INTRODUCTION
Ticks are the most prevalent cattle ectoparasites worldwide, especially in tropical and subtropical areas [1]. In Egypt, Rhipicephalus annulatus is the most common cattle-infesting tick. Tick-borne diseases, babesiosis, and anaplasmosis present serious constraints to animal productivity of particularly exotic cattle breeds and their crosses. Chemical acaricides are used extensively to control the ticks. Incorrect dilution, application methods, and extensive pressure on a particular compound are the main factors that accelerate acaricide resistance [2]. Synthetic pyrethroid and deltamethrin resistance was recorded in Rhipicephalus microplus and R. annulatus, respectively, in many countries [3–5]. In addition, chemical acaricides have toxic effects on nontarget species besides meat and milk residues; therefore, there is a massive need to develop eco-friendly effective acaricides [6–8]. For many years, plant EOs have been studied widely as one of the natural acaricides [9]. EOs are composed of terpenoids, monoterpenoids (C10), and sesquiterpenoids (C15). Terpenoid hydrocarbons are hydrophobic, a property associated with protein deactivation and enzyme inhibition activities especially acetylcholinesteras which is the target of many chemical acaricides [10]. In comparison with chemical products, EOs have advantages such as low toxicity to livestock and safety to the environment. Among reported natural acaricides from EOs, thymol and eucalyptus oils were found effective in managing deltamethrin-resistant R. annulatus infestation in cattle [11–13]. In continuation of our search for natural acaricides, larvicidal activity of EOs from five medicinal plants, namely, Coriandrum sativum leaves (cilantro, 1), Citrus aurantium leaves (orange, 2), Tagetes erecta flowers (3), Pelargonium graveolens herb (geranium, 4), and Ocimum basilicum leaves (sweet basil, 5), were evaluated against R. annulatus larvae using larval packet test (LPT). Gas chromatography-mass spectrometry (GC-MS) analysis was performed for the EOs to identify their major components and rationalize their activity.
MATERIALS AND METHODS
Essential oils (EOs)
Five EOs were purchased from a volatile oil factory, in Beni-Suef Governorate, Egypt. The oils were C. sativum leaves (cilantro, 1), C. aurantium leaves (orange, 2), T. erecta flowers (Tagetes, 3), P. graveolens herb (geranium, 4), and O. basilicum leaves (sweet basil, 5). Different concentrations of EOs (10%, 5%, 2.5%, and 1.25%) were prepared in ethanol (95% in water) as a diluent. EOs lethal effects on R. annulatus larvae were evaluated by calculating the concentrations that kill 99% (LC99), and lethal concentrations that kill 50% (LC50).
GC-MS analysis
Mass spectra were recorded using Shimadzu GCMS-QP2010 (Tokyo, Japan) Column (Rtx5MS fused, 30 m × 0.25 mm id × 0.25 μm film thickness) (Restek, USA) equipped with a split–splitless injector was used at 45°C isothermal temperature at for 2 minutes then programmed to 300°C at a rate of 5°C/minute kept isothermal at 300°C for 5 minutes. The injector temperature was 250°C. The helium carrier gas flow rate was 1.41 ml/minute. All the mass spectra were recorded applying the following conditions: (equipment current) filament emission current, 60 mA; ionization voltage, 70 eV; and ion source, 200°C. Diluted samples (1% v/v) were injected with split mode (split ratio 1: 15). The identification of components was based on the National Institute of Standards and Technology library attached to the GC-MS instrument. Compounds were recognized by relating their peak retention indices and mass spectral fragmentation patterns to those of the known compounds available in the library.
Acaricidal activity LPT
Larvicidal activities were evaluated by using the modified larval packet technique (LPT) as previously described [14]. Various concentrations of the tested EOs were prepared. Approximately 100 7-day-old larvae were placed on the center of 7 × 7 cm filter papers then 100 μl of the test solutions were added then enclosed to form packets with clips. Control groups were treated with ethanol (95%). There were five replicates for each concentration. Finally, mortality was determined after packet incubation at 27°C–28°C and 80%–90% relative humidity for 24 hours.
Statistics
For the acaricidal study, statistical analysis of data was performed using Statistical Package for Social Science [SPSS for Windows (IBM), version 22, Chicago, USA] to determine if variables differed between treatments. Analysis of variance tests and subsequent Duncan’s multiple range tests were applied to determine the differences between means. Results were presented as means. Probability values (p < 0.05) were considered significant. The effective concentration (LD50) with a 95% confidence interval was calculated (SPSS version 22).
RESULTS AND DISCUSSION
The acaricidal efficacy of EOs was assessed by estimating R. annulatus larvae mortality percentage (Table 1) where the acaricidal activity of cilantro, orange leaf, and Tagetes flower EOs against R. annulatus is reported here for the first time. LC99 indicates the lethal concentrations that kill 99% of larvae; the smaller the concentration the more potent the EO. EO from cilantro is considered the most potent among the tested oils, (LC99 = 2.77 μg/ml), followed by orange leaf (3.78 μg/ml), Tagetes flower (6.05 μg/ml), geranium herb (10.07 μg/ml), and finally sweet basil (13.46 μg/ml). Major components in each EO were determined using GC-MS (Tables 2–6, Figs. 1,2 and 3) and compared with the reported data.
Acaricidal activity and chemical composition of cilantro leaf EO
LPT showed 100% mortality at 5% and 10% concentrations with LC50 of 1.46 and LC99 of 2.77 μg/ml. 2.5% concentration still showing potent activity (96.67% ± 1.67% larval mortality) GC-MS analysis of cilantro EO (Table 2, Fig. 2) showed the major components detected were 2(E)-decenal (49.72%), decanal (21.47%), β-linalool (9.08%), and 2-dodecenal (5.98%) and its GC-MS data agree to some extent with the data reported by Silva et al. [15] on leaf EO that showed the same major constituent but with different percentages; 32.23% 2(E)-decenal, 13.97% linalool, 7.51% (E)-2-dodecenal, and 6.56% (E)-2-tetradecenal. In addition, Shavandi et al. [16] reported that 2(E)-decenal (19.6%), 1-decanol (26.0%), E-2-tetradecenal (7.0%), decanal (6.6%), and E-2-dodecenal (5.4%) percentage, respectively, while Delaquis and Stanich [17] reported that linalool (25.9%) and (E)-2-decenal (20.2%) are the most abundant component followed by decanal (8.4%) and (E)-2-decenol (7.9%).
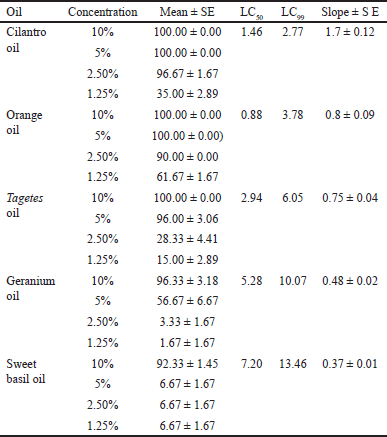 | Table 1. Mean mortality percentage, lethal concentrations (LC50 and LC99) of the tested oils against R. annulatus larvae. [Click here to view] |
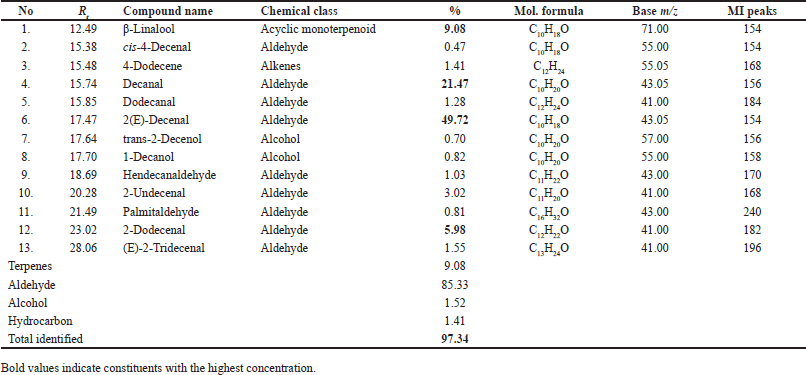 | Table 2. Major constituents of cilantro (C. sativum) leaves EO, family Apiaceae, were analyzed by GC-MS. [Click here to view] |
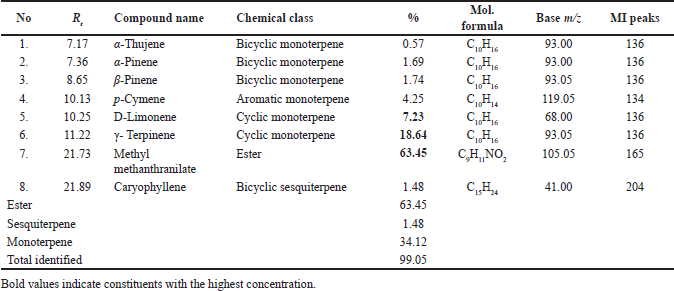 | Table 3. Major constituents of orange (C. aurantium) leaves EO, family Rutaceae, were analyzed by GC-MS. [Click here to view] |
Coriander leaf EO acaricidal activity against R. annulatus is reported here for the first time and it showed potent activity. The oil also has 100% acaricidal activity against red mite Dermanyssus gallinae De Geer [18], 100% nematicidal activity (2 mg/ml) against Bursaphelenchus xylophilus [19], and 100% Tribolium castaneum egg mortality at 20 mg/ml (96 hours exposure) and 90% repellent activity to the adults at 12 mg/ml [20].
Acaricidal activity and chemical composition of orange leaf EO
LPT showed 100% mortality at 5% and 10% concentrations as cilantro with LC50 of 0.88 and LC99 of 3.78 μg/ml. 2.5% concentration still showing potent activity (90.00% ± 0.0% larval mortality). GC-MS analysis of orange leaf EO (Table 3, Fig. 2) showed the major components detected were methyl methanthranilate (63.45%), γ-terpinene (18.64%), and D-limonene (7.23%). GC-MS data showed different compositional patterns compared to the previous studies except for the percentage of D-limonene. Khalid et al. [21] studied the effects of geographical locations of Egypt on EO composition from leaves and flowers and showed that plant source has significant variation in orange EO composition where sabinene (33.8%–44.9%) and terpinen-4-ol (15.6%–22.6%), where the major constituents followed by Δ-3-carene (9.3%–12.4%) and limonene (5.5%%–8.3%). In another study, major components of leaf EO were terpinen-4-ol (14.1%), limonene (10.18%), β-pinene (8.73%), and trans-sabinene hydrate (8.21%) [22].
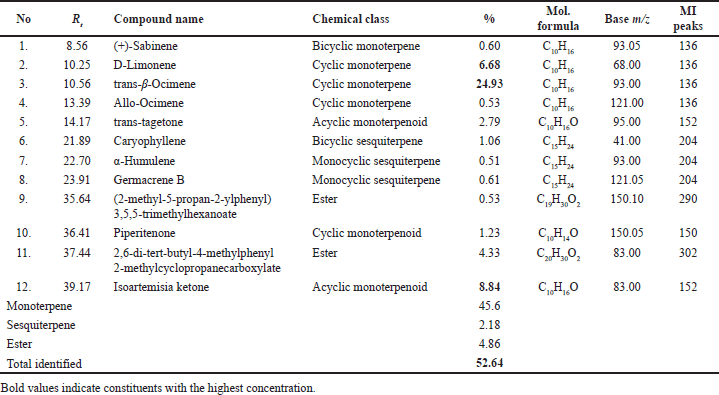 | Table 4. Major constituents of the Tagetes (T. erecta) flower EO, family Astraceae, were analyzed by GC-MS. [Click here to view] |
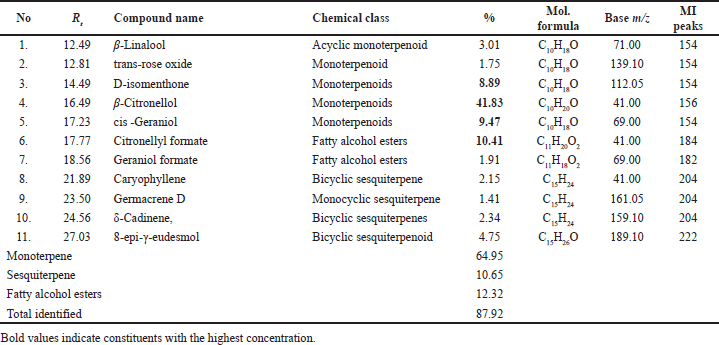 | Table 5. Major constituents of the geranium (P. graveolens) herb EO, family Geraniaceae, were analyzed by GC-MS. [Click here to view] |
d-Limonene and peel oil from different Citrus species were reported to have acaricidal activity against R. microplus, related species to R. annulatus where peel oil from Citrus maxima (mature and immature fruits) and Citrus reticulata exhibited two times stronger acaricidal than d-limonene on female tick. Citrus sinensis, C. maxima (mature and immature fruits), Citrus hystrix, Citrus suncris, and C. reticulata exhibited 1.5 times more larvicidal activity than d-limonene [23]. Depending on the acaricidal activity of d-limonene, orange oil activity may be attributed to the synergistic action of its constituents also minor components may contribute to the activity.
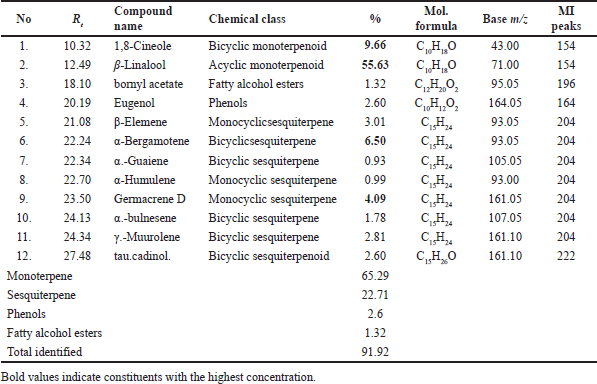 | Table 6. Major constituents determined by GC-MS analysis of sweet basil leaves EO (O. basilicum). [Click here to view] |
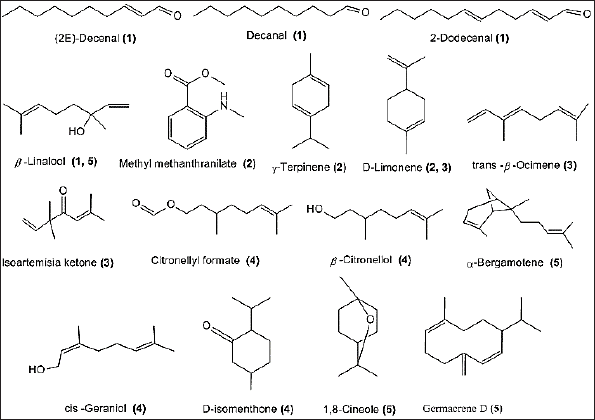 | Figure 1. Chemical structure of major compounds identified in the EO of (1) cilantro leaves, (2) orange leaves, (3) Tagetes flower, (4) geranium herb, and (5) sweet basil herb. The number between brackets indicates the plant source. [Click here to view] |
Acaricidal activity and chemical composition of Tagetes flower EO
LPT showed 100% mortality at 10% concentration and about 96.00% at 5% concentration with LC50 of 2.94 and LC99 of 6.05 μg/ml, while 2.5% showed low larval mortality % (28.33 ± 4.41). Our chemical investigation of T. erecta flower EO, showed that the major constituents were trans-β-ocimene (24.93%), D-limonene (6.68%), and isoartemisia ketone (8.84%) (Table 4, Fig. 2). EO showed variation in its main components according to plant source. Reported EO composition of fresh flowers collected in México were piperetone (19.2%), b-caryophyllene (15.2%), and (E)-ocimene (13.7%), and also limonene (11.7%) was detected [24], and that is collected from Italy were piperitone 28.9%, terpinolene 5.8%, phyllene 3.8%, and limonene 3.5% [25], while flowers collected from Nigeria, characterized by the presence of 1, 8-cineole (23.1%) as the major constituents followed by α-pinene (11.8%), α-terpineol (10.7%), and piperitone (8.0%) [26].
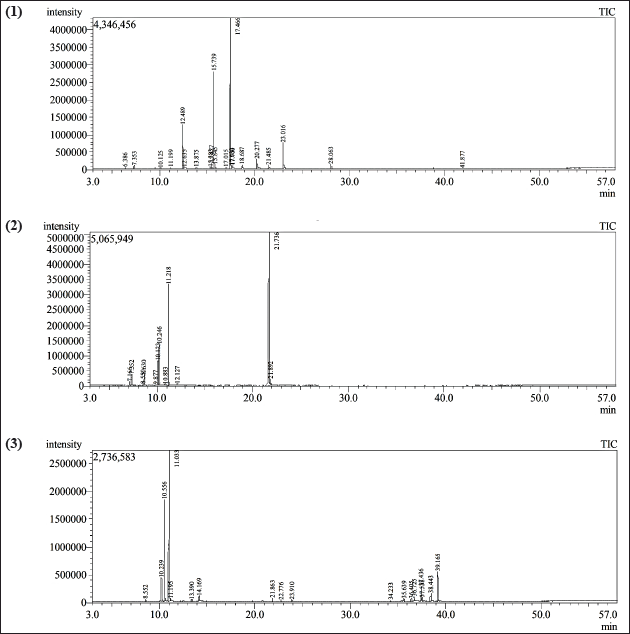 | Figure 2. GC-MS chromatogram of the EO of (1) cilantro leaves, (2) orange leaves, and (3) Tagetes flower. [Click here to view] |
The Brazilian T. erecta L. leaves essential showed schistosomicidal effects after 24 hours against Schistosoma mansoni with 50 μg/ml minimum inhibitory concentration and parasites death and coupled pairs separation at 100 μg/ml after 24 hours [27]. In addition, EO was reported to have antifungal activity against Aspergillus terreus and Colletotrichum falcatum [28].
Acaricidal activity and chemical composition of geranium herb EO
LPT showed 96.33% mortality at 10% concentration and 56.67 at 5% concentration with LC50 of 5.28 and LC99 of 10.07 μg/ml, while 2.5% concentration showed very low larval mortality % (3.33 ± 1.67). GC-MS analysis of geranium oil (P. graveolens, family Geraniaceae) revealed the presence of β-citronellol (41.83%), citronellyl formate (10.41%), geraniol (9.47%), D-isomenthone (8.89%), β-linalool (3.01%) (Table 5, Fig. 3). These major GC-MS detected components were consistent with the previous studies [29] but with different percentages, namely, β-citronellol (44.5%), geraniol (13.7%), citronellyl formate (7.3%), β-linalool (3.9%), and D-isomenthone (3.5%).
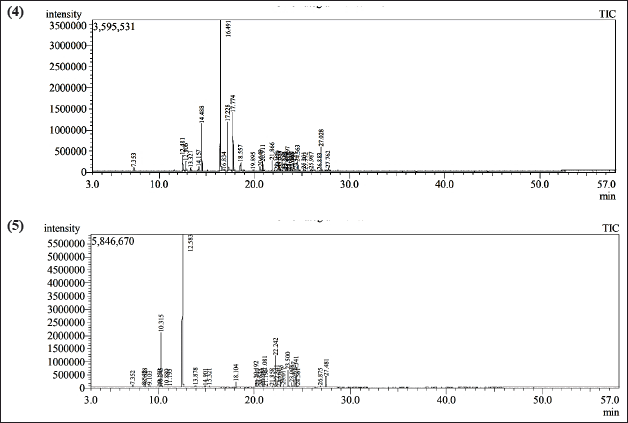 | Figure 3. GC-MS chromatogram of the EO of (4) geranium herb and (5) sweet basil herb. [Click here to view] |
EO of geranium leaves was reported to have acaricidal activity against different stages of R. annulatus, where LC50 of geranium EO, its nanoemulsion, and its combination with the sesame oil against the adult ticks were 7.53%, 5.60%, and 1.91%, respectively, and against larvae were 3.435%, 1.688%, and 0.944%, respectively. In addition, in-vivo tested nanoemulsion and geranium-sesame oil combination showed a significant reduction in tick burden 3 weeks post application to 87.97% and 74.83%, respectively [30]. In addition, geranium EO inhibited the oviposition of R. microplus by 97% at 10% concentration [31].
Acaricidal activity and chemical composition of sweet basil EO
LPT showed 92.33% mortality at 10% concentration with LC50 of 7.2 and LC99 of 13.46 μg/ml, while 5% other concentrations were inactive (only 6.67% ± 1.67% larval mortality). GC-MS analysis of sweet basil oil (O. basilicum, family; Lamiaceae) revealed that β-Linalool (55.63%) was the major component in addition to 1,8-cineole (9.66%), α-bergamotene (6.50%), and germacrene D (4.09%) (Table 6, Fig. 3). Reported data on sweet basil leaf oil presented that the major compounds detected were linalool (30.61%) and estragole (20.04%), followed by a nearly equal percentage of α-farnesene, eugenol, and 1,8-cineole [32]. In addition, GC-MS analysis of EO extracted from three varieties of basil showed that the most common compounds detected in var. Nu Far were linalool (52.2%), estragole (18.2%), and sabinene (6.71%), and in var. Jolina were linalool (43.9%), eugenol (11.2%), and α-bergamotene ( 9.19%), and var. in Aroma were linalool (48.2%), sabinene (8.99%), and eugenol (8.71%) [33]. Linalool was the dominant component in the reported data which is in agreement with our chemical study of the oil.
Aboelhadid et al. [34] studied the larvicidal and repellent efficacy of the oil and its nanocomposite (O. basilicum EO/layered double hydroxide) against R. annulatus and its results showed 100% larval mortality by the oil at 300 μl/ml and by nanocomposite at 200 μl/ml. Oil and nanocomposite have 100% adult mortality, prevent egg deposition and eggs hatching at a dose of 300 μl/ml [34], but oil did not show any acaricidal activity on 10-day-old R. microplus larvae [32], while eugenol which was detected as a minor compound (2.60%) in our study reported having 100% acaricidal activity at 2% against R. microplus larvae [35]. Oil has been widely investigated against other insects and showed a high lethal effect on adult mosquitoes (93%–95%) [36], Eutetranychus orientalis (Klein), eggs number reduction with 100–87.5 oviposition deterrence indices at 2%–0.5% against Tetranychus urticae (Koch) and E. orientalis mites [37]; also, methanol extract of the leaves and flowers have insecticidal activity on larvae of the Egyptian cottonworm (Spodoptera littoralis) with 1.7 μg/ml lC50 [38] and yellow fever mosquito (Aedes aegypti) with 3.7%–5.1% LC50 for I–IV instar larvae and 5.449% LC50 of pupae [39].
CONCLUSION
Our results recommended the use of EOs of five medicinal plants; cilantro leaves, orange leaves, Tagetes flower, geranium herb, and sweet basil herb as environment-friendly acaricides for R. annulatus control tick control. EO from cilantro is considered the most potent among the tested oils; (LC99 = 2.77 μg/ml), followed by orange leaf (3.78 μg/ml), Tagetes flower (6.05 μg/ml), geranium herb (10.07 μg/ml), and finally sweet basil (13.46 μg/ml). The acaricidal activity of cilantro, orange leaf, and Tagetes flower EOs against R. annulatus are reported here for the first time. The high mortality percentage caused by these oils will shed light on natural alternatives for tick control which will have both economic and environmental impact and encourage us to pursue more future work to get pharmaceutical products.
LIST OF ABBREVIATIONS
EOs: essential oils; GC-MS: gas chromatography-mass spectrometry; LC50: lethal concentrations that kill 50%; LC99: lethal concentrations that kill 99%; LPT: larval packet test; NIST: National Institute of Standards and Technology; SE: standard error.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data. A.M., H. A. and W. A. choose the oils and designed the experiments. H. A. E. A. and A.O. analyzed the oils and interpreted the data, K.H. and K.H. collected the ticks and grew the larvae. W. A, K. H. and K. H. made the larvicidal activity, analyzed the data and made the statistical analysis. All authors were involved in writing, review and editing and gave the final approval of the version to be published.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Yadav N, Upadhyay R. Tick saliva toxins, host immune responses and its biological effects. Int J Pharm Pharm Sci. 2021;13(8):9–19.
2. Abbas RZ, Zaman MA, Colwell DD, Gilleard J, Iqbal Z. Acaricide resistance in cattle ticks and approaches to its management: the state of play. Vet Parasitol. 2014;203(1–2):6–20. CrossRef
3. Rodríguez-Vivas RI, Hodgkinson JE, Trees AJ. Resistencia a los acaricidas en Rhipicephalus (Boophilus) microplus: situación actual y mecanismos de resistencia. Rev Mex Cienc Pecu. 2012; 3:9–24.
4. Sharma AK, Kumar R, Kumar S, Nagar G, Singh NK, Rawat SS, et al. Deltamethrin and cypermethrin resistance status of Rhipicephalus (Boophilus) microplus collected from six agro-climatic regions of India. Vet Parasitol. 2012;188(3–4):337–45. CrossRef
5. Aboelhadid S, Arafa WM, Mahrous LN, Fahmy MM, Kamel AA. Molecular detection of Rhipicephalus (Boophilus) annulatus resistance against deltamethrin in middle Egypt. Vet Parasitol Reg Stud Rep. 2018;13:198–204. CrossRef
6. Tabari MA, Youssefi MR, Maggi F, Benelli G. Toxic and repellent activity of selected monoterpenoids (thymol, carvacrol and linalool) against the castor bean tick, Ixodes ricinus (Acari: Ixodidae). Vet Parasitol. 2017;245:86–91. CrossRef
7. Khater H, Hendawy N, Govindarajan M, Murugan K, Benelli G. Photosensitizers in the fight against ticks: safranin as a novel photodynamic fluorescent acaricide to control the camel tick Hyalomma dromedarii (Ixodidae). Parasitol Res. 2016;115(10):3747–58. CrossRef
8. Aboelhadid SM, Arafa WM, Wahba A, Mahrous LN, Ibrahium SM, Holman PJ. Effect of high concentrations of lufenuron, pyriproxyfen and hydroprene on Rhipicephalus (Boophilus) annulatus. Vet Parasitol. 2018;256:35–42. CrossRef
9. Iori A, Grazioli D, Gentile E, Marano G, Salvatore G. Acaricidal properties of the essential oil of Melaleuca alternifolia Cheel (tea tree oil) against nymphs of Ixodes ricinus. Vet Parasitol. 2005;129(1–2):173–6. CrossRef
10. Ryan M, Byrne O. Plant-insect coevolution and inhibition of acetylcholinesterase. J Chem Ecol. 1988;14:1965–75. CrossRef
11. Arafa WM, Abolhadid SM, Moawad A, Abdelaty AS, Moawad UK, Shokier KA, et al. Thymol efficacy against coccidiosis in pigeon (Columba livia domestica). Prev Vet Med. 2020;176:104914. CrossRef
12. Arafa WM, Aboelhadid SM, Moawad A, Shokeir KM, Ahmed O, de León AAP. Control of Rhipicephalus annulatus resistant to deltamethrin by spraying infested cattle with synergistic eucalyptus essential oil-thymol-deltamethrin combination. Vet Parasitol. 2021;290:109346. CrossRef
13. Goudoum A, Ngamo T, Ngassoum M, Mbofung C. Biodegradation of insecticidal compounds of Clausena anisata and Plectranthus glandulosus essential oils applied as protectant on stored grains. J Agric. 2012;7(2):165–71.
14. Arafa WM, Aboelhadid SM, Moawad A, Shokeir KM, Ahmed O. Toxicity, repellency and anti-cholinesterase activities of thymol-eucalyptus combinations against phenotypically resistant Rhipicephalus annulatus ticks. Exp Appl Acarol. 2020;81(2):265–77. CrossRef
15. Silva F, Domeño C, Domingues FC. Coriandrum sativum L.: characterization, biological activities, and applications. Nuts and seeds in health and disease revention. ELSEVIER ACADEMIC PRESS: London, UK; 2020. pp 497–519.
16. Shavandi MA, Haddadian Z, Ismail MHS. Eryngium foetidum L. Coriandrum sativum and Persicaria odorata L.: a review. JASR. 2012;2(8):410–26.
17. Delaquis PJ, Stanich K. Antilisterial properties of cilantro essential oil. J Essent Oil Res. 2004;16(5):409–14. CrossRef
18. Torres-Cabra E, Lagos-López MI. Evaluation of essential coriander (Coriandrum sativum L.) oil against the red poultry mite (Dermanyssus gallinae) (De Geer, 1778) (Acari: Dermanyssidae) under laboratory conditions. Cienc Tecnol Agropecuaria. 2019;20(1):53–68.
19. Kim J, Seo S-M, Lee S-G, Shin S-C, Park I-K. Nematicidal activity of plant essential oils and components from coriander (Coriandrum sativum), oriental sweetgum (Liquidambar orientalis), and valerian (Valeriana wallichii) essential oils against pine wood nematode (Bursaphelenchus xylophilus). J Agric Food Chem. 2008;56(16):7316–20. CrossRef
20. Islam M, Hasan MM, Xiong W, Zhang S, Lei C. Fumigant and repellent activities of essential oil from Coriandrum sativum (L.) (Apiaceae) against red flour beetle Tribolium castaneum (Herbst) (Coleoptera: tenebrionidae). J Pest Sci. 2009;82(2):171–7. CrossRef
21. Khalid KA, Essa EF, Ismaiel HM, Elsayed AA. Effects of geographical locations on essential oil composition of navel orange leaves and flowers. J Essent Oil Bear Plants. 2020;23(1):139–48. CrossRef
22. Hamdan DI, Mohamed ME, Abdulla RH, Mohamed SM, El-Shazly AM. Anti-inflammatory, insecticidal and antimicrobial activities and chemical composition of the essential oils of different plant organs from navel orange (Citrus sinensis (L.) Osbeck var. Malesy) grown in Egypt. J Med Plant Res. 2013;7(18):1204–15. CrossRef
23. Chungsamarnyary N, Jansawan W. Acaricidal activity of peel oil of Citrus spp. on Boophilus microplus. Agric Nat Resour. 1996;30(1):112–7.
24. Pérez Gutierrez R, Hernández Luna H, Hernández Garrido S. Antioxidant activity of Tagetes erecta essential oil. J Chil Chem Soc. 2006;51(2):883–6. CrossRef
25. Marotti M, Piccaglia R, Biavati B, Marotti I. Characterization and yield evaluation of essential oils from different Tagetes species. J Essent Oil Res. 2004;16(5):440–4.
26. Ogunwande IA, Olawore NO. The essential oil from the leaves and flowers of “African Marigold,” Tagetes erecta L. J Essent Oil Res. 2006;18(4):366–8. CrossRef
27. Tonuci LR, Melo NId, Dias HJ, Wakabayashi KA, Aguiar GP, Aguiar DP, et al. In vitro schistosomicidal effects of the essential oil of Tagetes erecta. Rev Bras Farmacogn. 2012;22:88–93. CrossRef
28. Singh G, Singh OP, De Lampasona M, Catalan CA. Studies on essential oils. Part 35: chemical and biocidal investigations on Tagetes erecta leaf volatile oil. Flavour Fragr J. 2003;18(1):62–5. CrossRef
29. Nardoni S, Ebani VV, D’Ascenzi C, Pistelli L, Mancianti F. Sensitivity of entomopathogenic fungi and bacteria to plants secondary metabolites, for an alternative control of Rhipicephalus (Boophilus) microplus in cattle. Front Pharmacol. 2018;9:937. CrossRef
30. Ibrahium SM, Aboelhadid SM, Wahba AA, Farghali AA, Miller RJ, Abdel-Baki A-AS, et al. Preparation of geranium oil formulations effective for control of phenotypic resistant cattle tick Rhipicephalus annulatus. Sci Rep. 2022;12(1):1–13. CrossRef
31. Pazinato R, Volpato A, Baldissera MD, Santos RC, Baretta D, Vaucher RA, et al. In vitro effect of seven essential oils on the reproduction of the cattle tick Rhipicephalus microplus. J Adv Res. 2016;7(6):1029–34. CrossRef
32. Martinez-Velazquez M, Castillo-Herrera GA, Rosario-Cruz R, Flores-Fernandez JM, Lopez-Ramirez J, Hernandez-Gutierrez R, et al. Acaricidal effect and chemical composition of essential oils extracted from Cuminum cyminum, Pimenta dioica and Ocimum basilicum against the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasitol Res. 2011;108(2):481–7. CrossRef
33. Wang HV, Pickett LJ, Faraone N. Repellent and acaricidal activities of basil (Ocimum basilicum) essential oils and rock dust against Ixodes scapularis and Dermacentor variabilis ticks. Exp Appl Acarol. 2022;86(4):583–98. CrossRef
34. Aboelhadid SM, Mahran HA, El-Ela FAI, Shokeir KM, Henedy KH, Gadelhaq SM, et al. Synthesis of nanocomposites layered double hydroxide via Ocimum basilicum and its acaricidal efficacy against multi-resistance Rhipicephalus annulatus tick. J Biomed Nanotechnol. 2020;16(12):1765–75. CrossRef
35. Hüe T, Cauquil L, Fokou J, Dongmo P, Bakarnga-Via I, Menut C. Acaricidal activity of five essential oils of Ocimum species on Rhipicephalus (Boophilus) microplus larvae. Parasitol Res. 2015;114(1):91–9. CrossRef
36. Umerie S, Anaso H, Anyasoro L. Insecticidal potentials of Ocimum basilicum leaf-extract. Bioresour Technol. 1998;64(3):237–9. CrossRef
37. Refaat M, Momen F, Amer S. Acaricidal activity of sweet basil and French lavender essential oils against two species of mites of the family Tetranychidae (Acari: Tetranychidae). Acta Phytopathol Entomol Hung. 2002;37(1–3):287–98. CrossRef
38. Pavela R, Chermenskaya T. Potential insecticidal activity of extracts from 18 species of medicinal plants on larvae of Spodoptera littoralis. Plant Prot Sci. 2004;40(4):145–50. CrossRef
39. Murugan K, Murugan P, Noortheen A. Larvicidal and repellent potential of Albizzia amara Boivin and Ocimum basilicum Linn against dengue vector, Aedes aegypti (Insecta: Diptera: Culicidae). Bioresour Technol. 2007;98(1):198–201. CrossRef