INTRODUCTION
Liver disorders are globally distributed with a high burden of disease costs, it is estimated that about 2 million cases of liver disorders occur every year, these liver disorders consist of many liver diseases (mainly inflammatory hepatitis, viral hepatitis, liver cirrhosis, and jaundice), these liver diseases- if untreated- will lead to many complications which may end with death [1]. Unfortunately, there are few drugs that are used in the treatment and management of liver disorders, most of these drugs are mainly used for alleviating symptoms of some liver disorders and their complications [2]. Despite that many herbs and natural products have shown marked hepatoprotective activity, but only silymarin (a flavono-lignan mixture obtained from Silybum marianum, Asteraceae) is widely available in the market as a hepatoprotective agent, also few natural compounds were isolated and characterized from their natural sources, hence there is a need to isolate more natural compounds with hepatoprotective activity [3,4].
Capparis decidua Edgwe (Forssk.) (Capparidaceae) is a woody plant that is widely distributed in Sudan and other tropical and sub-tropical countries [5], it has many pharmacological uses, many studies showed that it possesses anti-inflammatory, and analgesic, anti-rheumatic, anti-arthritis, anti-gout, anti-platelet aggregation, anti-diabetic, hypolipidemic, antioxidant, anti-bacterial, anti-fungal, anti-viral, and anthelmintic properties [6–11]. Many traditional uses have been recorded, the plant is used in various cases such as asthma, cough, rheumatism, swellings, toothache, stomach problems, intermittent fever, jaundice, dysentery, cardiac diseases, and some skin inflammations [12].
The hepatoprotective activity of the stem part of C. decidua had extensively been studied, both methanolic and aqueous extracts of its stem part were administered orally at doses 200 and 400 mg/kg on CCl4-induced hepatotoxicity rats, and it was found that both extracts have significant hepatoprotective activity when compared with the standard silymarin, the study suggested that this significant hepatoprotective activity may due to the presence of tannins, flavonoids, sterols, saponins, coumarins, and alkaloids [13]. In our previous study, the methanolic extract of C. decidua stem was bio-assay guided fractionated successively into dichloromethane (DCM), ethylacetate, n-butanol, and aqueous fractions, these fractions were examined on CCl4- induced hepatotoxicity rats at dose 500 mg/kg i.p., it was found that DCM and ethyl acetate fractions showed significant hepatoprotective activity when compared with standard silymarin, while n-butanol fraction showed moderate activity, but the aqueous fraction was inactive, the : 2,2-diphenyl-1-picrylhydrazyl free radical scavenging activity (RSA) of our fractions were matching with their hepatoprotective activity, the fractions showed 86, 92, 53, and 14 %RSA, respectively, while the active standard (propyl gallate) showed 92 %RSA [14], so to continue with our previous work, the aim of this study is to develop gas chromatography/mass spectrometry (GC/MS), high-performance thin layer chromatography (HPTLC)-fingerprint profile and in silico docking of both DCM and ethylacetate active fractions of methanolic extract of C. decidua stem part, as well as identification of some hepatoprotective active compounds present in both fractions.
MATERIALS AND METHOD
Materials
Plant material
The plant material (stem part of C. decidua, Capparidaceae) was collected from Shambat (Khartoum North) in September 2019, the plant material was authenticated by the taxonomist (Dr. Yahia Sulaiman) in the Herbarium of Medicinal and Aromatic Plants Research Institute (MAPRI), Khartoum, Sudan. The voucher herbarium specimen was deposited in the MAPRI (Sudan).
Chemicals and reagents
All chemicals and reagents used in this study were of analytical grade quality and were obtained from certified sources. DCM, methanol, n-butanol, sulphuric acid, and formic acid were obtained from SDFCL-India. Ethylacetate and toluene were obtained from Romil-England. Ethanol and chloroform were obtained from Duksan-South Korea. Vanillin was obtained from Loba-chemie-India. Rutin, quercetin, kaempferol, ferulic acid, oleanolic acid, and β-sitosterol were obtained from Sigma-Aldrich (USA).
Equipment
GC/MS equipment (Perkin Elmer model Clarus 600 T combined with single quadrapole mass spectrometer) obtained from PerkinElmer Co. (USA). Precoated silica gel 60 F254 plates are used for TLC and HPTLC techniques (Merck, Germany). CAMAG glass twin trough chamber for development (20 × 20) plus Camag Linomat-IV applicator for HPTLC technique plus CAMAG HPTLC scanner III (CAMAG, Muttenz, Switzerland).
Method
Preparation of the plant extracts
The collected plant material (C. decidua stem) was dried and ground into coarse powder form, the total weight was about 1.5 kg, it was macerated firstly by DCM and next by methanol 80%, and the extraction process was repeated for both solvents several times by changing with fresh solvent each time, both extracts were collected and dried under reduced pressure, they have kept in tightly closed containers and stored at 2°C–8°C.
Fractionation of the methanolic extract of C. decidua
About 100 g of dried methanolic extract was taken and suspended in distilled water (up to 250 ml), the solution was transferred into the separatory funnel (500 ml) and about 100 ml of DCM was added; the mixture was shaken gently and allowed to stand till two immiscible layers were separated, the DCM layer was removed and the process was repeated by adding another fresh solvent several times till the DCM layer became colorless; this procedure was repeated with ethylacetate and n-butanol; finally the four fractions were dried under reduced pressure and stored in tight closed containers at 2°C–8°C.
GC/MS analysis of DCM and ethyl acetate fractions
GC/MS analysis was carried out by using the Perkin Elmer model Clarus 600 T combined with a single quadrapole mass spectrometer. The chromatographic column was an Elite 5-MS column (30 m × 0.25 mm × 0.25 μm film thickness), while the carrier gas was high purity helium, 1 μl of DCM and ethyl acetate fractions samples were injected individually by splitless injector (split ratio 1:20), the injector temperature was 280°C. The temperature was set initially at 40°C (held for 1 minute), then was increased up to 150°C at 10°C/minute, then was further increased to 300°C at 10°C/minute. The flow rate was adjusted at 1 ml/minute. The peaks of the compounds were identified by comparing the spectra with that of the NIST 2005 (National Institute of Standard and Technology Library) and Wiley 2006 library. 29 minutes was the total time required for analyzing both samples.
TLC profile
About 15 mg of each active fraction (DCM and ethylacetate fractions) was taken and dissolved in about 3 ml of DCM and ethylacetate solvents, respectively, pre-coated silica gel 60 F254 plates were prepared (2.5 × 10 cm) and a spot from each fraction in separate plates was applied, several mobile phases had been tried and were prepared in well closed chromatographic jars, these mobile phases had been left for at least 20 minutes for saturation, after that all pre-coated plates had been placed inside the chromatographic jars, after development process the plates were then removed, dried, and detected under daylight, short and long UV lamps (254 and 366 nm, respectively), after that the plates were sprayed by vanillin/H2SO4 (1 g of vanillin was dissolved in 80 ml of methanol 80%, and the volume was completed to 100 ml by adding H2SO4 60%), they were then heated inside the oven for 10 minutes. The most proper mobile phase which gave the appropriate separation was selected for each fraction for HPTLC analysis.
HPTLC profile of DCM and ethylacetate fractions
The samples of active fractions were prepared by dissolving 20 mg from each fraction in 1 ml of methanol. The HPTLC pre-coated plates were adjusted to 20 × 20 cm, the silica gel used was 60 F254, the selected mobile phases were toluene: ethylacetate: methanol 9:1:1 for DCM active fraction, and toluene: ethylacetate: formic acid: n-butanol 5:4:1:1 for ethylacetate active fraction. The prepared samples were filtered and 8 μl of each sample was applied on each plate. Each plate was eluted to a distance of 8 cm at room temperature (25°C) in a specific developed solvent system. The samples were applied as bands (6 mm wide and 8 mm apart) by using a Camag Linomat IV sample applicator equipped syringe size of 25 μl with nitrogen flow providing a delivery speed of 150 nl/second from the syringe. The standard samples of reference compounds with known hepatoprotective activity were used as markers, they were rutin, quercetin, kaempferol, ferulic acid, oleanolic acid, and β-sitosterol (the stock solution was prepared by taking accurately 2 g from each compound was dissolved in a small amount of methanol, then the volume was completed to 25 ml of methanol in a volumetric flask). The plates were developed in a Camag twin trough glass tank pre-saturated with the mobile phase. The plate was developed horizontally in the Camag horizontal developing chamber at room temperature. The scanning was carried out under short and long wavelengths (254 and 366 nm), each plate was heated at 100°C and derivatized by vanillin/H2SO4 using CAMAG HPTLC scanner III. In addition, the peak areas were recorded by using WIN CATS 1.2.3. software program. For the preparation of the HPTLC fingerprint profile for DCM active fraction, the above procedure was repeated with a marker consisting of a mixture of β-sitosterol and oleanolic acid. The calibration curves of β-sitosterol and oleanolic acid were prepared accordingly with the above-mentioned procedure by using their standard solutions with aliquot concentrations ranging from 200–1,400 ng/spot.
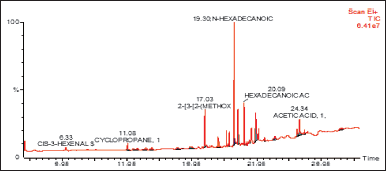 | Figure 1. GC/MS spectrum of DCM fraction of methanolic extract of C. decidua stem part. [Click here to view] |
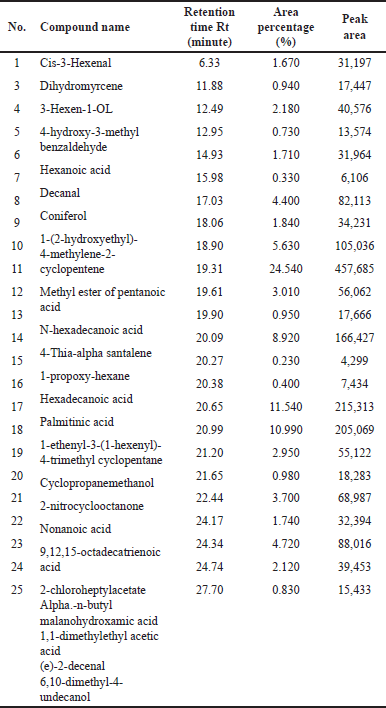 | Table 1. GC/MS data of DCM fraction of methanolic extract of C. decidua stem part. [Click here to view] |
Validation of HPTLC method
International Council for Harmonization (ICH) guidelines (2005) were regarded for the validation analytical process to confirm precision, repeatability, and accuracy. The instrumental precision was checked by repeated scanning (n = 6) of the same spot of either β-sitosterol or oleanolic acid (400 ng/spot) and expressed as a percentage relative standard deviation (% RSD). The repeatability of the method was performed by analyzing 400 ng/spot of either β-Sitosterol or oleanolic acid on the TLC plate (n = 6) and expressed as % RSD. Variability of the method was also studied by analyzing various aliquots of standard solutions containing 600, 800, and 1,000 ng/spot of either β-Sitosterol or oleanolic acid on the same day (i.e., intra-day precision) and on different days (i.e., inter-day precision). The accuracy of the method was assessed by performing the recovery study method at four different ascending concentration levels (starting with 0%, 50%, 100%, and 150% addition of either β-Sitosterol or oleanolic acid).
Quantification of β-sitosterol and oleanolic acid present in DCM active fraction
Accurately, 20 μl of suitably diluted sample solution of DCM active fraction was applied on a pre-coated plate (n = 3), the plate was developed and scanned as mentioned in section 4.2.5, then the peak areas were recorded and the amounts of β-Sitosterol and oleanolic acid were calculated by using the calibration curve.
In silico docking of the active phyto-chemical compounds
The protein ligands responsible for hepatotoxicity (and hence for hepatoprotective activity) were determined by literature review, and the structures of the selected proteins were determined and downloaded from www.uniprot.org and from research collaboratory for structural bioinformatics–protein data bank (www.rcsb.org), the three-dimensional chemical structures of the selected active phytochemical compounds were downloaded from PubChem web site (www.pubchem.ncbi.nlm.gov). The selected proteins were initially prepared before docking by removing all the bad steric clashes using AMBER 99 force field; MMFF94s force field parameters were adjusted for the small molecules. Next, docking studies were performed by the molecular operating environment program for each ligand as described, while other parameters were kept default (i.e., the necessary chain was deleted, the scoring function was London force and 10 conformers of the ligand were retained with highest and best score by default). Finally, the scoring configuration of the ligand-target complexes was selected on energetic grounds (Kcal/Mol), and the obtained results were presented in tabular and image forms.
RESULTS
GC/MS technique
(Fig. 1) and (Table 1) revealed the GC/MS data analysis of DCM fraction of the methanolic extract of C. decidua stem part, while (Fig. 2) and (Table 2) revealed GC/MS data analysis of ethyl acetate fraction of methanolic extract of C. decidua stem part.
TLC technique
TLC profile of DCM active fraction
Toluene: ethylacetate: methanol (9:1:1) was the most proper mobile phase among many examined mobile phases for the DCM active fraction (Fig. 3), the isolated spots in the plate (precoated silica gel 60 F254) were seen under daylight, under short UV (254 nm), under long UV (366 nm), were exposed to ammonia vapour and were finally sprayed by vanillin/H2SO4 followed by heating in an oven for 10 minutes.
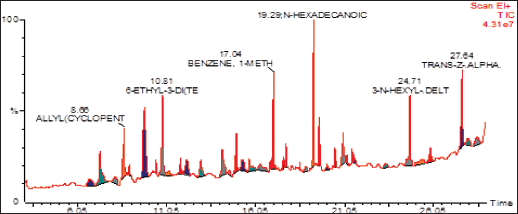 | Figure 2. GC/MS spectrum of ethyl acetate fraction of methanolic extract of C. decidua stem part. [Click here to view] |
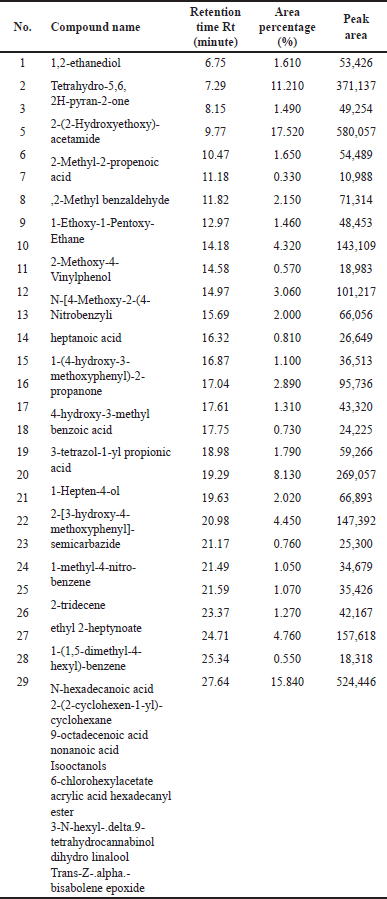 | Table 2. GC/MS data of ethyl acetate fraction of methanolic extract of C. decidua stem part. [Click here to view] |
TLC profile of ethylacetate active fraction
Several mobile phases were attempted to obtain the best-isolated spots, it was observed that to get the best-isolated spots without tailing, this was achieved by applying two dimensional TLC method (precoated silica gel 60 F254: 20 × 20 cm) with toluene: ethylacetate: formic acid: n-butanol: chloroform (5:4:1:1:0.5) and then by toluene: ethylacetate: formic acid: n-butanol (5:4:1:1) after rotating the plate 90o (Fig. 4).
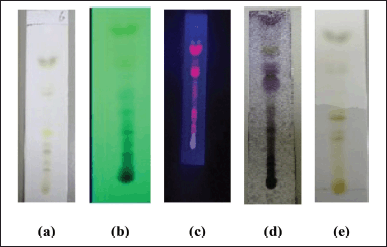 | Figure 3. TLC profile of DCM active fraction of methanolic extract of C. decidua stem part; (a): The plate as seen in daylight; (b): The plate as seen under short UV (254 nm); (c): The plate as seen under long UV (366 nm); (d): The plate after spraying by vanillin/H2SO4 and heating for 10 minutes in the oven; (e): The plate after exposing to ammonia vapor. [Click here to view] |
 | Figure 4. Two dimensional TLC profile of ethylacetate active fraction of methanolic extract of C. decidua stem part; the mobile phase was toluene: ethylacetate: formic acid: n-butanol: chloroform (5:4:1:1:0.5) then after 90o rotation, the plate was developed by toluene: ethylacetate: formic acid: n-butanol (5:4:1:1); (a): The plate as seen under short UV (254 nm); (b): The plate as seen under long UV (366 nm); (c): The plate after spraying by vanillin/H2SO4 and heating for 10 minutes in the oven. [Click here to view] |
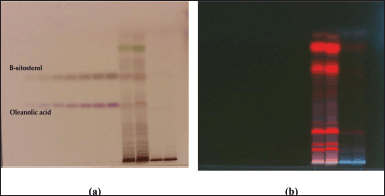 | Figure 5. HPTLC profile of DCM active fraction of methanolic extract of C. decidua stem and marker containing a mixture of standard compounds of oleanolic acid and β-sitosterol; (a): The plate under white light illumination after derivatization by vanillin/ H2SO4 reagent; (b): The plate under long UV 366 nm. [Click here to view] |
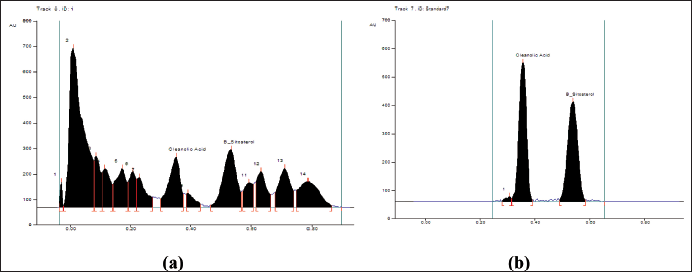 | Figure 6. (a): The peak areas of isolated compounds from DCM active fraction of methanolic extract of C. decidua stem part by HPTLC; (b): Peak areas of isolated standard compounds from the marker containing oleanolic acid and β-sitosterol by HPTLC. [Click here to view] |
 | Figure 7. Three-dimensional graph showing the peak areas of isolated compounds from DCM active fraction of methanolic extract of C. decidua stem part and the marker of standard compounds (oleanolic acid and β-sitosterol) by HPTLC. [Click here to view] |
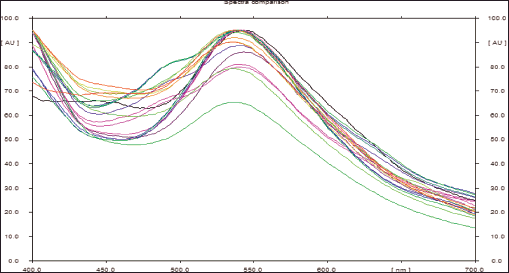 | Figure 8. Spectra comparison of isolated known compounds (oleanolic acid and β-sitosterol) from DCM active fraction of methanolic extract of C. decidua stem part and the marker by HPTLC. [Click here to view] |
HPTLC fingerprint profile
The mobile phase applied with DCM active fraction was toluene: ethyl acetate: methanol 9:1:1; while the mobile phase applied with ethylacetate active fraction was toluene: ethylacetate: formic acid: n-butanol 5:4:1:1. For both active fractions, a number of standard compounds with known hepatoprotective and antioxidant activities were selected and developed in each plate (precoated silica gel 60 F254), these selected compounds were rutin, quercetin, kaempferol, ferulic acid, oleanolic acid and β-sitosterol. After development, both plates were developed, and scanned and the peak areas were recorded by using WIN CATS 1.2.3. software program. DCM active fraction revealed the presence of oleanolic acid and β-sitosterol, while ethylacetate active fraction does not show any presence of these selected standard compounds. Hence, the procedure was re-chromatogramed again by applying DCM active fraction with a marker containing a mixture of β-sitosterol and oleanolic acid (Figs. 5–8).
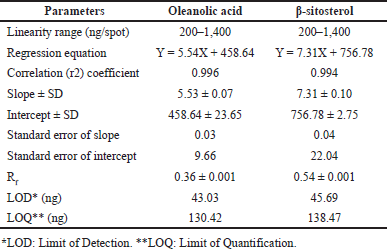 | Table 3. Rf-values and linear regression data for the calibration curve of oleanolic acid and β-sitosterol (n = 6). [Click here to view] |
Validation methods in terms of precision, accuracy, and repeatability were performed according to ICH guidelines 2005 (International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use, which is recommended by regulatory bodies of the European Union, Japan, and the USA), validation methods were applied to confirm that the isolated compounds from DCM active fraction were specifically oleanolic acid and β-sitosterol, and their Rf-values were calculated. Their calibration curves were determined. The recovery, precision, and robustness of the proposed HPTLC method were also determined. In addition, the quantification method was also applied according to ICH guidelines to estimate their concentrations in the DCM active fraction, all results are shown in (Tables 3–7).
In silico docking of selective active compounds
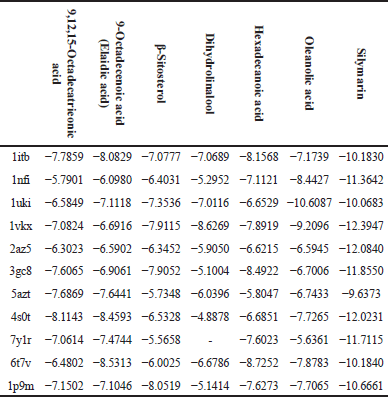
The following table (Table 8) represents the docking binding energy (Kcal/Mol) of the selected active phytochemical compounds with the selected protein ligands responsible for hepatotoxicity.
Figures 9 and 10 display the docking results of hexanoic acid binding to the active sites of 1itb, 6t7v, and 3gc8.
In addition, Figures 11 and 12 display the docking results of 9-octadecenoic acid (Elaidic acid) binding to the active sites of 1itb, 6t7v, and 5azt.
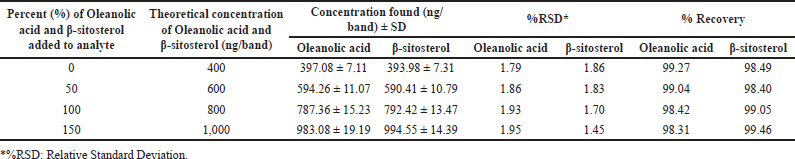 | Table 4. Recovery as accuracy studies of the proposed HPTLC method (n = 6). [Click here to view] |
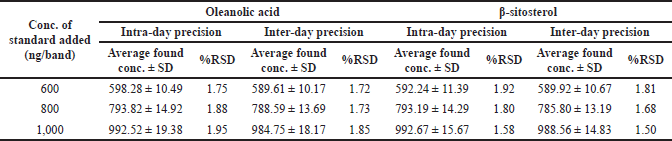 | Table 5. Precision of the proposed HPTLC method (n = 6). [Click here to view] |
 | Table 6. Robustness of the proposed HPTLC method (n = 6). [Click here to view] |
 | Table 7. Oleanolic acid and β-sitosterol content in DCM active fraction of methanolic extract of C. decidua stem part (n = 3). [Click here to view] |
 | Table 8. Docking binding energy (Kcal/Mol) of active phytochemical compounds on targeted receptors. [Click here to view] |
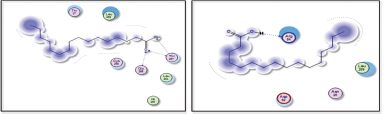 | Figure 9. Docking results of hexadecanoic acid binding to the active sites of 1itb: predicted interactions and binding modes. [Click here to view] |
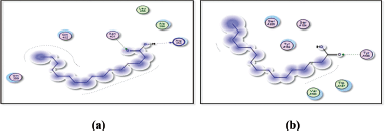 | Figure 10. Docking results of hexadecanoic acid binding to the active sites of (a): 6t7v; and (b): 3gc8: Predicted interactions and binding modes. [Click here to view] |
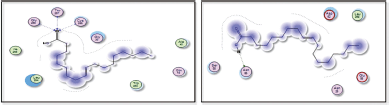 | Figure 11. Docking results of 9-octadecenoic acid (elaidic acid) binding to the active sites of 1itb: Predicted interactions and binding modes. [Click here to view] |
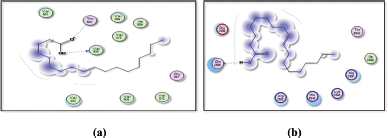 | Figure 12. Docking results of 9-octadecenoic acid (elaidic acid) binding to the active sites of (a): 6t7v; and (b): 5AZT: Predicted interactions and binding modes. [Click here to view] |
Furthermore, Figures 13 and 14 depict the docking results of oleanolic acid binding to the active sites of 1nfi, 1uki, and 1vkx.
The docking result of β-sitosterol binding to the active site of 1p9m as well as the docking result of 9,12,15-octadecatrienoic acid binding to the active site of 5azt are represented in Figures 15 and 16, respectively.
1itb: IL-1 receptor; 1nfi: p65 (NF-kappa B transcription factor binds to DNA as a heterodimer of 65 kDa); 1uki: inhibits JNK; 1vkx: the crystal structure of NF-kb; 2az5: TNF-α, 3gc8: p38 (p38 mitogen-activated protein kinases); 5azt: PPAR-α (Peroxisome proliferator-activated receptor-alpha); 4s0t: PXR (Pregnene X receptor factor); 7y1r: TGF-β (Transforming Growth Factor-beta); 6t7v: Nrf2 (Nuclear factor erythroid 2- related factor 2); 1p9m: IL-6α receptor.
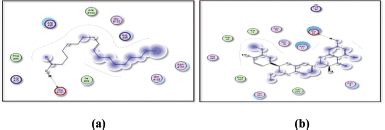 | Figure 13. Docking results of oleanolic acid binding to the active sites of (a): 1nfi; and (b): 1uki: Predicted interactions and binding modes. [Click here to view] |
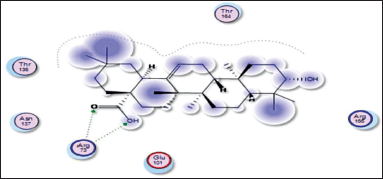 | Figure 14. Docking result of oleanolic acid binding to the active site of 1vkx: predicted interactions and binding modes. [Click here to view] |
DISCUSSION
Capparis decidua stem part has many traditional uses, treatment of jaundice is one of its traditional uses [13,14]. The hepatoprotective activity of the stem part of C. decidua was tested against CCl4-induced hepatotoxicity rats, the methanolic extract revealed significant hepatoprotective activity, this alcoholic extract was fractionated successively by DCM, ethyl acetate, n-butanol, and aqueous fractions, only DCM and ethyl acetate fractions revealed strong hepatoprotective and antioxidant activities [14], hence they were both selected in this study to determine and prove the active compounds by using GC/MS, HPTLC finger print profile and in silico docking techniques.
GC/MS chromatogram for both DCM and ethyl acetate fractions showed presence of 25 and 29 peaks, respectively, indicating presence of 25 and 29 compounds; in DCM fraction (Fig. 1 and Table 1), the highest percentage was n-hexadecanoic acid (Rt = 19.31, 24.54%), this compound was also present in ethyl acetate fraction (Rt = 19.29, 8.13%), which ranked as 4th compound over 29 compounds in its amount content (Fig. 2 and Table 2), the first three compounds were an oxygenated hydrocarbons; a previous study had analysed the hepatoprotective active ethanolic extract of Cryptolepis buchanani stem part against paracetamol-induced hepatotoxicity in rats by GC/MS, their results showed dominant presence of oxygenated and phenolic hydrocarbons, in which many of these compounds have hepatoprotective, antioxidant and anti-inflammatory properties, the interesting point was the presence of high amount of n-hexadecanoic acid which ranked as 3rd over 20 compounds (11.44%), also octadecanoic acid was also observed in their study and it was detected in our ethyl acetate fraction (Rt = 20.98, 4.45%) [15]. Moreover, searching in the literature for any biological activities of the detected compounds in DCM and ethyl acetate fractions showed that hexadecanoic acid, 9,12-octadecadienoic acid, and linalool have hepatoprotective, antioxidant, anti-inflammatory, and immunomodulatory properties [16–18], our fractions showed the presence of hexadecanoic acid, 9-octadecenoic acid, 9,12,15-octadecatrienoic acid and dihydro linalool. Hexadecanol and octadecanol were identified and characterized before in C. decidua [19], also hexadecanoic acid and 9,12,15-octadecatrienoic acid were detected before in C. decidua seeds [9,11], so this is the first time to identify hexadecanoic acid and 9,12,15-octadecatrieonic acid in C. decidua stem part. Hence, we can say that the hepatoprotective and antioxidant activities of DCM and ethyl acetate fractions may probably be due to the presence of these compounds and other compounds like phenolic hydrocarbons, oxygenated hydrocarbons and carboxylic acids which were detected in both fractions, so further analysis by using other chromatographic techniques and in silico docking method are recommended to prove our findings.
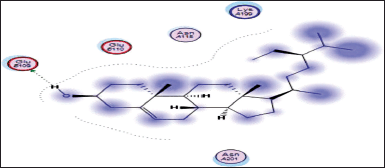 | Figure 15. Docking result of beta-sitosterol binding to the active site of 1p9m: predicted interactions and binding modes. [Click here to view] |
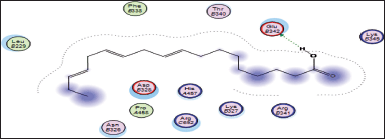 | Figure 16. Docking result of 9,12,15-octadecatrienoic acid binding to the active site of 5azt: predicted interactions and binding modes. [Click here to view] |
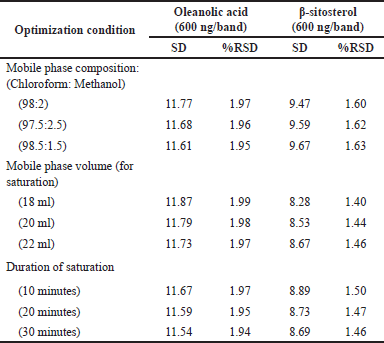
HPTLC is considered an important technique used in the standardization and quality control of phyto-pharmaceuticals, any medicinal plant should be authenticated to exclude adulteration and HPTLC can be used for this purpose, also checking the presence of active constituents and quantifying their concentrations are very important in the preparation of phyto-pharmaceuticals, these are also achieved by HPTLC [20]. TLC technique was firstly used to choose the most proper mobile phases (Figs. 3 and 4); to identify the active compounds responsible for hepatoprotective activity in both fractions, we selected some known hepatoprotective and antioxidant active standard compounds and used them as markers, it was revealed that DCM active fraction contains β-sitosterol and oleanolic acid (Figs. 5–8), while ethylacetate active fraction does not showed presence of tested standard compounds, so it was concluded that the hepatoprotective activity of ethylacetate active fraction was due to presence of compounds other than tested standard compounds, and hence both fractions are not totally identical. The Rf-values of β-sitosterol and oleanolic acid were 0.54 and 0.36, respectively. The identification of β-sitosterol and oleanolic acid occurred in DCM active fraction were proved by validation and quantifying methods according to ICH guidelines 2005 [21]; generally, validation method is always repeated in terms of precision and accuracy to confirm that the identified compounds are really β-sitosterol and oleanolic acid, so the linearity range used for β-sitosterol and oleanolic acid were 200–1,400 ng/spot with r2 values = 0.994 and 0.996, respectively (n = 6), their calibration curves and regression equations were determined as the data shown in (Table 3), the limit of detection of β-sitosterol and oleanolic acid were 45.69 and 43.03 ng, respectively; the mean percent recovery of β-sitosterol and oleanolic acid at different concentrations were 98.85% and 98.76%, respectively (Table 4); also the method was precise because the percentages of relative standard deviation (%RSD) of intra-day precision of β-sitosterol and oleanolic acid were ranged between 1.58–1.92 and 1.75–1.95, respectively, with ascending concentrations (i.e., 600, 800, and 1,000 ng/band), while the %RSD of inter-day precision of both compounds were ranged between 1.50–1.81 and 1.72–1.85, respectively, with the same ascending concentrations (Table 5); the robustness of the proposed HPTLC technique was also precise after using different compositions of the mobile phase chloroform: methanol with different volumes and different times of saturation (n = 6) as shown in (Table 6). By using the quantifying method, and with the aid of the obtained calibration curves, it was observed that the mean contents (w/w) of β-sitosterol and oleanolic acid were 9.21 and 7.73 μg/mg of dry fraction weight, respectively (Table 7). β-sitosterol and oleanolic acid are steroidal (phytosterol) and pentacyclic triterpenoids, respectively, sometimes both compounds may occur in glycosidal form in the form of saponins [22]. They are widely distributed and abundant in many plants [23,24].
β-sitosterol was isolated before from the seeds, fruit husks, flowers, roots, and root barks of C. decidua [25], also it was identified and quantified before by HPTLC from the stem part of C. decidua [26], it was proved in many studies that it possesses strong antioxidant and anti-inflammatory properties with moderate hepatoprotective activity, also it possesses anti-pyretic, immuno-modulatory, anti-diabetic, hypocholesterolaemic, anti-bacterial, and anti-cancer properties [18,25,27].
Many studies proved that oleanolic acid possesses strong hepatoprotective activity; in addition, it has antioxidant, anti-inflammatory, immuno-modulatory, α-glucosidase inhibitory, anti-hyperlipidemic, anti-bacterial, anti-fungal, anti-viral, anti-HIV, and anti-cancer properties [25,27,28], in China, oleanolic acid was marketed in oral formulations for the treatment of various liver disorders [28], it was suggested that its mode of action as hepatoprotective activity is achieved by inhibiting toxicants activation and enhancing the body defense system, this is an interpretation that the hepatoprotective, antioxidant and anti-inflammatory properties of oleanolic acid are synergistic triple strength for treatment of many liver disorders [24]. According to our knowledge, this is the first time to identified oleanolic acid in C. decidua stem part. Therefore, we suggest that the hepatoprotective and antioxidant activities of DCM fraction are probably due to the presence of oleanolic acid, β-sitosterol, hexadecanoic acid, 9,12,15-octadecatrienoic acid, and other phenolic hydrocarbon acids, while we suggest the hepatoprotective and antioxidant activities of ethyl acetate fraction is probably due to the presence of hexadecanoic acid, 9-octadecenoic acid, and other phenolic hydrocarbon acids, to get the whole clear picture, further analysis such as liquid chromatography–mass spectrometry (LC/MS) and nuclear magnetic resonance (NMR) are recommended to identify and characterize other isolated compounds from both DCM and ethylacetate active fractions.
Generally, triterpenoids (such as β-sitosterol and oleanolic acid) are very important natural hepatoprotective agents, because about 350 natural triterpenoidal compounds with hepatoprotective activity were reviewed between 1975 and 2016 [29], so these important natural compounds should be considered in the field of drug discovery and design for searching for new compounds with potential hepatoprotective activity.
The selected active phytochemical compounds for in silico docking were 9,12,15-octadecatrieonic acid, 9-octadecenoic acid (elaidic acid), β-sitosterol, dihydrolinalool, hexadecanoic acid, and oleanolic acid; silymarin was selected as a positive control because it is known hepatoprotective agent. The selected proteins were according to their mechanisms of action either by activation or signaling transcriptions which finally lead to liver injury, some of these proteins are inflammatory mediators, so by inhibiting these proteins targets by some drugs is considered potential for improving liver injury which was stimulated by free radicals and/or some foreign toxic substances such as CCl4 and paracetamol [30]. The negatively charged binding energy (Kcal/Mol) indicates that the compound has low energy binding and, hence possesses hepatoprotective properties, all tested phyto-chemical compounds showed negatively charged binding energy which means that they have hepatoprotective activity but with various degrees, silymarin was the lowest binding energy with all targeted proteins except against 1uki protein (JNK inhibitor) (Table 8).
Hexadecanoic acid, being a hydrophobic molecule, may interact with hydrophobic regions or residues in the proteins. This could involve interactions with nonpolar amino acid side chains or hydrophobic pockets within the protein structures. Van der Waals forces, including London dispersion forces, can occur between hexadecanoic acid and the protein. These forces contribute to the overall stability of the complex through attractive interactions between the electron clouds of hexadecanoic acid and the protein’s atoms. Hexadecanoic acid contains a carboxyl group (−COOH), which can potentially form hydrogen bonds with specific amino acid residues such as arginine in the protein. Hexadecanoic acid contains a carboxylic acid group, which could potentially form hydrogen bonds with arginine residues in the active site of 6t7v protein (Nrf2) (Fig. 10a). Hexadecanoic acid has the potential to engage in hydrogen bonding with specific amino acid residues in the 3gc8 protein (p38 mitogen-activated protein kinase) (Fig. 10b), along with Dipole–Dipole interactions, this is because the carbonyl group possesses a substantial dipole moment resulting from the difference in electronegativity between the oxygen and carbon atoms. In addition, the oxygen atom within the carbonyl group can function as a hydrogen bond acceptor.
9-octadecenoic acid (elaidic acid), being a fatty acid with a hydrophobic hydrocarbon tail, may interact with hydrophobic residues or regions in the active site of 1itb, 6t7v, and 5azt proteins (IL-1 receptor, Nrf2, and peroxisome proliferator-activated receptor-α (PPAR- α), respectively) (Figs. 11 and 12). These interactions occur when nonpolar regions of elaidic acid interact with nonpolar amino acid side chains, forming a hydrophobic pocket. Hydrophobic interactions can play a crucial role in the binding affinity and stability of the complex. Elaidic acid may form hydrogen bonds with asparagine in the active site of IL-1 receptor (1itb), hydrogen bonding interactions can provide additional stability to the complex. Van der Waals interactions can occur due to temporary fluctuations in electron distribution and can contribute to the overall stability of the fatty acid-protein complex. The docking results revealed distinct binding modes for elaidic acid and hexadecanoic acid within the active site of the IL-1 receptor. Elaidic acid formed a combination of hydrophobic interactions, hydrogen bonding, and Van der Waals contacts. The trans double bond in elaidic acid allowed for favorable π-stacking interactions with aromatic residues in the active site of the IL-1 receptor, contributing to the stability of the complex. In contrast, hexadecanoic acid exhibited primarily hydrophobic interactions with IL-1 receptors. The straight-chain structure of hexadecanoic acid allowed for extensive hydrophobic contacts with nonpolar amino acid residues within the binding pocket of the IL-1 receptor. Van der Waals interactions played a critical role in stabilizing the complex, while the absence of a double bond limited the potential for π-stacking interactions observed with elaidic acid. The differential interactions of elaidic acid and hexadecanoic acid with IL-1 receptor suggest distinct binding modes and potential functional implications. The trans double bond in elaidic acid introduces geometric constraints and enables specific interactions that may influence its biological activity. On the other hand, the lack of a double bond in hexadecanoic acid allows for a more extended hydrophobic surface, facilitating favorable hydrophobic interactions. Elaidic Acid contains a carboxylic acid group (−COOH), which could potentially form hydrogen bonds with Valine residues in the active site of 6t7v protein (Nrf2), and glutamine residues in 5azt protein (PPAR- α). It is important to note that elaidic acid is a dietary fatty acid and not a known inhibitor of COX-2 like Celecoxib. Nevertheless, considering general principles, the following interactions and SAR could be explored:
Regarding SAR, it is important to note that elaidic acid is not specifically designed as a COX-2 inhibitor, and its SAR as a ligand for COX-2 has not been extensively studied; modifications to the fatty acid chain length, the presence of double bonds, or the introduction of functional groups could be explored to assess their effects on COX-2 binding and inhibitory activity.
Oleanolic acid, being a small molecule, can potentially interact with proteins through various mechanisms, including hydrogen bonding, hydrophobic interactions, and electrostatic interactions. Oleanolic acid contains a carboxylic acid group (−COOH), which may establish hydrogen bonds with glutamic acid residues in the active sites of both the 1nfi and 1uki proteins (p65 and JNK inhibitor, respectively) (Fig. 13), as well as the arginine residue in the 1vkx protein (NF-kb) (Fig. 14). Dipole–Dipole Interactions are possible with oleanolic acid because of the difference in electronegativity between the oxygen and carbon atoms; the carbonyl group has a significant dipole moment. Furthermore, the oxygen atom in the carbonyl group can act as a hydrogen bond acceptor.
The docking studies between beta-sitosterol and 1p9m (IL-6α) (Fig. 15) provide insights into their potential binding interactions. Although experimental validation is necessary, the analysis of the docking results can offer a preliminary understanding of the structure activity relationship (SAR) and possible interactions. One possible interaction is the formation of hydrogen bonds between the hydroxyl group (−OH) of beta-sitosterol and glutamic acid within the binding site of the IL-6α receptor. These hydrogen bonds can play a crucial role in stabilizing the ligand-protein complex. In addition to hydrogen bonding, hydrophobic interactions are likely to occur between the hydrophobic regions of beta-sitosterol and the hydrophobic residues of the IL-6α receptor. These interactions involve nonpolar groups and contribute to the overall binding affinity and stability of the complex.
The carboxylic acid group (−COOH) of 9,12,15-octadecatrienoic acid has the capacity to create hydrogen bonds with the glutamic acid found in 5azt protein (PPAR-α) (Fig. 16).
The results obtained by in silico docking are useful prediction tools for future plans of drug development, so we recommend considering our active compounds for drug development (SAR) on the tested proteins, proteins which are responsible for hepatotoxicity, also we recommend to consider and examine any presence of synergism (as hepatoprotective and antioxidant activities) if these compounds were combined together as in our case in DCM and ethyl acetate fractions of the methanolic extract of C. decidua stem.
CONCLUSION
GC/MS chromatogram for both DCM and ethyl acetate fractions showed the presence of 25 and 29 compounds, respectively, our fractions showed the presence of considerable contents of hexadecanoic acid, 9-octadecenoic acid, 9,12,15-octadecatrienoic acid and dihydrolinalool, which they have various degrees of hepatoprotective and antioxidant activities, this is the first time to identify hexadecanoic acid in C. decidua stem.
β-sitosterol and oleanolic acid were isolated and identified from the DCM fraction by HPTLC, the two lead triterpenoidal natural compounds are known hepatoprotective and antioxidant agents, this is the first time to isolate and identify oleanolic acid from C. decidua stem, this validated HPTLC fingerprint profile method can be used for standardization and quality control of the active hepatoprotective DCM fraction, the two lead compounds should be considered in the field of drug development and design of hepatoprotective activity, LC/MS and NMR methods should also be considered in the future to identify other unknown compounds present in DCM and ethylacetate fractions.
All tested phyto-chemical compounds identified by GC/MS and HPTLC revealed negatively charged binding energy by in silico docking, which means that they have hepatoprotective activity but with various degrees. Hexadecanoic acid showed significant predicted binding properties with Nrf2, p38 mitogen-activated protein kinase, and IL-1 receptor proteins. 9-octadecenoic acid (elaidic acid) showed significant predicted interactions with IL-1 receptor, Nrf2, and PPAR-α proteins. Oleanolic acid showed significant predicted binding with p65, JNK, and NF-kb. Hexadecanoic acid showed significant predicted binding properties with Nrf2, p38 mitogen-activated protein kinase, and IL-1 receptor proteins. 9-octadecenoic acid (elaidic acid) showed significant predicted interactions with IL-1 receptor, Nrf2, and PPAR-α proteins. Oleanolic acid showed significant predicted binding with p65, JNK, and NF-kb. β-sitosterol showed significant predicted interactions with IL-6α protein, while 9,12,15-octadecatrienoic acid revealed significant binding with PPAR-α protein. So we recommend considering our active compounds for the field of drug development (SAR) and the presence of synergism if they are combined and administered together.
ACNOWLEDGEMENT
The authors extend their appreciation to Prince Sattam bin Abdulaziz University for funding this research work through the project number (PSAU/2023/03/25508).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(01):151–71. CrossRef
2. Reddy VJS, Deval Rao G, Mallikarjuna K. A review on hepatoprotective activity of some medicinal plants. Int J Innov Pharm Res. 2014;5(02):395–404.
3. Kumar SV, Sanjeev T, Ajay S, Kumar SP, Anil S. A review on hepatoprotective activity of medicinal plants. Int J Adv Res Pharm Biosci. 2012;2(01):31–8.
4. Jannu V, Baddam PG, Boorgula AK, Jambula SR. A review on hepatoprotective plants. Int J Drug Dev Res. 2012;4(03):1–8.
5. Dhakad P, Sharma P, Kumar S. A review on ethnobiological and medicinal potential of Capparaceae family plant: Capparis decidua (Forssk.) Edgwe. Adv Pharmacol Pharm. 2016;4(03):27–39. CrossRef
6. Mohammed MS, Khalid HS, Muddathir AK, Siddiqui NA, Ali M. A novel germacranolide sesquiterpene lactone with anti-inflammatory effect from Capparis decidua (Forsk.). Int J Res Pharm Chem. 2012;2(04):1073–7.
7. Dev SK, Shukla A, Choudhury PK, Singh GK. Analgesic and anti-nociceptive activity of hydroethanolic extract of Capparis decidua Linn. Asian J Pharm Pharmacol. 2015;1(01):40–4.
8. Mohammed MS, Khalid HS, Muddathir AK, Tahir KEH, Osman B, Osman WJA, et al. Effect of two sesquiterpene lactones from Capparis decidua (Forsk.) on arachidonic acid and adenosine diphosphate-induced platelets aggregation. J Phytopharm. 2014;3(03):176–9.
9. Zia-El-Haq M, Cavar S, Qayum M, Imran I, de Feo V. Compositional studies: antioxidant and anti-diabetic activities of Capparis decidua (Forssk.) Edgwe. Int J Mol Sci. 2011;12:8846–61. CrossRef
10. Dahiya R, Vaghela JS. Evaluation of anti-asthmatic activity of Capparis decidua. Asian J Pharm Clin Res. 2022;15(02):94–7. CrossRef
11. Nazar S, Hussain MA, Khan A, Muhammad G, Tahir MN. Capparis decidua Edgwe (Forssk.): a comprehensive review of its traditional uses, phytochemistry, pharmacology and nutrapharmaceutical potential. Arab J Chem. 2020;13:1901–16. CrossRef
12. Rasheed HMF, Jabeen Q. Pharmacological role of Capparis decidua (Forssk.) Edgwe in preventing cyclophosphamide-induced myelosuppression and modulating innate and adaptive immune response. Dose Response. 2022;20(03):15593258221123672. CrossRef
13. Ali SA, Al-Amin TH, Mohamed AH, Gameel AA. Hepatoprotective activity of aqueous and methanolic extracts of Capparis decidua stem against carbon tetrachloride induced liver damage in rats. J Pharmacol Toxicol. 2009;4(04):167–72.
14. Mirghani M, Khalid HS, Mohammed MS, Mohamed AB, Ali A, Osman W, et al. Hepatoprotective and free radical scavenging activities of methanol extract fractions of Capparis decidua Edgwe (Forssk.) (Capparidaceae). Afr J Pharm Pharmacol. 2020;14(08):316–30. CrossRef
15. Padmalochana K, Rajan MSD, Lalitha R, Sivasankari H. Evaluation of the antioxidant and hepatoprotective activity of Cryptolepis buchanani. J Appl Pharm Sci. 2013;3(02):099–104. CrossRef
16. Shah MD, D’Souza UJA, Iqbal M. The potential protective effect of Commelina nudiflora L. against carbon tetrachloride (CCl4)-induced hepatotoxicity in rats, mediated by suppression of oxidative stress and inflammation. Environ Health Prev Med. 2017;22(01):1–19. CrossRef
17. Thakur AV, Ambwani S, Ambwani TK, Ahmad AH, Rawat DS. Evaluation of phytochemicals in the leaf extract of Clitoria ternatea Willd. Through GC/MS analysis. Trop Plant Res. 2018;5:200–6. CrossRef
18. Maran BAV, Iqbal M, Gangadaran P, Ahn BC, Rao PV, Shah MD. Hepatoprotective potential of Malaysian medicinal plants: a review on phytochemicals, oxidative stress and antioxidant mechanisms. Molecules. 2022;27(05):1533. CrossRef
19. Ali MZ, Mehmood MH, Saleem M, Akash MSH, Malik A. Pharmacological evaluation of Euphorbia hirta, Fagonia indica and Capparis decidua in hypertension through in-vivo and in-vitro assays. Heliyon. 2021;7(10):e08094. CrossRef
20. Mohamed NS, Mohamed MS, Mothana RA, Osman WJ, Khalid HS. HPTLC fingerprint profiles and UPLC-MS identification of potential antioxidant fractions and compounds from Ambrosia maritima L. and Ammi majus L. Afri J Biotechnol. 2020;19(05):249–58. CrossRef
21. Anonymous. ICH harmonized tripartite guideline (2005) validation of analytical procedures: text and methodology Q2(R1). International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland, November 2005. Available from: https://www.fda.gov/regulatory-information/search- fda-guidance- documents/q2r1-validation-analytical-procedures-text-and-methodology-guidance-industry.
22. Castellano JM, Ramos-Romero S, Perona JS. Oleanolic acid: extraction, characterization and biological activity. Nuterients. 2022;14(03):623. CrossRef
23. Bahat AH, Alia A, Rather GM, Kumar B. Isolation and characterization of beta-sitosterol from the rhizomes of Arisaema utile and its evaluation of antioxidant activity. Int J Sci Res Biol Sci. 2019;6(02):111–8. CrossRef
24. Wan XL, Lu YF, Xu SF, Wu Q, Liu J. Oleanolic acid protects against the hepatotoxicity of D-galactosame plus endotoxin in mice. Biomed Pharmacother. 2017;93:1040–6. CrossRef
25. Sharma P, Sharma DK. Medicinal value of three common plants of Rajasthan, India: review. J Med Plants Stud. 2018;6(01):096–101.
26. Rathee S, Mogla OP, Rathee P, Rathee D. Quatification of β-sitosterol using HPTLC from Capparis decidua (Forssk.) Edgwe. Der Pharma Chem. 2010;2(04):86–92.
27. Balogun O, Ojerinde OS, Afolabi EO, Alemika TE. Isolation and characterization of β-sitosterol, oleanolic, 19-dehydroursolic and yarumic acids from Plectransthus esculentus leaves and tubers. J Pharm Bioresour. 2022;19(01):43–50. CrossRef
28. Liu J. Review article: pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49(02):57–68. CrossRef
29. Xu GB, Xiao YH, Zhang QY, Zhou M, Liao SG. Hepatoprotective natural triterpenoids. Eur J Med Chem. 2018;145:691–716. CrossRef
30. Ullah H, Khan A, Bibi T, Ahmad S, Shehzad O, Ali H, et al. Comprehensive in vivo and in silico approaches to explore the hepatoprotective activity of poncirin against the paracetamol toxicity. Naunyn Schmiedebergs Arch Pharmacol. 2022;395(02):195–215. CrossRef