INTRODUCTION
There is a global increase in chronic diseases such as hypertension, obesity, diabetes, and cancer [1]. Hypertension is the most prevalent cardiovascular disease (CVD) with a serious public health concern in both industrialized and developing countries [2]. It is characterized by an increase in blood pressure (BP) in the arteries. The normal systolic and diastolic BP during rest are 100–140 and 60–90 mmHg, respectively. BP that is consistently above 140/90 mmHg indicates hypertension [3]. It has also been implicated to be responsible for other medical conditions such as myocardial infarction, hypertensive retinopathy, convulsion, elevated sugar level [4]. According to previous study, CVDs is responsible for 5% of disability-adjusted life years [2,5]. Many emerging countries, particularly urban populations, are claimed to have prevalence levels that are comparable to those seen in affluent ones [3].
Cocoa (Theobroma cacao L.) is a member Sterculiaceae family and is native to the tropical rain forest of the eastern Andean slopes [6]. It is a perennial crops principally utilized in the manufacturing of chocolate. Cocoa pod husk (CPH) accounts for more than 70% of the total weight. Cocoa is rich in polyphenols such as flavonoids. Chocolate flavonoids boost nitric oxide (NO) production, increase vasodilation, and minimize endothelial dysfunction. Hence, they are beneficial to hypertensive [7].
Due to the concerns about the cost and negative effects of long-term use of synthetic substances, there is increased interest in natural compounds and their derivatives as safer alternatives as functional foods or nautraceuticals [4]. Plants rich in polyphenols have been reported to contain biologically active compounds which offer beneficial protection against chronic degenerative diseases [8]. Cocoa extract has been reported to contain gamma-aminobutyric acid which is a natural amino acid that aids in reducing BP and calming of nerves [9]. However, BP lowering properties of CPH polyphenol has not been scientifically validated. As a result, the aim of this research was to investigate antihypertensive activity of polyphenol-rich fraction of T. cacao pod husk in L-nitro-arginine-methyl-ester (L-NAME)-induced hypertensive male rats as well as appraising computational prediction of drug-like bioactive lead in ethyl acetate fraction of Theobroma cacao pod husk (EAF-TCPH).
MATERIALS AND METHODS
Chemicals and reagents
Captopril was procured from Bristol Pharmaceutical Company (BPC, UK). L-NAME from ElabScience, Texas, USA. Electrosphygmomanometer was a product of Kent Scientific (CODA, Kent Scientific, USA). All other reagents utilised in this research were of analytical grade and were prepared in all-glass apparatus with distilled water.
Collection of sample
The freshly processed CPHs were obtained/collected from a Cocoa plantation at Oko, Surulere LGA, Oyo State, Nigeria. The voucher samples were authenticated at the University of Ilorin, Plant Biology’s Herbarium Unit where a sample was deposited and assigned voucher number UIH 001/786.
Methods used
Preparation of sample extract
Fresh CPHs were air-dried for 15 days to prevent loss of bioactive agents due to irradiation and then pulverized to powdered form using mechanical blender. The sample (100 g of CPH) was soaked in 80% methanol (1:10 w/v) for 48 hours. Then, mixture was filtered and the filtrate obtained was concentrated using rotary evaporator, dried with water-bath at 45°C, and kept in the refrigerator [i.e., methanolic extraction of cocoa pod husk (MCPH)] (Sample 1).
Solvent fractionation of methanol extract of Theobroma cacao pod husk (TCPH)
The sample 1 (above) was subjected to exhaustive solvent/solvent partitioning using organic solvents (n-hexane and ethylacetate) in order of their polarity index. Briefly, 10 g of the dried MCPH extract was dissolved in 20 ml distilled water (1/2 w/v %). Thereafter, the mixture was poured into a separating funnel, this was followed by the addition of 100 ml of n-hexane. The mixture was agitated and set aside until the mixture was separated into three partitioned layers of n-hexane extract, water extract and crude residue of the MCPH extract in the funnel. Each layer was collected into different conical flask. The residue of the crude extract was then again poured into the funnel and 100 ml of n-hexane was again added to continue the partitioning. The process was repeated continuously with the n-hexane solvent until a clear n-hexane solvent appeared. The entire residue obtained from the n-hexane partitioning was further partitioned with ethylacetate solvent, as done with the n-hexane solvent until a clear ethyl acetate solvent appeared in the separating funnel. Each of the portioned fractions (n-hexane and ethylacetate) obtained was then concentrated using a rotary evaporator to remove the solvents. The ethyl acetate yield was subjected to high performance liquid chromatography (HPLC) in order to profile the bioactive components of the sub-fraction (EAF-TCPH) as described by Afolabi et al. [10]. The yields of the fractions were calculated with the ethylacetate fraction having lesser yield (%) than the hexane fraction.
Animals’ treatment protocol
Thirty-six Healthy male Wistar rats weighing 200 ± 1.20 g used for this research were obtained from the Animal Housing Unit of the Department of Biochemistry, University of Ilorin, Ilorin, Kwara State, Nigeria. They were kept in plastic cage in a well-ventilated house condition with free access to rat pellets and water ad libitum. They were allowed to acclimatize for 1 week prior the commencement of the study.
Ethical clearance
Ethical clearance on the use and proper handling of laboratory animals in line with United Nation guidelines was approved by the University of Ilorin Ethical Review Committee (UERC) with the number UERC/ASN/2018/1380.
Induction of hypertension
High BP was induced in the Wistar rats following the exposure to oral dose of 40 mg/kg body weight (bw) L-Name for 3 weeks (21 days). Hypertension was confirmed in rats in the fourth week by measuring an increase in the systolic and diastolic BP in the rats by tail plethysmography using an electrosphygmomanometer. Experimental rats were considered hypertensive at ≥140 mmHg systolic and ≥90 mmHg diastolic.
Animal grouping
The animals used (36 male Wistar rats) in this study were randomly grouped into six groups of six (n = 6) rats as follows; and the choice of extract dosing regimen was informed based on the pilot studies carried out prior the study.
Group 1: Control group (Paraffin oil only);
Group 2: L-NAME induced untreated (Positive control);
Group 3: L-NAME induced + 12.5 mg/kg EAF-TCPH;
Group 4: L-NAME induced + 25 mg/kg EAF-TCPH;
Group 5: L-NAME induced + 50 mg/kg EAF-TCPH;
Group 6: L-NAME induced + 25 mg/kg Captopril.
Note: The doses of EAF-TCPH used were determined during the pilot study and were calculated based on the individual rat’s bw in relation to the chosen concentration.
Molecular docking
The HPLC-identified compounds and Captopril (PDB: 44093) were subjected to docking with the PubChem CID: ACE proteins (PDB: 3BKK) obtained from the protein database. This docking process was performed using Schrodinger’s Grid-Based Ligand Docking with Energetics software, as outlined in the study by Friesner et al. [11] and Mahmoud et al. [12]. The X-ray crystal structure of ACE was acquired from the Protein Data Bank (https://www.rcsb.org/), and subsequently processed using Glide’s protein preparation wizard [13].
Statistical analyses
Data were analyzed using one-way ANOVA, followed by Tukey’s test for post-hoc analysis and graphical representation of results performed using GraphPad Prism 8.5 version (GraphPad Software, San Diego, CA). All values were expressed as mean ± SEM (n = 6). Statistical differences were considered at p < 0.05.
RESULTS
Effect of the administration of EAF-TCPH on systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial volume of L-NAME-induced hypertensive rats
The effect of administration of various doses of sub-fraction of EAF-TCPH on the SBP, DBP and mean arterial volume of hypertensive rats is displayed in Table 1. There was a significant increase (p < 0.05) in the BP of rats administered 40 mg/kg bw of L-NAME for 3 weeks, while there was a significant decrease (p < 0.05) in the SBP, DBP and mean arterial volume in the hypertensive rats following 6 weeks of treatment with the various doses of sub-fraction of EAF-TCPH.
HPLC-identification of bioactive components of EAF-TCPH
The chromatogram of sub-fraction of EAF-TCPH is represented in Figure 1. Theobromine, caffeine, resveratrol, flavanol, flavan-3-ol, procyanidin, catechin, epicatechin, epigallocatechin and theaflavin were the compounds identified. Theaflavin has the highest retention time, while catechin has the highest area (Table 2).
In silico analysis of angiotensin-1 converting enzyme (PDB: 3BKK) in complex with captopril, theaflavin and procyanidin
Figures 2 and 3 shows hydrogen bonding, hydrophobic and binding energy interacion of theaflavin and capropril (standard drug) with angiotensin-1-converting enzyme (ACE) respectively. There were several hydrogen interactions observed in theaflavin at Ser355, His387, Tyr66, Glu143 of ACE. His387,Val518, Val63 are involved in the formation of hydrophobic interactions. Val518 in theaflavin were involved in π-π stack interactions. Gln281, His513, Phe457, His383, Tyr523 and Phe527, His383, Tyr523 and Phe527 are involved in hydrogen bonding. Molecular docking of sub-fraction of TCPH using AutoDock Vina scoring function algorithm shows that procyanidin and theaflavin had the highest binding energy at the binding pocket of angiotensin-1 converting enzyme when compared to the co-crystalized ligand captopril (Table 3).
DISCUSSION
The importance of cocoa in vasodilation, which aids in the management of hypertension by lowering BP has been well-reported previously [14]. These earlier reports emphasized on the presence of bioactive components in cocoa as the basis for this acclaimed activity [15]. Also, health benefit from the intake of tea or chocolate has also been attributed to the consumption of flavan-3-ols and flavonoids. Numerous studies have suggested that the intake of flavan-3-ols attenuates the risk of CVDs, which included; hypertension, coronary heart diseases, myocardial infarction and stroke. Mechanistic in vitro studies confirmed that flavan-3-ols modulate NO production or breakdown, lipid metabolism and platelet function [16–18]. In our study (Table 1), a noteworthy decrease in both SBP and diastolic blood pressure (DBP) was observed in rats administered 12.5 and 25 mg/kg bw of a sub-fraction from EAF-TCPH. This reduction was comparable to the group that received 25 mg/kg bw of captopril. This effect may be attributed to the high polyphenol content found in this sub-fraction (Fig. 1 and Table 2), specifically the flavanol monomer/polymer, as indicated by ?y?elewicz et al. [19]. These findings are supported by a prior study which demonstrated that compounds like catechin and epicatechin have vasodilatory effects by stimulating NO-synthase, leading to an increase in NO and subsequently soluble guanylate cyclase (SGC) activity in the blood vessels [20].
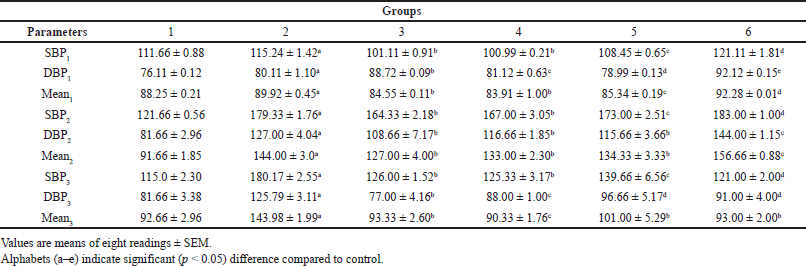 | Table 1. Effect of EAF-TCPH on the SBP, DBP and mean arterial volume of L-NAME- induced hypertensive rats. [Click here to view] |
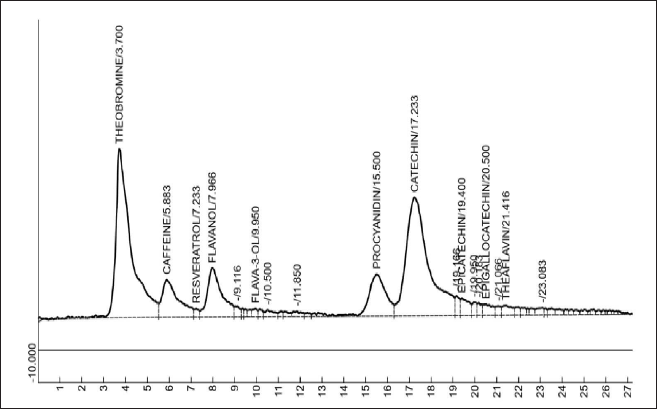 | Figure 1. HPLC-fingerprints of identified bioactive constituents of EAF-TCPH. [Click here to view] |
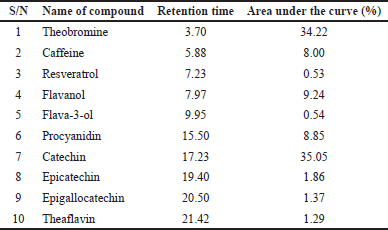 | Table 2. HPLC-identified phytoconstituents of EAF-TCPH. [Click here to view] |
The process by which guanosine triphosphate is transformed into cyclic guanosine-3′,5′-monophosphate is expedited by SGC, thereby instigating the expansion of blood vessels and enhancing blood flow [21]. In a similar vein, polyphenols found in CPHs are recognized for their role in safeguarding against oxidative stress, inhibiting platelet aggregation, promoting insulin sensitivity, and reducing lipid peroxidation [22]. Collectively, these mechanisms may contribute to the reduction of BP and the potential mitigation of CVDs. The major bioactive compounds, including theobromine, caffeine, resveratrol, flavanol, flavan-3-ols, procyanidin, catechin, epicatechin, epigallocatechin, and theaflavin, found in the methanol extract of sub-fractions from TCPH, have been documented for their ability to enhance antioxidant enzymes. Additionally, they have shown a capacity to reduce malondialdehyde levels, thereby playing a crucial role in combating reactive oxygen species, which have been linked to the development of various diseases, including hypertension [23]. The observed reduction in BP attributed to the bioactive components within the EAF-TCPH sub-fraction of TCPH may also be attributed to their ability to inhibit pro-inflammatory cytokines [24].
Acute intake of caffeine has been associated with elevation of BP both in the normotensive and hypertensive individuals [25]. In contrast chronic intake has been associated with protective effects in the development of insulin resistance, glucose intolerance and hypertension [26,27]. Resveratrol, a naturally occurring flavonoid found in EAF-TCPH has previously been reported to have cardioprotective, vasoprotective, antioxidant, anti-vasoconstriction, antidiabetic and neuroprotective properties [28]. Resveratrol has been reported to increase the synthesis of NO (a vasodilator) via the activation protein kinase. Vasodilation lowers BP by lowering peripheral resistance [29]. Theobromine on the other hand has also been reported to have vasodilating properties; thus, playing crucial roles in hypertension. Mechanistically, theobromine was reported to inhibit phosphodiesterase and block the activities of adenosine receptors [30,31]. This mechanism has been reported to be beneficial in hypertensives by preventing obstruction of blood vessels and vascular resistance [32].
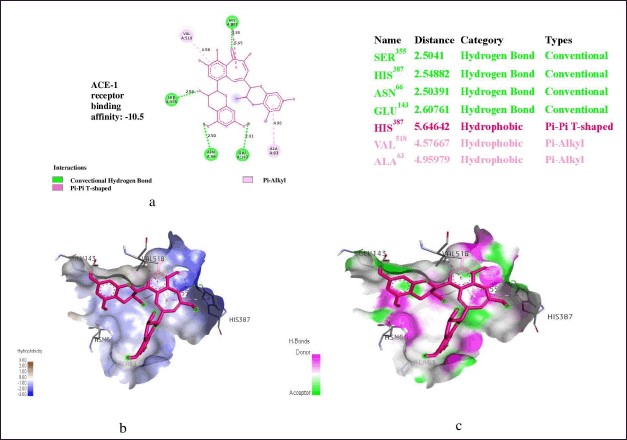 | Figure 2. (a) Binding energy. (b) hydrophobic, and (c) hydrogen bonding interactions of theaflavin with angiotensin-1 converting enzyme. [Click here to view] |
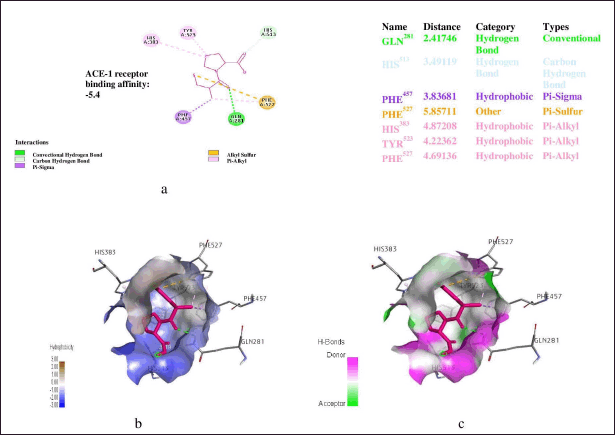 | Figure 3. (a) Binding energy, (b) hydrophobic; and (c) hydrogen bonding interactions of captopril with angiotensin-1 converting enzyme. [Click here to view] |
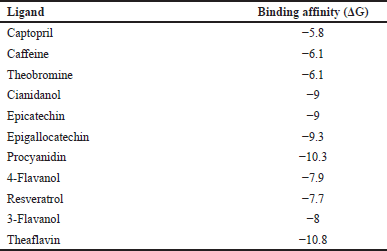 | Table 3. Binding affinities of HPLC-identified compounds from EAF-TCPH and Captopril using AutoDock Vina scoring function algorithm. [Click here to view] |
Similarly, the interactions of these bioactive compounds in lowering BP were also investigated in this study (Figs. 2 and 3; Table 3). The binding sites of the ligands were predicted using P2Rank, a machine learning method that predicts local chemical neighbourhood ligand ability based on points placed on a solvent-accessible protein surface. Points that has high ligand ability score were used to create ligand binding sites. In this study, the first ranked binding pocket of ACE1 has probability of 0.988 with a score of 89.84 compared to the second ranked pocket which has a low probability of 0.279 and a score of 8.27. This implies that the first ranked pocket is the most likely binding site of ligands.
The ligand-based and structural-based (receptor-based) are virtual screening approaches commonly utilized in the discovery of prospective lead compounds against a potential target [33,34]. In this study, all the identified compounds have higher binding affinities than captopril (standard drug); however, two lead compounds (procyanidin and theaflavin) of the 10 compounds had extremely high binding affinities. This implies that the selected designed compounds would be a potential target for drug design against hypertension compared to captopril already available in markets.
The amino acid residues involved in ligand interactions of captopril after molecular docking simulation study are Gln281, His513, Phe457, His383, Tyr523 and Phe527, His383, Tyr523 and Phe527. Hydrogen bonds are the most common directed intermolecular interactions in biological complexes, and they account for the majority of molecular recognition specificity. Because of their severe distance and geometric restrictions, hydrogen bonds are used in medication design to gain specificity. The binding of captopril to ACE-1 resulted in Gln281 hydrogen-donating interaction. Previous study showed that the contribution of hydrogen bonds to free energy is dependent on the local environment. Solvent-exposed hydrogen link contributes more to net interaction energy than a concealed hydrophobic pocket hydrogen bond [35]. Thus, the increased number of hydrogen interactions observed in theaflavin (Ser355, His387, Tyr66,Glu143) may greatly contribute or explained the higher binding affinities or free energy observed in both of the ligands compared to captopril. In previous studies, heavy atoms in N–H…O, N–H…N, and O–H…O hydrogen bonds were shown to be separated by similar median lengths of roughly 3.0. The bond distances for lead complexes were investigated in this study. Theaflavin (Ser355: 2.5041, His387: 2.54882, Tyr66: 2.50391,Glu143: 2.60761) are within the required threshold of median distance of 3.0 Å [36].
Hydrophobic contacts are by far the most common interactions in protein–ligand complexes, they account for 66, 772 connections. The most popular group is formed by an aliphatic carbon in the receptor and an aromatic carbon in the ligand, accounting for about 42, 000 interactions [37]. This shows that aromatic rings frequently inhibits small molecules. In fact, 76% of marketed medications have one or more aromatic rings, with benzene as the most frequent ring system [38]. Leucine side-chains are the most frequent involved in hydrophobic interactions, followed by valine, isoleucine, and alanine side-chains [36]. In this study, theaflavin (His387,Val518, Val63) are involved in the formation of hydrophobic interactions which precisely entails alkyl, π-alky and π-π alkyl types of bond of interactions which are responsible for 3-dimensional structural shape of the complex. More precisely, the π-π stack are the third most common protein-ligand interactions involving aromatic rings. The amino acid residue Val518 in theaflavin involved in π-π stack interactions has been indicated in previous works to enormously contribute to protein-ligand recognition, thus aiding drug design [39,40].
CONCLUSION
The findings from this research highlight the potential therapeutic value of sub-fraction of ethylacetate from the methanol extract of Theobroma cacao pod husk (EAF-TCPH) and the computer-aided interactions of its bioactive compounds (such as theaflavin, epigallocatechin, procyanidin, and epicatechin). The EAF-TCPH showed remarkable antihypertensive and antioxidant properties in both in vitro experiments and L-NAME induced hypertensive rats which could possibly be credited to the presence of HPLC-identified constituents present in the extract. Therefore, EAF-TCPH might be useful as alternative in the management of hypertension.
ACKNOWLEDGMENTS
The authors hereby appreciate the management of Afe Babalola University and University of Ilorin where larger parts of the work were carried out and for creating an enabling environment for the attainment of this research study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There was no financial assistance received for the study either from governmental or non-governmental body.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical clearance on the use and proper handling of laboratory animals in line with United Nation guidelines was approved by the University of Ilorin Ethical Review Committee (UERC) with the number UERC/ASN/2018/1380.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Hernández-Ledesma B, del Mar Contreras M, Recio I. Antihypertensive peptides: production, bioavailability and incorporation into foods. Adv Colloid Interface Sci. 2011;165(1):23–35. CrossRef
2. Bu X, Xie Z, Liu J, Wei L, Wang X, Chen M, et al. Global PM2. 5-attributable health burden from 1990 to 2017: estimates from the Global Burden of disease study 2017. Environ Res. 2021;197:111123. CrossRef
3. Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cífková R, Dominiczak AF, et al. Hypertension (Primer). Nat Rev Dis Primers. 2018;4(1):18014. CrossRef
4. Adeoye RI, Joel EB, Igunnu A, Arise RO, Malomo SO. A review of some common African spices with antihypertensive potential. J Food Biochem. 2022;46(1):e14003. CrossRef
5. Safiri S, Sepanlou SG, Ikuta KS, Bisignano C, Salimzadeh H, Delavari A, et al. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenter Hepatol. 2019;4(12):913–33. CrossRef
6. Ashrafuzzaman M, Sarwar AK. Species diversity of Sterculiaceae at Bangladesh Agricultural University Botanical Garden and their ethnobotanical uses. Asian J Res Bot. 2021;5(4):1–8.
7. Álvarez-Cilleros D, Ramos S, Goya L, Martín MÁ. Colonic metabolites from flavanols stimulate nitric oxide production in human endothelial cells and protect against oxidative stress-induced toxicity and endothelial dysfunction. Food Chem Toxicol. 2018;115:88–97. CrossRef
8. Hayat M, Abbas M, Munir F, Hayat MQ, Keyani R, Amir R. Potential of plant flavonoids in pharmaceutics and nutraceutics. J Biomol Biochem. 2017;1(1):12–7.
9. Diana M, Quílez J, Rafecas M. Gamma-aminobutyric acid as a bioactive compound in foods: a review. J Funct Foods. 2014;10:407–20. CrossRef
10. Afolabi OB, Oloyede OI, Agunbiade OS, Obafemi TO, Aline B, Obajuluwa A, et al. HPLC-DAD profiling and inhibitory potentials of ethylacetate and aqueous extracts of Talinum triangulare on key enzymes linked to type-2 diabetes (α-amylase and α-glucosidase) and oxidative stress (monoamine oxidase). Egypt J Basic Appl Sci. 2019;6(1):99–110. CrossRef
11. Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47(7):1739–49. CrossRef
12. Mahmoud DE, Faraag AH, Abu El-Wafa WM. In vitro study on the potential fungicidal effects of atorvastatin in combination with some azole drugs against multidrug resistant Candida albicans. World J Microbiol Biotechnol. 2021;37:1–3. CrossRef
13. Repasky MP, Shelley M, Friesner RA. Flexible ligand docking with Glide. Curr Protoc Bioinform. 2007;18(1):8–12. CrossRef
14. Muniyappa R, Hall G, Kolodziej TL, Karne RJ, Crandon SK, Quon MJ. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am J Clin Nutr. 2008;88(6):1685–96. CrossRef
15. Tanghe A, Heyman E, Lespagnol E, Stautemas J, Celie B, Op ‘t Roodt J, et al. Acute effects of cocoa flavanols on blood pressure and peripheral vascular reactivity in type 2 diabetes mellitus and essential hypertension. Nutrients. 2022;14(13):2692. CrossRef
16. Duncan AJ, Graham W, Tommasi EN, Gallagher PE, Tallant EA, Chappell MC, et al. Muscadine grape extract improves exercise capacity and reduces oxidative stress in aging transgenic (mRen2) 27 hypertensive female rats. FASEB J. 2017;31:793–12. CrossRef
17. Fayeulle N, Vallverdu-Queralt A, Meudec E, Hue C, Boulanger R, Cheynier V, et al. Characterization of new flavan-3-ol derivatives in fermented cocoa beans. Food Chem. 2018;259:207–12. CrossRef
18. Samaniego E, Anitescu C, Goswami S, Nguyen-Thanh VM, Guo H, Hamdia K, et al. An energy approach to the solution of partial differential equations in computational mechanics via machine learning: concepts, implementation and applications. Comput Methods Appl Mech Eng. 2020;362:112790. CrossRef
19. ?y?elewicz D, Krysiak W, Oracz J, Sosnowska D, Budryn G, Nebesny E. The influence of the roasting process conditions on the polyphenol content in cocoa beans, nibs and chocolates. Food Res Inter. 2016;89:918–29. CrossRef
20. Mangels DR, Mohler III ER. Catechins as potential mediators of cardiovascular health. Arterioscler Thromb Vasc Biol. 2017;37(5):757–63. CrossRef
21. Follmann M, Griebenow N, Hahn MG, Hartung I, Mais FJ, Mittendorf J, et al. The chemistry and biology of soluble guanylate cyclase stimulators and activators. Angew Chem Int Ed. 2013;52(36):9442–62. CrossRef
22. Hügel HM, Jackson N, May B, Zhang AL, Xue CC. Polyphenol protection and treatment of hypertension. Phytomedicine. 2016;23(2):220–31. CrossRef
23. Sinha N, Kumar Dabla P. Oxidative stress and antioxidants in hypertension–a current review. Curr Hypertens Rev. 2015;11(2):132–42. CrossRef
24. Van Beusecum JP, Barbaro NR, Smart CD, Patrick DM, Loperena R, Zhao S, et al. Growth arrest specific-6 and Axl coordinate inflammation and hypertension. Circul Res. 2021;129(11):975–91. CrossRef
25. Rhee JJ, Qin F, Hedlin HK, Chang TI, Bird CE, Zaslavsky O, et al. Coffee and caffeine consumption and the risk of hypertension in postmenopausal women. Am J Clin Nutr. 2016;103(1):210–17. CrossRef
26. Spaeth AM, Goel N, Dinges DF. Cumulative neurobehavioral and physiological effects of chronic caffeine intake: individual differences and implications for the use of caffeinated energy products. Nutr Rev. 2014;72(suppl_1):34–47. CrossRef
27. Beaumont R, Cordery P, Funnell M, Mears S, James L, Watson P. Chronic ingestion of a low dose of caffeine induces tolerance to the performance benefits of caffeine. J Sports Sci. 2017;35(19):1920–7. CrossRef
28. Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. 2014;34(10):837–43. CrossRef
29. Lan F, Weikel KA, Cacicedo JM, Ido Y. Resveratrol-induced AMP-activated protein kinase activation is cell-type dependent: lessons from basic research for clinical application. Nutrients. 2017;9(7):751. CrossRef
30. Babar A, Bujold E, Leblanc V, Lavoie-Lebel É, Paquette J, Bazinet L, et al. Changes in endothelial function, arterial stiffness and blood pressure in pregnant women after consumption of high-flavanol and high-theobromine chocolate: a double blind randomized clinical trial. Hypertens Pregnancy. 2018;37(2):68–80. CrossRef
31. Mladenovic K, Root Y, Ramanathan D. UHPLC-HRMS analysis of theobromine in theobroma cacao and its products. J Nutr. 2018;8(6):1000737. CrossRef
32. Alencar AK, Montes GC, Barreiro EJ, Sudo RT, Zapata-Sudo G. Adenosine receptors as drug targets for treatment of pulmonary arterial hypertension. Front Pharmacol. 2017;8:858. CrossRef
33. Clark DE. What has virtual screening ever done for drug discovery?. Expert Opin Drug Discov. 2008;3(8):841–51. CrossRef
34. Cereto-Massagué A, Ojeda MJ, Valls C, Mulero M, Garcia-Vallvé S, Pujadas G. Molecular fingerprint similarity search in virtual screening. Methods. 2015;71:58–63. CrossRef
35. Shoichet BK. No free energy lunch. Nat Biotechnol. 2007;25(10):1109–10. CrossRef
36. de Freitas RF, Schapira M. A systematic analysis of atomic protein–ligand interactions in the PDB. Medchemcomm. 2017;8(10):1970–81. CrossRef
37. Ritchie TJ, Macdonald SJ. Physicochemical descriptors of aromatic character and their use in drug discovery: miniperspective. J Med Chem. 2014;57(17):7206–15. CrossRef
38. Taylor S, Dirir O, Zamanian RT, Rabinovitch M, Thompson AR. The role of neutrophils and neutrophil elastase in pulmonary arterial hypertension. Front Med. 2018;5:217. CrossRef
39. Tsuzuki S, Honda K, Uchimaru T, Mikami M, Tanabe K. Origin of attraction and directionality of the π/π interaction: model chemistry calculations of benzene dimer interaction. J Am Chem Soc. 2002;124(1):104–12. CrossRef
40. Salonen LM, Ellermann M, Diederich F. Aromatic rings in chemical and biological recognition: energetics and structures. Angew Chem Int Ed. 2011;50(21):4808–42. CrossRef