the Indian Council of Medical Research (ICMR); [File No: RMBH/FW/2020/32] India.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Zea-Vera A, Ochoa TJ. Challenges in the diagnosis and management of neonatal sepsis. J Trop Pediatr. 2015;61(1):1–13.
2. Iroh Tam PY, Bendel CM. Diagnostics for neonatal sepsis: current approaches and future directions. Pediatr Res. 2017;82(4):574–83.
3. Sharma D, Farahbakhsh N, Shastri S, Sharma P. Biomarkers for diagnosis of neonatal sepsis: a literature review. J Matern Fetal Neonatal Med. 2018;31(12):1646–59.
4. Delanghe JR, Speeckaert MM. Translational research and biomarkers in neonatal sepsis. Clin Chim Acta. 2015;451:46–64.
5. Ramasethu J, Kawakita T. Antibiotic stewardship in perinatal and neonatal care. Semin Fetal Neonatal Med. 2017;22(5):278–83.
6. Ruan L, Chen GY, Liu Z, Zhao Y, Xu GY, Li SF. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: a meta-analysis and systematic review. Crit Care. 2018;22(1):316.
7. Gilfillan M, Bhandari V. Neonatal sepsis biomarkers: where are we now? Res Rep Neonatol. 2019;9:9–20.
8. Cizmeci MN, Kara S, Kanburoglu MK, Simavli S, Duvan CI, Tatli MM. Detection of cord blood hepcidin levels as a biomarker for early onset neonatal sepsis. Med Hypotheses. 2014;82:310–2.
9. Chauhan N, Tiwari S, Jain U. Potential biomarkers for effective screening of neonatal sepsis infections: an overview. Microb Pathog. 2017;107:234–42.
10. Zou Q, Wen W, Zhang X-C. Presepsin as a novel sepsis biomarker. World J Emerg Med. 2014;5:16–19.
11. Chenevier-Gobeaux C, Borderie D, Weiss C, Mallet-Coste T, Claessens YE. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin Chim Acta. 2015;450:97–103.
12. Bellos I, Fitrou G, Pergialiotis V, Thomakos N, Perrea DN, Daskalakis G. The diagnostic accuracy of presepsin in neonatal sepsis: a meta-analysis. Eur J Pediatr. 2018;177:625–32.
13. Iskander A, Arthamin MZ, Indriana K, Anshory M, Hur M, Di Somma S. Comparison between presepsin and procalcitonin in early diagnosis of neonatal sepsis. J. Matern-fetal Neonatal Med. 2019;32:3903–8.
14. Ozdemir AA, Elgormus Y. Diagnostic value of presepsin in detection of early onset neonatal sepsis. Am J Perinatol. 2017;34:550–6.
15. Kumar N, Dayal R, Singh P, Pathak S, Pooniya V, Goyal A. A comparative evaluation of presepsin with procalcitonin and CRP in diagnosing neonatal sepsis. Indian J Pediatr. 2019;86:177–9.
16. Parri N, Trippella G, Lisi C, Martino MD, Galli L, Chiappini E. Accuracy of presepsin in neonatal sepsis: systematic review and meta-analysis. Expert Rev Anti Infect Ther. 2019;17:223–32.
17. Sun B, Liang LF, Li J, Yang D, Zhao XB, Zhang KG. A meta-analysis of interleukin-6 as a valid and accurate index in diagnosing early neonatal sepsis. Int Wound J. 2019;16:527–33.
18. Qiu X, Zhang L, Tong Y, Qu Y, Wang H, Mu D. Interleukin-6 for early diagnosis of neonatal sepsis with premature rupture of membranes: a meta-analysis. Medic. 2018;97:e13146.
19. Shah BA, Padbury JF. Neonatal sepsis: an old problem with new insights. Virulence. 2014;5(1):170–8.
20. Singh M, Alsaleem M Gray CP. Neonatal Sepsis. StatPearls 2022. [cited 2022 Nov 19]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK531478/
21. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
22. Salameh JP, Bossuyt PM, McGrath TA, Thombs BD, Hyde CJ, Macaskill P, et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): explanation, elaboration, and checklist. BMJ. 2020;370:2632.
23. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
24. Molinaro AM. Diagnostic tests: how to estimate the positive predictive value. Neurooncol Pract. 2015;2(4):162–6.
25. Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31.
26. Freeman SC, Kerby CR, Patel A, Cooper NJ, Quinn T, Sutton AJ. Development of an interactive web-based tool to conduct and interrogate meta-analysis of diagnostic test accuracy studies: MetaDTA. BMC Med Res Methodol. 2019;19(1):81.
27. De?irmencio?lu H, Ozer Bekmez B, Derme T, Öncel MY, Canpolat FE, Tayman C. Presepsin and fetuin-A dyad for the diagnosis of proven sepsis in preterm neonates. BMC Infect Dis. 2019;19(1):695.
28. Rashwan NI, Hassan MH, Mohey El-Deen ZM, Ahmed AE. Validity of biomarkers in screening for neonatal sepsis—a single center-hospital based study. Pediatr Neonatol. 2019;60(2):149–55.
29. Ahmed AM, Mohammed AT, Bastawy S, Attalla HA, Yousef AA, Abdelrazek MS, et al. Serum biomarkers for the early detection of the early-onset neonatal sepsis: a single-center prospective study. Adv Neonatal Care. 2019;19(5):26–32.
30. Kumar N, Singh MK, Dayal R, Gupta S, Goyal A, Agarwal P, et al. Presepsin and IL-6 as potential markers of neonatal sepsis. J Neonatal. 2018;32:106–10.
31. Begg CB. Biases in the assessment of diagnostic tests. Stat Med. 1987;6(4):411–23.
32. Attia J. Moving beyond sensitivity and specificity: using likelihood ratio to help interpret diagnostic tests. Aust. Prescr. 2003;26:111–3.
33. Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56(11):1129–35.
34. Nur Ergor S, Yalaz M, Altun Koroglu O, Sozmen E, Akisu M, Kultursay N. Reference ranges of presepsin (soluble CD14 subtype) in term and preterm neonates without infection, in relation to gestational and postnatal age, in the first 28 days of life. Clin Biochem. 2020;77:7–13.
35. Stoma I, Karpov I, Uss A, Rummo O, Milanovich N, Iskrov I. Diagnostic value of sepsis biomarkers in hematopoietic stem cell transplant recipients in a condition of high prevalence of gram-negative pathogens. Hematol Oncol Stem Cell Ther. 2017;10(1):15–21.
36. Poggi C, Lucenteforte E, Petri D, De Masi S, Dani C. Presepsin for the diagnosis of neonatal early-onset sepsis: a systematic review and meta-analysis. JAMA Pediatr. 2022;176(8):750–8
37. Hashem HE, Abdel Halim RM, El Masry SA, Mokhtar AM, Abdelaal NM. The utility of neutrophil CD64 and presepsin as diagnostic, prognostic, and monitoring biomarkers in neonatal sepsis. Int J Microbiol. 2020;2020:8814892:1–13.
38. El-Madbouly AA, El Sehemawy AA, Eldesoky NA, Abd Elgalil HM, Ahmed AM. Utility of presepsin, soluble triggering receptor expressed on myeloid cells-1, and neutrophil CD64 for early detection of neonatal sepsis. Infect Drug Resist. 2019;12:311–19.
39. Lee J, Kim KW, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part II. Statistical methods of meta-analysis. Korean J Radiol. 2015;16(6):1188–96.
40. Shi X, Nie C, Shi S, Wang T, YangH, Zhou Y, et al. Effect comparison between Egger’s test and Begg’s test in publication bias diagnosis in meta-analysis: evidence from a pilot survey. Int J Res Stud Biosci. 2017;5:14–20.
41. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
42. Cortés JS, Losada PX, Fernández LX, Beltrán E, DeLaura I, Narváez CF, et al. Interleukin-6 as a biomarker of early-onset neonatal sepsis. Am J Perinatol. 2021;38(S 01):338–46.