INTRODUCTION
Dyslipidemia, an imbalance of lipids, is a major public health problem throughout the world [1]. This condition can lead to serious consequences, including cardiovascular disease, obesity, degenerative joint disorder, angina, and stroke, which could eventually lead to death [2]. Several studies have indicated that oxidative stress is associated with abnormally high levels of total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and low levels of high-density lipoprotein cholesterol (HDL-C) [3,4]. In addition, previous studies have reported that unhealthy eating habits or an unbalanced diet are major risk factors for the development of dyslipidemia [5]. High-fat consumption increases reactive oxygen species (ROS) production, including an increase in free radicals and a decrease in the activities of antioxidant enzymes [6,7]. Enhanced ROS production leads to abnormal fat accumulation, which in turn elevates oxidative stress in the plasma, disrupting cellular function and leading to damage [8].
Over the last 50 years, statins have been the first-choice drugs to reduce plasma lipid levels, especially LDL-C [9]. However, statins can cause unwanted effects such as muscle pain, fatigue, sleep problems, headache, low blood platelet levels, digestive problems, and mental fuzziness; these side effects have led to limitations in their use [10]. In addition, statin resistance and intolerance have been reported in some patients [11]. Extensive research is ongoing in search of potential strategies or novel candidates that can effectively control dyslipidemia.
Recently, there has been a growing interest in non-pharmacological strategies to manage hyperlipidemia, including physical exercise, dietary modification, weight control, and herbal medicine [12]. There is extensive evidence that medicinal plants and their extracts can effectively treat dyslipidemia with few or no side effects by exerting various pharmacological effects, including antioxidant activity [13,14].
The wild mushroom Astraeus hygrometricus (A. hygrometricus) of the Diplocystaceae family has long been used in traditional Indian medicine and traditional Chinese medicine [15,16]. Both in vitro and in vivo studies have revealed its hypoglycemic, anticandidal, immunoenhancement, anti-inflammatory, hepatoprotective, pro-splenocyte proliferation, cardioprotective, and antioxidant effects [17–22]. However, there is no published research on the potential of A. hygrometricus extract on serum lipid levels and the oxidative blood profile in the context of dyslipidemia. Therefore, we evaluated the ability of oral administration of A. hygrometricus extract to modulate oxidative biochemical markers, hyperlipidemia, and hyperglycemia in a rat model of dyslipidemia induced by a high-fat diet (HFD).
MATERIALS AND METHODS
Drugs and chemicals
A superoxide dismutase (SOD) assay kit (reference number: 19160), a malondialdehyde (MDA, a marker of lipid peroxidation) assay kit (reference number: MAK085), thiobarbituric acid (TBA), 5,5’-dithiobis-(2-nitrobenzoic acid), the Folin–Ciocalteu phenol reagent, and quercetin (QE) were procured from the Sigma-Aldrich (St. Louis, MO). A glutathione peroxidase (GPx) assay kit (reference number: 703102) was obtained from Cayman Chemicals (Ann Arbor, MI). A rat HbA1c assay kit was purchased from Crystal Chem (catalog # 80300, Elk Grove Village, IL). A rat insulin enzyme-linked immunosorbent assay (ELISA) kit was procured from Morinaga Institute of Biological Science Company Ltd. (Yokohama, Japan). Gallic acid was obtained from Merck (Darmstadt, Germany). All other chemical reagents or solutions were of analytical grade.
Preparation of A. hygrometricus extract
Astraeus hygrometricus was collected from the local Mae Tam Market in Phayao Province, Thailand, during the rainy season in June or early July 2023. The mushrooms were soaked in water and rubbed to remove any soil or dirt. Each mushroom was split in half and then placed in a single layer on dehydrator trays for 12 hours. Then, the mushrooms were dried in a hot air oven at 60°C for 48 hours. Dried mushrooms were blended and homogenized before maceration with 90% ethanol for 72 hours and filtering through a vacuum filter. The solvent was evaporated by rotary evaporation at 45°C before freeze-drying to reduce the amount of water in the extract. The ethanolic A. hygrometricus extract yield was 14.83%. It was stored in airtight bottles at 4°C for further analysis.
Assessment of the total phenolic content (TPC) and the total flavonoid content (TFC) of A. hygrometricus extract
The TPC of A. hygrometricus extract was determined with the Folin–Ciocalteu method [23], with a few modifications. In brief, 0.01 g of the dried extract was dissolved in 10 ml of ethanol and mixed well until homogeneous. Then, 0.25 ml of the supernatant was mixed with 0.75 ml of distilled water. Next, 1.25 ml of Folin–Ciocalteu reagent (diluted 10-fold) was added, and the mixture was allowed to incubate for 4 minutes at 25°C. Finally, 1 ml of 10% (w/v) sodium carbonate (Na2CO3) solution was added to the mixture, thoroughly shaken, and incubated for 60 minutes in the dark at room temperature. The absorbance at 760 nm was read with a spectrophotometer (Thermo Scientific GeneSys 20 model 4001/4, Netherlands). The TPC content is expressed as milligrams of gallic acid equivalents per gram of dry weight (mg GAE/g DW).
The TFC of A. hygrometricus extract was estimated using the aluminum chloride colorimetric method with QE as a standard [24]. First, 0.2 ml of the extract was dissolved and mixed with 0.15 ml of 5% sodium nitrite (NaNO2) and incubated for 5 minutes at 25°C. Then, 0.15 ml of 10% (w/v) aluminum chloride (AlCl3) was added, and the mixture was incubated in the dark for 5 minutes. After that, 1 l of 4% (w/v) sodium hydroxide (NaOH) was added and mixed thoroughly. After incubation for 15 minutes, the absorbance at 510 nm was measured with a spectrophotometer. The TFC is expressed as milligrams of QE per gram dry weight (mg QE/g DW).
Estimation of the antioxidant potential of A. hygrometricus extract
The antioxidant capacity of A. hygrometricus extract was determined with the diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, with some modifications [25]. Briefly, 500 µl of DPPH in 0.4 mM/ml ethanol was added to 500 µl of A. hygrometricus extract and incubated for 30 minutes at room temperature in the dark. The absorbance at 517 nm was determined with a spectrophotometer. The scavenging effect of A. hygrometricus extract was calculated according to the following equation:
DPPH scavenging effect (%) = [(A517 nm of control – A517 nm of sample) / A517 nm of control] × 100,
where A517 nm of sample is the absorbance at 517 nm of the DPPH solution mixed with A. hygrometricus extract, and A517 nm of control is the absorbance at 517 nm of the DPPH solution without extract.
Determination of the ferric reducing antioxidant power (FRAP) of A. hygrometricus extract
The FRAP of A. hygrometricus extract was assessed by using a slightly modified version of a published protocol [26]. The working FRAP reagent consisted of acetate buffer (300 mM), 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ, 10 mM) in hydrochloric acid, and ferric chloride (20 mM). One hundred microliters of the extract were allowed to react with 3 ml of fresh FRAP solution and 300 µl of water. After incubation for 30 minutes at 37°C, the absorbance at 593 nm was recorded. Trolox solution was used as a standard substance, and the results are presented as millimoles of Trolox equivalents per gram dry weight (mmol TE/g DW).
Preparation of the HFD
The HFD was prepared according to the method of Srinivasan and co-workers [27], with some modifications. The composition, expressed as the percent of total calories, was 60% fat, 20% protein, and 20% carbohydrate.
Experimental animal studies and protocol
The animal experiment was carried out in accordance with the guidelines of the National Institute of Health (NIH) (NIH publication 85–23, 1985) for the care and use of laboratory animals, and was approved by the Animal Ethics Committee of the University of Phayao (Approval no. 1-028-65).
Forty young male Sprague-Dawley rats weighing 220–250 g, supplied by Nomura Siam International Company Ltd. (Bangkok, Thailand), were used. The rodents were placed in polyacrylic cages (two rats/cage) and maintained at 22°C ± 2°C, relative air humidity of 55%–65%, and a 12-hours photoperiod. Before the experiment, all animals were fed with commercially available rat standard pellet diet and water ad libitum. After 7 days of acclimatization, the rats were randomly allocated into four groups, each with 10 rats:
- Group 1: Control group, rats were fed with the standard diet and treated with distilled water served as a vehicle.
- Group 2: HFD group, rats were fed the HFD.
- Group 3: HFD + simvastatin (10 mg/kg body weight), the positive control group.
- Group 4: HFD + A. hygrometricus extract (500 mg/kg body weight).
The A. hygrometricus extract dose was selected based on its in vitro antioxidant ability from our pilot study and the previous study by Biswas and Acharya [28]. In addition, our pilot study showed no mortality or signs and symptoms of toxicity in the experimental rats treated with 5,000 mg/kg body weight A. hygrometricus extract (data not shown). The starting dose of the extract was calculated based on one tenth of the severely toxic dose. Therefore, 500 mg/kg body weight of the extract was selected to examine its antihyperlipidemic and antihyperglycemic effects in the present study.
The rats received the specific treatment via a feeding tube once daily for a total of 63 days. The fasting blood glucose (FBG) levels, body weights, and food intake were recorded once per week until the end of the study. On day 63 of the experiment, the rats were fasted for 12 hours and then anesthetized with a single dose of thiobutabarbital sodium (80 mg/kg body weight, intraperitoneal). Blood was collected from the abdominal vein in blood collection tubes to analyze FBG; the serum insulin, hemoglobin (Hb), and glycated hemoglobin (HbA1c) levels; insulin resistance; the serum lipid profile; MDA (a lipid peroxidation end product); and SOD and GPx activities.
Determination of FBG, serum HbA1c, serum insulin levels, and insulin resistance
FBG was estimated using a glucometer (Beijing Yicheng Biology Electronic Technology Company Ltd., China). The serum HbA1c level was determined with a rat HbA1c assay kit. Serum insulin levels were estimated with a rat insulin ELISA kit. Insulin resistance was assessed with the homeostasis model assessment method (HOMA-IR). It was calculated using the Kirubananthan equation [29]:
HOMA-IR = [fasting insulin level (µU/ml) × fasting glucose level (mg/dl)] / 405.
Estimation of serum lipid profile
Rat whole blood was collected and allowed to clot for 45 minutes at room temperature before centrifugation. Then, it was centrifuged at 2,000 rpm for 30 minutes at 4°C. The serum was removed and used to assess the lipid profile. The TG, TC, and HDL-C levels were determined by using commercially available kits (Roche/Boehringer-Mannheim Diagnostics, Germany) on a Beckman Coulter Automatic Analyzer. The serum LDL-C level was calculated using the Friedewald equation [30]:
LDL-C (mg/dl) = [TC (mg/dl) − HDL-C (mg/dl) − TG (mg/dl)] / 5.
Evaluation of serum lipid peroxidation
The serum MDA level, a marker of lipid peroxidation, was determined with an MDA assay kit. MDA reacts with TBA to produce a pink product, the absorbance of which was measured at 532 nm with the microplate reader. The results are presented as nmol/mg protein.
Assessment of serum antioxidant enzyme activities
The effect of A. hygrometricus extract on the SOD enzymatic activity in rodents’ serum was measured with a SOD assay kit following the manufacturer’s protocol. The absorbance at 505 nm was measured with a microplate reader. The enzyme activity is presented as U/mg of protein.
The rat serum GPx activity was evaluated with a rat GPx assay kit according to the manufacturer’s guidelines. The absorbance at 340 nm was determined with a microplate reader. The GPx enzyme activity is expressed as U/mg of protein.
Statistical analysis
SPSS Statistics version 11.5 (SPSS Inc., Chicago, IL) was used for data analysis. The results are presented as the mean ± standard deviation (SD). Statistical analysis involved an unpaired Student’s t-test (for two groups) or analysis of variance followed by the Tukey post hoc test (for three or more groups). Pearson correlation coefficients were determined to assess the relationships between weekly body weight, food intake, and body weight gain. For all tests, the level of statistical significance was p < 0.05.
RESULTS
TPC, TFC, and antioxidant capacity of A. hygrometricus extract
The TPC and TFC of A. hygrometricus extract were 20.85 ± 0.31 mg GAE/g DW and 13.48 ± 0.24 mg QE/g DW, respectively. A. hygrometricus extract showed moderate DPPH radical scavenging activity (63.87% ± 1.73%). The FRAP of the extract was 0.0293 ± 0.0012 nmol TE/g extract. All results are presented in Table 1.
Effect of A. hygrometricus extract on body weight, body weight gain, and food intake
Figure 1A depicts the weekly average body weight for each experimental group during the 63-day experiment. At baseline, there was no significant difference in the average body weight among the groups. However, after 7 days the HFD group showed a significant increase (p < 0.001) in the mean body weight compared with the control group; this difference continued for the remainder of the experiment. These observations are similar to the results obtained in the rats treated with simvastatin and A. hygrometricus extract. The HFD + simvastatin and HFD + A. hygrometricus extract groups also showed a significant difference (p < 0.05) in average body weight compared with the control group. Although at day 56 there was a trend for a reduction in the average body weight of the HFD + simvastatin and HFD + A. hygrometricus extract groups compared with the HFD group, the difference was not significant.
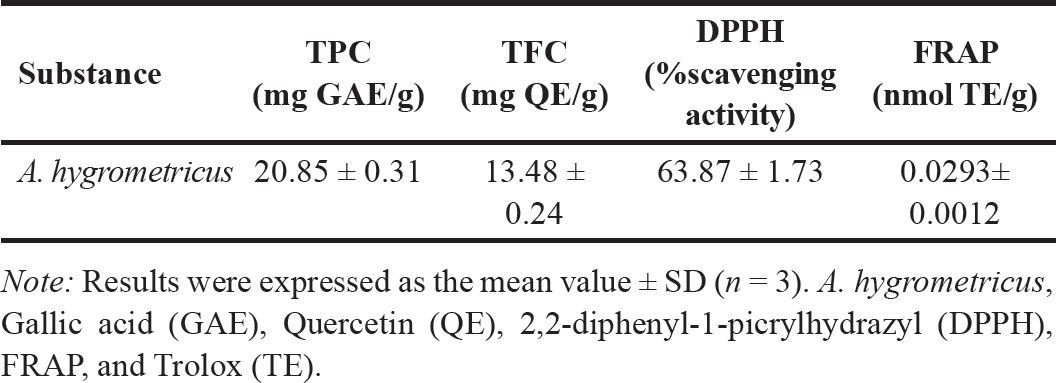 | Table 1. Total phenolic and flavonoid contents and antioxidant ability of the wild mushroom A. hygrometricus extract. [Click here to view] |
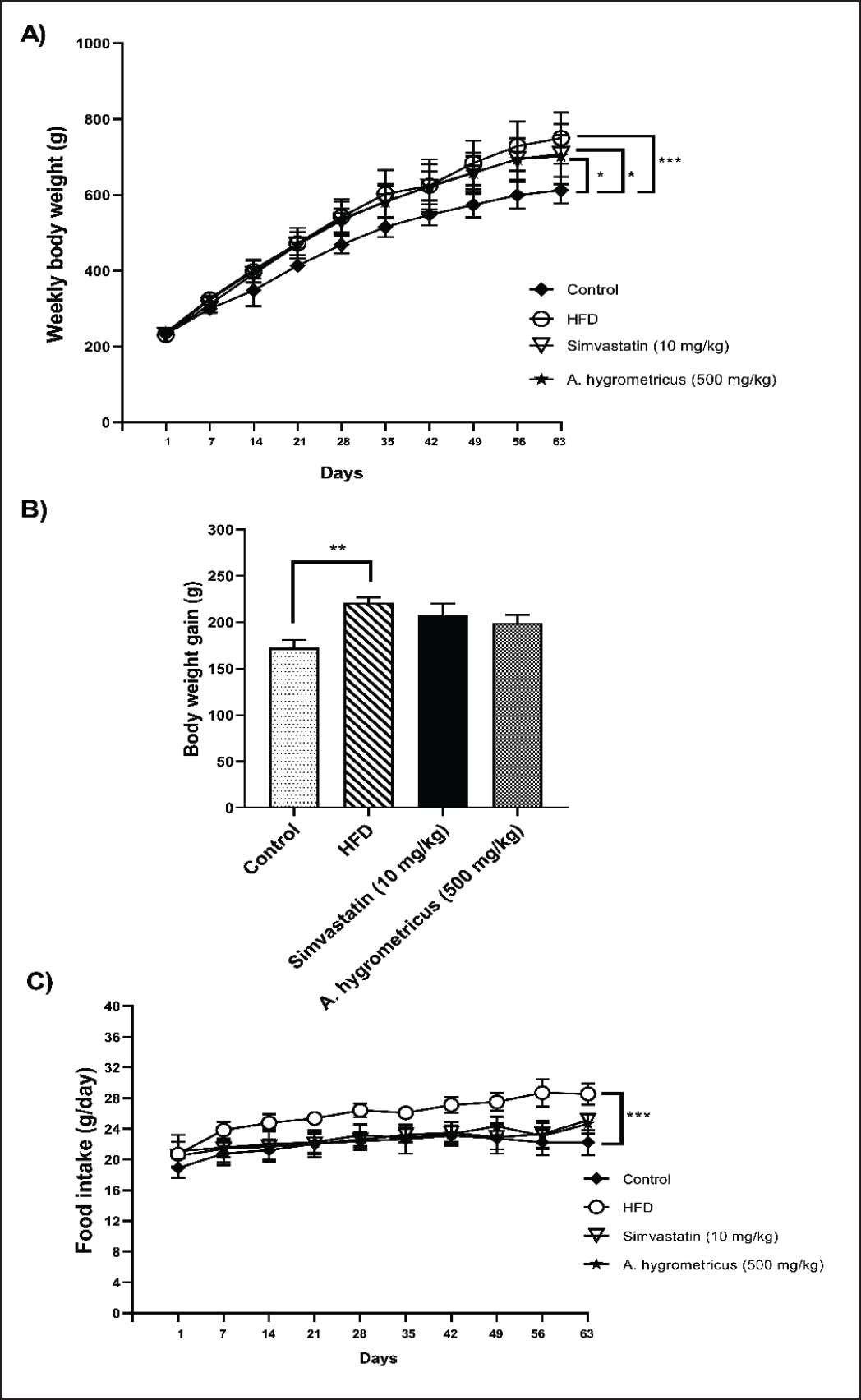 | Figure 1. (A) Effects of the wild mushroom A. hygrometricus extract on weekly body weight (g). (B) Body weight gain (g). (C) Food intake (g/day) in HFD induced hyperlipidemia rats. Data are shown in mean ± SD (n = 10/group). Asterisk (***) marked data are significantly different at p < 0.001, (**) denotes p > 0.01 and (*) denotes p > 0.05. [Click here to view] |
Figure 1B and C shows the relationships between weekly body weight, food intake, and body weight gain. The excess body weight of the HFD group was significantly higher (p < 0.01) than the control group, an outcome consistent with the increased daily food consumption in the HFD group (p < 0.001). Compared with the control group, there were no significant differences in the overall body weight gain and food intake for the HFD + simvastatin or HFD + A. hygrometricus extract groups.
Effect of A. hygrometricus extract on FBG, serum insulin, HbA1c levels, and HOMA-IR
Table 2 shows the FBG, serum insulin and HbA1c levels, and HOMA-IR data. The HFD group showed a significant increase (p < 0.01) in FBG and the serum insulin and HbA1c levels compared with the control group. HFD consumption also induced insulin resistance in the HFD group, denoted by a significant increase (p < 0.01) in HOMA-IR compared with the control group. FBG, the serum insulin, HbA1c levels, and HOMO-IR were dramatically decreased (p < 0.01) in the HFD + A. hygrometricus extract group compared with the HFD group.
Effect of A. hygrometricus extract on the serum lipid profile
The serum lipid profiles are shown in Figure 2A–D. The HFD group developed dyslipidemia, as indicated by significantly higher serum TC (p < 0.05), TG (p < 0.001), and LDL-C (p < 0.001) levels, and significantly lower HDL-C (p < 0.05) levels compared with the control group. In the HFD + simvastatin group, the serum TG and LDL-C levels were significantly decreased (p < 0.05) while the serum HDL-C levels were significantly increased (p < 0.01) compared with the HFD group. Moreover, the HFD + A. hygrometricus extract group showed significantly reduced serum TG (p < 0.05) and LDL-C (p < 0.01) levels, and significantly increased HDL-C levels (p < 0.05) compared with the HFD group. The TC levels in the HFD + simvastatin and HFD + A. hygrometricus extract groups were not significantly different compared with the HFD group.
Effect of A. hygrometricus extract on serum antioxidant enzyme activities and lipid peroxidation
As shown in Table 3, compared with the control group, the serum SOD and GPx activities were significantly decreased (p < 0.05) in the HFD group. However, SOD and GPx activities were markedly induced (p < 0.05) in the HFD + A. hygrometricus extract and HFD + simvastatin groups compared with the HFD group. Furthermore, the serum MDA levels were significantly elevated (p < 0.01) in the HFD group compared with the control group. Supplementation with either simvastatin or A. hygrometricus extract ameliorated this increase (p < 0.05).
DISCUSSION
Studies from several countries have shown that edible wild mushrooms such as A. hygrometricus are valuable foods with high nutritional value and numerous medicinal benefits [31,32]. We demonstrated that supplementation with 500 mg/kg body weight A. hygrometricus extract could decrease FBG, serum insulin and HbA1C levels, and HOMA-IR, and ameliorate the lipid profile by decreasing lipid peroxidation and increasing the activities of endogenous antioxidant enzymes in a rat model of dyslipidemia induced by an HFD. This extract did not cause mortality or adverse effects.
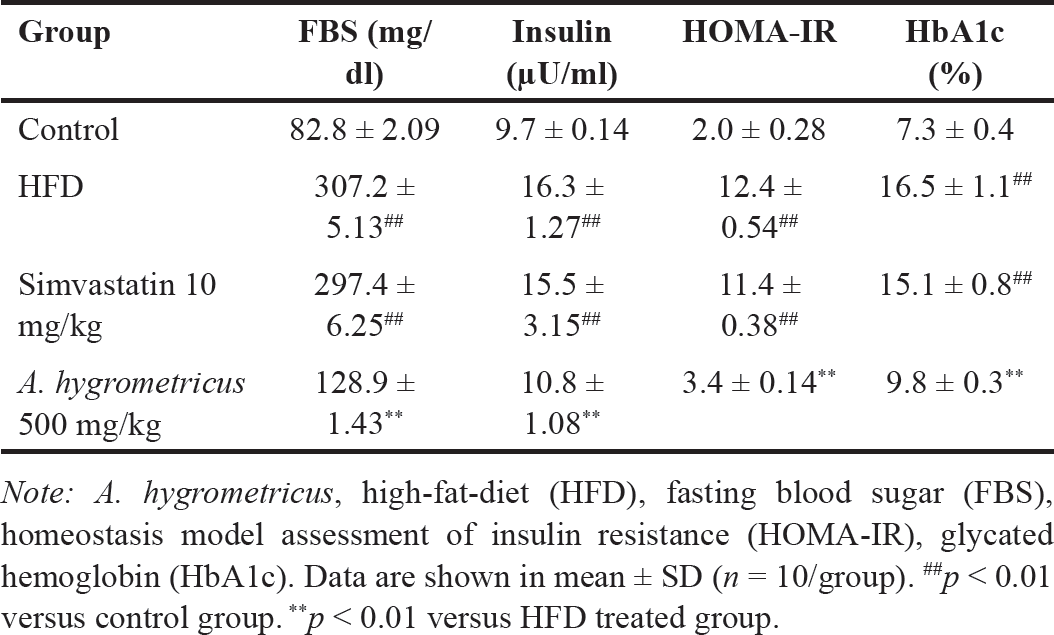 | Table 2. Effects of the wild mushroom A. hygrometricus extract on FBG, insulin, HOMA-IR, Hb, and HbA1c in a model of dyslipidemia by feeding rats an HFD. [Click here to view] |
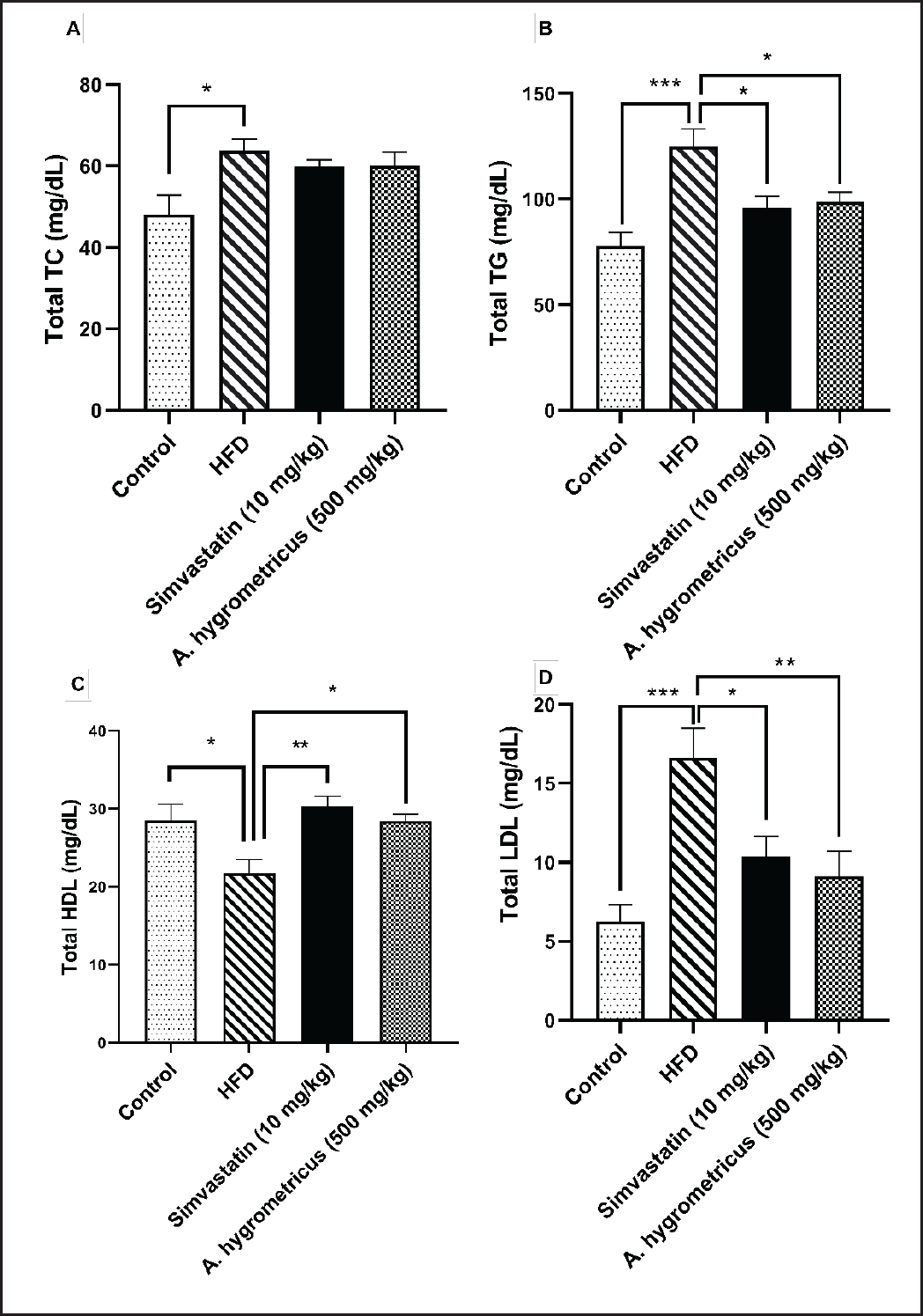 | Figure 2. (A) Effects of A. hygrometricus extract on serum TC, (B) TG, (C) serum HDL-C, and (D) low-density lipoprotein-cholesterol (LDL-C) in HFD induced hyperlipidemia rats. Data are shown in mean ± SD (n = 10/group). Asterisk (***) marked data are significantly different at p < 0.001, (**) denotes p > 0.01, and (*) denotes p > 0.05. [Click here to view] |
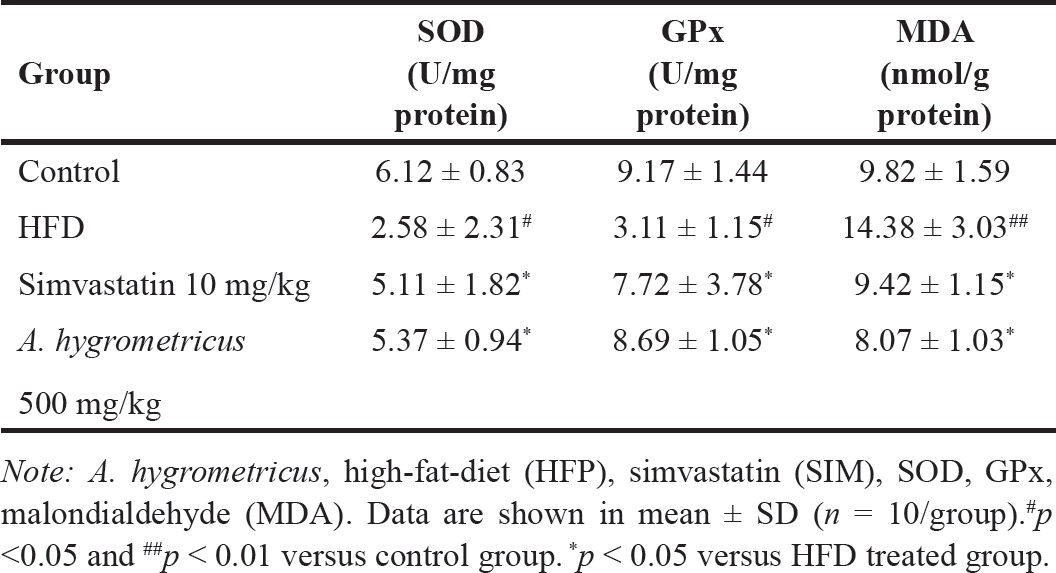 | Table 3. Effects of the wild mushroom A. hygrometricus extract on serum antioxidant enzymes activities and lipid peroxidation in HFD-induced hyperlipidemia rats. [Click here to view] |
Long-term excess consumption of a diet rich in saturated fat and cholesterol is positively associated with hyperglycemia and dyslipidemia [33]. Churuangsuk et al. [34] reported that an HFD is related to higher blood glucose and HbA1c levels in patients with type 2 diabetes. In addition, several studies have revealed that chronic overconsumption of HFD is mechanistically associated with the dysregulation of metabolic homeostasis, resulting in insulin resistance [35,36]. In our study, rats fed an HFD for 63 days developed hyperglycemia manifested by a significant increase in FBG, serum insulin, and HbA1c levels, which eventually led to insulin resistance. These results agree with a previous study revealing that long-term exposure to an HFD results in the development of hyperglycemia, glucose intolerance, and insulin resistance [37]. Chronic HFD feeding can also lead to hyperinsulinemia; hepatic steatosis; and dyslipidemia characterized by an increase in the plasma TC, TG, and LDL-C levels, but a decrease in HDL-C levels in rodents [38]. Similarly to our study, Sprague-Dawley rats fed an HFD presented greater body weight gain and food intake. The HFD-fed rats also successfully developed hyperglycemia, as indicated by significantly higher FBG and serum HbA1c levels. In addition, prolonged HFD consumption increased HOMA-IR due to prolonged exposure to high glucose and insulin levels. Moreover, the HFD-fed rats also developed dyslipidemia as indicated by significantly higher serum TC, TG, and LDL-C levels, but lower HDL-C concentrations.
Dietary supplement consumption is increasing in popularity throughout the world. Among the natural dietary supplements that have been studied are edible wild mushrooms, which are well known for their rich nutritional bioactive compounds as well as pharmacological effects especially antioxidant abilities [39]. A myriad of studies has revealed that there is a significant correlation between antioxidant capabilities and the TPC and TFC [40]. The notable antioxidant abilities of A. hygrometricus extract in the present study are consistent with the high TPC and TFC (Table 1). These results are also consistent with a previous study by Fong-in et al. [41], who reported that A. hygrometricus extract showed 45.72% DPPH radical scavenging activity and a FRAP of 0.41 g Fe (II)/kg DW. In addition, they reported a TPC and TFC of 2.33 g GAE/kg DW and 1.49 g CE/kg DW, respectively. The differences between our study and the study by Fong-in et al. [41] are probably due to variations in the extraction methods, climate conditions, and A. hygrometricus species.
A number of studies have proposed that the therapeutic and pharmacological properties of wild edible mushrooms could help treat and prevent several diseases including diabetes and hyperlipidemia [42]. Herein, A. hygrometricus extract at a dose of 500 mg/kg for 63 days exerted an antihyperglycemic effect, denoted by a marked reduction in serum HbA1c, FBG, and HOMA-IR in HFD-fed rats. Our results are consistent with the study by Biswas and Acharya [26], who reported that oral administration of A. hygrometricus extract could reduce blood glucose levels in diabetic mice. HOMA-IR is a parameter to predict insulin resistance for glucose uptake into cells. Insulin resistance is a condition in which the body’s cells or tissues stop responding to the hormone insulin, leading to higher insulin and blood glucose levels [43]. Based on our results, it could be primarily suggested that A. hygrometricus extract decreases blood glucose by reducing insulin resistance.
There is a strong correlation between insulin resistance and dyslipidemia: Insulin resistance can alter systemic lipid and lipoprotein metabolism, which results in the development of dyslipidemia [44]. There is a search for novel lipid-lowering agents that can also improve blood glucose, thus providing a treatment for hyperlipidemia and hyperglycemia. Surprisingly, A. hygrometricus extract could reduce markedly elevated TG and LDL-C levels and increase HDL-C levels in rats with dyslipidemia. Thus, A. hygrometricus extract possesses antihyperglycemic and antihyperlipidemic effects.
As mentioned above, consuming an HFD induces oxidative stress, hyperglycemia, and dyslipidemia; however, the precise mechanisms for increased oxidative stress in these conditions still are not completely understood [45]. HFD intake increases plasma blood glucose and free fatty acid (FFA) levels [46]. The increased blood glucose levels cause beta islet cells to continuously release insulin, while an increase in FFAs enhances lipid synthesis and intrahepatic diacylglycerol (DAG) accumulation, leading to the activation of protein kinase Cε (PKCε), which in turn inhibits insulin signaling and, finally, results in insulin resistance [47]. In addition, HFD provokes dyslipidemia through increased circulation of FFAs, FFA oxidation, mitochondrial dysfunction, and over-activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, accompanied by reduced expression of antioxidant enzymes and increased free radical production [5,48]. Thus, prevention or alleviation of oxidative stress seems to be a promising therapeutic approach to treating diabetes and hyperlipidemia.
Our results are consistent with the study by Matsuzawa-Nagata et al. [49], who demonstrated that excessive HFD consumption leads to the generation of oxidative stress in plasma, characterized by ROS overaccumulation, increased lipid peroxidation, and decreased enzymatic antioxidants. We found that the MDA level in the HFD group was higher than in the control group, suggesting that the increase in MDA contents could be attributed to increased intracellular ROS production and a deficient endogenous enzymatic antioxidant defense system. We also observed lower SOD and GPx activities in the HFD group, indicating that HFD feeding induced oxidative stress.
Our data confirmed that A. hygrometricus extract has strong antioxidant properties that help ameliorates hyperglycemia and dyslipidemia via modulating SOD and GPx activities and suppressing lipid peroxidation. It is worth noting that we used a crude extract containing several biologically active compounds including flavonoids, polyphenols, tannins, lycopene, phytic acid, vitamin C, carotenoids, and polysaccharides with broad pharmacological properties [50]. Thus, the antihyperglycemic and the antihyperlipidemic effects of the extract may occur through synergistic and/or additive interactions from various active compounds. In the future, it will be necessary to identify which active compound(s) in A. hygrometricus extract contribute to its antidiabetic and antihyperlipidemic activities.
The use of nutraceuticals and dietary supplementation is a promising complementary therapy in the prevention and treatment of type 2 diabetes and hyperlipidemia that has attracted a lot of attention. We clearly showed that A. hygrometricus extract exerts an antihyperglycemic effect by reducing blood glucose, and it also possesses an antihyperlipidemic effect by reducing TG and LDL-C but increasing HDL-C. It is interesting that the antihyperlipidemic effect of the extract was similar to that of simvastatin, an antihyperlipidemic drug used to lower TG and LDL-C levels [51]. This suggests that A. hygrometricus extract might have the same potential antihyperlipidemic effect as simvastatin. Notably, neither simvastatin nor A. hygrometricus extract could decrease the TC levels of HFD-fed rats, although they could lower the LDL-C levels. Simvastatin is a statin; it works by blocking an enzyme in the liver, namely HMG-CoA reductase, which is involved in hepatic cholesterol synthesis. Statins activate transcription of the LDL receptor gene, particularly in the liver, ultimately reducing LDL-C and blood cholesterol levels [52]. In this study, simvastatin did not reduce blood TC levels in the rats fed a high-fat diet. One possible explanation for this phenomenon might be related to the dose of simvastatin (only 10 mg/kg body weight). Therefore, the potency and efficacy of the drug cannot reach the plateau phase to reduce blood TC levels. Conversely, the exact mechanisms of A. hygrometricus extract may have an indirect effect on HMG-CoA reductase, thus explaining why serum TC levels did not change during the experiment.
CONCLUSION
In conclusion, A. hygrometricus extract has strong antioxidant potential: it improved blood glucose by reducing insulin resistance denoted by a reduction in FBG, serum insulin, and HbA1c, and HOMO-IR and corrected the lipid profile with a decrease in TG and LDL-C levels and increase in HDL-C levels in a rat model of dyslipidemia. These effects may occur via improving insulin sensitivity and suppressing oxidative stress in HFD rats with increased SOD and GPx activities and decreased MDA levels. Although the exact molecular mechanisms of A. hygrometricus extract need to be further investigated, in the future it could be employed in dietary supplements used to lower blood glucose and lipid levels.
ACKNOWLEDGMENTS
This research was supported by (i) University of Phayao, (ii) Thailand Science Research and Innovation (TSRI), and (iii) Plant Genetic Conservation Project Under the Royal Initiative of Her Royal Highness Princess Maha Chakri Sirindhorn (RSPG): (Grant No. PG66003).
AUTHOR CONTRIBUTIONS
WP conceived and designed the study, conducted the experiment, analyzed, and interpreted all data, and wrote the article; NK, PR, and DM conducted and shared the experiments. All authors have read and approved the final manuscript.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The animal experiment was carried out in accordance with the guidelines of the National Institute of Health (NIH) (NIH publication 85–23, 1985) for the care and use of laboratory animals, and was approved by the Animal Ethics Committee of the University of Phayao, Thailand (Approval no. 1-028-65).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Sunil C, Ignacimuthu S, Kumarappan C. Hypolipidemic activity of symplocos cochinchinensis S. Moore leaves in hyperlipidemic rats. J Nat Med. 2012;66:32–8.
2. Hill MF, Bordoni B. Hyperlipidemia. In: StatPearls. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559182/. Accessed 8 Nov 2023.
3. Roman Junior WA, Piato AL, Conterato GM, Wildner SM, Marcon M, Mocelin R, et al. Hypolipidemic effects of Solidago chilensis hydroalcoholic extract and its major isolated constituent quercetin in cholesterol-fed rats. Pharm Biol 2015;53(10):1488–95.
4. Tan BL, Norhaizan ME, Liew WP. Nutrients and oxidative stress: friend or foe? Oxid Med Cell Longev. 2018;2018:9719584.
5. Yuan Y, Liu Q, Zhao F, Cao J, Shen X, Li C. Holothuria leucospilota polysaccharides ameliorate hyperlipidemia in high-fat diet-induced rats via short-chain fatty acids production and lipid metabolism regulation. Int J Mol Sci. 2019;20:4738.
6. Charradi K, Elkahoui S, Limam F, Aouani E. High-fat diet induced an oxidative stress in white adipose tissue and disturbed plasma transition metals in rat: prevention by grape seed and skin extract. J Physiol Sci. 2013;63(6):445–55.
7. Chen HP, Zeng F, Li SM, Liu YL, Gong SY, Lv XC, et al. Spirulina active substance mediated gut microbes improve lipid metabolism in high-fat diet fed rats. J Funct Foods 2019;59:215–22.
8. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61.
9. Sizar O, Khare S, Jamil RT, et al. Statin medications. In: StatPearls https://www.ncbi.nlm.nih.gov/books/NBK430940/. Accessed 8 Nov 2023.
10. Ramkumar S, Raghunath A, Raghunath S. Statin therapy: review of safety and potential side effects. Acta Cardiol Sin. 2016;32(6):631–9.
11. Davies JT, Delfino SF, Feinberg CE, Johnson MF, Nappi VL, Olinger JT, et al. Current and emerging uses of statins in clinical therapeutics: a review. Lipid Insights 2016;9:13–29.
12. Nieves JP, Baum SJ. A review of the evidence for alternative and complementary medical approaches in the prevention of atherosclerotic cardiovascular disease and diabetes. Cardiovasc Endocrinol. 2017;6(1):39–43.
13. Mayer MA, Höcht C, Puyó A, Taira CA. Recent advances in obesity pharmacotherapy. Curr Clin Pharmacol. 2009;4:53–61.
14. Montaser MM, El-Sharnouby ME, El-Noubi G, El-Shaer HM, Khalil AA, Hassanin M, et al. Boswellia serrata resin extract in diets of Nile Tilapia, Oreochromis niloticus: effects on the growth, health, immune response, and disease resistance to Staphylococcus aureus. Animals (Basel) 2021;11(2):446.
15. Mallick SK, Swatilekha M, Bhutia SK, Maiti TK. Immunostimulatory properties of a polysaccharide isolated from Astraeus hygrometricus. J Med Food 2010;13:665–72.
16. Biswas G, Nandi S, Kuila D, Acharya K. A comprehensive review on food and medicinal prospects of Astraeus hygrometricus. Pharmacogn J. 2017;9(6):799–806.
17. Biswas G, Sarkar S, Acharya K. Free radical scavenging and anti-inflammatory activities of the extracts of Astraeus hygrometricus (Pers.) Morg. Lat Am J Pharm 2010b;29:549–53.
18. Biswas G, Rana S, Acharya K. Cardioprotective activity of ethanolic extract of Astraeus hygrometricus (Pers.) Morg. Pharmacol Online 2011a;2:808–17.
19. Biswas G, Sarkar S, Acharya K. Hepatoprotective activity of the ethanolic extract of Astraeus hygrometricus (Pers.) Morg. Dig J Nanomat Biostruc 2011b; 6:637–41.
20. Biswas G, Chatterjee S, Sarkar S, Acharya K. Evaluation of antioxidant and nitric oxide synthase activation properties of Astraeus hygrometricus (Pers) Morg. Int J Biomed Pharm Sci 2010;4(1):21–6.
21. Lai TK, Biswas G, Chatterjee S, Dutta A, Pal C, Banerji J. Leishmanicidal and anticandidal activity of constituents of Indian edible mushroom Asraeus hygrometricus. Chem Biodivers 2012;9:1517–24.
22. Shameem N, Kamili AN, Ahmad M, Masoodi FA, Parray JA. Radical scavenging potential and DNA damage protection of wild edible mushrooms of Kashmir Himalaya. J Saudi Soc Agric Sci. 2015;16(4):314–21.
23. Gong Y, Liu X, He WH, Xu HG, Yuan F, Gao YX. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta L.) residue. Fitoterapia 2012;83:481–9.
24. Woldegiorgis AZ, Abate D, Haki GD, Ziegler GR. Antioxidant property of edible mushrooms collected from Ethiopia. Food Chem 2014;157:30–36.
25. Phachonpai W, Tongun T. Cognition enhancing effects of Clausena lansium (Lour.) peel extract attenuate chronic restraint stress-induced memory deficit in rats. Heliyon. 2021;7(5):e07003.
26. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–6.
27. Srinivasan K, Patole PS, Kaul CL, Ramarao P. Reversal of glucose intolerance by pioglitazone in high fat diet-fed rats. Methods Find Exp Clin Pharmacol. 2004; 26(5):327–33.
28. Biswas G, Acharya K. Hypoglycemic activity of ethanolic extract of Astraeus hygrometricus (Pers.) Morg. in alloxan-induced diabetic mice. Int J Pharm Pharm Sci. 2013;5:391–4.
29. Kirubananthan G, Vijayan SG, Thangaraj A, Sundaram R. Antioxidant potential of theaflavin ameliorates the activities of key enzymes of glucose metabolism in high fat diet and streptozotocin-induced diabetic rats. Redox Rep. 2019;24(1):41–50.
30. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
31. Biswas G, Sagartirtha SS, Acharya K. Evaluation of pharmacognostic profile of Astraeus hygrometricus (Pers) Morg. J Botan Soc Bengal. 2009;63(1):25–30.
32. Pavel K. Chemical composition and nutritional value of European species of wild growing mushrooms: a review. Food Chem. 2009;113(1):9–16.
33. Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Scholmerich J, et al. Defining high-fat-diet rat models: Metabolic and molecular effect of different fat types. Mol Endocrinol. 2006;36:485–501.
34. Churuangsuk C, Lean MEJ, Combet E. Lower carbohydrate and higher fat intakes are associated with higher hemoglobin A1c: findings from the UK national diet and nutrition survey 2008–2016. Eur J Nutr. 2020;59(6):2771–82.
35. McGillicuddy FC, Reynolds CM, Finucane O, Coleman E, Harford KA, Grant C, et al. Long-term exposure to a high-fat diet results in the development of glucose intolerance and insulin resistance in interleukin-1 receptor I-deficient mice. Am J Physiol Endocrinol Metab. 2013;305(7):E834–44.
36. Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 2007;87(2):507–20.
37. Duiyan J, Yi X, Xin M, et al. Antiobesity and lipid lowering effects of the aflavins on high-fat diet induced obese rats. J Funct Foods. 2013;5:1142–50.
38. Rauf A, Joshi PB, Ahmad Z, Hemeg HA, Olatunde A, Naz S, et al. Edible mushrooms as potential functional foods in amelioration of hypertension. Phytother Res. 2023;37(6):2644–60.
39. Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants (Basel) 2019;11;8(4):96.
40. Muflihah YM, Gollavelli G, Ling YC. Correlation study of antioxidant activity with phenolic and flavonoid compounds in 12 Indonesian indigenous herbs. Antioxidants (Basel) 2021;10(10):1530.
41. Fong-in S, Khwanchai P, Prommajak T, Boonsom S. Physicochemical, nutritional, phytochemical properties and antioxidant activity of edible Astraeus odoratus mushrooms: effects of different cooking methods. Int J Gastron Food Sci. 2023;33: 100743.
42. Valverde ME, Hernández-Pérez T, Paredes-López O. Edible mushrooms: improving human health and promoting quality life. Int J Microbiol 2015;2015:376387.
43. Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 2022;7(1):216.
44. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122.
45. Ceriello A, Bortolotti N, Motz E, Pieri C, Marra M, Tonutti L, et al. Meal-induced oxidative stress and low-density lipoprotein oxidation in diabetes: the possible role of hyperglycemia. Metabolism 1999;48(12):1503–8.
46. Suk M, Shin Y. Effect of high-intensity exercise and high-fat diet on lipid metabolism in the liver of rats. J Exerc Nutrition Biochem. 2015;19(4):289–95.
47. Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cε and hepatic insulin resistance. Cell Metab 2012;15(5):574–84.
48. Le Lay S, Simard G, Martinez MC, Andriantsitohaina R. Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxid Med Cell Longev 2014;2014:908539.
49. Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, et al. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism 2008;57:1071–7.
50. Pavithra M, Sridhar KR, Greeshma AA, Tomita-Yokotani K. Bioactive potential of the wild mushroom Astraeus hygrometricus in South-west India. Mycology 2016; 7(4):191–202.
51. Dayar E, Pechanova O. Targeted strategy in lipid-lowering therapy. Biomedicines 2022;10(5):1090.
52. Edwards JE, Moore RA. Statins in hypercholesterolaemia: a dose-specific meta-analysis of lipid changes in randomised, double blind trials. BMC Fam Pract. 2003;4:18.