INTRODUCTION
The second most prevalent neurodegenerative condition after Alzheimer’s disease is Parkinson’s disease (PD). Postural instability, tremors, akinesia, rigidity, and bradykinesia are some of the clinical symptoms of PD [1]. It involves the loss of dopamine (DA)-producing nerve cells in an area of the brain called the substantia nigra (SN). This leads to dramatically reduced DA levels in the striatum, another brain region. The loss of these DA-releasing neurons and low DA levels in the striatum are key pathological hallmarks of PD. The formation of cytoplasmic inclusions and filamentous, primarily alpha-synuclein aggregations in the form of Lewy bodies or Lewy neurites is a pathological characteristic of PD [2]. The etiology of PD is complicated, incorporating both genetic and environmental factors. Previous research has found that genetic, environmental, and stochastic factors all contribute to the progression and development of PD [3]. PD arises from progressive and specific degeneration of dopaminergic neurons located in the substantia nigra pars compacta (SNpc), which reduces DA neurotransmitters in striatal projections and brainstem areas. This impairs the neural circuits responsible for motor activity and function [4]. Various factors have been linked to the etiology of PD, including oxidative stress, neuroinflammation, toxic factors, and genetic factors [4,5]. PD affects approximately 1% of individuals aged 60 and above, with its prevalence increasing to 1%–3% in individuals aged 80 and above, although these statistics do not account for undiagnosed cases [6]. PD has a male-to-female ratio of 3:2, with females experiencing a delayed onset due to the neuroprotective effect of estrogen on the nigrostriatal dopaminergic system [7].
Epidemiological studies suggest that several factors, such as genetic predisposition, oxidative stress, mitochondrial abnormalities, neuroinflammation, neurotoxicity, and aberrant protein accumulation [8–10], can increase the risk of developing PD. These factors can lead to apoptosis and the excessive death of dopaminergic neurons, which is a fundamental feature of neurodegenerative diseases throughout the disease. PD remains incurable, as existing medications cannot halt or reverse disease progression [11,12]. The SNpc are especially vulnerable to damage from the toxin rotenone (ROT). ROT can induce oxidative stress and the buildup of reactive oxygen species (ROS) which are toxic to cells [13]. Because of its high metabolic activity, the dopamine-producing neurons of the SNpc are particularly sensitive to the poisonous effects of rotenone and oxidative molecules. This selective toxicity makes the SNpc region of the brain highly susceptible to ROT-induced cell death [14]. Midbrain histology has shown decreased motor coordination responses and altered metabolic activities [15]. Other studies have also shown that the midbrain is susceptible to rot-induced oxidative stress [18,19]. Currently, Levodopa (LD) is the primary medication used to treat PD; it only provides limited symptomatic relief and can cause severe adverse effects such as hallucinations and dyskinesia with prolonged use. This limits its usefulness for PD treatment, and disease-modifying therapies for PD are not currently available [13,14]. Therefore, there is a need to investigate novel approaches that can delay PD onset or progression. Previous studies suggest that herbal medicines exhibit antioxidative, anti-inflammatory, and neuroprotective properties, indicating their potential efficacy for PD treatment.
The plants Ficus religiosa and Oxalis corniculata, which belong to the family Moraceae and Oxalidaceae families, respectively, have the potential to treat a wide range of diseases, including but not limited to cancer, dysmenorrhea, hepatitis, respiratory disorders, edema, scabies, including PD. Ficus religiosa contains compounds such as flavonoids, tannins, and phenolic acids that exhibit antioxidant effects to counteract oxidative stress. This plant also has anti-inflammatory actions that can help suppress chronic neuroinflammation associated with PD progression.
Similarly, O. corniculata is rich in antioxidants such as vitamin C, quercetin, and linoleic acid. It also demonstrates anti-inflammatory activity. Both oxidative damage and neuroinflammation are implicated in the selective loss of dopaminergic neurons in PD. By targeting these key mechanisms involved in neurodegeneration, extracts from F. religiosa and O. corniculata may protect against neuronal cell death. Their complementary antioxidant and anti-inflammatory actions could potentially have synergistic benefits. However, their combined effects have not been explored. This study investigates their potential synergistic actions, differing from single extract studies. Ficus religiosa and O. corniculata were selected for their antioxidant and anti-inflammatory properties relevant for mitigating PD pathology.
The primary objective is to elucidate the combined neuroprotective effects of F. religiosa and O. corniculata extracts in an ROT-induced mouse model of PD. Additive therapeutic potential was assessed by evaluating neurobehavioral function, neurochemicals, neurotransmitters, and brain histology. This may provide preclinical evidence supporting further research into multitargeted plant extracts for PD treatment.
MATERIALS AND METHODS
Collection and preparation of O. corniculata extract
Oxalis corniculata plants were collected from MDU, Rohtak, Haryana, India, and shade-dried for 3–8 weeks at room temperature before being mechanically ground into a coarse powder that was filtered through a 40-mesh to achieve a consistent particle size. A Soxhlet apparatus was then used to continuously extract the plant material by heating it with ethanol at 65°C–70°C. The obtained extracts were concentrated using a rota flash evaporator at low pressure to completely remove the ethanol solvent, and the final O. corniculata extract was stored at −20°C until further use.
Collection and preparation of F. religiosa extract
Leaves of the F. religiosa plant were collected from M.D.U Rohtak and shade-dried before being coarsely powdered using a mechanical grinder. The powdered plant material then underwent a 72-hour extraction process using a Soxhlet apparatus with petroleum ether (60°C–80°C) as the solvent. The concentrated extract was transferred to a rotary evaporator to completely remove the solvent. The extract was then dried at ambient temperature (45°C–50°C) to obtain the dried extract, which was stored until further use.
Experimental animals
Adult Swiss albino mice (25–30 g) of either sex, were procured from L.U.V.A.S. animal house, Hisar, India. Mice were housed in standard opaque polypropylene cages, 6%, with dust-free paddy husk bedding material and were maintained on a 12-hour light/dark cycle with provided ad libitum access to water and food at Maharshi Dayanand University’s registered animal house in Rohtak, Haryana. The Committee for the Purpose of Control and Supervision of Experiments on Animals, animal welfare division, government of India, established guidelines for all experimental procedures, which were carried out by them. Mice were acclimatized to lab conditions for 7 days before experimentation.
Chemicals and reagents
ROT, Carbidopa, and LD were purchased from Sigma Aldrich chemicals (Bangalore, India), 5,5-dithiol-bis-(2-nitrobenzoic acid) was procured from Fluka, India, and Nitro blue tetrazolium chloride from Loba Chemicals. Petroleum ether, trichloroacetic acid, thiobarbituric acid (TBA), glutathione (GSH) reduced, Ethylenediaminetetraacetic acid, and sodium dodecyl sulfate were obtained from Loba Chemicals (India), Greiss reagent, and DA hydrochloride from SRL Chemicals (India). The remaining chemicals and reagents were of analytical grade. All compounds were prepared in DW as stock solutions, and the dilutions were made fresh on the day of the experiment.
Experimental design
The animals were divided into six groups (n = 6 per group). ROT dissolved in 1% dimethyl sulfoxide (DMSO) was administered subcutaneously at a dose of 1.5 mg/kg on alternate days for 21 days to groups II–VI.
Group I: Served as control
Group II: Received ROT + DMSO
Group III: Received ROT + F. religiosa extract (200 mg/kg, p.o)
Group IV: Received ROT + O. corniculata extract (500 mg/kg, p.o)
Group V: Received ROT + F. religiosa extract (200 mg/kg, p.o) + O. corniculata extract (500 mg/kg, p.o)
Group VI: Received ROT + Sinemet (100 mg/kg, p.o)
Neurobehavioral tests [pole, beam walk, wire hanging, and open field tests (OFT)] were performed on days 1,7,14, and 21. On day 22, the mice were euthanized, their brains dissected, and subjected to neurochemical analysis [malondialdehyde (MDA), total protein content (TPC), superoxide dismutase (SOD), catalase (CAT), GSH, and nitrite (NO)] and neurotransmitter estimation (DA and glutamate). Histopathological examination of the brains was also carried out as mentioned in Figure 1.
Assess of the motor function of mice
Open field test
The OFT assessed exploratory behavior and general locomotor activity in mice. Before the experiment, mice were given an adaptation period to acclimate to the novel environment. Subsequently, a mouse was placed in the central area of the arena, and the experiment duration for each mouse was 5 minutes. To eliminate any excretions or scent signals, the open field was thoroughly cleaned after each animal and left to air dry. The arena’s exploratory trace and total distance were recorded using SMART v.3.0 software as shown in Figure 3 [20].
Wire hanging test
Crawley’s [21] wire-hanging test also referred to as the horizontal bar test, this test evaluated forelimb grip strength and motor coordination. Each mouse has suspended forelimbs on a steel wire (25 × 0.2 × 25 cm). The latency period, representing the grasped time on the wire was measured for a 60 seconds maximum cut-off time [21].
Pole test
The pole test assessed balance and motor coordination in mice. To conduct this test, mice were positioned on a horizontal wooden rod measuring 50 cm in length and 1 cm in diameter. The time required for each mouse to descend from the pole to the floor was recorded as an indicator of motor coordination. Each mouse was tested 3 times, and the Avg. time was calculated for further analysis. The pole test was performed in a quiet and well-lit room to minimize the effect of external factors on the animals’ performance [22].
Beam walking test
The beam walk test is a well-established method to assess fine motor coordination and balance skills. The experimental apparatus consists of an acrylic round beam (1.5 cm in diameter and 60 cm in length) placed horizontally over two vertical supports (60 cm high) above a circular tank (150 cm in diameter). Each mouse had three successive trials while being positioned perpendicular to the beam’s center. Recorded the duration it took for each mouse to cross the entire length of the beam, with a cut-off time of 60 seconds for each trial. This method was previously described in the literature [23].
Neurochemical analysis
Tissue preparation for neurochemical analysis
Animals were euthanized in a CO2 Chamber, and their brains were promptly excised and rinsed with ice-cold normal saline solution (0.9% NaCl). Subsequently, the brain tissue was homogenized using ice-cold 0.1 M phosphate buffer with a pH of 7.4 at a ratio of 19 times the weight of the tissue. The resulting homogenates were then subjected to centrifugation at 4,000 rpm for 15 minutes. The supernatants and cell pellets were carefully separated into aliquots and stored at −20°C in a deep freezer for subsequent neurochemical analysis.
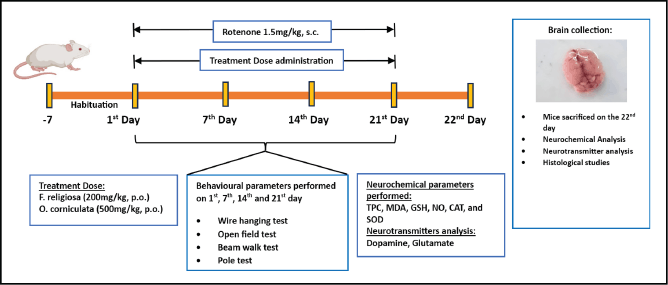 | Figure 1. A schematic representation of the experimentation design. [Click here to view] |
 | Figure 2. Effect of F. religiosa and O. corniculata on OFT in the ROT-induced PD mice. Values were expressed as Mean ± SEM. Data were analyzed by repeated measures of two-way ANOVA Tukey post hoc analysis. Each time point has six groups and n = 6 for each group. [Click here to view] |
Measurement of TPC
Alkaline copper solution (5 ml) will be added to 1 ml of homogenate tissue of mice brain which is allowed to stand for 10 minutes. 0.5 ml diluted Folin’s reagent (1:1) will be then added and the tubes will be shaken to mix the solution. After 30 minutes, the absorbance will be read at 750 nm against the appropriate blank.
Calculations
Protein will be calculated using a standard curve prepared with a standard curve expressed as μg/ml protein [24].
Measurement of MDA
Free radicals are generated during lipid peroxidation, and a mixture of peroxides is formed as primary products, which eventually break down to form carbonyl compounds. One such carbonyl compound is MDA, which reacts with TBA to produce a colored product. The ultimate absorbance of this colored product is measured at 540 nm, which provides an estimation of lipid peroxidation. MDA is a widely accepted secondary product of lipid peroxidation used for measuring oxidative stress in various biological samples [25].
Measurement of GSH
GSH levels were determined in the obtained supernatant. 1 ml of the supernatant was mixed with Ellman’s [26] reagent and phosphate buffer, and the resulting mixture was measured for absorbance at 412 nm using a spectrophotometer. The resulting GSH levels were calculated and presented as nanomoles per gram of moist tissue [26].
Measurement of NO
The presence of NO was assayed spectrophoto-metrically by the Greiss reagent. Greiss reagent was diluted in an amount equivalent to collected supernatants before being left at room temperature for 10 minutes. At 550 nm, the reaction’s absorbance will be evaluated in comparison to a properly prepared blank solution of distilled water (DW). NO concentrations were determined by utilizing a calibration curve for sodium NO, and they were measured in micrograms per mg protein [27].
Measurement of CAT
The reaction mixture contained 1.0 ml of 0.01 M (pH) phosphate buffer, 0.1 ml of supernatant, and 0.4 ml of 2 M H2O2. The addition of 2 ml of the dichromate acetic acid reagent (5% potassium dichromate and glacial acetic acid will be combined in a 1:3 ratio) stopped the reaction. At 620 nm, absorbance was calorimetrically quantified [28].
Measurement of SOD
The procedure developed by Kakkar et al. [29] will be used to measure the activity of SOD. The suppression of NADH production serves as the basis for the SOD assay. The process will be stopped by the addition of glacial acetic acid after 90 seconds of incubation. The reaction’s final color will be extracted into a layer of butanol and measured at 560 nm [29].
Neurotransmitter estimation
Estimation of striatal DA level
DA levels in mouse brains were quantified by UV spectrophotometry. The brain was weighed and then homogenized in 3 ml solution of HCl-Butanol solution (0.85 ml of 37% HCl in 1 l of n-butanol) at 0°C for 1 minute. The resulting homogenate was centrifuged at 2,000 rpm for 10 minutes at 0°C. Then, 0.25 ml of 0.1 M HCl and 2 ml of heptane were added to a tube containing a 0.8 ml phase of supernatant. The mixture was vigorously shaken for 10 minutes before centrifugation to separate the two phases, and the aqueous phase was retained for analysis [30,31].
To measure DA levels, 1 ml of the prepared supernatant was mixed with 1.5 ml of ferric chloride and 1.5 ml of potassium ferricyanide in 25 ml of DW. After incubation for 30 minutes, the absorbance of the developed color was quantified at 735 nm via a UV-visible double-beam spectrophotometer [32,33].
Estimation of glutamate level
Glutamate level in the tissue homogenate was determined using the method given by Bernt and Bergmeyer [34] A specific area of the brain was meticulously weighed before being homogenized with the two parts by weight of perchloric acid to determine the amount of glutamate in the brain. After that, the homogenate was centrifuged for 10 minutes at 3,000 rpm. After centrifugation, 1 ml of phosphate buffer solution was added to 3 ml of the supernatant fluid to bring the pH to 9.0. After standing in an ice bath for 10 minutes, the solution was filtered using flute filter paper. At a wavelength of 340 nm, the filtered solution’s absorbance was measured. To account for any background interference, a blank reading at 340 nm was taken concurrently. Glutamate levels are expressed as μg/g of proteins [34].
Histology
The brain tissue samples were subjected to preservation in a 10% formalin solution and subsequent fixation with paraffin. Once fixed, the tissue underwent slicing into 5 μm sections utilizing a microtome. To aid in visual examination, the tissue sections were stained using hematoxylin and eosin dyes. The stained sections were then subjected to analysis through microscopic observation facilitated by a camera-equipped microscope system.
Statistical analysis
The analysis software GraphPad Prism v 9.5.1 was used for all calculations. Data from at least three independent experiments were used to calculate the mean and standard error of the mean (SEM) for behavioral test results. An analysis of variance with one-way (ANOVA) was used to determine the statistical differences between the groups. Statistics were judged significant at a value of 0.05.
RESULTS
Behavioral observations: evaluate and observe the impact on motor function
Open field test
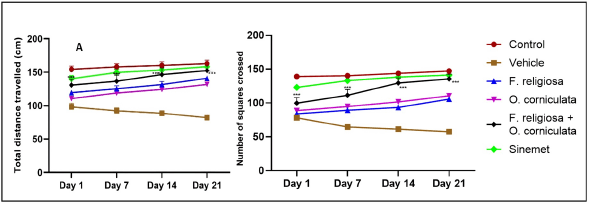
The open field track plots of mice in the control operation group, ROT group, ROT + F. religiosa group, ROT + O. corniculata group, ROT + F. religiosa + O. corniculata group, and ROT + Sinemet group are shown in Figure 2, while the total distance covered by mice in the six groups is presented in Figure 2A. Compared with the disease vehicle group, the open field movement distance of mice in the combination-treated group was significantly increased (***p < 0.001).
The results indicated that the increased exploratory behavior and locomotor activity in the combination treatment group suggests that the F. religiosa and O. corniculata extracts may help mitigate the dopaminergic neuron loss and motor deficits induced by ROT. The improved open-field parameters correlate with the observed recovery of DA levels and reduction in neuroinflammation markers.
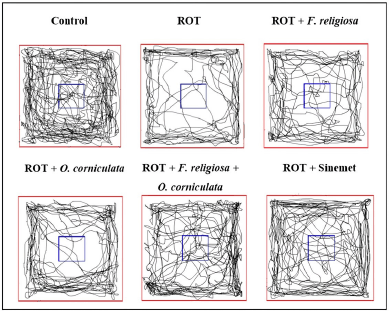 | Figure 3. The impact of ROT on the behavioral performance of mice was observed in the open-field test. This test was conducted within a specifically designed chamber that measured 40 × 40 × 35 cm and was equipped with an advanced automated video tracking system. To evaluate the lasting consequences of ROT, a meticulous selection process was employed to choose representative samples of the mice’s movement path traces on the 21st day after their final treatment. The total distance traveled and the number of crossings were recorded as shown in Figure 2 . [Click here to view] |
Pole test
The time to reach the floor or bradykinesia, in mice, was evaluated, and the study revealed that it significantly increased in the vehicle-treated group from day 1 (12.27 ± 0.494), day 7 (14.04 ± 0.408), day 14 (14.805 ± 0.644), and day 21 (16.38 ± 0.387), respectively, as compared to the control group from day 1 (9.04 ± 0.351), day 7 (8.36 ± 0.604), day 14 (8.195 ± 0.604), and day 21 (7.85 ± 0.383), respectively. In addition, a significant interaction between treatment and time was found, indicating that the effect of treatment varied across different time points. A combination dose of F. religiosa + O. corniculata (8.93 ± 0.485) in the treated group exhibits a significantly decreased increase in time to reach the floor of mice at day 21 as compared to other treated groups except for the standard drug Sinemet (8.54 ± 0.604) as shown in Figure 4. The result indicated that the shortened time-to-descend in the pole test implies that the combination extract treatment may help improve motor coordination impairments in ROT-induced PD mice. This could be attributed to the enhanced DA levels and attenuated oxidative stress resulting from the extract administration.
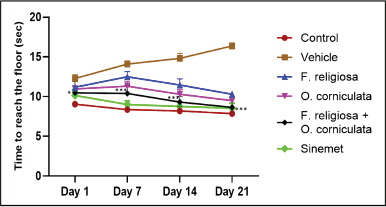 | Figure 4. Effect of F. religiosa and O. corniculata on pole test in the ROT-induced PD mice. Values were expressed as Mean ± SEM. Data were analyzed by repeated measures of two-way ANOVA Tukey post hoc analysis. Each time point has six groups and n = 6 for each group. [Click here to view] |
Beam walk test
The beam balancing activity or time to cross the beam by mice significantly increased in the vehicle-treated group from day 1 (23 ± 1.196), day 7 (25.31 ± 1.43), day 14 (26.32 ± 1.538), and day 21 (28 ± 1.55), respectively, as compared to a control group from day 1 (13.98 ± 0.589), day 7 (13.26 ± 0.974), day 14 (12.98 ± 0.938), and day 21(11.52 ± 0.841), respectively. In addition, a significant interaction between treatment and time was found, indicating that the effect of treatment varied across different time points. A combination dose of F. religiosa + O. corniculata (14.525 ± 0.545) in the treated group exhibits significantly improved balancing activity as well as reaching time to cross the beam at day 21 as compared to other treated groups except for the standard drug Sinemet (13.16 ± 0.786). The result indicated that the reduced time taken to cross the beam in the combination treatment group indicates better balance and motor skills as shown in Figure 5. This demonstrates the potential of the dual extracts to alleviate PD-associated motor deficits linked to nigrostriatal DA depletion.
Wire hanging test
The latency time, or time to fall from a horizontal wire in mice, was evaluated, and the study revealed that it significantly decreased in the vehicle-treated group from day 1 (94 ± 3.80), day 7 (88.33 ± 3.80), day 14 (54.5 ± 2.59), and day 21 (28.83 ± 1.351), respectively, as compared to the control group from day 1 (183.3 ± 7.70), day 7 (196.1 ± 8.45), day 14 (191 ± 9.11), and day 21(192.16 ± 7.94), respectively. In addition, a significant interaction between treatment and time was found, indicating that the effect of treatment varied across different time points. A combination dose of F. religiosa and O. corniculata (167 ± 4.60) treated mice exhibited significantly improved grip strength at day 21 as compared to other treated groups except for the standard drug Sinemet (170.33 ± 6.96). The result indicated that the improved wire grasping ability and muscle strength in extract-treated mice suggests that F. religiosa and O. corniculata administration may help mitigate the motor impairments induced by ROT possibly by reducing oxidative damage and restoring dopaminergic neuronal health as shown in Figure 6.
Effect of F. religiosa and O. corniculata on neurochemical analysis
The administration of a specific compound caused clear changes in various neurochemical markers present within the brain, encompassing TPC, SOD, CAT, GSH, and MDA (Fig. 7). Notably, in a particular experimental group associated with a specific condition, the levels of TPC, CAT, SOD, and GSH exhibited statistically significant reductions (***p < 0.001) when compared to a control group displaying normal characteristics. However, an intervention involving the administration of a combination of two substances, namely F. religiosa (200 mg/kg) and O. corniculata (500 mg/kg), elicited a substantial upsurge in the levels of TPC, GSH, CAT, and SOD (***p < 0.001) when compared to the aforementioned experimental group. Moreover, in the group subjected to the primary compound under investigation, a marked elevation (***p < 0.001) was observed in the levels of MDA and NO, which were subsequently ameliorated upon the application of F. religiosa and O. corniculata at various dosage levels, and the standard medication (Sinemet).
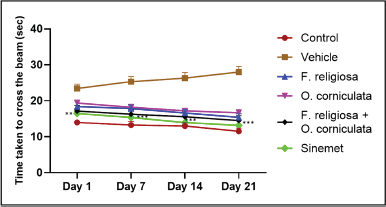 | Figure 5. Effect of F. religiosa and O. corniculata on beam walk test in the ROT-induced PD mice. Values were expressed as Mean ± SEM. Data were analyzed by repeated measures of two-way ANOVA Tukey post hoc analysis. Each time point has six groups and n = 6 for each group. [Click here to view] |
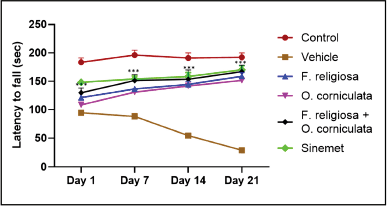 | Figure 6. Effect of F. religiosa and O. corniculata on wire hanging test in the ROT-induced PD mice. Values were expressed as Mean ± SEM. Data were analyzed by repeated measures of two-way ANOVA Tukey post hoc analysis. Each time point has six groups and n = 6 for each group. [Click here to view] |
Effect of F. religiosa and O. corniculata on neurotransmitter levels
ROT administration revealed a pronounced decrease (***p < 0.001) in DA and a rise in glutamate levels (***p < 0.001) when collated with the normal (control) group. The combinational-treated group, i.e., F. religiosa + O. corniculata (200; 500 mg/kg) elevated (***p < 0.001) the levels of DA, while a considerable decline (***p < 0.001) in the level of glutamate was noted when collated to the ROT group (Fig. 8).
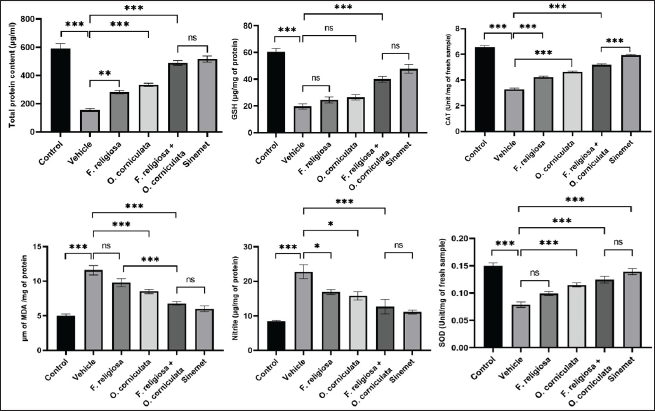 | Figure 7. Effect of F. religiosa and O. corniculata on TPC, GSH, CAT, MDA, NO, and SOD levels in the ROT-induced PD mice. The data were expressed as the Mean ± SEM. Statistical analyses were performed using one-way ANOVA followed by Tukey’s post hoc test; *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the ROT group. [Click here to view] |
Histology of brain tissues
The examination of brain tissue histology revealed the presence of typical neuronal architecture, including glial cells, neutrophils, and blood vessels, in the (A) normal control group. Conversely, the disease group (B) vehicle (ROT) exhibited the presence of distinct plaques and neurofibrillary tangles. Notably, in the individual treatment groups administered with extracts of F. religiosa and O. corniculata at specific doses (200 and 500 mg/kg) (Group C and D), the presence of plaques and neurofibrillary tangles was observed. However, it is interesting to highlight that in the combinational group (E) treated with a combination of ROT, F. religiosa, and O. corniculata, a noticeable reduction in the number of plaques and neurofibrillary tangles was observed. Furthermore, it is noteworthy that the standard treatment group (F) receiving Sinemet displayed enhanced neural architecture in comparison to the other groups (Fig. 9).
DISCUSSION
The results showed that ROT administration significantly increased oxidative stress, inflammation, and motor impairments in mice. Treatment with F. religiosa and O. corniculata extracts alone or in combination significantly attenuated these effects. The combination therapy group showed a greater reduction in oxidative stress, inflammation, and motor impairments compared to the groups treated with either F. religiosa or O. corniculata extract alone. This may be due to targeting multiple pathological mechanisms underlying PD progression.
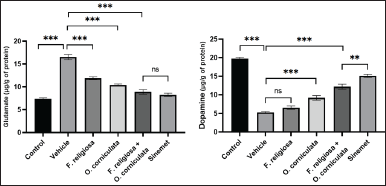 | Figure 8. Effect of F. religiosa and O. corniculata on neurotransmitters, i.e., DA and glutamate levels in the ROT-induced PD mice. The data were expressed as the Mean ± SEM. Statistical analyses were performed using one-way ANOVA followed by Tukey’s post hoc test; *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the ROT group. [Click here to view] |
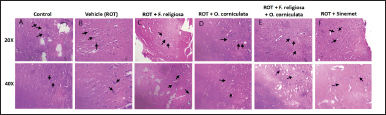 | Figure 9. A representative photomicrograph of SN for all tested groups: (A) control; (B) ROT (ROT) only; (C) ROT + F. religiosa (200 mg/kg); (D) ROT + O. corniculata (500 mg/kg); (E) ROT + F. religiosa + O. corniculata; and (F) ROT + Sinemet. [Click here to view] |
Oxidative stress and neuroinflammation are key factors contributing to the motor impairments and neurodegeneration seen in PD. ROT is known to induce oxidative damage and neuroinflammation leading to apoptosis of dopaminergic neurons. The antioxidants such as flavonoids, coumarins, and phenolic acids in F. religiosa and O. corniculata can counteract these effects by scavenging ROS and suppressing pro-inflammatory mediators. This helps mitigate ROT-induced motor coordination deficits.
The incremental benefits of the extracts likely accumulated over the 21-day treatment period, explaining the maximum effect observed on day 21. The sustained administration allowed time for the antioxidant, anti-inflammatory, and neuroprotective actions to fully manifest in improving neuronal health and motor function.
The crucial measurable characteristics in the OFT include the number of crossings, and the total distance traveled. Reduced exploratory (locomotor) activity in the open field is frequently regarded as a sign of increased emotionality, anxiety, and locomotor activity [35]. The present study revealed that F. religiosa, O. corniculata, and co-administration of F. religiosa and O. corniculata significantly altered different behavioral activities. The decreased locomotor activities in the vehicle group were possibly due to decreasing levels of DA and increased levels of glutamate stimulation due to the induction of PD, with a significant reduction in the total number of squares crossed and total distance travelled, whereas administration of F. religiosa per se and combination with O. corniculata significantly increased the total number of squares crossed. However, the administration of F. religiosa and O. corniculata has also suggested an increase in the total number of squares crossed, but the effect was more apparent in the combination of F. religiosa and O. corniculata. However, these results suggest that a combination treatment of F. religiosa and O. corniculata elevates exploratory behavior in an open-field test.
The wire-hanging test is employed to assess muscle strength [21]. A decline in grip strength would likely impact performance in behavioral tests. The difference in the time of fall from the hanging wire between the vehicle and extract-treated groups was used as an indicator of muscle strength. The administration of the plant extract resulted in a noticeable increase in the time of fall in the wire-hanging test.
The present study utilized the Beam walk test to examine the impact of F. religiosa and O. corniculata on motor coordination and balance. The findings of this study demonstrate that combined doses of F. religiosa and O. corniculata significantly enhanced motor coordination and balance (p 0.05). Previous studies on motor coordination and beam balance in PD have indicated that the plant extract, which is rich in antioxidants, improves these behavioral parameters. The enhancement of motor coordination and beam balance by the extracts of F. religiosa and O. corniculata may be attributed to their antioxidant properties, which are derived from compounds such as flavonoids, cinnamic acid derivatives, coumarins, tocopherols, and phenolic acids. These antioxidants are effective in scavenging ROS.
Based on the data and the obtained results, it can be concluded that the extracts of F. religiosa and O. corniculata improved learning and memory performance in mice in various experimental series. This improvement is likely a result of reducing oxidative stress through the activation of antioxidant defense mechanisms. The beneficial effects of F. religiosa and O. corniculata extracts involve the modulation of cholinergic neurotransmission. Therefore, it holds potential as a therapeutic agent for the treatment of neurodegenerative diseases, including Alzheimer’s disease, Parkinsonism, and other forms of dementia and memory decline.
CONCLUSION
In conclusion, this study has unveiled the remarkable neuroprotective potential of F. religiosa and O. corniculata, particularly when administered in combination, within the context of a ROT-induced mouse model of PD. The key findings, which include improvements in motor function, a reduction in oxidative stress, an increase in DA levels, and a noticeable attenuation of neurodegeneration, underscore the promise of multicomponent herbal therapies for PD and related neurodegenerative disorders. The observed synergistic actions, believed to be due to the simultaneous targeting of multiple sites and mechanisms of action, hold immense promise in enhancing therapeutic outcomes. The potential reduction in toxicity over higher doses of single extracts further strengthens the case for combination therapy. However, it is essential to emphasize that while preclinical evidence is encouraging, the transition to clinical trials in PD patients represents the pivotal step toward realizing these therapeutic benefits. There are many challenges and complexities while moving from preclinical studies to clinical studies including the need to account for individual variability, ethical considerations, regulatory approvals, and resource allocation, which cannot be underestimated. Rigorous randomized controlled trials are the cornerstone for validating the safety and efficacy of this novel treatment approach in the clinical setting, ultimately determining its real-world impact on improving the lives of PD patients. Therefore, the journey from the laboratory to the clinic is a formidable yet essential one, and it is our hope that the remarkable potential demonstrated here, particularly in the context of combination therapy, will pave the way for more effective treatments in the fight against neurodegenerative diseases especially for PD.
LIST OF ABBREVIATIONS
DTNB, 5,5-dithiol-bis-(2-nitrobenzoic acid); DW, Distilled water; F. religiosa, Ficus religiosa; H & E, Hematoxylin-eosin; LD, levodopa; O. corniculata, Oxalis corniculata; PD, Parkinson’s disease; ROT, Rotenone; SNpc, Substantia nigra pars compacta.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJEs) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The experimental protocols (IAEC/MDU/2022/09) were approved by the Institutional Animal Ethics Committee (IAEC) of the Maharshi Dayanand University Rohtak, Haryana, India.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Erro R, Stamelou M. The motor syndrome of Parkinson’s disease. Int Rev Neurobiol. 2017;132:25–32. CrossRef
2. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–40. CrossRef
3. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093–118. CrossRef
4. Cacabelos R. Parkinson’s disease: from pathogenesis to pharmacogenomics. Int J Mol Sci. 2017;18(3):551. CrossRef
5. Kilzheimer A, Hentrich T, Burkhardt S, Schulze-Hentrich JM. The challenge and opportunity to diagnose Parkinson’s disease in midlife. Front Neurol. 2019;10:1328. CrossRef
6. Cerri S, Mus L, Blandini F. Parkinson’s disease in women and men: what’s the difference? J Parkinsons Dis. 2019;9(3):501–15. CrossRef
7. Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–44. CrossRef
8. Sherer TB, Betarbet R, Greenamyre JT. Environment, mitochondria, and Parkinson’s disease. Neuroscientist. 2002;8(3):192–7. CrossRef
9. Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50(5):1346–50. CrossRef
10. Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, et al. Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem Biophys Res Commun. 1989;163(3):1450–5. CrossRef
11. Wu T, Fang X, Xu J, Jiang Y, Cao F, Zhao L. Synergistic effects of ginkgolide B and protocatechuic acid on the treatment of Parkinson’s disease. Molecules. 2020;25(17):3976. CrossRef
12. Singh N, Pillay V, Choonara YE. Advances in the treatment of Parkinson’s disease. Prog Neurobiol. 2007;81(1):29–44. CrossRef
13. Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302(5646):819–22. CrossRef
14. Fahn S, Sulzer D. Neurodegeneration and neuroprotection in Parkinson disease. Neuro Rx. 2004;1(1):139–54. CrossRef
15. Testa CM, Sherer TB, Greenamyre JT. ROT induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res. 2005;134(1):109–18. CrossRef
16. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. CrossRef
17. Chen Y, Hou Y, Ge R, Han J, Xu J, Chen J, et al. Protective effect of roscovitine against ROT-induced parkinsonism. Restor Neurol Neurosci. 2018;36(5):629–38. CrossRef
18. Olanow CW, Koller WC. An algorithm (decision tree) for the management of Parkinson’s disease: treatment guidelines. Neurology. 1998;50(3):S1–57. CrossRef
19. Jankovic J. Motor fluctuations and dyskinesias in Parkinson’s disease: clinical manifestations. Mov Disord. 2005;20(11):11–6. CrossRef
20. Atiq A, Lee HJ, Khan A, Kang MH, Rehman IU, Ahmad R, et al. Vitamin E analog trolox attenuates MPTP-induced parkinson’s disease in mice, mitigating oxidative stress, neuroinflammation, and motor impairment. Int J Mol Sci. 2023;24(12):9942. CrossRef
21. Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835(1):18–26. CrossRef
22. Han B, Zhao H. Effects of hydroxysafflor yellow a in the attenuation of MPTP neurotoxicity in mice. Neurochem Res. 2010;35(1):107–13. CrossRef
23. Meng XH, Liu P, Wang H, Zhao XF, Xu ZM, Chen GH, et al. Gender-specific impairments on cognitive and behavioral development in mice exposed to fenvalerate during puberty. Toxicol Lett. 2011;203:245–51. CrossRef
24. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75. CrossRef
25. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8. CrossRef
26. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. CrossRef
27. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 126(1):131–8. CrossRef
28. Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–94. CrossRef
29. Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21(2):130–2.
30. Kumar AS, Gandhimathi R. Effect of Guettarda speciosa extracts on biogenic amines concentrations in rat brain after induction of seizure. Int J Pharm Pharm Sci. 2009;1(Suppl 1):237–43.
31. Pusa RT, Satyavati D, Dasari R. Effect of Jessica (A Polyherbal Formulation) on the levels of neurotransmitters in the brain of rats. Int J Pharm Sci Rev Res. 2014;24(2):37–42.
32. Gopi C, Sastry VG, Dhanaraju MD. Effect of novel phenothiazine derivatives on brain dopamine in Wistar rats. Beni Suef Univ J Basic Appl Sci. 2019;8(1):1–9. CrossRef
33. Liu L, Li J, Li Q. A novel method for the determination of tiopronin by using potassium ferricyanide as spectroscopic probe reagent in pharmaceutical and urine samples. J Anal Chem. 2012;67(1):41–6. CrossRef
34. Bernt E, Bergmeyer HU. L-Glutamate: determination with glutamic dehydrogenase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Cambridge, MA: Academic Press; 1965. pp. 384–97. CrossRef
35. Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature. 2016;534(7609):688–92. CrossRef