INTRODUCTION
Nuclear medicine is a pivotal domain within the medical field, harnessing the power of small quantities of radioactive isotopes to facilitate the diagnosis and treatment of a wide array of medical conditions. Central to the customary nuclear medicine procedures is introducing radioisotopes into the body, achieved through diverse means such as intravenous injection, inhalation of gases or aerosols, or even in conjunction with food. These radioisotopes navigate through the targeted regions within the body, emitting gamma rays as a form of energy. These emitted gamma rays are meticulously captured by specialized cameras and processed by advanced computer systems, culminating in intricate organ images [1].
Nuclear medicine imaging unfurls a treasure trove of unique and unparalleled insights that often elude alternative imaging techniques. This inherent uniqueness holds the potential to unveil diseases in their nascent stages, empowering healthcare professionals to swiftly and accurately diagnose patients. With the aid of nuclear imaging methods, visualizing organs such as bones, the heart, thyroid, and liver becomes a seamless endeavor, enabling the early detection of anomalies. Moreover, in certain instances, radiation itself becomes a therapeutic tool capable of treating afflicted organs or tumors. The fundamental distinction of nuclear medicine lies in its focus on capturing images based on the dynamic physiological processes within the body, contrasting sharply with other imaging modalities such as computed tomography scans and magnetic resonance imaging , which primarily emphasize anatomical structures.
In the realm of developed nations, nuclear medicine diagnoses transpire at an approximate annual rate of 1.9%, whereas therapeutic applications employing radioisotopes occur at a frequency of roughly one-tenth of this rate. Globally, more than 10,000 hospitals have integrated radioisotopes into their medical repertoire, with approximately 90% of these applications dedicated to diagnostic purposes. Among the pantheon of radioisotopes used for diagnostic tasks, technetium-99 reigns supreme, facilitating an astonishing 35 million procedures annually, constituting about 80% of all nuclear medicine procedures worldwide. Notably, using radioisotopes for diagnostic purposes exhibits an annual growth rate exceeding 10% [2].
In recognition of the significant strides in diagnostic and therapeutic methodologies, many tertiary hospitals have established dedicated nuclear medicine departments as specialized units. Commonly harnessed radioisotopes for medical imaging in these departments encompass Technetium-99m, Iodine-123 and 131, Thallium-201, Gallium-67, Fluorine-18, and Indium-111. The distinctive characteristics of radioisotopes, determined by their mass number and half-life, render them formidable tools for medical imaging. However, it is paramount to underscore that the potency of radioisotopes, specifically their ability to emit high-energy gamma rays, necessitates stringent adherence to precise dosage administration and appropriate techniques. Deviations from these meticulous procedures can have adverse consequences, ranging from contamination to detrimental health effects among those involved. Contamination arises when ionizing radiation-emitting materials are inadvertently deposited on individuals or surfaces, leading to potential internal radiation exposure if radioactive substances infiltrate the body. The approach to decontamination varies depending on several factors, including the location, type of isotope, half-life, radiation mode, and nature of the contamination [3].
While nuclear medicine holds immense promise for enhancing healthcare diagnostics and treatments, it is not without its perils. High-profile cases like the slow and agonizing poisoning of Alexander Litvinenko serve as stark reminders of the dangers posed by radioactive materials. The 26 radioactive isotopes of polonium used in the murder of Mr. Litvinenko are exceedingly lethal, with a potency 250 billion times greater than hydrocyanic acid. Similar radiological poisonings have afflicted investigative journalists (Yuri Shchekochikhin) and business people (Romam Tsepov), underscoring the life-threatening implications of radioisotope mishandling [3].
A distressing statistic emerges in the broader healthcare landscape: more than 450 radioisotope contamination-related deaths were reported in Europe, all linked to the manual handling of radioisotopes [2]. The gravest concern with using radioisotopes in nuclear medicine lies in the potential for adverse health impacts when proper protocols are not followed. Tissue damage, skin burns, nausea, and severe conditions such as leukemia and lung cancer stand as chilling possibilities, with the ultimate consequence being the loss of life [4].
A gamut of incidents in nuclear medicine practice can result in unjustified radiation exposure to patients and healthcare providers. These unfortunate occurrences encompass procedures performed on the wrong patient, the incorrect procedure carried out on the correct patient, erroneous activity administered to a patient for a specific procedure, unwarranted exposure of radioisotopes to pregnant or lactating female patients, incorrect or failed image acquisition or processing necessitating repeat studies, improper use of shielding or personal protective equipment, loss of a sealed radioactive source, and radioactive contamination of the environment.
Errors leading to mis-administration include instances where the wrong single-dose syringe or multidose vial is selected, the contents of vials or syringes are inaccurately labeled, confusion arises regarding units of measured activity (e.g., MBq or mCi), and the risk of mistaken patient identity increases, particularly when patients share similar names. In the context of diagnostic procedures, the low radiation dose resulting from mis-administration is unlikely to cause injury or complications to patients or staff. However, studies in this field have illuminated the potential for staff members to receive significant radiation doses to their hands while preparing and injecting radiopharmaceuticals, doses that may indeed lead to adverse effects [5].
The healthcare industry, cognizant of the devastating impact of preventable medical errors, is incessantly striving to enhance safety for both patients and healthcare workers, seeking avenues to eradicate medical errors wherever possible. The essential tenet in this endeavor is to substitute manual methods with state-of-the-art robotic or automated technologies that minimize human errors [6]. In today’s cutthroat healthcare arena, where hospitals are relentlessly dedicated to minimizing expenses and optimizing efficiency, the strategic automation of traditionally manual processes emerges as a paramount facet of performance enhancement strategies [7].
As the healthcare sector persistently seeks ways to curtail expenses and augment operational efficiency, the strategic integration of automation into formerly manual workflows assumes unprecedented importance. This trend underscores the fundamental realization that incorporating automated systems represents an invaluable asset in achieving heightened efficiency and cost-effectiveness to flourish in the dynamic healthcare milieu [4].
Looking ahead, the trajectory of automation portends its integration into every specialized domain within healthcare. This forward-looking shift anticipates a future where automation serves as a linchpin in diminishing labor costs, augmenting diagnostic precision, mitigating human errors in treatment procedures, and fostering a safer working environment for medical staff. The promise of automation extends beyond enhancing patient care; it holds the potential to revolutionize the entire healthcare industry landscape by harmonizing cutting-edge technology with the quest for optimal care delivery [8].
In the realm of nuclear medicine, where the ramifications of human errors resonate more profoundly than in typical medical disciplines, the imperative for automation becomes all the more pronounced. Automated drug dispensing system (ADDS) transcends conventional manual techniques by prioritizing safety through the automated preparation, loading, and dispensing of radiopharmaceutical agents in sterile IV syringes [9].
Research endeavors undertaken to evaluate the efficacy of ADDSs in enhancing safety have yielded invaluable insights. A study conducted at the University Hospital UZ Brussels, Belgium, honed its focus on assessing the exposure of nuclear medicine department staff and technicians to the radioisotope 18FDG. The investigation juxtaposed the levels of extremity exposure experienced by staff members during the manual techniques of loading and dispensing with the exposure levels, ensuring the adoption of an automated drug dispensing and injecting system [10].
The results of this comparative analysis stand as a testament to the significance of automation. The study’s findings unequivocally demonstrate that the implementation of an ADDS precipitates a substantial reduction in extremity doses, which in turn, significantly diminishes the risk of skin contamination among staff members. Remarkably, the reduction surpasses a staggering 95%. This marked reduction in extremity exposure, a direct corollary of automated drug dispensing technology, underscores the system’s efficacy in substantially mitigating radiation exposure risks for nuclear medicine staff and technicians.
In lieu of the manual preparation and loading of radioisotopes, the application of robotic technology through ADDS serves a dual purpose: shielding nuclear medicine personnel from potential exposure to radioactive elements while ensuring patients receive precise and calibrated dosages. ADDS implementation guarantees the maintenance of dosage accuracy within the nuclear medicine domain and permits dose customization based on immediate requirements, eliminating the need for pre-prepared dosages. This approach effectively curtails the decay of nuclear elements and preserves the quality of administered dosages [11].
This research study centers on enhancing technology and safety measures for patients and staff involved in radiopharmaceutical handling. This objective is achieved by introducing an ADDS within the nuclear medicine department of a tertiary care hospital. Throughout the study, a comprehensive assessment is conducted to gauge the financial and operational feasibility of the new ADDS system within a selected tertiary care environment.[12]
Integrating advanced technologies into healthcare settings has garnered considerable attention, driven by the overarching goal of enhancing precision, efficiency, and patient care. ADDS has emerged as a standout innovation in medication management in clinical settings. Recent years have witnessed a plethora of studies exploring the integration of ADDS into various medical departments, including the sphere of nuclear medicine, where the precise administration of radiopharmaceuticals assumes paramount importance.[13]
Within the domain of nuclear medicine, the administration of radiopharmaceuticals mandates exacting precision to ensure patient safety and an accurate diagnosis. Existing literature underscores the potential advantages of ADDS in this regard. Notable manufacturing companies such as RESCUEDOSE, OMNICELL, and TEMA SINERGIE have meticulously developed ADDS solutions to streamline medication administration. These systems are engineered to reduce human errors, enhance workflow efficiency, and minimize the risks inherently associated with manual drug dispensing.
This study conducted within the Nuclear Medicine Department of a tertiary care teaching hospital aligns with the growing body of literature emphasizing technology’s integration to augment patient care. Similar research endeavors have been undertaken in diverse medical settings, consistently highlighting the positive impact of ADDS on medication administration accuracy, the reduction of adverse events, and the holistic optimization of processes.
Furthermore, the focus on staff acceptability is a pivotal determinant of the long-term effectiveness of any technological intervention. Previous research within healthcare settings underscores the significance of factors influencing staff members’ perception and adoption of new technologies. These factors encompass the adequacy of training, ease of use, perceived benefits, and concerns regarding potential disruption to established workflows.
Within the ambit of nuclear medicine, a domain relatively underexplored in this context, the present study contributes valuably by examining the feasibility, acceptability, and potential challenges associated with ADDS implementation in the Nuclear Medicine Department of a tertiary care teaching hospital.
The landscape of healthcare brims with an ever-mounting emphasis on technology-driven enhancements, particularly in the realm of medication administration. The current study’s deliberate focus on ADDS implementation within the Nuclear Medicine Department aligns seamlessly with the broader trend of harnessing automation to enrich patient care. In addition, the meticulous examination of staff members’ acceptance and concerns provides profound insights into the practical viability of such implementations. By shedding light on the application of ADDS in a specific medical context, this study effectively bridges a critical gap in the existing literature, thus contributing substantively to the body of knowledge to elevate healthcare processes through technological advancements.
The central thrust of this study revolves around examining the costs intertwined with implementing an ADDS, which spans both operational and administrative expenses. Simultaneously, the research delves deeply into the feasibility and degree of acceptance of the ADDS among the staff of the nuclear medicine department.
METHODOLOGY
Study design and setting
We conducted a cross-sectional study within the Nuclear Medicine Department of a tertiary care teaching hospital over a period of six months. Data were collected from staff members employed in the Nuclear Medicine Department and manufacturing companies specializing in ADDSs, namely RESCUEDOSE, OMNICELL, and TEMA SINERGIE.
Participants
Inclusion Criteria: The study included staff members actively working in the Nuclear Medicine Department of the selected hospital.
Exclusion Criteria: Staff members who expressed unwillingness to participate in the study or were unavailable during data collection for any other reason were excluded.
Data collection
The study comprised two distinct sections designed to fulfil our objectives.
Assessment of expenses and cost-effectiveness
In the initial section, we comprehensively analysed the expenses associated with implementing the ADDS. We conducted a market search and consulted biomedical engineers to select international manufacturing companies. We collected detailed information through email exchanges and Skype interviews, including total costs, specifications, maintenance expenditures, and product availability in the Indian market. Subsequently, data obtained from the sales managers of the selected three companies underwent a rigorous comparative analysis, highlighting discrepancies in cost aspects and specifications.
This section’s subsequent focus was to assess each model’s financial viability. We calculated the payback period for each of the three company models to determine the investment recovery rate, aiding in selecting the model exhibiting the fastest recuperation of the initial investment.
After a thorough evaluation of the cost-specification comparison and in-depth payback period calculations, we made a conclusive determination to select the most optimal manufacturing company. Our recommendation for a suitable ADDS for the Nuclear Medicine Department of the chosen tertiary care hospital was based on extensive market research, including websites, articles, and input from biomedical engineers.
To gauge the financial viability of the chosen model, a meticulous calculation of the breakeven point was conducted. This involved utilizing the extensive financial data collected from a tertiary care teaching hospital’s finance and Nuclear Medicine Department.
Manufacturing companies of ADDS
In the current international market, three well-known companies manufacture the best-in-class ADDS, especially for handling hazardous radioisotopes.
1. RESCUEDOSE.co, Haifa, Israel.
2. OMNICELL, Florida, United States of America.
3. TEMA SINERGIE, Malpighi, Faenza RA, Italy.
Data gathering from manufacturing companies
We obtained details regarding cost, specifications, user experience, maintenance, and quality certifications through correspondence with sales managers and representatives of each company via email, Skype interviews, and comprehensive web searches.
Dissemination and acceptability assessment
The second section of the study involved disseminating the data acquired from the manufacturing companies to both the Nuclear Medicine Department and the administrative sectors of the chosen hospital.
This phase included identifying the necessity and assessing the acceptability of the newly introduced ADDS. We adopted a comprehensive approach, using observations and in-depth interviews conducted via a structured questionnaire. This survey encompassed all staff members and trainees actively engaged within the Nuclear Medicine department. Ethical clearance for the study was obtained from the Institutional Ethical Committee of KMC & KH with approval number IEC 246/2017. Dated 26/04/2017.
RESULTS
Comparison of ADDS models
Several vital technical specifications and features were considered in evaluating three ADDS models—RESCUEDOSE, OMNICELL, and TEMA SINERGIE (Table 1). RESCUEDOSE demonstrated excellence in handling various compounding capabilities, including transfers, dilutions (including QS), and reconstitutions, with high-end accuracy. OMNICELL focused primarily on dilutions and reconstitutions and offered fully programmable dosing by drug, while TEMA SINERGIE specialized in direct dilution and reconstitution.
All three systems shared a minimum dose requirement of 0.5 ml, ensuring precision in medication preparation. RESCUEDOSE utilized gravimetric controls, providing exceptional accuracy. OMNICELL offered full drug programmability, while TEMA SINERGIE’s dose accuracy was not specified. RESCUEDOSE boasted a yield greater than 95%, OMNICELL exceeded 90%, and TEMA SINERGIE did not provide information on yield.
Regarding sterility, RESCUEDOSE complied with ISO 14644-1 Class 3, OMNICELL with Class 5, and TEMA SINERGIE with Class 7 cleanroom standards. RESCUEDOSE featured a negative pressure ISO 3 airflow loading chamber with no recirculation. OMNICELL employed a positive pressure compounding chamber with no recirculation, while TEMA SINERGIE utilized a positive pressure balanced chamber without recirculation.
RESCUEDOSE offered the highest throughput, with the ability to process up to 40 preparations per hour, making it suitable for high-volume compounding needs. OMNICELL and TEMA SINERGIE both offered up to 35 preparations per hour, catering to medium- to high-volume requirements. In terms of size and weight, RESCUEDOSE was the lightest at 660 kg with the dimensions 1.01 × 1.25 × 1.42 m, followed by OMNICELL at 850 kg with the dimensions 1.71 × 1.13 × 2.32 m, and TEMA SINERGIE at 1,100 kg with the dimensions 1 × 1.53 × 1.96 m. These differences in size could impact the space requirements within a healthcare facility.
Regarding power supply and networking, RESCUEDOSE operated on 220 VAC/15 A power and utilized RJ45 Ethernet for networking, while OMNICELL used 110 VAC/30 A (America) or 110 VAC/50 A (North America) power, also employing RJ45 Ethernet. TEMA SINERGIE operated on 110 VAC/50 A (North America) power and used RJ45 Ethernet for networking.
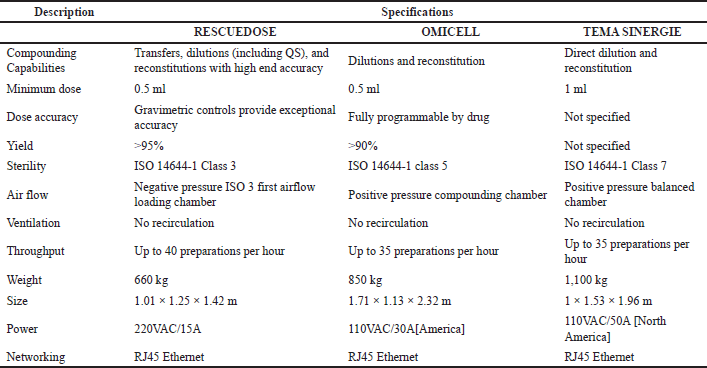 | Table 1. From the detailed comparison of three ADDS models from three companies based on common technical specifications and other features found out that each model of ADDS have different specifications. [Click here to view] |
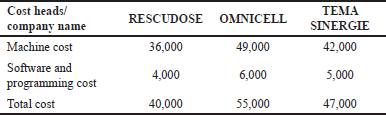 | Table 2. Cost comparison. [Click here to view] |
Cost comparison
In a detailed cost comparison (Table 2), RESCUEDOSE emerged as the most budget-friendly option, costing $40,000, making it an excellent choice for smaller healthcare organizations, or those looking to minimize upfront expenses. OMNICELL, offering advanced features, came at a higher total cost of $55,000, making it suitable for facilities with more extensive needs and resources. TEMA SINERGIE, striking a balance between cost and functionality, presented a competitive option with a total cost of $47,000, making it a competitive choice for organizations seeking both affordability and quality.
Payback period analysis
To assess the financial feasibility of each machine, the payback period was calculated using the formula mentioned above. RESCUEDOSE had the shortest payback period at 1.46 years, indicating a rapid return on investment. OMNICELL’s payback period was slightly longer at 1.92 years, justifiable for facilities seeking advanced features. TEMA SINERGIE presented a balanced payback period of 1.6 years, making it a competitive choice for those seeking affordability and quality.
Based on these findings, RESCUEDOSE was selected as the preferred model due to its low cost, fast return on investment, high-end specifications, and suitability for the hospital’s requirements. It also has certifications under the Asian Regional Cooperative Council for Nuclear Medicine (ARCCNM), safety clearance from the International Atomic Energy Agency (IAEA), and is a certified manufacturer of robotic drug dispensing systems for handling radiopharmaceuticals in the Asian region. CERMET Certification for quality assurance, easy availability, and transportation to India Compared to other vendors.
Additional features and maintenance
RESCUEDOSE’s partnership with US-based ec2 Software Solutions to integrate its robotics with ec2’s Bio Dose medical software boost aims to produce a system that automates the entire nuclear medication-dispensing process, from calculating doses to administering the drug. RESCUEDOSE’s management software is user-friendly and easily integrated with existing medical IT infrastructure. It offers remote dispensing capabilities, enhancing its utility. The total cost of RESCUEDOSE, including all components, was approximately $42,716.14 (Table 3).
A dedicated field engineering team supports maintenance for RESCUEDOSE. Daily, weekly, and monthly cleaning protocols are recommended, similar to primary engineering controls. The machine has a 3-year warranty, with additional comprehensive maintenance contract (CMC) charges applicable beyond the initial warranty period. CMC charges apply during the 4th, 5th, and 6th years as 3.5%, 4.5%, and 5.5% of the equipment’s original cost, ranging from $1,500 to $2,500. The maintenance protocol of RESCUEDOSE is mentioned in Table 4, where P refers to performed.
The breakeven point for RESCUEDOSE was calculated to be 1,668 procedures per month or 55 procedures per day, indicating the volume required to achieve breakeven within the machine’s payback period of 1.46 years.
 | Table 3. Details of the cost of RESCUEDOSE ADDS (according to Israel value added taxes authority 8% tax for exporting goods). [Click here to view] |
 | Table 4. Maintenance protocol of RESCUEDOSE. [Click here to view] |
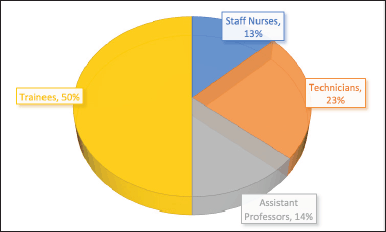 | Figure 1. Number of staff handling dangerous radioisotopes in department. [Click here to view] |
Figure 1 illustrates the number of staff members handling dangerous radioisotopes in the department, indicating the need for an ADDS to enhance safety. Figure 2 shows the common radioisotopes used in the Nuclear Medicine Department, with Tc-99 being the most commonly employed for nuclear imaging procedures.
Staff perceptions
In assessing staff perceptions gathered via the interview, it was found that a significant portion of staff (59%) favored the hospital’s implementation of the new ADDS in the department. Only 36% expressed concerns about potential financial constraints, while 5% did not offer a distinct opinion.
Most staff (50%) working in the nuclear medicine department had less than three years of experience, while only 22% had more than ten years of experience handling radioisotopes. All the staff (100%) showed the need for an ADDS to handle dangerous isotopes in the nuclear medicine department, with 86% acceptance of the implantation of a new ADDS.
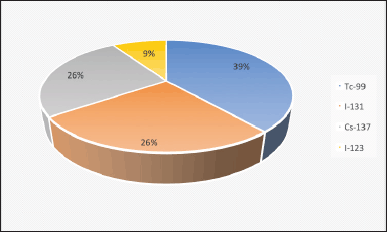 | Figure 2. Commonly using radioisotopes in the department. [Click here to view] |
DISCUSSION
In this section, we delve into the results and implications of our feasibility analysis for implementing an ADDS in the nuclear medicine department of a resource-limited country. Our evaluation focused on three distinct ADDS models: RESCUDOSE, OMNICELL, and TEMA SINERGIE.
Compounding capabilities and specifications
Our analysis revealed that RESCUDOSE excels in compounding capabilities, dose accuracy, and sterility compared to OMNICELL and TEMA SINERGIE. The high precision and reliability of RESCUDOSE make it a favorable choice for ensuring the accurate administration of radiopharmaceuticals in nuclear medicine. The significance of dose accuracy and sterility cannot be overstated in a medical context, particularly in radiopharmaceutical applications where precision is paramount.
Throughput and efficiency
Throughput emerged as a critical factor in our evaluation. It directly impacts the operational efficiency of the nuclear medicine department. Our data clearly showed that RESCUDOSE outperforms the other models in this regard, with a capacity of 40 preparations per day, while OMNICELL and TEMA SINERGIE lag at 30 preparations per day. This higher throughput of RESCUDOSE translates to faster and more efficient medication preparation, which can be vital in critical patient care situations.
Machine specifications and performance
We also considered factors such as the weight of the machine, its physical size, power requirements, and networking capabilities. Collaborating with biomedical engineers, we reached a conclusive comparison of specifications and performance. Based on these categories, our analysis identified RESCUDOSE as the most feasible and best-suited model for our nuclear medicine department. Its compact size, lower power requirements, and efficient networking capabilities align well with the department’s needs and limitations.
Financial implications
Turning our attention to the financial aspects, we drew insights from previous studies conducted in different healthcare settings. In Spain, a comprehensive cost-benefit analysis encompassed factors such as capital investment, staff costs, inventory expenses, and drug use policies. The results of this study indicated that expenses associated with implementing ADDSs were offset by significant cost savings, mainly driven by drug use policies. This underscores the potential financial benefits of adopting automated systems in critical care and emergency units.
A parallel study in France highlighted the economic implications of implementing ADDSs in intensive care units. It substantially reduced nursing staff time spent on medication-related tasks, allowing for more patient-centered interactions. These findings emphasize the transformative potential of automated systems in healthcare environments, improving operational efficiency and patient care quality.
Enhancing medication safety
Another French study focused on the impact of automated drug distribution systems on medication error rates within a geriatric unit. After implementing an automated system, it revealed a 53% reduction in medication administration errors. Reducing errors is particularly crucial for elderly patients who require precise and accurate medication management.
In the UK, research explored strategies for shielding hands during the preparation and injection of radiopharmaceuticals. Syringe shields significantly reduced finger doses and lowered dose rate peaks. These findings underscore the importance of technology and protective measures in enhancing safety and accuracy within healthcare practices.
Radiation safety and efficiency
Studies conducted in Belgium and the UK examined the impact of automated dispensing and injecting systems during positron emission tomography (PET) procedures in nuclear medicine. These studies revealed substantial reductions in whole-body and extremity doses for staff, highlighting automation’s efficiency and safety benefits in radiopharmaceutical handling.
Radiation safety in nuclear imaging
A review article from India stressed the importance of radiation safety in nuclear imaging and radionuclide therapy. It called for heightened awareness and meticulous implementation of safety measures, emphasizing the need to keep radiation exposure levels as low as reasonably achievable for all stakeholders.
Efficiency in radiopharmaceutical handling
Research from India focused on time-reducing exposure containment during 18-FDG master vial dispensing. The study introduced an improved method for dispensing FDG 18, reducing procedure time and limiting radiation exposure to technologists. These findings highlight the significance of adopting improved techniques for handling radioactive materials to enhance radiation safety practices.
User acceptance and recommendations
We assessed the acceptability of new ADDSs among nuclear medicine staff through interviews and questionnaires. Most staff expressed a positive attitude and willingness to adopt this technology, indicating the importance of staff acceptance for successful integration.
Recommendations for implementation
Our research project has identified RESCUDOSE as a financially and operationally feasible ADDS model for our nuclear medicine department. To proceed with implementation, we recommend conducting a comprehensive market and technical feasibility study to ensure alignment with market demands and hospital capabilities.
Strategic marketing efforts, including informative brochures and pamphlets, can generate interest in automated technology among the public, attracting individuals seeking nuclear medicine procedures and services.
We also stress the importance of regular awareness sessions on radioactive safety and occupational hazards for hospital staff to enhance staff well-being and foster a safety culture.
Finally, considering the potential for enhanced efficiency and precision, we propose that implementing ADDS can significantly benefit our nuclear medicine department, especially with the impending introduction of PET scan services.
Limitations and future directions
While our study demonstrates the feasibility of implementing ADDS in our department, we acknowledge that its findings are limited to our specific context. Future research should explore the broader applicability of ADDS in different hospital types and settings.
In the ever-evolving healthcare landscape, automation emerges as a strategic step toward a safer, more efficient, and more patient-centric future. ADDSs have the potential to revolutionize healthcare practices by enhancing safety, minimizing errors, and optimizing resource utilization. By embracing automation, healthcare institutions can provide more accurate diagnoses and safer treatments and elevate the quality of patient care, aligning with the evolving demands of modern healthcare.
CONCLUSION
Scientific evidence strongly supports the multifaceted nature of selecting a pharmaceutical compounding system in healthcare facilities. Carefully evaluating unique needs, budget constraints, and the available scientific literature is essential for making informed decisions. Minimizing upfront expenses with systems like RESCUEDOSE remains a viable strategy that does not compromise medication safety. Simultaneously, advanced features and customization, as OMNICELL exemplifies, are crucial in high-complexity healthcare settings.
A balanced approach, as seen with TEMA SINERGIE, aligns with scientific consensus by considering both cost and functionality, optimizing resource allocation for pharmaceutical compounding while ensuring medication precision and patient safety. Each of these pharmaceutical compounding systems boasts its own strengths and areas of specialization. RESCUEDOSE excels in accuracy and throughput, making it suitable for high-demand settings. OMNICELL offers precise dosing and flexibility in drug programmability. Meanwhile, TEMA SINERGIE, though providing direct dilution and reconstitution, may lack certain detailed specifications.
When choosing among these systems, healthcare facilities must carefully evaluate their specific compounding needs, space constraints, and budgetary considerations. The choice ultimately hinges on whether precision, sterility, or throughput are the highest priorities within a particular pharmacy or hospital setting.
A cost comparison among RESCUEDOSE, OMNICELL, and TEMA SINERGIE illustrates that each system has a unique pricing structure. RESCUEDOSE stands out as an affordable choice, thus accessible to many healthcare facilities. OMNICELL, though relatively pricier, may offer advanced programming and features that justify the investment for some organizations. Meanwhile, TEMA SINERGIE positions itself as a cost-effective solution with competitive pricing, making it a compelling option for those seeking a balance between cost and functionality.
The selection of a pharmaceutical compounding system in healthcare facilities should be an informed decision driven by carefully considering budgetary constraints, specific needs, and the desired level of automation, precision, and integration with existing systems. The scientific evidence underscores the importance of this multifaceted approach in enhancing patient safety, medication accuracy, and operational efficiency in the healthcare setting.
ACKNOWLEDGEMENT
The authors thank the Manipal Academy of Higher Education for their support throughout the study.
AUTHOR CONTRIBUTIONS
All authors of this manuscript have made substantial contributions to the research process. They collectively participated in the conception and design of the study, acquisition of data, and the analysis and interpretation of the results. Each author actively contributed to drafting the article and revising it critically for important intellectual content. Furthermore, all authors have agreed to submit this work to the current journal, provided their final approval for the version to be published, and have committed to being accountable for all aspects of the work. This authorship conforms to the requirements and guidelines set forth by the International Committee of Medical Journal Editors (ICMJE). Each author has met the criteria for authorship as outlined by the ICMJE, ensuring that they have played a significant role in the development and completion of this research.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest related to the publication.
ETHICAL APPROVALS
The Ethical clearance for the study was obtained from the Institutional Ethical Committee of KMC & KH with approval number IEC 246/2017. Dated 26/04/2017.
DATA AVAILABILITY
All data generated and analyzed during the course of this research are included in this article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Cherry Simon R, Sorenson James A, Phelps Michael E. Physics in nuclear medicine. Amsterdam, The Netherlands: Elsevier; 2012.
2. Radioisotopes in medicine | Nuclear Medicine—World Nuclear Association. [cited 2023 Dec 4]. Available from: https://world-nuclear.org/information-library/non-power-nuclear-applications/radioisotopes-research/radioisotopes-in-medicine.aspx
3. Mas JC, Johnson SG. A patient’s guide to nuclear medicine pocedures: English–Spanish. J Nucl Med Technol. 2008;36(3):169–169. CrossRef
4. Covens P, Berus D, Vanhavere F, Caveliers V. The introduction of automated dispensing and injection during PET procedures: a step in the optimisation of extremity doses and whole-body doses of nuclear medicine staff. Radiat Prot Dosimetry. 2010;140(3):250–8. CrossRef
5. Whitby M, Martin CJ. Investigation using an advanced extremity gamma instrumentation system of options for shielding the hand during the preparation and injection of radiopharmaceuticals. J Radiol Prot. 2003;23(1):79. CrossRef
6. RescueDose Ltd. [cited 2023 Dec 4]. Available at: https://rescuedose.co/#
7. Andrés JLP, Gómez CG, Sansalvador MH, Walsh AV. Cost-benefit analysis of the implementation of automated drug-dispensing systems in critical care and emergency units. Farm Hosp. 2003;27(1):4–11.
8. Kheniene F, Bedouch P, Durand M, Marie F, Brudieu E, Tourlonnias MM, et al. Economic impact of an automated dispensing system in an intensive care unit. Ann Fr Anesth Reanim. 2008;27(3):208–15. CrossRef
9. Cousein E, Mareville J, Lerooy A, Caillau A, Labreuche J, Dambre D, et al. Effect of automated drug distribution systems on medication error rates in a short-stay geriatric unit. J Eval Clin Pract. 2014;20(5):678–84. CrossRef
10. Tandon P, Prakash D, Kheruka SC, Bhat NN. Radiation safety guide for nuclear medicine professionals. Berlin, Germany: Springer Nature; 2022.
11. Rao VVSP, Manthri R, Hemalatha P, Kumar VN, Azhar M. Time reducing exposure containing 18 fluorine flourodeoxyglucose master vial dispensing in hot Lab: omega technique. Indian J Nucl Med. 2016;31(2):158. CrossRef
12. Chapuis C, Roustit M, Bal G, Schwebel C, Pansu P, David-Tchouda S, et al. Automated drug dispensing system reduces medication errors in an intensive care setting. Crit Care Med. 2010;38(12):2275–81. CrossRef
13. Mather L, Molyneaux H, Lebouthillier A, Guerrette-Daigle L, Robichaud S, Leblanc-Cormier G, et al. Factors affecting nurses’ attitudes toward an automated dispensing unit: a cross sectional quantitative study. Can J Nurs Informatics. 2012;7:2012.