INTRODUCTION
CA medications are widely used in the treatment of infections caused by bacteria resistant to wide-spectrum β-lactam antibiotics; some of them are included in the World Health Organization priority list of infectious diseases [1,2]. CA is a soft β-lactam antibiotic with a strong inhibitory effect on β-lactamase enzymes and it is produced by the filamentous Gram-positive bacterium Streptomyces clavuligerus as a secondary metabolite; the complete pathway (known as the clavam pathway) derives from the condensation of arginine and glyceraldehyde-3-phosphate, as anabolic precursors [2,3]. Investigations regarding the performance of CA production processes are focused on the two main restrictions for achieving high product concentrations: (i) the cultivation process and the metabolic bottlenecks and (ii) downstream processing [2]. Although the metabolic restrictions to reach high CA titers are the first difficulty in the CA production process, downstream processing is compromised by the degradation of the CA molecule in aqueous media, presumably by following a hydrolytic reaction mechanism catalyzed by both, acid and alkaline species [4,5].
The selection of separation processes is based on the physicochemical properties of the product and cultivation broth, e.g., solubility, polarity, and dielectric constant, which allows for a good recovery performance of CA from the aqueous phase. In the case of CA, separation of the product of interest from cultivation broths could be carried out by solid–liquid separation operations, liquid–liquid extraction, and precipitation in the form of clavulanate [2,6,7]. Furthermore, the high costs associated with the separation/purification operations reveal the need for deeper studies regarding the feasible alternatives for CA recovery from cultivation broths. In this research, we re-evaluated the potential of two distinct operations for CA recovery: first, the adsorption of CA through the utilization of the anion exchange resin Amberlite IRA 400 within the recovery process, and second, the application of liquid–liquid extraction systems. Results showed that there is still room for further improvements in CA recovery using both, adsorption and extraction, as the most advantageous techniques.
MATERIALS AND METHODS
Strain, media, and culture conditions
The commercial strain S. clavuligerus ATCC 27064 was used in this study. Mycelium cultures were stored as 20% (v/v) glycerol stocks at −80ºC. S. clavuligerus ATCC 27064 was cultivated in baffled shake flasks in defined glycerol-sucrose-proline-aspartate (GSPA) and complex isolate soy protein (ISP) media as described by Pinilla et al. and Gómez-Rios et al. [4,8,9]. The cultivation supernatants containing CA were separated and the biomass was discarded. For the separation and purification assays, the concentration of CA in supernatants was adjusted after high performance liquid chromatography (HPLC) analysis, either by adding potassium clavulanate or by dilution. Central composite rotational experimental designs (CCRDs) were performed having CA recovery as the response variable.
For CA production, either in complex ISP or defined GSPA media were inoculated with a pre-culture medium at 10% (v/v), as needed. All S. clavuligerus cultures were performed in 250-baffled Erlenmeyer flasks containing 50 ml of medium. Cultures for CA production were incubated for 144 hours, at 220 rpm and 28ºC and all experiments were performed in triplicate. CA quantification was performed in 300 μl samples by HPLC (Agilent 1200, Agilent Technologies, Waldbrom, Germany) equipped with a Diode Array Detector at 312 nm, using a reverse-phase ZORBAX Eclipse XDB-C18 (4.6 × 150 mm, 18 μm Agilent Technologies, Palo Alto, CA, USA) column; 94% (v/v) KH2PO4 (50 mM, pH 3,2); and a 6% (v/v) methanol solution were used as mobile phase at 0.7 ml/minute. CA was imidazole-derivatized at a ratio of 1:3; the reaction was kept at 28°C for 15 minutes [4].
CA separation by liquid–liquid extraction
The extraction of CA from the cultivation broth of S. clavuligerus cultures was carried out at a laboratory scale; for this, two experimental designs were developed to find the best process conditions. The procedure is summarized in Figure 1a. At the end of the culture, biomass was separated and discarded, then extraction was performed with an organic solvent to minimize the degradation probability since no hydrolytic mechanism is possible in organic media. Four organic solvents were initially screened for CA separation from aqueous media based on the chemical structure of CA and polarity; two of them had an ester functional group (ethyl acetate and butyl acetate); and two with ether functional groups (polyetal100® and PolyDiox®). Once, the best solvent for the extraction was selected, a first central composite rotatable experimental design (CCRD) was used to assess the effect of temperature (10°C–15°C) and pH (2.0–7.0) in single and double extraction operations.
A second CCRD was used for investigating the CA recovery by varying the CA initial concentration in the typical range obtained during submerged cultivations of S. clavuligerus, i.e., 100–900 mg/l [2,7]. The extraction aqueous/organic phase ratio varied between1:1 and 1:3 considering the limits of viability of the operation at industrial scale. Additional experiments were performed in the range of highest concentrations (500–1,000 mg/l), as those obtained typically in bioreactor operation and using lower solvent ratios. In addition, the effect of pretreatment with nonpolar solvent before CA extraction was also assessed as an alternative for elimination of possible interfering substances from the cultivation broth.
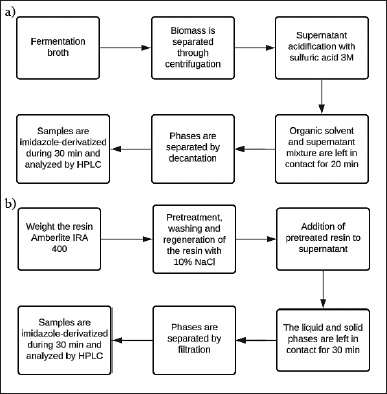 | Figure 1. Schematic representation of procedures performed for the CA separation. (a) Implementation of liquid–liquid extraction of CA from fermentation broths. (b) CA adsorption using ion exchange resin IRA 400. [Click here to view] |
CA separation by adsorption with anion exchange resin
For comparison between the two separation techniques, experiments, using the anion exchange resin IRA 400 for the adsorption process was considered. The process is summarized in Figure 1b. Similarly, the effect of temperature and pH on CA adsorption was studied. At the best condition of pH and temperature, the experimental design considered CA initial concentration in the cultivation broth, and solid–liquid ratio of adsorbent to supernatant. The selection of the contact time was based on the dynamics of adsorption obtained for CA samples at 100 mg/l. The desorption was evaluated after filtration and mixing with a NaCl solution of 10% (w/v) with a solid/liquid ratio of 45% [6,10,11]. The resulting mixture was incubated at 22°C and 240 rpm for 135 minutes. The resin in the solution was left in contact at 220 rpm and 10°C for 50 minutes. All experiments were performed in triplicate and a negative control was considered.
RESULTS AND DISCUSSION
Separation processes are complex and the selection of an appropriate approach depends on a thorough understanding of the properties of the molecules to be separated, operating conditions, environmental impact, and economics [12]. The complexity of separation processes is due to a number of factors that must be carefully considered. One of them is the nature of the molecules; since the physical and chemical properties of the molecules such as polarity, charge, and solubility are crucial characteristics in the process, as well as the instability of the molecules [13]. CA is sensitive to pH and temperature when dissolved in aqueous solution conditions, thus requiring gentle separation methods that allow to capture of the product from the fermentation broths to minimize the degradation by hydrolysis [5]. The recovery of CA from cultivation broths is limited by mass transfer phenomena, which can cause a product loss of up to 70% in techniques such as adsorption, liquid–liquid extraction, and two-step precipitation [5,14–16]. The high variability of CA yields reported by different authors suggest that recovery at different environmental conditions and methods is not fully understood [2]. The clinical importance of CA and the characteristic low yield of the bioprocess motivate the continuous study of process engineering aspects applied to S. clavuligerus cultivation and CA recovery.
Liquid–liquid extraction systems for CA recovery
Influence of operating variables on CA liquid extraction
Comparison of extraction performance with organic solvents reflected that ethyl acetate and butyl acetate given their polarity and dielectric constant favored the CA recovery, yielding 49.09% and 46.70%, respectively. The higher selectivity of ethyl acetate toward CA might be caused by the low solubility of this solvent in the aqueous phase during the extraction process. In addition, ethyl acetate has a lower boiling point than the other solvents used, which favors the precipitation of CA in the later stages of the process, at the time of recovery in its clavulanate salt form. Nonpolar or poorly soluble molecules, such as ethyl acetate, do not interact at all with the aqueous phase, allowing in the process that, instead of dissolving, they form separate layers when placed in an aqueous medium [17,18]. The ethers polyetal100 and PolyDiox rendered a poor CA extraction yield, this is in agreement with previous results obtained by Mancilha et al. [14] using ethyl acetate as a solvent for extraction.
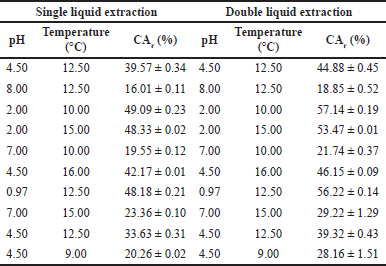
Table 1 shows the CA recovery for single and double extractions with ethyl acetate as the solvent with the highest affinity with CA. The values varied from 16.01% (pH: 8.0 and T: 12.5°C) to 49.09% (pH: 2.0 and T: 10°C). CA is more stable in acidic conditions, since less acid catalytic activity is presented in the degradation reaction [5,19]. It was observed that the acidic medium favored the extraction process while alkaline conditions decreased the CA recovery. Some authors have reported about CA instability under alkaline environmental conditions [10,20] and it becomes extremely unstable when the pH exceeds 8.0 [10,21].
As observed in Table 1, the best experimental conditions for CA liquid–liquid extraction were pH 2.0 and 10°C. At low pH, beta-lactam antibiotics, including CA, usually have a protonated carboxylate group causing its low solubility in water and facilitating the organic-solvent extraction [15,20,22]. CA is a weak acid with pKa equal to 2.5; only the undissociated acid can be extracted by an organic solvent. At higher pH values the carboxylate group is deprotonated and charged, rendering the CA water soluble (clavulanate form) [22]. The results obtained showed a better percentage of CA recovery at low temperatures; it was also observed that the CA degradation rate increased significantly with increasing temperature.
 | Table 1. Effect of pH and extraction temperature on CA recovery (CAr) from aqueous solutions by liquid extraction with ethyl acetate. [Click here to view] |
A second extraction with fresh solvent allowed to increase in the percentage of CA recovery from the cultivation broth. The values varied from 18.85% (pH: 8.00 and T: 12.50°C) to 57.14% (pH: 2.0 and T: 10°C). These results showed a 10% increase in the recovery of CA with respect to the process with a single extraction. However, the higher the volume of solvent used, the more diluted the CA solution would become, which increases the cost of the process at the moment of concentrating the organic phase for the subsequent precipitation processes.
Although higher stability of CA is observed under slightly acidic conditions and at low temperatures (10°C), at higher temperatures the partitioning of the system, favors the separation. This is observed at 16°C, 12.5°C, and 9°C at the same pH conditions (4.5), the recoveries of CA were 46.15%, 39.32%, and 28.16%, respectively. The extraction at temperatures below the room conditions would increase the energy demands, therefore higher temperatures and shorter process times would impact possitively the utilities requirement of the separation process. The required time for reaching the equilibrium during the extraction process was studied by sampling at regular intervals up to 70 minutes. After 10 minutes of contact between phases, the CA concentration reached the equilibrium point; undoubtedly, shorter extraction times favors CA yield since the degradation possibilities are reduced. Time is a determining factor due to the high CA instability.
Influence of CA initial concentration on its recovery from the culture medium
Using the best process conditions found, the effect of initial CA concentration (CA0) in the broth and the aqueous/organic phase ratio on CA recovery was studied. These factors were considered because they are directly related to the cost of the process and the size of the equipment. For scaling-up purposes, these parameters have the strongest influence on the bioprocess economy. The results are presented in Table 2.
 | Table 2. Effect of initial CA concentration (CAo) and solvent ratio on CA recovery (CAr) from aqueous solutions by liquid extraction with ethyl acetate. [Click here to view] |
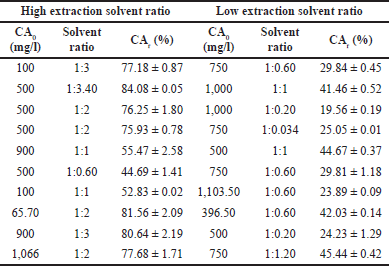
Although high solvent ratios (>2) allowed to extract up to 80% of the CA present in the cultivation broth, high solvent volumes could increase considerably the cost of the operation at industrial scale, as more energy would need to be implemented to remove the solvent and hence, to concentrate the recovered CA. In contrast, the use of low volumes of organic solvent (ratios 0.20–0.60) led to 40% less CA extracted from the broth (see Table 2), with respect to that achieved when using high volumes of organic solvent.
Interestingly, at the very low solvent ratio, the formation of micelles in an unstable emulsion of ethyl acetate in water at ratios below 0.1 favored a high CA extraction, leading to yields comparable to those observed when using the 0.6 ratio. The results suggest an unfavorable effect on the recovery percentage as the concentration of CA in the cultivation broth increases when maintaining the same aqueous/organic phase ratio, which is in agreement with the fact that higher solvent ratio would increase the extraction yield, but with a consequent high dilution. Extraction at an aqueous to the organic ratio of 1:0.6, as a feasible condition, led to recoveries of 42.03%, 29.81%, and 23.89% at 396.5, 750, and 1,103.50 mg/l of CA initial concentrations, respectively. The observed effect of CA concentration in decreasing the final recovery in the organic phase can be explained by the critical micellar concentration, which is the minimum point where micelles start to form in a liquid solution. In aqueous-organic extraction systems, the micelles formation is favored by low temperatures and low concentrations as shown by Martino et al. [23] for extraction of amino-acids from aqueous solutions.
Pretreatment with nonpolar solvent as a strategy for increasing CA extraction
The complex and defined media were used to analyze how the complex mixture of nutrients/metabolites could affect CA extraction. To decrease the interference of compounds other than CA in the extraction process, and given the polar nature of the antibiotic, a pre-treatment consisting of contact with a nonpolar solvent (petroleum ether) was evaluated (see Table 3). Since the extraction with a nonpolar solvent is a pre-treatment preceding the extraction with ethyl acetate, low organic solvent ratios were preferred (0.2–1.0).
 | Table 3. Percentage of CA recovered when a pretreatment strategy is applied to the cultivation broth. [Click here to view] |
The use of an equal aqueous to organic solvent ratio, Table 3, showed a favorable increase in the recovery of CA (~17%), and by decreasing the purification solvent to only 20% the yield of the purification process increased by 10%. It was observed that when the process considered a double extraction, using the pretreatment with the nonpolar organic solvent up to 60.37% of recovery can be obtained. Mancilha et al. [14] reported up to 35% CA recovery, also using ethyl acetate as a solvent for extraction, thus indicating the effectiveness of the strategies implemented in the process. Before the pretreatment with the nonpolar solvent, recovery percentages were 55.37% for the defined medium and 54.07% for the complex medium. Once the cultivation broth was exposed to the pretreatment, a recovery of 60.37% was attained for the complex medium, which contained fewer impurities, and therefore, higher selectivity for CA.
Nonpolar solvents, such as many hydrocarbons (e.g., benzene, hexane, and ethane), or organic solvents such as ether, do not exhibit miscibility with the aqueous phase (cultivation broths) due to differences in the intermolecular forces and polarities of the substances involved. As a result, nonpolar molecules tend to group together, giving rise to separate phases in the presence of water, which leads to the absence of miscibility between nonpolar solvents and aqueous media. However, some impurities, such as proteins and amino acids, are soluble in the organic phase, which enables significant transfer of these impurities to the petroleum ether. This transfer reduces interference when recovering the compound of interest (CA) in the cultivation broth, resulting in an increased yield in the extraction process.
Thus, the best experimental conditions for CA liquid–liquid extraction are pH 2.0 and 10°C. The results obtained showed a better percentage of CA recovery at low temperatures and high solvent ratios (>2) that allowed to extract up to 80% of the CA present in the cultivation broth. However, the high solvent volumes could increase considerably the cost of the operation at industrial scale, as more energy would need to be implemented to remove the solvent and hence, to concentrate the recovered CA. In contrast, the use of low volumes of organic solvent (ratios 0.20–0.60) led to 40% less CA extracted from the broth, with respect to that achieved when using high volumes of organic solvent.
 | Table 4. Effect of pH and extraction temperature on CA recovery (CAr) from aqueous solutions by using adsorption with Amberlite® IRA 400. [Click here to view] |
CA recovery from cultivation broths by anion exchange adsorption
Influence of operating variables on CA adsorption
In the present study, CA separation tests were carried out using the anion exchange resin Amberlite IRA 400, and the effect of operating variables such as pH, temperature, solid/liquid ratio, adsorption, and desorption kinetics, on CA separation from cultivation broths. The adsorption processes have received special attention in bioprocesses since biomolecules may be selectively adsorbed within a range of solid materials, allowing the exploitation of these properties in the extraction steps of the target molecule [24]. Adsorption processes strongly depend on the nature and surface area of the adsorbent, nature of adsorbate, temperature, contact time of adsorbent and adsorbate, nature of medium, and the pH of the adsorption medium [21]. For the case of CA extraction, temperature, and pH have been confirmed as the variables that have major incidence in the extraction yield.
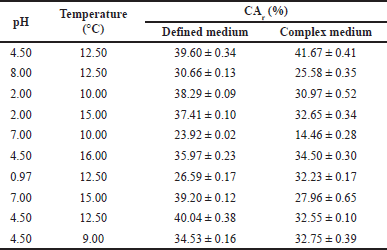
Table 4 shows the CA adsorption when varying pH and temperature in defined and complex media as the sole separation process. The CA recovery varied from 23.92% (pH: 7 and T: 10°C) to 40.04% (pH: 4.50 and T: 12.50°C) when the defined medium was used, and from 14.46% (pH: 7.0 and T: 10.0°C) to 41.67% (pH: 4.50 and T: 12.50°C) when using the complex medium. As observed and likewise the extraction process, the acidic conditions and low temperatures favored the adsorption process of CA. The results obtained in the defined cultivation broth were higher than those from the complex medium; this might be the result of interferences caused by amino acids and proteins present in the medium, which do not allow the CA molecules to effectively bind to the active sites of the anion exchange resin.
It has been hypothesized that compounds such as phosphate, sulfate, amino acids, small peptides, clavam metabolites, and cephamycin C might also attach to the resin. Yepes et al. [6] reported that amino acids such as tyrosine, histidine, proline, and lysine might also interfere with the adsorption processes. This interference limits the optimal recovery of CA, implying that the presence of these compounds could negatively affect the recovery efficiency in the process, generating a rapid saturation of all available sites for CA adsorption on the anion exchange resin.
It can be further observed (Table 4) that small variations in temperature and pH led to a significant increase in the adsorption capacity of the resin, as the temperature increases there is a decrease in the values of the effective diffusion coefficient [20]. This phenomenon might be caused by an increase in the frequency of molecular collisions between the CA ionic groups and the charged sites of the resin, as argued by Barboza et al. [25]. Recently, Yepes et al. [6] demonstrated that CA adsorption adjusts to the Langmuir model. The low values of the kinetic constant suggested a negative effect of temperature on CA adsorption as an exothermic process (?H° = −29.15 kJ/mol). This phenomenon has been explained by the increase in the frequency of molecular collisions between the CA ion groups and the loaded sites of the resin, resulting in a more pronounced desorption.
Several authors have reported the operational advantages of using Amberlite® IRA 400 resin as one of the most effective adsorbents in terms of capacity and speed of CA adsorption, compared to other materials such as zeolites, which require more time to reach equilibrium [10,11,20]. It is important to note that temperature does not affect the time required to reach dynamic equilibrium in the resin since this time depends on the concentrations of CA in the cultivation broth and its adsorption on the specific sites of the resin, as well as the maximum adsorption capacity, which for the case of CA, it is not affected by the temperature (Fig. 2) [11].
Figure 2 displays the dynamics of the adsorption process while approaching the equilibrium condition. As observed, 46.3% of CA was recovered during the first 10 minutes of the process, indicating the rapid saturation of the sites available for CA adsorption. The process generates a great ionic interaction force or binding force between the resin and the CA molecules, showing, therefore, that the maximum adsorption capacity was limited by the operational conditions. Finally, 61.3% of the CA was recovered from the cultivation broth after 50 minutes. According to Yepes et al. [6], the best capacity of IRA 400 resin for CA adsorption is 0.641 mgCA/gresin, with a maximum recovery at equilibrium corresponding to 55%. Similarly, Costa et al. [10] employed extractive cultivation using IRA 400 ion-exchange resin, obtaining a CA recovery of 78%; thus, increasing the cumulative CA concentration by 248% compared to the control without product removal. The increase in CA productivity when it is adsorbed from the cultivation broth supports the hypothesis that high CA concentration may inhibit antibiotic biosynthesis and justifies its application for extractive cultivation.
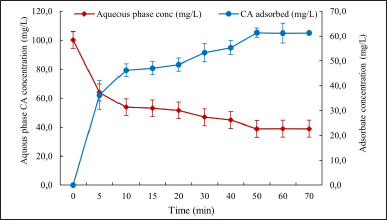 | Figure 2. Dynamics of the adsorption process while approaching the equilibrium condition. [Click here to view] |
Influence of CA initial concentration and solid/liquid ratio on the adsorption process
The limitation in the amount of resin in an adsorption process can be attributed to several fundamental reasons, one of which is that each adsorbent has a finite adsorption capacity, i.e., a maximum amount of adsorbed substance that it can retain on its surface under specific conditions. Once this capacity is reached, the resin can no longer adsorb any more substance and must be regenerated or replaced [26]. Alternatively, adsorption is a dynamic equilibrium process, meaning that there is a constant struggle between molecules that adsorb on the adsorbent surface and those that desorb from the surface and return to the liquid phase [21]. When the maximum adsorption capacity is reached, the equilibrium shifts toward desorption, and the amount adsorbed no longer increases. In addition, using an excessive amount of resin would be costly and inefficient, as it would require higher investments in equipment, more time for regeneration, and higher energy consumption for the process. Therefore, it is important to find a balance between the amount of resin used and the required adsorption capacity, in the particular case of CA, a high concentration could quickly occupy the active sites of the anion exchange resin implemented, which would generate a very low efficiency in the process by being able to recover all the CA molecules that are in the cultivation broth, resulting in a low yield of the process [21,27,28].
The adsorption was favored by increasing the adsorbent-to-liquid ratio, see Table 5. Thus, the highest separation was attained in the range of 40%45% solid/liquid ratio, adsorbing a mean of 47.70% of CA present in the broth. The CA concentration in the liquid does not significantly influence the mass of CA adsorbed since the available sites in the resin for the adsorption are the main factors associated with the separation. The resin is highly selective for CA, showing no significant difference between the adsorption capacity on defined and complex medium, i.e., complex medium components do not reduce the mass transfer driving force for CA adsorption.
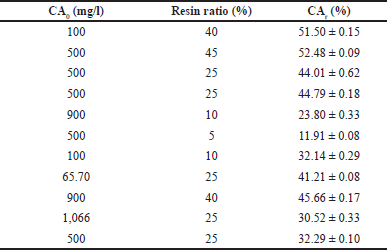 | Table 5. Effect of the concentration and solid/liquid ratio on adsorption percentages of CA using the anion exchange resin IRA 400. [Click here to view] |
Optimizing the amount of resin used according to the concentration of CA to be treated is important for an efficient design and operation of the separation process. In cases of very low CA concentrations, i.e., when a large amount of resin and a limited amount of CA are available (in very dilute solutions), there is a risk that the resin will not reach its full saturation capacity due to the lack of CA to occupy all the available active sites. This situation would result in an underutilization of the adsorption capacity of the resin, resulting in increased operating costs and resin regeneration processes. On the other hand, in reverse situations where high concentrations of CA are found in cultivation broths, and a limited amount of resin is available, rapid saturation of the resin could occur. In this case, the resin would reach its maximum adsorption capacity before having adsorbed a significant amount of the CA present in the solution. As a result, the resin would need to be replaced frequently, resulting in increased operating costs and technical complexity of the process.
Desorption process of CA from anion exchange resin
The desorption of CA retained from the anion exchange resin was carried out by elution (Fig. 3). The process involved the addition of 10% (w/v) of NaCl solution at 22°C and 240 rpm for a duration of 135 minutes [10,29,30].
Desorption kinetics depends on various factors, including resin type, CA concentration, NaCl concentration, and temperature. Figure 3 shows the CA desorption dynamics. As observed, most of the adsorbate was released during the first 15 minutes of elution, reaching its maximum concentration at 75 minutes, which is in agreement with the data already reported by Barboza et al. [11] and Costa et al. [10]. Larger times are required for desorption processes compared to that of adsorption, possibly due to the strong ionic interaction that is established between the resin and CA. This interaction overcomes the adhesive strength between the resin and the chloride ion, which comes from the NaCl solution used for elution in the desorption process [20]. The best thermal condition for adsorption (10°C) differs from the desorption temperature (22°C), since the dissociation of the resin-CA complex is actually an endothermal process, in contrast to the adsorption. Almeida et al. [31] reported that desorption follows Arrhenius dependence respect to temperature suggesting that increasing the agitation state of the molecules, allows the ionic CA molecules to be more easily displaced from the sites where they are located.
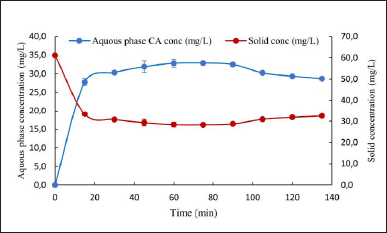 | Figure 3. Result of the desorption of CA obtained from S. clavuligerus cultures using complex cultivation broths. [Click here to view] |
As observed, there were some losses between the adsorption and desorption process, due to the fact that the adsorption agent (anion exchange resin IRA 400) presented some limitations both regarding the cultivation broth and in the saline solution, used at the moment of CA desorption. The presence of high concentrations of negatively charged ions (Cl-) in salt solutions can lead to ionic competition with the resin exchange sites. This can decrease desorption efficiency by interfering with the release of adsorbed CA [32]. Likewise, the high concentration of ions (Cl−) in the salt solution could accelerate the saturation of the active sites of Amberlite IRA 400, limiting its ability to completely desorb the adsorbed CA.
In addition to NaCl, there are other ionic solutions that can be used to desorb CA from the resin, such as sodium hydroxide or potassium chloride. It is essential to consider the specific requirements and limitations of the process, such as the stability of the CA under alkaline conditions, which precludes the use of hydroxides [33,34]. It is also necessary to consider the compatibility of the desorbing solution with downstream processes and the general purification requirements of the recovered CA.
The adsorption process was clearly favored by increasing the adsorbent-to-liquid ratio. Data has shown that the highest separation was attained in the range of 40%–45% solid/liquid ratio, adsorbing a mean of 47.70% of CA present in the broth. However, the adsorption process requires further studies, since the yields obtained were low due mainly to the desorption equilibrium, showing a considerable loss of CA when compared to the liquid–liquid extraction using ethyl acetate as solvent.
A nonlinear model for prediction of CA recovery by liquid extraction
Response surface methodology is a set of techniques used to model and analyze problems in which a variable of interest is influenced by at least two quantitative factors [35]. Among the strategies studied to achieve the highest recovery of CA from the cultivation broth, it was found that due to optimization in process time, the raw material to be used, and reduction of environmental pollution in a possible industrial scale process, liquid extraction was presented as one of the best alternatives to implement. The results obtained in the treatments performed for the extraction showed higher values for obtaining CA with respect to the recovery by the adsorption method, taking the latter into account and adding the complexity of the process, liquid extraction was chosen as the best alternative, and therefore, the optimization was carried out by means of the simulation tool. Therefore, a statistical analysis of the process was performed in the R software, to evaluate the effect of the variables CA concentration in the cultivation broth and aqueous/organic phase ratio, on the CA extraction yield.
As a predicted effect of the control variables on the response variable, the aqueous/organic phase ratio factor was expected to have a greater effect on CA extraction yield. The experimental runs were carried out in random order, so that the possible environmental and temporal effects were distributed equally, between treatments. Table 6 shows each of the estimated effects and interactions of the variables CA concentration in the cultivation broth and the aqueous/organic phase ratio on the percentage of CA recovery. Also shown is the standard error of each of the effects, which measures their sampling error.
From the analysis of variance, it was observed that all the effects were influential, given that their p-values were less than α = 0.05. It could be seen that both main effects were significant. Among the effects of double interactions, the most significant was contributed by that of concentration [35]. In the analysis of variance, Table 6, it was observed that the highest p-value is given by the double interaction between the controlled variables, with a value of 0.023, lower than the predefined significance value, which indicates that it is statistically significant and, therefore, all interactions should be taken into account. The accuracy of the prediction of the percentage of CA as a response variable was measured by the quality of the model fit and was determined by the adjusted coefficient of determination .
The value of adjusted by degrees of freedom was 0.922; thus, the model obtained explains 92% of the variability of the CA extraction percentage versus the factor’s concentration and liquid ratio between the aqueous/organic phase, and it is possible to implement it as a tool to predict the behavior of the CA extraction percentage. The liquid–liquid ratio between the aqueous/organic phase presented a strongly linear effect, and although its quadratic factor was significant, it constituted the largest effect of the model (Fig. 4). Concentration presented a more pronounced curvature effect, and in summary, the overall behavior of the effects was not linear, given that, the quadratic factors were significant. The fitted model is represented by the following equation:
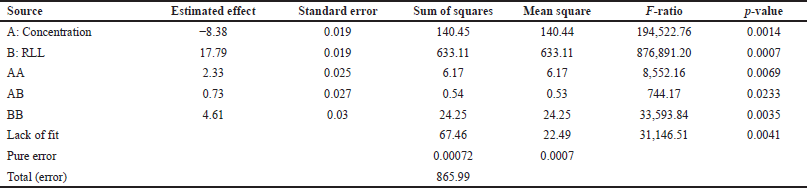 | Table 6. Analysis of effects and variance for percentage of CA recovery. [Click here to view] |
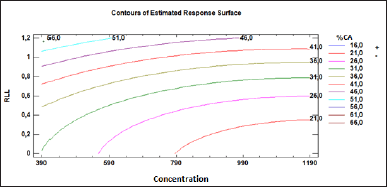 | Figure 4. Contours plot of estimated response surface. [Click here to view] |
where represents the percentage of CA extraction. and represent the controlled variables; concentration and liquid ratio between the aqueous/organic phase, respectively. It should be noted that for the above equation, the variables take their real values.
An analysis of the contour curves (Fig. 4) indicated that the region of the highest yield of CA is associated with the high liquid ratios between the aqueous/organic phases studied here. The fitted model of regression predicted the maximum CA extraction or the optimum point (54.53%) by using a liquid ratio of 1:1.20 and a concentration of 396.45 mg/l, so the predicted value was equal to the experimental value and confirms the validity of the obtained model.
CONCLUSION
This study aimed to evaluate the performance of different recovery alternatives for the separation of CA from supernatants of S. clavuligerus cultivations at the laboratory scale. To carry it out, the study evaluated the potential of two distinct operations for CA recovery: first, the adsorption of CA through the utilization of the anion exchange resin Amberlite IRA 400, and second, the application of liquid–liquid extraction systems, considered as controlled variables temperature, pH, aqueous phase/extraction agent or adsorbent ratio, and CA concentration in the cultivation broth; the CA recovery yield was taken as the response variable.
In summary, CA adsorption was enhanced by increasing the adsorbent-to-liquid ratio. Consequently, the most effective separation occurred within the 40%–45% solid/liquid ratio range, leading to the adsorption of an average of 47.70% of CA present in the broth. Despite this, the overall adsorption process fell short of being entirely satisfactory for CA recovery, as the yields obtained were relatively low, indicating a significant CA loss (approximately 25%) compared to liquid–liquid extraction using ethyl acetate (which achieved a 60.37% recovery for the complex medium). Based on the results, a potential contributing factor to this outcome could be the interferences resulting from impurities adhering to the active sites of the resin, leading to a reduction in adsorption capacity. The extraction strategy potentially allows up to 80% of product recovery. Meeting this condition would enable the retrieval of concentrated CA for subsequent precipitation and purification stages, thereby enhancing overall process efficiency and resulting in a clear reduction in both cost and environmental impact.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The authors acknowledge the financial support of Universidad de Antioquia (CODI). Grant number PRG2017-15946.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Gómez-Ríos D, Ramírez-Malule H. Bibliometric analysis of recent research on multidrug and antibiotics resistance (2017–2018). J Appl Pharm Sci. 2019;9(05):112–6. CrossRef
2. López-Agudelo VA, Gómez-Ríos D, Ramirez-Malule H. Clavulanic acid production by Streptomyces clavuligerus: insights from systems biology, strain engineering, and downstream processing. Antibiotics. 2021;10(1):84. CrossRef
3. Ramirez-Malule H, López-Agudelo VA, Gómez-Ríos D, Ochoa S, Ríos-Estepa R, Junne S, et al. TCA cycle and its relationship with Clavulanic acid production: a further interpretation by using a reduced genome-scale metabolic model of Streptomyces clavuligerus. Bioengineering. 2021;8(8):103. CrossRef
4. Gómez-Ríos D, Ramírez-Malule H, Neubauer P, Junne S, Ríos-Estepa R. Data of clavulanic acid and clavulanate-imidazole stability at low temperatures. Data Brief. 2019;23:103775. CrossRef
5. Gómez-Ríos D, Ramírez-Malule H, Neubauer P, Junne S, Ríos-Estepa R. Degradation kinetics of clavulanic acid in fermentation broths at low temperatures. Antibiotics. 2019;8(1):6. CrossRef
6. Yepes J. Mejoramiento de la producción de ácido clavulánico mediante el cultivo de Streptomyces clavuligerus en fermentación extractiva, usando biorreactores operados en lote alimentado. Tesis de magister. Medellín, Colombia: Universidad de Antioquia; 2020.
7. Ser HL, Law JW, Chaiyakunapruk N, Jacob SA, Palanisamy UD, Chan KG, et al. Fermentation conditions that affect clavulanic acid production in Streptomyces clavuligerus: a systematic review. Front Microbiol. 2016;7:522. CrossRef
8. Pinilla L, Toro LF, Avignone-Rossa C, Peñuela M, Rios-Estepa R. Streptomyces clavuligerus strain selection for clavulanic acid biosynthesis: a study based on culture composition effects and statistical analysis. Dyna. 2018;85(205):111–8. CrossRef
9. Gómez-Rios D, Ramírez-Malule H, Ochoa S, Ríos-Estepa R. Rational selection of culture medium for clavulanic acid production by Streptomyces Clavuligerus based on a metabolic modeling approach. Agric Nat Resour. 2022;56(2):267–76. CrossRef
10. Costa CL, Badino AC. Overproduction of clavulanic acid by extractive fermentation. Electron J Biotechnol. 2015;18:154–60. CrossRef
11. Barboza M, Almeida M, Hokka C. Influence of temperature on the kinetics of absorption and desorption of clavulanic acid by ionic exchange. Biochem Eng J. 2003;14:19–26. CrossRef
12. Abdo T, Kumar V. An overview of membrane science and technology. Nanomater Polym Membranes. 2016;1–23. CrossRef
13. Abreu DC, Figueiredo KC. Bromelain separation and purification processes from pineapple extract. Braz J Chem Eng. 2019;36(2):1029–39. CrossRef
14. Mancilha M, Guimaraes G, Nardi J, Oliveira J, Hirata D. Optimization of liquid—liquid extraction step for clavulanic acid from fermentation broth using solvent mixtures. Quim Nova. 2014;37(8):1335–41. CrossRef
15. Brites L, Oliveira J, Barboza M, Hokka C. Effect of physicochemical properties of solvents on clavulanic acid extraction from fermentation broth. Lat Am Appl Res. 2012;42(1):65–70.
16. Carvalho V, Brandão JF, Brandão R, Rangel-yagui CO, Couto JA, Converti A, et al. Stability of clavulanic acid under variable pH, ionic strength and temperature conditions. A new kinetic approach. Biochem Eng J. 2009;45:89–93. CrossRef
17. Raven PH, Johnson GB, Mason KA, Losos JB, Singer SR. The nature of molecules and properties of water. In Biology. 10th ed. New York, NY: McGraw-Hill; 2014. pp 17–30.
18. Reece JB, Urry LA, Cain ML, Wasserman SA, Minorsky PV, Jackson RB. Water and life. In Campbell biology. 10th edition. San Francisco, CA: Pearson; 2011. pp 44–54.
19. Bersanetti PA, Almeida RM, Barboza M, Araújo ML, Hokka CO. Kinetic studies on clavulanic acid degradation. Biochem Eng J. 2005;23(1):31–6. CrossRef
20. Mayer AF, Deckwer WD. Simultaneous production and decomposition of clavulanic acid during Streptomyces clavuligerus cultivations. Appl Microbiol Biotechnol. 1996;45(2):41–6. CrossRef
21. Forte MB, Luna EC, Pastore HO, Rodrigues MI, Filho FM. Evaluation of clavulanic acid adsorption in MgAl-layered double hydroxide: kinetic, equilibrium and thermodynamic studies. Adsorp Sci Technol. 2012; 30(1):65–80. CrossRef
22. Hirata DB, Oliveira JH, Leão KV, Rodrigues MI, Ferreira AG, Giulietti M, et al. Precipitation of clavulanic acid from fermentation broth with potassium 2-ethyl hexanoate salt. Sep Purif Technol. 2009;66(3):598–605. CrossRef
23. Martino M. Tensoactivos. CMC a temperaturas bajas o moderadas. Influencia sobre la CMC de la presencia de compuestos neutros o polares: alanina. Trabajo de grado, España: Universidad de la Laguna; 2020.
24. Luz DA, Rodrigues AK, Silva FR, Torres AE, Cavalcante CL, Brito ES, et al. Adsorptive separation of fructose and glucose from an agroindustrial waste of cashew industry. Bioresour Technol. 2008;99:2455–65. CrossRef
25. Barboza M, Almeida R, Hokka C. Kinetic studies of clavulanic acid recovery by ion exchange chromatography. Bioseparation. 2002;10(4–5):221–7. CrossRef
26. Seader JD, Henley J, Roper DK. Separation process principles, chemical and biochemical operations. New York, NY: John Wiley & Sons; 2011.
27. Barboza M, Silva C, Cuel M, Barreto V, Hokka CO. Separation of clavulanic acid from fermented broth of amino acids by an aqueous two-phase system and ion exchange adsorption. N Biotechnol. 2012;29(3):428–31. CrossRef
28. López VA, Gómez-Ríos D, Ramirez-Malule H. Clavulanic acid production by Streptomyces clavuligerus. Antibiotics. 2021;10(1):84. CrossRef
29. da Silva KC, Souza AT, Badino AC, Pedrolli DB, Cerri MO. Screening of medium constituents for clavulanic acid production by Streptomyces clavuligerus. Braz J Microbiol. 2018;49(4):832–9. CrossRef
30. Saudagar PS, Survase SA, Singhal RS. Clavulanic acid: a review. Biotechnol Adv. 2008;26:335–51. CrossRef
31. Almeida R, Barboza M, Hokka C. Continuous clavulanic acid adsorption process. Appl Biochem Biotechnol. 2003; 108(1–3):867–80. CrossRef
32. da Silva CS, Cuel MF, Barreto VO, Kwong WH, Hokka CO, Barboza M. Separation of clavulanic acid from fermented broth of amino acids by an aqueous two–phase system and ion–exchange adsorption. N Biotechnol. 2012;29(3):428–31. CrossRef
33. Scott MJ. Principles of ion exchange technology. Amsterdam, Netherlands: Elsevier Science; 2003.
34. Chen G, Liu Y, Cao Y. Recovery of chromate ions from industrial wastewater using ion exchange resins: a review. J Ind Eng Chem. 2018; 66:1–13.
35. Gutiérrez H, Salazar R. Elementos de inferencia estadística: experimentos con uno y dos tratamientos. In Análisis y Diseño de Experimentos. 2th ed. New York, NY: McGraw-Hill; 2008.