INTRODUCTION
As of 2030, it is estimated that 51% of the population will be obese, nearly a triple increase since 1975 [1] and is a result of an energy imbalance between increased calorie consumption and decreased energy expenditure [2]. An increasingly sedentary lifestyle, industrialization, transportation mechanization, urbanization, and consumption of processed foods contribute to obesity [3]. Obesity-related liver diseases are associated with non-alcoholic fatty liver disease (NAFLD), starting with simple steatosis (fatty liver), and progressing to nonalcoholic steatohepatitis and cirrhosis [4]. Similarly, obesity-related kidney dysfunction, including glomerular hyperfiltration, albuminuria, and renal fibrosis, can progress to chronic kidney disease (CKD), and end-stage renal disease [5].
In animal studies, high-fat diets (HFDs) have been commonly used to persuade obesity [6]. These diets contribute to excessive caloric intake and lead to increased adiposity, insulin resistance, dyslipidemia, and inflammation, all of which play significant roles in the development of obesity-related complications of the liver and kidney [1]. Additionally, it is important to note that obesity is related to systemic inflammation releasing the inflammatory cytokine Interleukine-6(IL-6) and suppressing the synthesis of anti-inflammatory cytokine Interleukine-10(IL-10) [7]. Adiposopathy, resulting from excessive energy intake, primarily affects adipose tissue in overweight/obese individuals, preceding to the deposition of fat in the liver. This buildup of fat causes lipotoxicity and the generation of reactive oxygen species. Consequently, a fatty liver exacerbates resistance to insulin and disrupts the metabolism of lipids, creating a cycle of metabolic dysfunction [8]. In the kidneys, the macrophages oxidize lipoprotein and transform into foam cells releasing inflammatory cytokines which ultimately reduce the rate of glomerular filtration, and impair the renal function [9].
Exercise has emerged as a non-intrusive alternative method for managing and preventing obesity [10] along with demonstrated positive effects on lipid profile, body weight reduction, and decreased fatty liver in NAFLD both in humans and murine obesity model [11]. Similarly, people with CKD are encouraged to engage in physical activity compatible with their heart health and tolerance, according to the Kidney Disease: Improving Global Outcomes report [12].
Research related to the effect of exercise on obesity, inflammation, and its associated complications on the liver and kidney tissues is not yet fully explored. The current study aims to investigate the effect of treadmill exercise as an intervention in alleviating obesity-related complications of the liver and kidney through an increase in serum IL-10 in a diet-induced obese rat model.
MATERIALS AND METHODS
Experimental animals
The study subject included 24 male Wistar rats (n = 6) weighing 150–200 g. The rats were provided with food and water and kept in a 12-hour light/dark cycle in institutions central animal research facility. The study protocol was approved by the Institutional Animal Care and Use Ethic Committee of Kasturba Medical College, Manipal Academy of Higher Education, Karnataka, India with approval number IAEC/KMC/66/2019.
Preparation of the experimental diets
The rats were given one of two diets: a normal chow diet (NCD) and a HFD. A NCD was acquired from the Amrut Laboratory Animal Feed (Pranav Afro Industry Ltd., Sangli, Maharashtra, India). Meanwhile lard was used to make the HFD procured from the local vendor [13]. The diet was prepared in accordance with AIN-93G diet [14].
Experimental design and treatment schedule
The animals were randomized into four groups (n = 6). Group 1: Normal control [NCD (15 g/day/kg)]; Group 2: HFD control [HFD (15 g/day/kg)]; Group 3:High fat diet obese[high fat diet (15 g/day/kg b.wt)] treated with atorvastatin (10 mg/kg b.wt in 0.5% CMC, orally) [15] and Group 4: HFD obese [HFD (15 g/day/kg)] treated with exercise. All rats consumed their respective diets for 11 months [16]. During the experimental period, body weight measurements were taken on a weekly basis. The blood samples of rats were collected after 12 hours fasting at the baseline (0 day) and then after 30 days and 90 days of the treatment. The serum was separated and stored at −80°C until further analyses. At the end of 90 days, the organs such as liver and kidney were collected through decapitation after anaesthesia with thiopental and the organs were stored in 10% formalin for histopathological examination.
Exercise regimen
On a thrice-weekly basis for a duration of 12 weeks (total of 36 sessions from 0 to 90 days of life), the rats underwent training on an animal treadmill (model: IITC life science:800 series Treadmill). The rats were subjected to assimilation training for 2 days and on the third day rats were adaptable to training. On the first and second days of exercise, the treadmill’s velocity was fixed at 5 m/minute for the first 10 minutes, followed by 9 m/minute for the next 10 minutes and 12 m/minute for the last 10 minutes. A treadmill velocity of 12 m/minute was maintained from the third and subsequent day of exercise [17].
Body weight analysis
An electronic weighing machine(model: DS-415Series) by Essae-Teraoka Ltd., India, was used to record the change in body weight [in grams] once a week throughout the experiment.
Biochemical investigations
STAR 20 Auto analyser (DC 19V 4.74A) by Rapid diagnostic PVT.LTD., India was used to carry out all the measurements such as the total cholesterol (TC), triglyceride (TG), and high-density lipoprotein (HDL). The levels of low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) were calculated using the formulae LDL = TC-HDL-VLDL and TG/5, respectively. The hepatic injury was assessed by estimating the levels of aspartate aminotransferase (AST), alanine transaminase (ALT) levels, and alkaline phosphatase (ALP) levels in the serum. The kidney function tests such as levels of urea (U), uric acid (UA), and creatinine (Cr) in the serum were estimated. All the kits were procured from the coral clinical systems, A Division of Tulip Diagnostics(P) Ltd., Goa, India, and the assays were carried out as per the kit instructions provided by the manufacturer.
Analysis of anti-inflammatory cytokine
The serum was analyzed for Rat IL-10 by enzyme-linked immunosorbent assay using Robonic ELISA plate reader analyzer.
Histopathological investigations
After euthanasia, the liver and kidney tissue samples were collected and immersed in 10% formalin for a period of 8 hours for fixation. A serial 5 mm slices of the liver and kidney were obtained and embedded in paraplast blocks for the analysis of fat accumulation. The sections were stained with hematoxylin stain by immersing the slides in 0.5% hematoxylin stain and were observed using Olympus life science solution CX33 microscope (under 200X and 400X).
Statistical analysis
Data were presented as Mean ± SD and were analyzed using Jamovi (Version 2.3) software. The normality of the distribution of the variables was assessed by the Shapiro–Wilk test. Repeated measures ANOVA with Greenhouse-Geisser correction was performed to check the changes between 0 and 30 days and 90 days across groups. The statistical significance among different sets was scrutinized followed by the post hoc, Tukey’s test. Statistical significance was determined at a 5% level of significance (p ≤ 0.05).
RESULTS
Body weight analysis
Before the experiment, the rats in both the control and HFD groups had similar body weights. However, after consuming the HFD for 11 months, the rats in the HFD groups exhibited a significant increase in body weight compared to the control group (p < 0.001). A significant reduction in body weight was observed in response to statin treatment both during the 30th (p < 0.05) and 90 days (p < 0.001) of treatment. The HFD caused a greater gain in body weight, but this increase was gradually mitigated by the exercise intervention in the HFD + Exercise group by the end of the 90-day experiment (p < 0.001) (Table 1).
Lipid profile analysis
The effect of exercise on lipid metabolism was evaluated by estimating the fasting serum lipid levels. HFD grouped animals showed a significant increase (p < 0.001) in TC, LDL, VLDL, and TG along with a significant decline (p < 0.001) in HDL levels compared with normal control Wistar rats. The statin and the exercise group showed a significant decrease (p < 0.001) in TC, LDL, VLDL, and TG. The levels of HDL were significantly elevated at 30 days (p < 0.05) and 90 days (p < 0.001) of exercise intervention (Fig. 1).
Serum liver function analysis
The levels of AST, ALT, and ALP were found to be significantly(p < 0.001) increased indicating liver injury in the groups fed HFD when compared to the NC group given NCD. However, exercise intervention significantly(p < 0.001) mitigated the liver damage by reflecting the decreased levels of the enzyme in serum both at 30 and 90 days of intervention. A more significant finding was that the enzyme levels were comparable to the normal controls with no statistical significance at the end of 90 days. While the levels of markers were significantly lower in the statin group compared with HFD, the levels were still at the higher end during the 30 and 90 days of treatment, indicating that further intervention or lifestyle changes might be needed to bring them down to a more desirable range (Table 2).
 | Table 1. Effect of treadmill exercise on the body weight [grams] in Wistar rats at 30 days and 90 days of intervention. [Click here to view] |
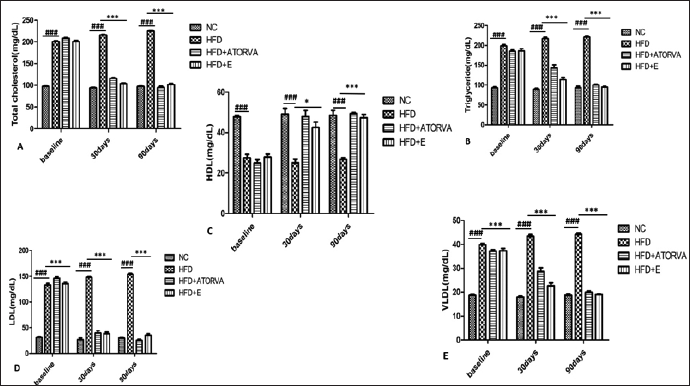 | Figure 1. Effects of exercise on the levels [mg/dl] of lipid profile in Wistar rats at 30 days and 90 days of intervention. [Click here to view] |
Serum renal function analysis
A significant (p < 0.001) increase in renal function tests such as U, UA, and Cr was observed in the HFD fed groups. There was no statistical significance observed at 30 d days for U (p = 0.144) and UA (p = 0.351) in HFD+E group when compared to HFD group. However, significance(p < 0.001) was observed at 90 days of exercise intervention in HFD+E group. Serum Cr levels were significant (p < 0.001) at both 30 days and 90 days in exercise group compared to HFD. Based on the findings, it can be inferred that significant improvement in renal function was observed only after a 90-day period of exercise intervention (Table 3).
Serum cytokine analysis
In comparison with the NC group, serum IL-10 level was significantly (p < 0.001) decreased in the HFD group, indicating that HFD induced systemic inflammatory response in the rats. In contrast, the level of serum anti-inflammatory cytokine IL-10 in the HFD group treated with Atorvastatin alone and exercise alone significantly (p < 0.001) increased. Our data illustrated the ameleorative action of exercise alone on the high-fat diet-induced inflammatory response by increasing IL-10 (Table 4).
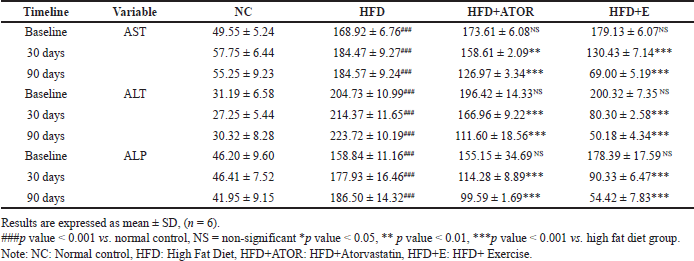 | Table 2. Effects of exercise on the levels of serum liver function parameters [IU/l] in Wistar rats at 30 days and 90 days of intervention. [Click here to view] |
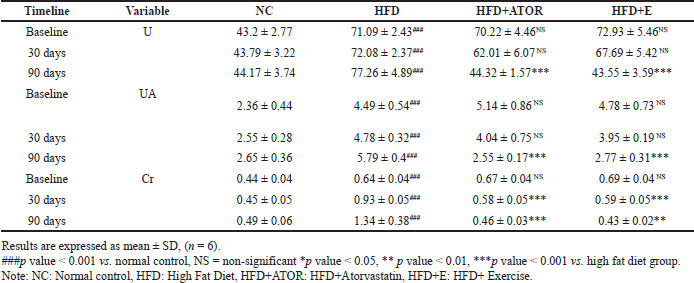 | Table 3. Effects of exercise on the levels of serum kidney function parameters [mg/dl] in Wistar rats at 30 days and 90 days of intervention. [Click here to view] |
 | Table 4. Effects of exercise on the levels of serum IL-10[pg/ml] in Wistar rats at 30 days and 90 days of intervention. [Click here to view] |
Histopathology
The NC group showed intact parenchyma with central venule and portal vein branches at the periphery with unremarkable intervening hepatocytes (Fig. 2A); the HFD group showed congested central venule and sinusoids. The intervening hepatocytes showed intracytoplasmic micro vesicular fat droplets (Fig. 2B). The HFD + ATORVA (Fig. 2C) and HFD + E (Fig. 2D) groups showed congested central venule and sinusoids. The intervening hepatocytes appear normal, with very occasional intracytoplasmic micro vesicular fat droplets.
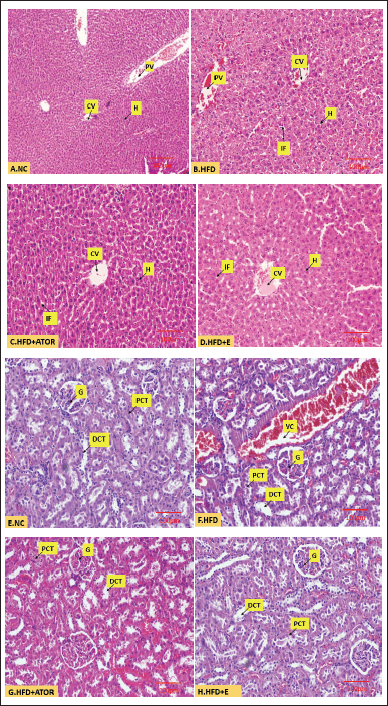 | Figure 2. Microscopic image of hematoxylin and eosin-stained A. normal liver tissue (10x). B. Liver tissue showing intracellular fat deposits (40x) C. Liver tissue after treatment with Statin (40x). D. Liver tissue after treatment with exercise (40x). E. Normal kidney tissue (10x) F. Kidney tissue with endocapillary congestion (40x). G. Kidney tissue after treatment with Statin (40x) H. Kidney tissue after treatment with exercise (40x). [Click here to view] |
In the NC group, the study showed renal cortex with cellular glomeruli and adjoining tubular system (Fig. 2E); the HFD diet group showed renal cortex with cellular glomeruli with endocapillary congestion and adjoining tubular system showing luminal hyaline cast with congestion of stromal arterioles (Fig. 2F). The HFD + ATOR(Fig. 2G) and HFD + E (Fig. 2H) group showed renal cortex with cellular glomeruli with endocapillary congestion and adjoining tubular system appearing unremarkable with congestion of the stromal arterioles.
DISCUSSION
HFDs contribute to the rise in obesity rates. Exercise interventions can help counteract obesity induced by such diets [18]. Atorvastatin, a common lipid-lowering drug, is known for its benefits in both animal models and patients [19]. Both atorvastatin and exercise offer distinct yet interconnected strategies for managing obesity and improving cardiovascular health [20]. Here, we investigated the effects of treadmill exercise on liver and kidney health in a rat model of diet-induced obesity.
In this study, the 90-day treadmill exercise intervention reduced the body weight to 40% when compared to the HFD group. Whereas the atorvastatin significantly reduced the body weight up to 39% compared to the HFD group. By 30 days, only 11.28% and 10.93% decrease in bodyweight was noticed with respect to atorvastatin and exercise intervention respectively. Importantly, exercise is an accessible approach, unlike statin medication with associated side effects. It is noteworthy that Li et al. [21] also observed in their study a significant decrease in body weight after a 90-day exercise intervention in obese mice induced by an HFD.
The HFD leads to the development of dyslipidemia in HFD groups, a condition characterized by abnormal levels of lipids in the blood [16]. The results obtained after the intervention of exercise given at 30 and 90 days were highly significant (p < 0.001) in improving lipid markers such as TC, TG, HDL, LDL, and VLDL. Specifically, it was found that exercise significantly improved HDL levels (p < 0.001) when compared to the group that only received HFD after 90 days, whereas during 30 days only p < 0.05 significance was noted with respect to improving the HDL levels in the exercise intervention group. Notably, these outcomes were comparable to the effects of atorvastatin. Emami et al. [22] also observed the results similar to the present study. The metabolic demands of active muscles stimulate the oxidation of fatty acids, leading to an enhanced release of free fatty acids (FFAs) and glycerol into the bloodstream. The FFAs can divert to the energy expenditure metabolism [23]. This metabolic effect of exercise might be the possible explanation for the observed improvement in lipid profiles with exercise.
The liver function test results revealed signs of liver injury in the group subjected to HFD, with elevated levels of AST and ALT when compared to the control group. This increase in ALT levels can be attributed to hepatic damage and the findings are supported by previous studies [19]. After 30 days of exercise as intervention, the reduction of 27.18%, 59.91%, and 49.36% was noted with respect to AST, ALT, and ALP, respectively. However, the group that underwent exercise (HFD + E) exhibited a remarkable reduction in liver injury markers AST, ALT, and ALP levels up to 62%, 77%, and 70%, respectively, when compared to the HFD group after 90 days. Moreover, the liver histology in the HFD + E group appeared to be normal (as shown in Fig. 2D) when compared to the histology of HFD group wherein the congested central venule and sinusoids, intracytoplasmic micro vesicular fat droplets were noted(Fig. 2B). The author Zang et al. [24] also observed the similar findings in his study. In contrast, atorvastatin treatment only led to a reduction of 31%, 50%, and 46%, respectively, at the end of the experiment. Furthermore, toward the end of the experiment, rats in the atorvastatin group displayed muscle stiffness, a phenomenon not observed in the exercise intervention group. Additionally, there were instances of mortality in the atorvastatin group, while no such adverse effects were observed in the exercise group. These findings collectively suggest that exercise intervention may present a more favourable approach for managing obesity when compared to atorvastatin treatment.
Renal function tests are valuable in detecting the presence of kidney disease, assessing the kidneys’ response to treatment, and evaluating the advancement of renal disease [25] and renal recovery has emerged as a multidisciplinary approach with physical activity being a key component in preventing CKD [26].The HFD group showed increase levels of serum UA, U, and Cr. Elevated serum Cr levels in obesity indicates a correlation between obesity and impaired kidney function [27].The similar findings were noted by Pereira et al. [28]. Fortunately, rats fed with HFD and exercise training after 90 days showed a significant (p < 0.001) decrease in sCr from the baseline. Consistently, serum UA and U were significantly (p < 0.001) decreased in the HDF+E group from the baseline [29] after 90 days. During 30 days of exercise as an intervention, no significance was noted in terms of U and UA levels when compared to the HFD groups. Renal histopathological examination of the HFD diet group (Fig. 2F) showed renal cortex with cellular glomeruli with endocapillary congestion and adjoining tubular system showing luminal hyaline cast with congestion of stromal arterioles whereas the group treated with exercise showed the unremarkable normal articulate of the kidney tissue (Fig. 2H).
During obesity, there is a notable decrease in the expression of the anti-inflammatory mediator IL-10 [7]. The same is been proved in our study as a significant decrease was noted in HFD groups. However, the exercise group significantly (p < 0.001) increased the expression of anti-inflammatory adipokine IL-10 to 95.51% in obese rats after 90 days and 53.38% after 30 days. These findings are in line with those observed by other researchers [7,30]. The obtained results are also comparable with effect of atorvastatin in high fat diet fed groups. It can also be further added that IL-10 deficiency exacerbates liver and kidney complications in obesity, leading to lipid build-up, abnormal histopathology, inflammation, fibrosis, and impaired functions [31]. Hence, in our study, we not only observed the benefits of exercise intervention on biochemical and histological indices over time, but we also demonstrated that a longer duration of 90 days is more effective than 30 days.
CONCLUSION
Treadmill exercise showed significant benefits in mitigating weight gain, improving dyslipidemia, liver functions, and renal functions, and also in reducing hepatic steatosis, and renal congestion along with improving the serum IL-10 levels in HFD-induced obese Wistar rats. Given its affordability and minimal side effects, exercise presents a promising approach in managing obesity and associated metabolic complications, offering valuable insights into its positive effects on liver and kidney function in this study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
The study protocol was approved by the Institutional Animal Care and Use Ethic Committee of Kasturba Medical College, Manipal Academy of Higher Education, Karnataka, India with approval number IAEC/KMC/66/2019.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. Biomed Pharmacother. 2021 May 1;137:111315. CrossRef
2. Sharma AM, Padwal R. Obesity is a sign–over-eating is a symptom: an aetiological framework for the assessment and management of obesity. Obes Rev. 2010 May;11(5):362–70. CrossRef
3. Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015 Jul;33:673–89. CrossRef
4. Maurice J, Manousou P. Non-alcoholic fatty liver disease. Clin Med. 2018 Jun;18(3):245. CrossRef
5. Kovesdy CP, Furth S, Zoccali C, World Kidney Day Steering Committee. Obesity and kidney disease: hidden consequences of the epidemic. Physiol Int. 2017 Mar;104(1):1–4. CrossRef
6. Wang CY, Liao JK. A mouse model of diet-induced obesity and insulin resistance. Mtor: Methods Mol Biol. 2012;821:421–33. CrossRef
7. Gotoh K, Inoue M, Masaki T, Chiba S, Shiraishi K, Shimasaki T, et al. Obesity-related chronic kidney disease is associated with spleen-derived IL-10. Nephrol Dial Transplant. 2013 May 1;28(5):1120–30. CrossRef
8. Machado MV. Aerobic exercise in the management of metabolic dysfunction associated fatty liver disease. Diabetes Metab Syndr Obes. 2021 Aug 11:3627–45. CrossRef
9. Luo K, Bian J, Wang Q, Wang J, Chen F, Li H, et al. Association of obesity with chronic kidney disease in elderly patients with nonalcoholic fatty liver disease. Turk J Gastroenterol. 2019 Jul;30(7):611–5. CrossRef
10. Pagnotti GM, Styner M, Uzer G, Patel VS, Wright LE, Ness KK, et al. Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity. Nat Rev Endocrinol. 2019 Jun;15(6):339–55. CrossRef
11. Farzanegi P, Dana A, Ebrahimpoor Z, Asadi M, Azarbayjani MA. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): roles of oxidative stress and inflammation. Eur J Sport Sci. 2019 Aug 9;19(7):994–1003. CrossRef
12. Clyne N, Anding-Rost K. Exercise training in chronic kidney disease—effects, expectations and adherence. Clin kidney J. 2021 Apr 1;14(Supplement_2):113–14. CrossRef
13. Sasidharan SR, Joseph JA, Anandakumar S, Venkatesan V, Ariyattu Madhavan CN, Agarwal A. An experimental approach for selecting appropriate rodent diets for research studies on metabolic disorders. BioMed Res Int. 2013 Jan 1;2013:752870. CrossRef
14. Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997 May 1;127(5):838S–41S. CrossRef
15. Wu F, Cui M, Wang S, Yu C, Yin W, Li J, et al. Effect of berberine on pharmacokinetics and pharmacodynamics of atorvastatin in hyperlipidemia rats. Xenobiotica. 2023 Dec 2;53(12):644–52. CrossRef
16. Sheik SM, Bakthavatchalam P, Shenoy RP, Hadapad BS, Nayak D, Biswas M, et al. Anti-hyperglycemic, anti-hyperlipidemic, and anti-inflammatory effect of the drug Guggulutiktaka ghrita on high-fat diet-induced obese rats. J Ayurveda Integr Med. 2022 Jul 1;13(3):100583. CrossRef
17. Zhang Q, Wu Y, Zhang P, Sha H, Jia J, Hu Y, et al. Exercise induces mitochondrial biogenesis after brain ischemia in rats. Neuroscience. 2012 Mar 15;205:10–7. CrossRef
18. Alvarez-Monell A, Subias-Gusils A, Mariné-Casadó R, Belda X, Gagliano H, Pozo OJ, et al. Restricted cafeteria feeding and treadmill exercise improved body composition, metabolic profile and exploratory behavior in obese male rats. Sci Rep. 2022 Nov 15;12(1):19545. CrossRef
19. Esmail M, Anwar S, Kandeil M, El-Zanaty AM, Abdel-Gabbar M. Effect of Nigella sativa, atorvastatin, or L-Carnitine on high fat diet-induced obesity in adult male Albino rats. Biomed Pharmacother. 2021 Sep 1;141:111818. CrossRef
20. Cavero-Redondo I, Deeks JJ, Alvarez-Bueno C, Jolly K, Saz-Lara A, Price M, et al. Comparative effect of physical exercise versus statins on improving arterial stiffness in patients with high cardiometabolic risk: a network meta-analysis. PLoS Med. 2021 Feb 16;18(2):1003543. CrossRef
21. Li L, Wei Y, Fang C, Liu S, Zhou F, Zhao G, et al. Exercise retards ongoing adipose tissue fibrosis in diet-induced obese mice. Endocr Connect. 2021 Mar 1;10(3):325–35. CrossRef
22. Emami SR, Jafari M, Haghshenas R, Ravasi A. Impact of eight weeks endurance training on biochemical parameters and obesity-induced oxidative stress in high fat diet-fed rats. J Exerc Nutr Biochem. 2016 Mar 3;20(1):29. CrossRef
23. Muscella A, Stefàno E, Lunetti P, Capobianco L, Marsigliante S. The regulation of fat metabolism during aerobic exercise. Biomolecules. 2020 Dec 21;10(12):1699. CrossRef
24. Zhang Y, Gu M, Wang R, Li M, Li D, Xie Z. Dietary supplement of Yunkang 10 green tea and treadmill exercise ameliorate high fat diet induced metabolic syndrome of C57BL/6 J mice. Nutr Metabo. 2020 Dec;17:1–5. CrossRef
25. Gounden V, Bhatt H, and Jialal I. “Renal Function Tests,” In StatPearls, Treasure Island, FL: StatPearls Publishing, 2023. [cited 2023 Oct 03] [Online]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK507821/
26. Hoshino J. Renal rehabilitation: exercise intervention and nutritional support in dialysis patients. Nutrients. 2021 Apr 24;13(5):1444. CrossRef
27. Pengrattanachot N, Cherngwelling R, Jaikumkao K, Pongchaidecha A, Thongnak L, Swe MT, et al. Atorvastatin attenuates obese-induced kidney injury and impaired renal organic anion transporter 3 function through inhibition of oxidative stress and inflammation. Biochim Biophys Acta Mol Basis Dis. 2020 Jun 1;1866(6):165741. CrossRef
28. Pereira RO, Muller CR, de Nascimento NR, Fonteles MC, Evangelista FS, Fiorino P, Farah V. Early consumption of high-fat diet worsens renal damage in spontaneously hypertensive rats in adulthood. Int J Physiol Pathophysiol Pharmacol. 2019;11(6):258.
29. Dupas J, Feray A, Guernec A, Pengam M, Inizan M, Guerrero F, et al. Effect of personalized moderate exercise training on Wistar rats fed with a fructose enriched water. Nutr Metab. 2018 Dec;15:1–2. CrossRef
30. Kesherwani V, Chavali V, Hackfort BT, Tyagi SC, Mishra PK. Exercise ameliorates high fat diet induced cardiac dysfunction by increasing interleukin 10. Front Physiol. 2015 Apr 22;6:145778. CrossRef
31. Kim DH, Chun SY, Lee E, Kim B, Yoon B, Gil H, et al. IL-10 deficiency aggravates renal inflammation, fibrosis and functional failure in high-fat dieted obese mice. Tissue Eng Regen Med. 2021 Jun;18:399–410. CrossRef