INTRODUCTION
A diverse array of plants has been employed in traditional medicine since ancient times, with a substantial proportion specifically harnessed for the alleviation of inflammatory conditions such as rheumatic diseases and diabetes [1]. For example, in South America, Baccharis. trimera, Annona neosalicifolia, and Artemisia absinthium L. CTES0030264 are used frequently for the treatment of diabetes and Dysphania ambrosioides, Sida cordifolia, and Maytenus ilicifolia as anti-inflammatory agents [2].
Inflammation is a protective reaction that contributes to the return to a homeostatic state after injury. Innate and adaptive immune cells participate in this response [3]. In this context, monocytes/macrophages play a significant role in inflammation, defense mechanisms, and removal of damaged cells. Excessive responses of these cells, on the other hand, can lead to chronic inflammatory conditions including autoimmune diseases, atherosclerosis, and cancer, among others. Overproduction of inflammatory mediators by monocytes, such as pro-inflammatory cytokines, reactive nitrogen species, and reactive oxygen species, characterizes this response [4]. In addition, lymphocytes are highly activated under chronic inflammatory conditions [5]the histology of inflamed tissue begins to resemble that of peripheral lymphoid organs, which can be referred to as lymphoid neogenesis or formation of tertiary lymphoid tissues. Lymphocyte recruitment to inflamed tissues is also reminiscent of lymphocyte homing to peripheral lymphoid organs. In the latter, under physiological conditions, homing receptors expressed on lymphocytes adhere to vascular addressin expressed on high endothelial venules (HEVs. Thus, overactivation of innate and adaptive responses can lead to chronic inflammation and autoimmune diseases.
Currently used anti-inflammatory drugs are effective; however, they have some disadvantages, such as undesirable adverse effects and unresponsiveness to treatment in certain patients [6]. Therefore, alternative treatment options are needed, and the study of plants with anti-inflammatory activity is relevant because of their potential pharmacological activity.
Baccharis (Asteraceae) is a widespread genus in the Americas and some species are commonly named “carqueja” [7]. Baccharis species are used in South American traditional medicine to treat gastrointestinal and liver disorders, infections, fever, and inflammation [8].
The Baccharis genus has been associated with numerous biological activities, including antioxidant, antibacterial, and antifungal properties [7,9–11]. Furthermore, previous studies have recognized potential anti-inflammatory effects [12–16]; however, its impact on the immune system has not been extensively analyzed.
Baccharitrimera is one of the most studied species within the genus Baccharis. The anti-inflammatory activity of B. trimera has been demonstrated in murine models of inflammation [12–14,17]. However, studies of its anti-inflammatory activity in human cells are limited.
Baccharis. punctulata has rarely been studied. It has been reported that B. punctulata exhibits anti-inflammatory properties in a murine model of 12-O-tetradecanoyl-phorbol-13-acetate-induced inflammation [18]. In addition, B. punctulata has been shown to decrease lymphocyte proliferation [19].
Therefore, in the present study, we analyzed the immunomodulatory activity of two species of the Baccharis genus, B. punctulata and B. trimera, in in vitro models of inflammation using monocytes and splenocytes, encompassing the assessment of both innate and adaptive immune responses.
MATERIALS AND METHODS
Plant material and sample preparation
Baccharis punctulata DC and B. trimera (Less.) DC were collected from Ñemby, Paraguay, and San Lorenzo, Paraguay, respectively. Taxonomical evaluations were performed by botanists and voucher specimens (B. trimera: Voucher N° 4088 and B. punctulata: Voucher N° 4302) were deposited in the Herbarium-FCQ of the Department of Botany, Facultad de Ciencias Químicas, Universidad Nacional de Asunción (UNA). The aerial parts of the collected plants were used for extract preparation. The plant material was air-dried and ground into a fine powder using a cutting mill. To obtain the extracts, 200 g of the powder was suspended in HPLC-grade methanol (1.5 l) and sonicated three times for 30 minutes (in-house method). The extraction procedure was repeated thrice, after which the extracts were filtered. A rotary evaporator (RVO 400 SD; Boeco, Germany) was used to remove the solvent. The yield was calculated considering the initial powdered dry material as the starting point (B. trimera: 17.1 g, 5.04%, and B. punctulata: 24.12 g, 6.96%). Plant methanol extracts were chemically characterized as previously described by our group [19]. The extracts were resuspended in dimethyl sulfoxide (DMSO; Sigma, USA) at a concentration of 10 or 100 mg/ml for further experiments.
Cells and cell culture
In this study, THP-1 (human acute monocytic leukemia) cells and splenocytes isolated from murine spleens were used. These cellular models were used to evaluate the immunomodulatory activity of the extracts on monocytes and lymphocytes, which are the primary cells involved in the innate and adaptive immune responses, respectively. To investigate the effect of B. punctulata and B. trimera extracts on innate immunity, their effects on human monocytic cells were determined using an in vitro model of lipopolysaccharide (LPS)-induced inflammation.
THP-1 cells were cultured at 37°C in 5% CO2 in Roswell Park Memorial Institute (RPMI) medium (Gibco, USA), 10% fetal bovine serum (Gibco, Brazil), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (Gibco, USA). Splenocyte isolation was performed as described in Coligan et al. [20] with minor modifications. Briefly, the spleen of 6–8-week-old healthy female Swiss albino mice were surgically removed in a sterile manner (under a laminar flow cabinet). The spleens were then gently pressed and subsequently subjected to a 70 μm cell strain. After that, the cells were incubated in red blood cell lysis using an ammonium chloride-potassium solution, washed with 1× phosphate buffer saline, and cultured in RPMI medium (Gibco, USA), 10% fetal bovine serum (Gibco, Brazil), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (Gibco, USA). Cell viability was assessed by trypan blue staining. The study was approved by the Ethics Research Committee of Facultad de Ciencias Químicas, UNA (N° 798/21).
Measurement of cell viability
THP-1 cells were seeded in 96-well plates (Corning Costar®, USA) at a density of 2 × 105 cells/well. The cells were treated with 10, 25, 50, 100, or 200 μg/ml of the extract for 24 hours. Cell viability was measured using an MTT colorimetric assay. Splenocytes, isolated as previously described, were cultured at a density of 5 × 106 cells/ml and 200 μl/well in a 96-well plate in RPMI medium in the presence of increasing extract concentrations. Resazurin was added 48 hours later and incubated for 24 hours at 37°C and 5% CO2. After this time, cell viability was determined by absorbance measurements at 570 and 600 nm on a MultiskanTM GO (Thermo Scientific, Waltham, USA). Cells cultured in medium alone or 0.1% DMSO (vehicle control) were used as controls.
Determination of soluble CD14 (sCD14) levels
LPS induces monocyte activation and subsequent production of pro-inflammatory mediators such as cytokines and sCD14 [21–23] Cells (2 × 105 cells/well) were seeded in 96-well plates and pretreated for 15 minutes with the methanol extracts, followed by 1 μg/ml LPS (E. coli 0111:B4, Sigma, USA) for 24 hours. Cells treated with DMSO and cells pretreated with DMSO and stimulated with LPS were used as the negative and positive controls, respectively. sCD14 levels in the culture medium were assessed using an enzyme-linked immunosorbent assay (ELISA) assay (Quantikine ELISA, R&D Systems, USA) following the manufacturer’s instructions. The optical density of each well was determined at 450 nm using a microplate reader (Multiscan GO; Thermo Fisher Scientific Waltham, USA).
Reverse transcription-polymerase chain reaction
THP-1 cells at a density of 1 × 106 cells/ml were treated with the methanolic extracts (15 minutes), followed by stimulation with LPS (1 μg/ml) for 4 hours as reported previously [24]. RNA extraction was performed using TRIzol reagent (Thermo Fisher Scientific, USA) and subsequently processed with DNase RQ1 (Promega, USA) at 37°C for 30 minutes. cDNA synthesis was performed with Moloney Murine Leukemia Virus Reverse Transcriptase (Promega®, USA), 100 μM Oligo dT, and dNTPs. cDNA was amplified (StepOnePlus real-time PCR system, Thermo Fisher Scientific, USA), using a SsoAdvanced Universal SYBR Green Supermix (BioRad, USA), and primers (Table 1).
A negative PCR control (with no template) was included. Relative mRNA expression was determined by the 2−ΔΔCt method [29], using RPL13A as a normalizing gene.
Proliferation assay
Splenocytes (5 × 106 cells/ml) were seeded in RPMI medium and stimulated with 20 μg/ml concanavalin A (ConA) in the presence of extracts. After 48 hours of treatment, resazurin (AlamarBlue®) was added, and the absorbance at 570 and 600 nm was measured 24 hours later on MultiskanTM GO (Thermo Scientific, Waltham, USA). The proliferation index was calculated using the following equation:
Eox = molar extinction coefficient of oxidized resazurin; λ1 = 570 nm; λ2 = 600 nm.
Measurement of NO production
Griess assay [30] was performed using the culture supernatant of splenocytes treated with or without the extract for 15 minutes, followed by stimulation with LPS (10 μg/ml) for 18 hours. The cell culture medium and Griess solution (0.1% N-1-naphthyl ethylenediamine dihydrochloride and 1% sulfanilamide in 5% phosphoric acid) were mixed in a 1:1 ratio and incubated for 15 minutes. The absorbance was measured at 545 nm (Multiscan GO, Thermo Fisher Scientific, USA). A standard curve was prepared by dissolving increasing concentrations of sodium nitrite (Cicarelli, Argentina) in the RPMI culture medium. The NO concentration was determined by extrapolation to the standard curve.
Statistical analyses
The results are expressed as mean ± standard deviation and statistical significance was determined by one-way analysis of variance (ANOVA) and Dunnett’s post-test (p < 0.05) using GraphPad Prism 8.01 (GraphPad Software Inc., USA).
RESULTS AND DISCUSSION
Cytotoxicity on THP-1 monocytic cells by B. punctulata and B. trimera extracts
First, the effect of the extracts on cell viability was determined by treating THP-1 cells with different concentrations of B. punctulata or B. trimera extracts. The species had no cytotoxic effects at 10, 25, or 50 μg/ml (Fig. 1A and B) compared with cells treated only with the vehicle (DMSO) used to dissolve the plant extract. It should be noted that unstimulated cells (with culture medium only) showed no difference in viability from cells treated with the vehicle (DMSO). Based on these results, concentrations of up to 50 μg/ml were selected for subsequent assays.
Effects of B. punctulata and B. trimera extracts on LPS-induced soluble CD14 and cytokines production in monocytic cells
THP-1 cells treated with the inflammatory stimulus LPS showed a significant increase in sCD14 production compared to unstimulated cells (Fig. 2). This indicated that the inflammation model functioned properly.
In addition, THP-1 cells were pretreated with vegetal extracts before stimulation with LPS. The levels of sCD14 in the culture supernatant were subsequently measured, which revealed a decrease in sCD14 production in cells that were pretreated with the extracts and stimulated with LPS, compared to cells that were only treated with LPS. Therefore, treatment with B. punctulata or B. trimera markedly decreased LPS-induced sCD14 production (Fig. 2A and B), suggesting an immunomodulatory effect of these extracts on human monocytic cells. These results indicate a decrease in monocyte activation in the presence of the extracts compared to cells stimulated with LPS alone and without treatment with plant extracts. This suggests that the species modulates innate immunity.
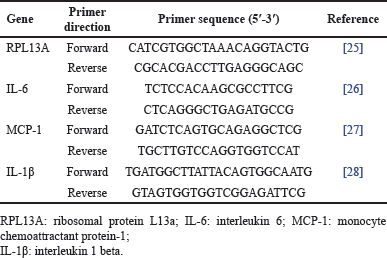 | Table 1. List of primers used for qPCR. [Click here to view] |
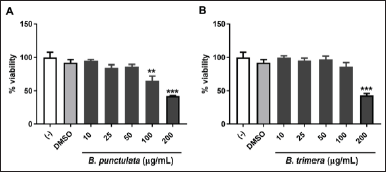 | Figure 1. Effects of B. punctulata and B. trimera on cell viability. THP-1 cells were treated with B. punctulata (A) or B. trimera (B) extract for 24 hours. Cell viability was determined using an MTT assay. ANOVA: *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle (DMSO) control. [Click here to view] |
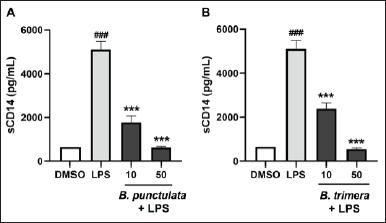 | Figure 2. Inhibitory effects of B. punctulata and B. trimera extracts on the production of sCD14 in LPS-stimulated human monocytes. THP-1 cells were treated with B. punctulata (A) or B. trimera (B) extract in the presence of LPS (1 μg/ml) for 24 hours. ANOVA, ###p < 0.001 versus untreated control; ***p < 0.001 versus LPS-treated cells. [Click here to view] |
LPS acts as a stimulus that triggers the production of inflammatory cytokines and chemokines in monocytes/macrophages [22]. THP-1 cells were pretreated with B. punctulata or B. trimera extracts and subsequently stimulated with LPS. The cells were then collected, and RNA extraction and RT-qPCR were performed to evaluate IL-1, IL-6, and MCP-1 mRNA levels. Baccharipunctulata significantly reduced LPS-induced IL-6 and MCP-1 mRNA levels compared to those in cells stimulated with LPS (Fig. 3), indicating its anti-inflammatory effects. Similarly, the B. trimera extract reduced LPS-induced IL-1β and IL-6 mRNA levels (Fig. 4). Together, these results suggest that the extracts lead to a reduction in innate immune response. These findings are in line with the results observed in another species, B. dracunculifolia, which decreased IL-1β, IL-6, and IL-10 levels in murine macrophages [15].
It should be noted that there were some differences in the effects of the species on cytokine expression: B. punctulata reduced IL-6 and MCP-1 mRNA levels, and B. trimera reduced IL-1β and IL-6 mRNA levels, compared to those in cells stimulated with LPS alone. One explanation for this phenomenon is that different compounds were identified in these extracts in a previous study conducted by our group [19]. In our previous study, we showed that the methanol extract of B. trimera contained apigenin and genkwanin [19]. The flavonoid apigenin has been shown to decrease IL-6 and Il-1β [31]. In addition, genkwanin has anti-inflammatory activity and decreases IL-6 levels [32]. These compounds may be responsible for the action of the extract on the production of pro-inflammatory cytokines. In the B. punctulata methanol extract, trans-phytol and di-O-caffeoylquinic acid were previously identified by our group [19]. Di-O-caffeoylquinic acid and phytol have been shown to inhibit the production of the pro-inflammatory cytokines IL-6 and TNF-α [33,34]. Furthermore, di-O-caffeoylquinic acid has been shown to suppress the expression of inflammatory mediators such as nitric oxide(NO), prostaglandin E2, NO synthase, and cyclooxygenase-2 and to inhibit carrageenan-induced edema in vivo [33]. Therefore, these compounds could be responsible for the effects of the B. punctulata extract. These compounds have been documented to decrease the activation of the transcription factor NF-κB, which stimulates the expression of pro-inflammatory cytokines and other inflammatory mediators [33,35]. Consequently, it is hypothesized that the mechanism of action of B. punctulata extract involves inhibition of NF-κB activation.
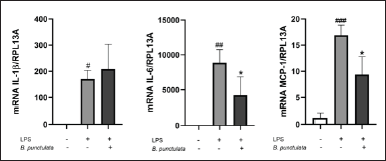 | Figure 3. Inhibition of cytokine production by the B. punctulata extract in LPS-stimulated human monocytes. THP-1 cells were treated with B. punctulata extract in the presence of LPS (1 μg/ml) for 4 hours. mRNA levels were analyzed by RT- RT-qPCR and the 2-ΔΔCt method (each figure depicts fold change). ANOVA, #p < 0.05, ##p < 0.01, ###p < 0.001 versus untreated control; *p < 0.05, versus LPS-treated cells. [Click here to view] |
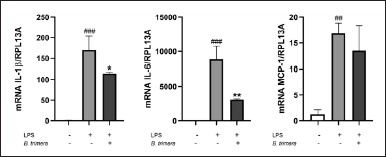 | Figure 4. Inhibition of cytokine production by the B. trimera extract in LPS-stimulated human monocytes. THP-1 cells were treated with B. trimera extract in the presence of LPS (1 μg/ml) for 4 hours. mRNA levels were analyzed by RT- RT-qPCR and the 2-ΔΔCt method (each figure depicts fold change). ANOVA, #p < 0.05, ##p < 0.01, ###p < 0.001 versus untreated control; *p < 0.05, ***p < 0.001 versus LPS-treated cells. [Click here to view] |
Cytotoxicity on splenocytes and effects of B. punctulata and B. trimera extracts on cell proliferation
To gain a more comprehensive understanding of the effects of the extracts on immune cells, we evaluated the activities of B. punctulata and B. trimera in murine splenocytes. The extracts had no cytotoxic effects on splenocytes at concentrations of up to 50 μg/ml (Fig. 5A and B).
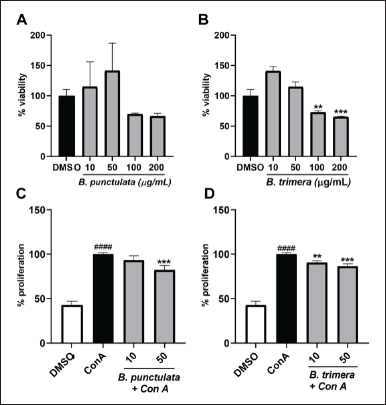 | Figure 5. Inhibitory effects of B. punctulata and B. trimera extracts on the proliferation of ConA-stimulated murine splenocytes. A-B. Effects of the extracts on cell viability. Splenocytes were treated with B. punctulata (A) or B. trimera (B) extract for 72 hours. ANOVA, **p < 0.01, *** p < 0.001 versus vehicle (DMSO) control. C-D. Splenocytes were treated with B. punctulata (A) or B. trimera (B) extract in the presence of ConA (20 μg/mL) for 72 hours. Splenocyte proliferation was determined using the Alamar Blue assay. ANOVA, ###p < 0.001 versus DMSO control; ***p < 0.001 versus ConA-treated cells. [Click here to view] |
ConA is a promoter of T lymphocyte division [36]. Therefore, ConA-induced splenocyte proliferation was determined as a marker of adaptive immunity [37]. Both species exhibited a decrease in the proliferation of cells induced by ConA (Fig. 5C and D), which further corroborated the anti-inflammatory activity of these extracts.
In accordance with the present results, previous studies have shown that B. trimera inhibits phytohaemagglutinin-induced human lymphocyte proliferation [14,19]. In addition, B. punctulata and B. trimera have been associated with a reduced production of IFN-γ, a cytokine produced by lymphocytes [19]. Our results and previous data suggest that B. punctulata and B. trimera affect T-lymphocyte proliferation.
Determination of B. punctulata and B. trimera effect on NO production
The effects of LPS-induced NO production were determined as representative indicators of the inflammatory response. Baccharipunctulata and B. trimera extracts decreased LPS-induced NO production (Fig. 6). These results indicate that both extracts decreased the inflammatory response pathway in murine splenocytes.
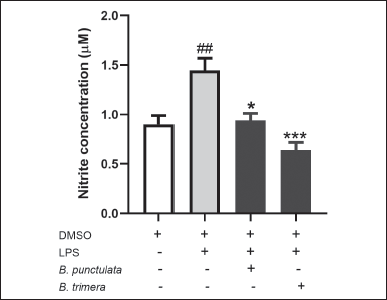 | Figure 6. Effect of B. punctulata and B. trimera extracts on the production of nitric oxide (NO) in LPS-stimulated splenocytes. Cells were treated with B. punctulata or B. trimera extracts (50 μg/ml) in the presence of LPS (10 μg/ml) for 18 hours. NO production was determined by the Griess method. Data are expressed as the mean ± SD. ANOVA. ##p < 0.01 significance versus untreated control; *p < 0.05, versus LPS-treated cells. [Click here to view] |
NO, produced by immune cells, is an important mediator of the immune response. However, uncontrolled NO release can damage or destroy the host tissue. In the present study, B. punctulata and B. trimera reduced the LPS-induced NO production. Similarly, other Baccharis species (B. obtusifolia, B. latifolia, B. pentlandii, and B. subulate) have been shown to inhibit NO production [38]. Inhibition of iNOS activity, responsible for NO production, has been suggested to be beneficial for the treatment of pathologies involving inflammation [39]. Thus, B. punctulata and B. trimera present this potential action as immunomodulators of inflammatory response.
CONCLUSION
Evaluation of the activity of plant extracts on immune cells is a necessary step to recognize their potential in the management of inflammatory disorders such as autoimmunity, chronic infections, and allergies. In this study, we found that B. punctulata and B. trimera have anti-inflammatory activities, acting on both innate and adaptive immune responses. Our findings are significant because they show how these species globally modulate the inflammatory response.
In conclusion, the extracts tested in this study exhibited significant anti-inflammatory activity, acting on the innate and adaptive immune responses. Therefore, these species have important potential for the anti-inflammatory modulation of the inflammatory response.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study was approved by the Ethics Research Committee of Facultad de Ciencias Químicas, UNA (N° 798/21).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Ghasemian M, Owlia S, Owlia MB. Review of anti-inflammatory herbal medicines. Adv Pharmacol Sci. 2016;2016:9130979. CrossRef
2. Kujawska M, Schmeda-Hirschmann G. The use of medicinal plants by Paraguayan migrants in the Atlantic Forest of Misiones, Argentina, is based on Guaraní tradition, colonial and current plant knowledge. J Ethnopharmacol. 2022;283:114702. CrossRef
3. Medzhitov R. The spectrum of inflammatory responses. Science. 2021;374(6571):1070–5. CrossRef
4. Kapellos TS, Bonaguro L, Gemünd I, Reusch N, Saglam A, Hinkley ER, et al. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol. 2019;30(10):1–13. CrossRef
5. Sakai Y, Kobayashi M. Lymphocyte “homing” and chronic inflammation. Pathol Int. 2015;65(7):344–54. CrossRef
6. Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther [Internet]. 2017;39(11):2216–29. CrossRef
7. Ramos Campos F, Bressan J, Godoy Jasinski VC, Zuccolotto T, Da Silva LE, Bonancio Cerqueira L. Baccharis (Asteraceae): chemical constituents and biological activities. Chem Biodivers. 2016;13(1):1–17. CrossRef
8. Abad MJ, Bermejo P. Baccharis (Compositae): a review update. Arkivoc. 2006 Sep 5;2007(7):76–96. CrossRef
9. Pádua B da C, Silva LD, Rossoni JV, Humberto JL, Chaves MM, Silva ME, et al. Antioxidant properties of Baccharis trimera in the neutrophils of Fisher rats. J Ethnopharmacol. 2010;129(3):381–6. CrossRef
10. Gabaglio S, Alvarenga N, Cantero-González G, Degen R, Ferro EA, Langjahr P, et al. A quantitative PCR assay for antiviral activity screening of medicinal plants against Herpes simplex 1. Nat Prod Res. 2021;35(17):2926–30. CrossRef
11. Carrizo SL, Zampini IC, Sayago JE, Simirgiotis MJ, Bórquez J, Cuello AS, et al. Antifungal activity of phytotherapeutic preparation of Baccharis species from argentine Puna against clinically relevant fungi. J Ethnopharmacol. 2020;251:112553. CrossRef
12. Gené RM, Cartañá C, Adzet T, Marín E, Parella T, Cañigueral S. Anti-inflammatory and analgesic activity of Baccharis trimera: identification of its active constituents. Planta Med. 1996;62(3):232–5. CrossRef
13. Nogueira NPA, Reis PA, Laranja GAT, Pinto AC, Aiub CAF, Felzenszwalb I, et al. In vitro and in vivo toxicological evaluation of extract and fractions from Baccharis trimera with anti-inflammatory activity. J Ethnopharmacol. 2011;138(2):513–22. CrossRef
14. Paul EL, Lunardelli A, Caberlon E, De Oliveira CB, Santos RCV, Biolchi V, et al. Anti-inflammatory and immunomodulatory effects of Baccharis trimera aqueous extract on induced pleurisy in rats and lymphoproliferation in vitro. Inflammation. 2009;32(6):419–25. CrossRef
15. Bachiega TF, de Sousa JPB, Bastos JK, Sforcin JM. Immunomodulatory/anti-inflammatory effects of Baccharis dracunculifolia leaves. Nat Prod Res. 2013 Sep;27(18):1646–50. CrossRef
16. Burgos C, Alfonso L, Ferro E, Langjahr P. Immunomodulatory activity of species of the genus Baccharis. Rev Paraguaya Reumatol. 2022;8(1):45–50. doi: 10.18004/rpr/2022.08.01.45
17. de Oliveira CB, Comunello LN, Lunardelli A, Amaral RH, Pires MGS, da Silva GL, et al. Phenolic enriched extract of Baccharis trimera presents anti-inflammatory and antioxidant activities. Molecules. 2012;17(1):1113–23. CrossRef
18. Ascari J, de Oliveira MS, Nunes DS, Granato D, Scharf DR, Simionatto E, et al. Chemical composition, antioxidant and anti-inflammatory activities of the essential oils from male and female specimens of Baccharis punctulata (Asteraceae). J Ethnopharmacol. 2019;234:1–7. CrossRef
19. Burgos C, Alvarenga N, Heiderich H, Florentín-pavía M, Sotelo PH, Carpinelli MM, et al. Immunomodulatory effects of three species of Baccharis on human peripheral blood mononuclear cells. Trop J Nat Prod. 2021;1055–9. CrossRef
20. Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W. Current protocols in immunology. Hoboken, NJ: John Wiley and Sons; 1991. Available from: https://www.wiley.com/en-au/Current+Protocols+in+Immunology+-p-9780471522768
21. Ma WT, Gao F, Gu K, Chen DK. The role of monocytes and macrophages in autoimmune diseases: a comprehensive review. Front Immunol. 2019;10:1–24. CrossRef
22. Ngkelo A, Meja K, Yeadon M, Adcock I, Kirkham PA. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and G iα dependent PI-3kinase signalling. J Inflamm. 2012;9:2–8. CrossRef
23. Zanoni I, Granucci F. Role of CD14 in host protection against infections and in metabolism regulation. Front Cell Infect Microbiol. 2013;3(32):1–6. CrossRef
24. Chanput W, Mes J, Vreeburg RAM, Savelkoul HFJ, Wichers HJ. Transcription profiles of LPS-stimulated THP-1 monocytes and macrophages: A tool to study inflammation modulating effects of food-derived compounds. Food Funct. 2010;1(3):254–61. CrossRef
25. Yamada S, Kotake Y, Demizu Y, Kurihara M, Sekino Y, Kanda Y. NAD-dependent isocitrate dehydrogenase as a novel target of tributyltin in human embryonic carcinoma cells. Sci Rep. 2014;4:5952. CrossRef
26. Wolf K, Schulz C, Riegger GAJ, Pfeifer M. Tumour necrosis factor-α induced CD70 and interleukin-7R mRNA expression in BEAS-2B cells. Eur Respir J. 2002;(2):369–75. CrossRef
27. Wang H, Yi T, Zheng Y, He S. Induction of monocyte chemoattractant protein-1 release from A549 cells by agonists of protease-activated receptor-1 and -2. Eur J Cell Biol. 2007;86(4):233–42. CrossRef
28. Ando Y, Kuroda A, Kusama K, Matsutani T, Matsuda A, Tamura K. Impact of serine protease inhibitor alpha1-antitrypsin on expression of endoplasmic reticulum stress-induced proinflammatory factors in adipocytes. Biochem Biophys Reports. 2021;26:100967. CrossRef
29. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;(4):402–8. CrossRef
30. Stich RW, Shoda LKM, Dreewes M, Adler B, Jungi TW, Brown WC. Stimulation of nitric oxide production in Macrophages by Babesia bovis. Infect Immun. 1998;66 (9):4130–6. CrossRef
31. Zhang X, Wang G, Gurley EC, Zhou H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in Macrophages. PLoS One. 2014;9(9):e107072. CrossRef
32. Gao Y, Liu F, Fang L, Cai R, Zong C, Qi Y. Genkwanin inhibits proinflammatory mediators mainly through the regulation of miR-101/MKP-1/MAPK pathway in LPS-activated macrophages. PLoS One. 2014;9(5):e96741. CrossRef
33. Jang G, Lee S, Hong J, Park B, Kim D, Kim C. Anti-inflammatory effect of 4,5-dicaffeoylquinic acid on raw264.7 cells and a rat model of inflammation. Nutrients. 2021;13(10):1–10. CrossRef
34. Zai JA, Khan MR, Mughal ZUN, Khan FS, Soomro N. Phytol anti-oxidative and anti-inflammatory effects in hydrogen peroxide challenged human PMBCs involves NF κ B pathway. Int J Emerg Technol. 2021;12(1):296–303.
35. Carvalho AMS, Heimfarth L, Pereira EWM, Oliveira FS, Menezes IRA, Coutinho HDM, et al. Phytol, a chlorophyll component, produces antihyperalgesic, anti-inflammatory, and antiarthritic effects: possible NFκB pathway involvement and reduced levels of the proinflammatory cytokines TNF-α and IL-6. J Nat Prod. 2020;83(4):1107–17. CrossRef
36. Dwyer JM, Johnson C. The use of concanavalin A to study the immunoregulation of human T cells. Clin Exp Immunol. 1981;46(2):237–49.
37. Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. 2010;125(2):S33–40. CrossRef
38. Abad MJ, Bessa AL, Ballarin B, Arag O, Gonzales E, Bermejo P. Anti-inflammatory activity of four Bolivian Baccharis species (Compositae). J Ethnopharmacol. 2006;103:338–44. CrossRef
39. Wong VC, Lerner E. Nitric oxide inhibition strategies. Futur Sci OA. 2015;1(1):FSO35. CrossRef