INTRODUCTION
A core principle of state medicine policy is to safeguard citizens’ health by establishing a healthcare-oriented policy for medicine circulation [1,2], promoting domestic drug production [3–8], and ensuring transparent and ethical governance and business practices in medicine circulation [9,10]. Allergic rhinitis (AR) is a prevalent global health issue, with varying prevalence rates of 2%–25% in children and 1%–40% in adults. Recent studies highlight an increasing AR prevalence, particularly in low-income countries, with environmental factors and atmospheric air playing a significant role in childhood AR development compared to internal factors. The issue of air pollution, stemming from industrial growth, chemical compound misuse, vehicle emissions, and associated health problems, disproportionately affects children, leading to reduced immunity, increased allergy rates, ENT issues, anemia, thyroid hyperplasia, and cardiovascular disorders [11].
AR is a prevalent pediatric condition with a significant impact on children’s quality of life and a potential precursor to bronchial asthma (BA). The relationship between atmospheric air and AR development remains a pressing concern in modern medicine, as current preventive measures fall short [12,13]. The economic burden of treating AR is compounded by indirect costs related to reduced productivity, surpassing those of BA. Some patients exhibit resistance to conventional pharmacotherapy, prompting the exploration of allergy-specific immunotherapy. The prevalence of allergen sensitization varies by geographic region, and modern surgical techniques offer noninvasive solutions for restoring nasal function [14]. Although AR is distinct from common physiological rhinitis, its onset typically occurs after the age of three to four, emphasizing the importance of timely intervention to prevent severe complications.
Around 40% of patients are diagnosed with BA due to its complications, with AR typically manifesting in children between ages 3 and 6, often progressing to a chronic form if left untreated [15]. AR in children is triggered by allergen exposure, including household factors such as dust and chemicals, germs from dental infections, dietary items such as eggs and cow’s milk, and airborne allergens in older children. Common inhaled allergens for children with BA, AR, or AR-induced BA include molds, dust mites, and pollen, with mold having a stronger association with AR [16,17]. Risk factors for these conditions include allergies, childhood eczema, family history of AR, passive smoking, and unhealthy habits, while breastfeeding provides protective benefits. Moderate to severe AR in children can lead to sleep disturbances, including nighttime sleep disruptions and daytime sleepiness, even with continuous treatment [18,19].
The frequency of drug-induced rhinitis depends on anti-inflammatory medication duration, and specific immunotherapy is used to prevent it in AR patients [20]. Intranasal corticosteroids are the preferred treatment for AR regardless of its cause. The rising prevalence of AR is concerning, but strategies for improved treatment and effective prevention are crucial in curbing its spread [21]. According to the World Health Organization, AR incidence has increased globally in the past 50 years, affecting 20–40% of the population. AR is more common in boys and peaks in teenagers aged 15–18. The WHO recommends sublingual immunotherapy as a viable alternative to injections for adults, highlighting the importance of allergen immunotherapy in children for immune system flexibility and preventive benefits [22].
Phenotypes of BA in children living in urban areas are grouped by allergic reaction. The severe form of BA is more common in children with severe allergies. However, the symptomatic BA phenotype has also been identified in children with mild allergies or AR [23]. One of the most crucial treatments for children with AR even today is the use of medicinal plants. Children with AR can be completely controlled with an accurate diagnosis, routine medical care, and the development of specialized immunotherapy. A new diagnosis and course of action for kids with AR will result from such an examination. In addition, it is based on recommendations made by the World Health Organization about AR and how it affects BA [24].
As the number of AR in children increases, so does the demand, need, and cost of the medications used to treat it. To date, no scientific research has been conducted in Uzbekistan on the pharmacoeconomic analysis of the availability of medicines used for AR in children in the population and therapeutic and prophylactic institutions. The aim of this study was to an assortment of medications used in AR in children, to identify an effective, low-cost group of medications by methods of comparative analysis and pharmacoeconomic analysis, and to give scientific recommendations for the optimization of supply.
MATERIALS AND METHODS
Data Collection and Sources. A retrospective analysis of the “Inpatient Medical Report,” product nomenclature, and prices of drugs used for AR in children was used. To study the direct and additional costs of treating the disease and to calculate the total cost of treating AR in children, the method of disease cost analysis was used.
Analysis Methods. The “Cost Minimization Analysis” recommended greater medical use of the lowest-cost medications among several drugs of equal therapeutic efficacy in the treatment of AR in children. This was used to compare the difference in cost between two or more alternative therapies.
Data Sources for Therapeutic Efficacy. The research utilized methods like Cost of Illness Analysis to examine the direct and indirect costs of mild, moderate, and severe AR. Cost Minimization Analysis compared medication costs between treatment alternatives offering equal therapeutic benefit. Cost-Effectiveness Analysis evaluated treatment costs together with outcomes like reduced AR attacks. The research found the majority of AR drugs were imported, and costs rose with disease severity. Domestic production of affordable options such as budesonide could optimize practices by decreasing costs and improving outcomes. Focusing on cost-efficiency can reduce economic losses and promote access to effective pediatric AR pharmacotherapy in Uzbekistan.
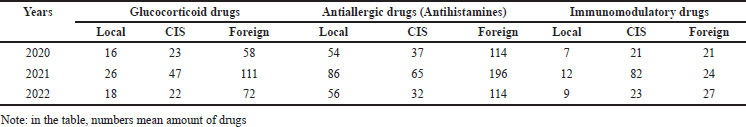
The number of registered drugs depending on the country of manufacture for 2020–2022 and the form of release of drugs used in AR were determined. A pharmacoeconomic analysis of drugs used for AR in children was conducted. An analysis of the registration of medicinal products used in AR was also carried out according to the form of the medicinal product, depending on the country of manufacture. The frequency of registration of international nonproprietary names of antihistamines, glucocorticoids, and immunomodulatory drugs was determined depending on the country of manufacture.
Cost minimization is calculated using the following formula [25]:
CMA=DC1–DC2 or CMA=(DC1+IC1)–(DC2+IC2), (1)
where CMA – cost difference indicator; DC1 – direct costs when using the first method; DC2 – indirect costs when applying the first method; DC1 and IC1, respectively, direct and indirect costs when applying the first method; DC2 and IC2 are direct and indirect costs, respectively, when applying the second method.
Patient Case Analysis. The history of AR in 142 children from the Republican Scientific Specialized Allergology Center was analyzed. Patients with BA and a moderate form of AR were observed for an average of 5 years at the Republican Scientific Specialized Allergology Center.
The costs of treating AR in children were also analyzed by comparing the use of the drugs “Momat Rhino” spray 50 mg/120 doses, Intermediary Firm: “ATM Partner,” Manufacturing Company: “Glenmark” and “Nasonex” spray 50 mg/120 doses, Intermediary Firm: “Novotek,” Manufacturing Company: “Schering-Plough” (Belgium). The costs of AR treatment in hospital conditions with moderate and severe severity of AR were determined.
The study examined the clinical and pharmacoeconomic efficacy of Budesonide 200 mcg/dose in the basic therapy of BA in children with AR. There were 120 moderately severe BA patients with AR under observation: 56 girls and 64 boys aged from 4 to 15 years, who were divided into two groups. Budesonide 200 mcg/dose a day was taken by BA patients in the first group of children with a severe course of AR (60 people). The duration of treatment was 60 days. The second group of moderately severe patients (60 people) received Budesonide 200 mcg/dose, and 60 capsules once a day. To evaluate and analyze the effect of the drug on the treatment of AR and BA in children, the frequency of choking attacks, coughing, wheezing, and subjective complaints of patients were observed. The patients were examined on the day of screening, on the 30th, 60th, and 90th days of treatment, and two weeks after the end of treatment.
Methodology for cost-effectiveness analysis. Pharmacoeconomic studies were determined using the method of “Cost-Effectiveness Analysis” (CEA). The cost-effectiveness ratio for each alternative CEA treatment regimen was calculated using the following formula:
CEA=(DC1+IC1)–(DC2+IC2)/Ef1-Ef2, (2)
where CEA is a cost-effectiveness ratio that shows the costs corresponding to each effect; DC1 – direct costs used in the first treatment; IC1 – indirect costs used in the first treatment; DC2 – direct costs used in the second treatment; IC2 – indirect costs used in the second treatment; Ef1 and Ef2 are the treatments used in the first and second methods, respectively [25].
RESULTS
Pharmacoeconomic analysis of ar treatment
Medicines, medical devices, and medical equipment approved for medical use are produced in the Commonwealth of Independent States (CISs) countries, and recorded in the State Register (Figure 1).
As seen in Figure 1, anti-allergy medications are registered by 36 pharmaceutical companies. Among them, 54 local medicines preparations and 112 medications from foreign manufacturers were registered. Table 1 shows the indicators of registration of antihistamines, immunomodulatory drugs, and glucocorticoids in the State Register.
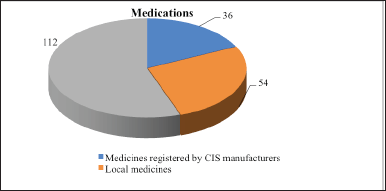 | Figure 1. Index of antiallergic agents used for AR in the state register of drugs approved for use in medical practice. Note: in the figure, numbers mean amount of drugs [Click here to view] |
The analysis of children’s medications for AR revealed that locally manufactured drugs include pills (65%), injection solutions (25%), and capsules (5%) among 6 pharmacy enterprises. In contrast, foreign manufacturers, represented by 11 enterprises, primarily offer pills (38%), syrups (8%), and capsules (6%). The registration data for anti-allergy (antihistamine) medications in 2022 indicates a predominance of imported drugs, with 72% used in pediatric AR being imports, including 54% from foreign countries and 18% from CIS countries, while local manufacturers account for 28%. Glucocorticoid drugs’ registration data for AR in 2022 shows a similar trend, with the majority being imported (88%), comprising 58% from foreign countries and 30% from CIS states, while local products constitute 12% of the glucocorticoid group. In accordance with the above information, according to the results of the analysis of antiallergic drugs used in AR, the largest share:
– 60% is in tablet form;
– 19% in solution for injection;
– 13% in syrup form.
Based on the analysis of glucocorticoid drugs used in AR (Table 2–3), according to the above information, by dosage form, the largest share:
– 37% is in the form of solution for injection;
– 25% is in the form of pill;
– 19% in the form of drops;
– 19% in the form of ointment.
According to the analysis of the international nonproprietary names of anti-allergy medicines used in AR, it was reported that the most registered drugs are cetirizine, chloropyramine, desloratadine, diphenhydramine, levocetirizine, ketotifen, and loratadine. Table 4 lists the 22 international nonproprietary names of anti-allergy medicines used in AR.
Table 5 presents an overview of glucocorticoid drugs based on an analysis of 15 international nonproprietary names. An analysis of the international nonproprietary names of glucocorticoid drugs used in AR reported that the most commonly registered medicines were dexamethasone, beclomethasone, hydrocortisone, prednisolone, mometasone, and methylprednisolone.
According to the results of the analysis of the international nonproprietary name of immunomodulatory drugs used in AR, Interferon alpha is the most registered. Analysis of dosage forms of immunomodulatory drugs registered in the State Register and produced in CIS countries yielded the following results. It was found that the main part of immunomodulators registered by 2 CIS pharmaceutical manufacturing companies is the solution for injection – 41%, pills – 23%, capsules – 18%, and dosage form syrup, which is not available from local manufacturing companies – 18%.
The next stage of the conducted analysis revealed the following results by country. In terms of registration of immunomodulatory drugs among foreign manufacturers India is the leader – 22%, followed by Turkey – 13%, Ukraine – 4%, and Russia – 4% among the CIS countries. The share of local manufacturers was 16%. By quantity, the most imported medicines – India – 127, followed by Turkey – 73, the CIS countries: 23 – Russia, 18 – Ukraine. The share of local manufacturers is 83.
 | Table 1. Indications for including different types of medicinal products in the State Register. [Click here to view] |
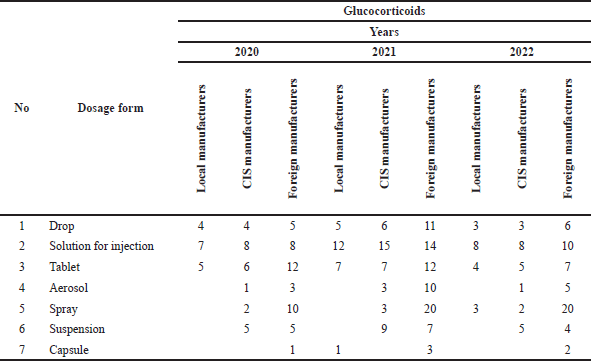 | Table 2. Analysis of glucocorticoid preparations manufacturers’ reporting by dosage form. [Click here to view] |
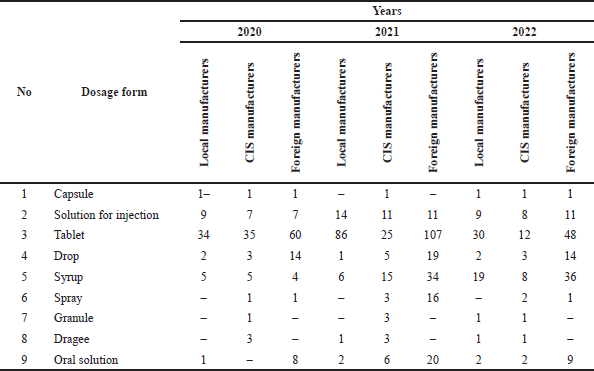 | Table 3. Analysis of the level of registration of manufacturers of antihistamines by dosage forms. [Click here to view] |
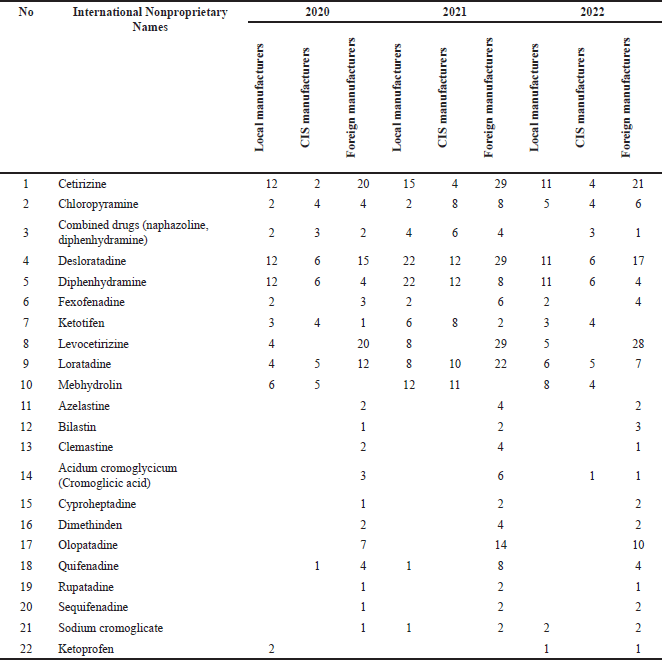 | Table 4. Results of the analysis of international nonproprietary names of antihistamines used in AR. [Click here to view] |
Pharmacoeconomic analysis of are treatment in uzbekistan.
Figure 2 shows the degree of severity of the course of AR in children of the Republican Scientific Specialized Allergology Center.
In turn, the analysis of AR in children by age and gender is represented in Figure 3–4.
Figure 5 examines 142 patients within the Republic of Uzbekistan when analyzing AR in children by administrative region: 73 patients from Tashkent city, 30 patients from Tashkent region.
Diagram 5 examines 142 patients within the Republic of Uzbekistan when analyzing AR in children by administrative region: 73 patients from Tashkent city and 30 patients from Tashkent region. Table 6 summarizes the cost analysis of outpatient AR treatment in children with mild disease, moderately severe disease, and severe disease. The “Cost of Illness Analysis” formula was used in this study to calculate costs.
Model 1 represents a cost-minimization analysis of the treatment of AR in children. Calculating the CMA indicator in the currency of the Republic of Uzbekistan (UZS), we obtain the following formula:
CMA=(DC1+IC1)–(DC2+IC2)=(1 129 536+1 227 550)–(1 227 550+1 227 550)=32 941.(3)
Imported medications account for the majority of the drugs used in the treatment of AR in children. According to the “Cost of Illness Analysis” of treatment of AR in children, treatment of mild form of the illness in outpatient conditions amounts to 268 000 UZS (21,60 USD), treatment of moderate form amounts to 330 800 UZS (26,46 USD), and the cost of treatment of severe form totals 420 800 UZS (33,66 USD) (Uzbekistani som, which is the official currency of Uzbekistan). The Budesonide efficacy according to the pharmacoeconomic analysis: savings per patient in Group 1 were 196 855 UZS (15,75 USD). According to the “Cost Minimization Analysis,” the medication Momat Rhino Spray 50 mg/120 doses is the optimal method.
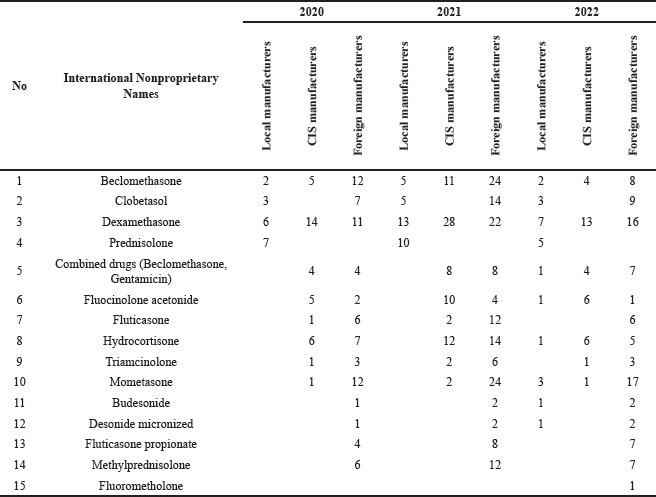 | Table 5. Results of analysis of glucocorticoids by international nonproprietary name. [Click here to view] |
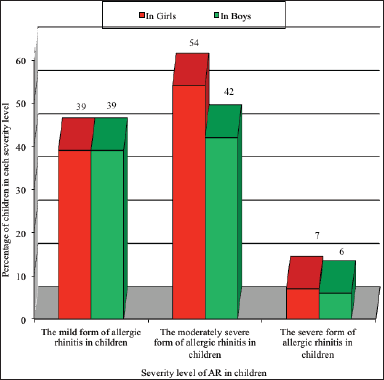 | Figure 2. Severity index of patients with AR in children. [Click here to view] |
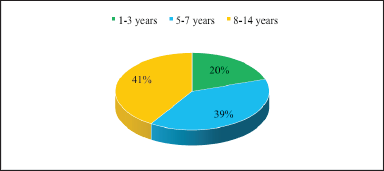 | Figure 3. Analysis of AR in children by age. [Click here to view] |
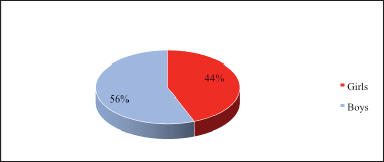 | Figure 4. Frequency of AR in girls and boys. [Click here to view] |
 | Table 6. “Cost of illness analysis” of AR treatment in children [Click here to view] |
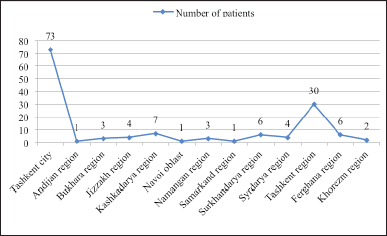 | Figure 5. Rate of patients (children) diagnosed with AR by regions. [Click here to view] |
Analysis of the costs incurred by patients during inpatient treatment
The analysis shows that an average of 1 547 722 UZS (123,80 USD) was spent on each patient during six inpatient bed days for the treatment of moderate forms of AR in children. Of this amount, 900 000 UZS (70,43 USD) per day were spent on medicines, 268 000 UZS (21,60 USD), on tests, 54 855 UZS (4,39 USD) on food, and 536 867 UZS (42,94 USD) on additional charges. When analyzing the costs spent by patients with a severe disease for 10 bed-days of inpatient treatment on average, it is shown that 2 721 936 UZS (217,73 USD) were spent for each patient. This amount includes 905 000 UZS (73,07 USD) for bed days, 224 536 UZS (17,96 USD) for medications, 77 400 UZS (6,19 USD) for medical tests, 150 000 UZS (12,00 USD) for food, and 1 150 000 UZS (91,99 USD) for additional expenses.
Throughout the observation periods, children in the first group exhibited symptoms of both AR and bronchial asthma (BA). These patients received glucocorticoid medications, specifically Budesonide, which proved well-tolerated and devoid of significant side effects. Budesonide demonstrated acute bronchodilator effects, enhanced specificity, and prolonged action. In the second stage of current research, the authors employed a “Cost-effectiveness analysis” approach. Simultaneously, the use of effective BA treatments alongside AR alleviated attacks, restored physical activity, and made “Budesonide 200” a foundational BA treatment for children, yielding favorable clinical outcomes and reducing both direct and indirect treatment costs.
The following criteria for pharmacoeconomic analysis of medical costs, and the sequence of calculations is given. “Cost-effectiveness analysis” is calculated according to the following formula:
CEA=(1 052 749+1 229 130)–(1 104 749+1 373 985)=2 281 879–2 478 734=196 855.(4)
In the pharmacoeconomic analysis of Budesonide, patients in Group 1 saved 196 855 UZS (15,75 USD) on average. Their average direct cost was 1 052 749 UZS (84,21 USD), with an average indirect cost of 1 229 130 UZS (98,32 USD), whereas in Group 2, these values were 1 104 749 UZS (88,37 USD) and 1 373 985 UZS (109,91 USD), respectively. Group 1 patients (Ef1) who received Budesonide showed slightly higher effectiveness compared to Group 2 (Ef2). This underscores the effectiveness and cost-effectiveness of using these drugs in the basic therapy of both BA and AR in children. In addition, a Cost Analysis for AR treatment in children revealed varying direct and indirect costs for mild, moderate, and severe forms of the disease, with a calculated cost savings of 32 941 UZS (2,63 USD) per patient for “Momat Rhino” compared to “Nazonex” in Cost Minimization Analysis. Overall, this approach not only led to cost savings of 196 855 UZS (15,75 USD) per patient for children with BA and AR treated with Budesonide but also restored physical activity and eliminated attacks, resulting in adequate clinical results and reduced treatment expenses.
DISCUSSION
Between 2020 and 2022, a significant portion of antihistamines, glucocorticoids, and immunomodulators for treating AR and BA in Uzbekistan were imported from foreign countries, particularly those far abroad and the CIS, rather than being domestically produced. In 2021, immunomodulating drugs imported from CIS countries surpassed those from far abroad. Glucocorticoids and antihistamines predominantly came from foreign countries. Regarding the preferred forms of drug release, pills, syrups, and capsules were most commonly used for AR, aligning with Alessandrini et al. findings [26]. Injectable solutions, notably glucocorticosteroids, constituted a significant portion of hormonal medications used in this study. Intranasal drugs were deemed effective for AR due to their localized impact and limited systemic and side effects, while liquid forms were suitable for children but contained substantial sugar content [27].
It was found that among the children with AR who were examined, the majority were city dwellers. It is possible that the greater number of AR patients in the city may be related to harmful emissions from production and other sources of air pollution. These factors may worsen the course of the disease and increase its incidence [28]. Children from 1 to 14 years old participated in this study, the majority of children with AR were from 5 to 14 years old, and boys predominated by gender. In the study of Abuziza et al. [29], the incidence of AR was higher among adults than among sick adolescents and children. In addition, AR was detected more often in men than in women. Research by Pakkasela et al. [30] showed that asthma incidence was highest between the ages of 0 and 9 and that the frequency and complexity of the disease tended to decrease with age. A. Widuri and V. Hidayya [31] reported the prevalence of AR in women in 23.9%, in men in 8.7% in their study. Certain data exhibit variations compared to the research findings in the Republic of Uzbekistan, necessitating additional investigation.
The choice of treatment for AR depends on the patient’s age, disease severity, and concurrent health issues. International guidelines recommend inhaled glucocorticoids and oral antihistamines for AR treatment [32]. However, improper use of older-generation antihistamines and premature discontinuation of nasal glucocorticoids often result in treatment ineffectiveness, chronicity, and side effects. Self-medication can exacerbate symptoms, prolong parental care, and increase healthcare system costs. In the United States, AR costs approximately $25 billion annually, with indirect costs constituting half of the total due to limited disease monitoring.
R. Tanjung et al [33] evaluated the cost-effectiveness of two treatments for gastroesophageal reflux disease (GERD) in class II inpatient patients at a police hospital in Bandung, Indonesia. Utilizing cost-effectiveness analysis with a hospital perspective focusing on direct costs and applying a 5% discount rate for year-to-year differences, the study found significant differences in both the length of hospital stay and total treatment costs between the two drugs, favoring pantoprazole as the more cost-effective option, especially for the majority female and over 40 years age group.
This study’s analysis of AR treatment costs at different severity levels aligns with Belhasen et al. findings [34], indicating that treatment expenses increase with AR severity, especially when comorbid bronchial asthma is present. Furthermore, it highlights the escalating direct and indirect costs in inpatient settings, primarily due to longer hospital stays associated with severe AR, leading to higher overall expenses.
First-generation antihistamines are generally not recommended for children due to their nonselectivity and potential to cause drowsiness and dizziness [35]. This study identified commonly registered antihistamines including cetirizine, chloropyramine, desloratadine, diphenhydramine, levocetirizine, ketotifen, and loratadine. Levocetirizine was found to be cost-effective based on clinical improvement when compared to fexofenadine, desloratadine, and montelukast [36]. Loratadine was the most frequently used oral antihistamine (3.48 defined daily dose or DHD) among all antihistamines (8.78 DHD), while intranasal glucocorticoids were led by budesonide (3.5 DHD) and mometasone furoate (2.25 DHD) [37]. These findings align with the analysis of registered antihistamines and glucocorticoids in Uzbekistan. The study also confirmed the effectiveness of mometasone furoate, a cost-effective medication for treating AR in children [38; 39].
Kang et al. [39] evaluated the cost-effectiveness of using toripalimab in combination with chemotherapy versus chemotherapy alone as the first-line treatment for patients with advanced esophageal squamous cell carcinoma within the Chinese healthcare system. The analysis considers costs, life-years, quality-adjusted life-years, and incremental cost-effectiveness ratio. The findings suggest that toripalimab plus chemotherapy is likely a cost-effective option compared to chemotherapy alone, with an ICER below the willingness-to-pay threshold commonly accepted in China. Sensitivity and subgroup analyses support the robustness of these conclusions.
When using budesonide in the treatment of children with BA and AR, positive dynamics of the disease were revealed. The children’s condition improved with each 30-day period of examination of the patients, and the effect of the treatment was stronger in the group with a severe course of the disease. According to GINA [40], budesonide, fluticasone, and beclomethasone are the most used and recommended corticosteroids in the treatment of AD. Taking into account the “Economic efficiency analysis” of the drug “Budesonide 200” and the clinical success in the treatment of BA and AR confirmed by the results of this study, the further use of this drug is effective and economically feasible.
Allergy-specific immunotherapy (ASIT), found to be more cost-effective than symptomatic therapy for AR, plays a significant role in reducing pharmaceutical usage and overall treatment costs, as demonstrated by Sanchez et al. [41]. Furthermore, the FDA acknowledges that generic drugs can be 30–80% cheaper than branded ones, although market restrictions exist [42, 43]. Although scientific studies often withhold financial information about drug registrations due to pharmaceutical company confidentiality, the frequency of registration is primarily tied to effectiveness rather than financial considerations. It is important to note that pharmaceutical companies can exert influence over healthcare providers’ and pharmacists’ medication choices [44]. Effective treatment, based on proper drug selection and duration of use, can help minimize the economic burden of AR in children. To promote economic growth and reduce material losses associated with AR and BA, focusing on domestic drug production and cost-effective alternatives while considering their effectiveness and safety is essential.
CONCLUSIONS
This study analyzed the registration and use of medicines for the treatment of AR (AR) in children in Uzbekistan. The study found that most antihistamines, glucocorticoids, and immunomodulators used for the treatment of AR in children were imported rather than domestically produced. Common dosage forms included tablets, syrups, and capsules, and injectable glucocorticoid solutions were also widely used.
In examining the costs of treating AR, the study found that total direct and indirect costs increased with the severity of the disease. In the mild form of the disease, the total costs amounted to 268 000 UZS (21,60 USD), and in the severe form of the disease – 420 800 UZS (33,66 USD). Cost minimization analysis showed that Momat Rhino nasal spray was the most affordable option, saving 32 941 UZS (2,63 USD) per patient compared to the alternative spray Nazonex. In addition, the study found that the use of budesonide to treat children with AR and asthma reduced costs by 196 855 UZS (15,75 USD) per patient compared to conventional treatment.
In light of these findings, the study recommends larger-scale domestic production and the inclusion of cost-effective drugs such as mebhydroline, chloropyramine, budesonide, and mometasone furoate in the essential medicines lists. Focusing on low-cost domestically produced medicines could optimize AR treatment practices, reduce economic losses, and improve access to pharmacotherapy for children with AR in Uzbekistan.
FUNDING
There is no funding to report.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the concept and design, acquisition of data, analysis, and interpretation of data, and took part in drafting the article and revising it. Also, all authors approved the final manuscript and agreed to be accountable for all aspects of the work.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments. A study was approved by the Republican Scientific Specialized Allergology Center, No. RP-428.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Serikbayeva EA, Zhakipbekov KS, Umurzakhova GZh, Datkhayev UM, Kauypova FE, Dyusembinova GA. Methods of assessing the possibility of forming a branch regional clusters in RK. Syst Rev Pharm. 2020; 11(6): 42-434.
2. Issatayeva N, Datkhayev U, Zhakipbekov K, Serikbayeva E, Umirzakhova G. Public-private partnership in the healthcare and pharmaceutical sectors of Kazakhstan: Problems and solutions. J Adv Res Law Econ. 2020; 11(3): 876-884.
3. Ashirov MZ, Datkhayev UM, Myrzakozha DA, Sato H, Zhakipbekov KS, Rakhymbayev NA, Sadykov BN. Study of cold-pressed tobacco seed oil properties by gas chromatography method. Sci World J. 2020; 2020: 8852724.
4. Tleubayeva MI, Abdullabekova RM, Datkhayev U?, Ishmuratova MY, Alimzhanova MB, Kozhanova KK, Seitaliyeva AM, Zhakipbekov KS, Iskakova ZB, Serikbayeva EA, Flisyuk EV. Investigation of CO2 extract of portulaca oleracea for antioxidant activity from raw material cultivated in Kazakhstan. Int J Biomat. 2022; 2022: 6478977.
5. Rakhymbayev N, Datkhayev U, Sagindykova B, Myrzakozha D, Zhakipbekov K, Iskakbayeva Z. Component composition and antimicrobial activity of subcritical CO2 extract of Ferula asafoetida L., growing in the territory of Kazakhstan. ScienceRise: Pharm Sci. 2023; 2(42): 82-91.
6. Biyashev KB, Kirkimbaeva ZS, Biyashev BK, Makbuz AZ, Bulegenova MD. Determination of the level of resistance of probiotic strain escherichia coli 64g to hydrochloric acid, bile and antimicrobial agents. Ecol Environ Conserv. 2019; 25(4): 1930-1933.
7. Turgumbayeva A, Ustenova G, Datkhayev U, Rahimov K, Abramavicius S, Tunaityte A, Zhakipbekov K, Kozhanova K, Tulemissov S, Ustenova O, Datkayeva G, Stankevicius E. Safflower (Carthamus Tinctorius L.) a potential source of drugs against cryptococcal infections, malaria and leishmaniasis. Phyt-Int J Exp Bot. 2020; 89(1): 137-146.
8. Datkhayev UM, Sakipova ZB, Ustenova GO, Zhakipbekov KS, Kozhanova KK, Kapsalyamova EN, Ibadullayeva GS, Tulemissov SK, Blatov RM. Validation of spectrophotometric method for determination of thiamazole in liquids by dissolution test for the transdermal form. Pharm Chem J. 2019; 53: 572-576.
9. Umurzakhova G, Sultanbekov A, Issatayeva N, Zhakipbekov K, Shopabaeva A, Shertaeva C, Datkhayev U. Communication skills as one of the main competences of pharmacists. Ann Trop Med Publ Health. 2018; 11(3): 62.
10. Datkhayev U, Shopabaeva A, Zhakipbekov K, Shertaeva C, Umurzakhova G, Sultanbekov A, Yerzhanova R, Tulegenova A. Determination of seasonal demand for pharmaceutical staff. Int J Pharm Sci Rev Res. 2016; 36(2): 105-111.
11. Suyunov ND. Allergic rhinitis: basic aspects of the disease and characteristics of drugs used in pharmacotherapy. Med J Uzbek. 2012; 1: 106-109.
12. Melnychaiko I, Andreychyn S. Biological therapy of severe bronchial asthma. Bulletin of Medical and Biological Research. 2023; 16(2): 86-92. https://doi.org/10.61751/bmbr.2706-6290.2023.2.86
13. Hasiuk OP. Pharmacological and morphological features and socioeconomic aspects of cannabidiol: A literature review. International Journal of Medicine and Medical Research. 2023; 9(1): 47-59
14. Balabolkin II, Terletskaya RN, Modestov AA. Allergic morbidity of children in modern environmental conditions. Sib Med Rev. 2015; 1: 63-67.
15. Belykh NA. Allergic rhinitis in children: Modern approaches to diagnosis, treatment and prevention. Mod Pediatr. 2015; 8(72): 22-28.
16. Giallongo A, Parisi GF, Licari A, Pulvirenti G, Cuppari C, Salpietro C, Marseglia GL, Leonardi S. Novel therapeutic targets for allergic airway disease in children. Drugs in Context. 2019; 8: 212590.
17. Parisi GF, Brindisi G, Indolfi C, Diaferio L, Marchese G, Ghiglioni DG, Zicari AM, Miraglia del Giudice M. Upper airway involvement in pediatric COVID-19. Pediatr Allergy Immunol. 2020; 31(26): 85-88.
18. Zhu C-H, Liu J-X, Zhao X-H. Risk factors of asthma among children aged 0-14 in Suzhou city. Chin J Prevent Med. 2012; 46(5): 456-459.
19. Loekmanwidjaja J, Carneiro ACF, Nishinaka MLT, Munhoes DA, Benezoli G, Wandalsen GF, Solé D. Sleep disorders in children with moderate to severe persistent allergic rhinitis. Brazilian Journal of Otorhinolaryngology, 2018; 84(2): 178-184.
20. Zhumambayeva S, Rozenson R, Morenko M, Shaidarov M, Zatonskikh V, Kazangapova A, Zhumadilova Z, Zhumambayeva R. The peculiarities of different types of chronic rhinitis in children and adolescents in Kazakhstan. Iran J Publ Health. 2013; 42(4): 374-379.
21. Yuan Z, Luo Z. Increasing prevalence of allergic rhinitis in China. Allergy, Asth Immun Res. 2019; 11(2): 156-169.
22. Zorica Ž, Ivana D-F, Snežana Ž. Current issues on sublingual allergen-specific immunotherapy in children with asthma and allergic rhinitis. Serb Arch Med. 2016; 144(5-6): 345-350.
23. Zoratti EM, Krouse RZ, Babineau DC, Pongracic JA, O’Connor GT, Wood RA, Hershey GKK, Kercsmar CM, Gruchalla RS, Kattan M, Teach SJ, Sigelman SM, Gergen PJ, Togias A, Visness CM, Busse WW, Liu AH. Asthma phenotypes in inner-city children. J Allerg Clin Immun. 2016; 138(4): 1016-1029.
24. Xin Y, Zhang Y, Lin Y. Progress in diagnosis and treatment of children allergic rhinitis. J Clin Otorhinolar Head Neck Surg 2015; 29(5): 400-403.
25. Suyunov ND, Zainutdinov HS. Clinical and pharmacoeconomic analysis: Basics and methods. Med J Uzbek. 2008; 4: 76-82.
26. Alessandrini E, Braco F, Scarpa M, Lupo M, Bonifazi D, Pignataro V, Cavallo M, Cullufe O, Enache C, Nafria B, Claverol J, Taeye LD, Vermelen E, Preston J, Tuleu C. Children preferences for formulation research via EPTRI (European paediatric translational research infrastructure). Pharm. 2021; 13(5): 730.
27. Diaferio L, Parisi GF, Brindisi G, Indolfi C, Marchese G, Ghiglioni DG, Zicari AM, Marseglia GL, Miraglia Del Giudice M. Cross-sectional survey on impact of paediatric COVID-19 among Italian paediatricians: Report from the SIAIP rhino-sinusitis and conjunctivitis committee. Ital. J. Pediatr. 2020; 46(1): 146.
28. Li CH, Sayeau K, Ellis AK. Air pollution and allergic rhinitis in symptom exacerbarion and strategies for management. J Asthm Allerg. 2020; 13: 285-292.
29. Abuziza A, Almatrafi MA, Alonazi AS, Zatari MH, Alqouzi SA, Mandili RA, Hawsawi WT, Aljohani RH. The prevalence, clinical picture, and triggers of allergic rhinitis in Saudi population: a systematic review and meta-analysis. J Asthm Allerg. 2022; 15: 1831-1849.
30. Pakkasela J, Ilmarinen P, Honkamaki J, Tuomisto LE, Andersen H, Piirila P, Hisinger-Molkanen H, Sovijarvi A, Backman H, Lundback B, Ronmark E, Kankaanranta H, Lehtimaki L. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm Med. 2020; 20(1): 9.
31. Widuri A, Hidayyat VAN. Differences in the prevalence of adults with allergic rhinitis by gender. Spring Nat. 2022; 55: 15-20.
32. Deshazo DR, Kemp SF, Corren J, Feldweg AM. Pharmacotherapy of allergic rhinitis. Uptodate. 2023. https://www.uptodate.com/contents/pharmacotherapy-of-allergic-rhinitis
33. Tanjung R, Wardati Y, Yulianingsih Y, Widyawati IE, Mustarichie R, Saptarini NM. Cost-effectiveness analysis of treatment in gastroesophageal reflux disease inpatient patients in Bandung, Indonesia. Int J Appl Pharm. 2023; 15(2): 141-144.
34. Belhasen M, Demoly P, Bloch-Hansen N, Toussi M, Ganse EV. Costs of perennial allergic rhinitis and allergic asthma increase with severity and poor disease control. Allerg. 2017; 72(6): 948-958.
35. Baharudin A, Latiff AHA, Woo K, Yap FB, Tang IP, Leong KF, Chin WS, Wang DY. Using patient profiles to guide the choise of antihistamines in the primary care setting in Malaysia: expert consensus and recommendations. Ther clin risk manag. 2019; 15: 1267-1275.
36. Goodman MJ, Jhaveri M, Saverno K, Meyer K, Nightengale B. Cost-effectiveness of second-generation antihistamines and montelukast in relieving allergic rhinitis nasal symptoms. Amer Health Drug Benef. 2008; 1(8): 26-34.
37. Suarez-Castanon C, Modrono-Riano G, Solis-Sanches G. Use of anti-allergic drugs in children. Allerg Immun. 2017; 45(5): 506-507.
38. Dai W, Zhen N, Qin X, Cao J. Effect of mometazone furoate with loratadine and montelukast sodium on inflammatory factors and pulmonary function in children with allergic rhinitis. Amer J Translat Res. 2022; 14(10): 1799-7207.
39. Kang S, Wang X, Pan Z, Liu H. Cost-effectiveness analysis of toripalimab plus chemotherapy for patients with advanced esophageal squamous cell carcinoma in China. Exp Rev Pharm Out Res. 2023; 24(2):285-292.
40. Global strategy for asthma management and prevention. 2022. https://ginasthma.org/gina-reports/
41. Sanchez J, Alvares L, Garcia E. Real-world study: drug reduction in children with allergic rhinitis and asthma receiving immunotherapy. Immunother. 2023; 15(4).
42. Meadows M. Saving money on prescription drugs. U.S. Food & Drug Administration. 2005.
43. Zhakipbekov K, Posylkina O, Zhumabayev N, Datkhayev U, Zhumabayev N, Almurzaeva A, Mukanova A. Analysis of the current state of the pharmaceutical market of the Republic of Kazakhstan. ScienceRise: Pharm Sci. 2023; 2(42): 57-67.
44. Ushakova IA, Dorokliova LP, Maliy VV, Dorokhov AV. Assessment of a pharmacy as a pharmaceutical service environment. Azerb Pharm Pharmacother J. 2020; 20(1): 24-30.