INTRODUCTION
Essential oils (EOs) are natural, volatile complex bio-compounds that have distinctive strong fragrances and are produced by aromatic plants [1–4]. Previously used for aromatherapy, flavoring agents, or preservatives in food, EOs are now predominantly used to treat a variety of infections as they possess antibacterial and antifungal activities [5–7]. The antimicrobial activity of EOs involves among others, the disruption of the cell membrane of microorganisms, interfering with their metabolic processes or inducing oxidative stress. This ability to exert an antimicrobial property is due to the presence of a wide range of bioactive compounds such as terpenoids, phenolics, and aldehydes [8]. Indeed, there are numerous in vivo and in vitro studies conducted that have shown different responses of microbes on treatment with EOs [9,10]. The biosynthesis of the bioactive compounds in the EOs is a complex and dynamic process that involves the coordinated action of multiple enzymes and pathways. The specific composition and chemical properties of EOs are determined by a combination of genetic, environmental, and developmental factors. Furthermore, there are variations in exhibiting this activity as different amounts of specific bioactive compounds vary and are widely dependent on the plant species, the part of the plant from which the oil is extracted, and the extraction method used.
However, there are few scientific studies validating the bacterial inhibition potential of these commercially available EOs. A body of evidence has shown that EOs consist of bioactive compounds which play an important role in exhibiting beneficial activities such as antiviral, antifungal, as well as antibacterial against resistant pathogens [11–14]. Current antimicrobial agents, which are regarded as the foundation of contemporary clinical medicine, are seriously threatened by the emergence of antimicrobial resistance in a number of bacteria [14]. The agricultural sector is no exception, to prevent food losses brought on by fungi, farmers today rely heavily on synthetic fungicides. The fungicide-resistant pathogens have also emerged, and concerns have been raised about their long-term effects on the environment and public health [15]. Drawing on this, the global trend is toward greener and more sustainable approaches to tackling these issues.
Similar studies were conducted previously [16,17] that reported on the antimicrobial potential of EOs. This study, therefore, aims to investigate the antimicrobial potential of commercially available 14 plant EOs—clove bud (Syzygium aromaticum), lemongrass (Cymbopogon citratus), Rosemary (Rosmarinus officinalis), Ylang-ylang (Cananga odorata), Cinnamon (Cinnamomum zeylanicum), Geranium (Pelargonium graveolens), Tea tree (Melaleuca alternifolia), Myrrh (Commiphora myrrha), Eucalyptus (Eucalyptus globulus), Peppermimt (Mentha piperita), Sweet basil (Ocimum basilicum), Thyme (Thymus vulgaris), Sage (Salvia officinalis), and Camphor (Cinnamomum camphora) on inhibition of Botrytis cinerea, a necrotrophic fungus, Fusarium oxysporum, a Gram-negative bacteria, Fusarium graminearum, a phytopathogenic fungus, and Collectotrichum gleosporoides, a fungus pathogen. Figure 1 represents a visual summary of the main findings of this research paper.
MATERIAL AND METHODS
Selection and purchasing of EOs
A total of 14 EOs were purchased from a reliable supplier in Johannesburg, South Africa. The selection of EOs was based on the literature on the antimicrobial effect of EOs against pathogenic microorganisms.
Selection of plant pathogenic fungi
A total of four plant pathogenic fungi were obtained from the South African National Collection of Fungi, Mycology Unit, Biosystematics Division, Plant Collection Institute (PPRI), Agricultural Research Council located at KwaMhlanga road, Pretoria, South Africa. Table 1 shows plant pathogenic fungi used in this study, their host, and geographic location.
Selection of pathogenic bacteria
A total of 10 different bacteria, 6 Gram-positive and 4 Gram-negative genera, were used in this study. The Gram-positive strains were Bacillus cereus (ATCC 19115), Enterococcus faecalis (ATCC 29212), Enterococcus faecium (ATCC 700221), Listeria monocytogenes (ATCC 19115), and Enterococcus gallinarum (ATCC 700425). Gram-negative bacteria included Salmonella enterica (MG663463), Mannheimia haemolytica, and Escherichia coli 0177 (ATCC 0177) as listed in Table 2. They were all collected from a reliable source. They were all stored in a refrigerator. These bacteria were selected based on their problematic effect on human health and the fact that they cause outbreaks and spread in hospitals.
Antifungal activity (Toxic medium assay)
Potato dextrose agar (PDA) was prepared aseptically and supplemented with Tween-20 (200 μl) as a surfactant. The EOs were evaluated at concentrations of 300, 500, and 1,000 ppm. The agar was poured into 90 mm Petri dishes to solidify. A 5 mm agar plug of fungal mycelia was inoculated in the center of each PDA plate. After 10 days, the mycelial growth was observed and measured (mm) with a ruler. Each test isolate and control were prepared in triplicates. The percentage inhibition of mycelial growth was determined according to the formula [18].
% inhibition = (C−T/C) × 100 (1)
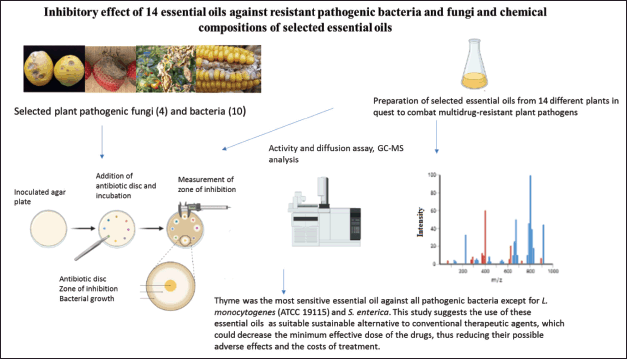 | Figure 1. Graphical abstract of the antimicrobial potential of EOs. [Click here to view] |
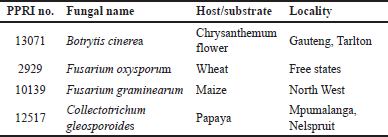 | Table 1. The pathogenic fungi used in this study and their host. [Click here to view] |
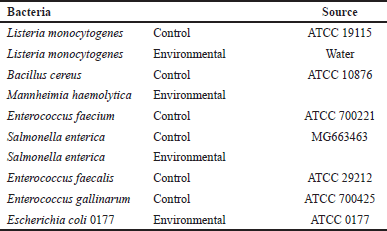 | Table 2. Shows pathogenic bacteria used in this study and their sources. [Click here to view] |
where C = growth in control plates and T = growth in test plates.
Antibacterial activity using disc diffusion assay
The antibacterial effect of tested EOs was determined by disc diffusion assay [19]. Bacterial species were spread in agar plates using sterile earbuds. Filter paper discs were soaked with each EO. The bacterial cultures with discs soaked with EOs were incubated at 37ºC for 24 hours, depending on the indicator microorganism. After incubation, the inhibition zones were measured. All experiments were performed in triplicate, and the results are presented as an average of three replications.
Gas chromatography mass spectrometry (GC-MS) analysis
An Agilent 6890N gas chromatography (GC) system was used to analyze the EO composition while being connected directly to a 5973 MS (Agilent Technologies, Santa Clara, CA). Using a split ratio of 200:1, 1 μl of the sample was injected at a pressure of 24.79 psi and a temperature of 250ºC. An HP-Innowax polyethylene glycol column measuring 60 m by 250 m and having a film thickness of 0.25 m was installed in the GC system (Agilent Technologies, Santa Clara, CA). Initially set to 60ºC for 10 minutes, the oven temperature program increased to 220ºC at a rate of 4ºC/minute and held for 10 minutes before increasing to 240ºC at a rate of 1ºC/minute. As a carrier gas, helium was used at a constant flow rate of 1.2 ml/minute. Electron impact at 70 eV was used to acquire spectra while scanning from 35 to 550 m/z. The MarkerLynxTM application manager software was used to import the exported GC-MS chromatograms and perform peak alignment and selection [20].
Statistical analysis
The results were expressed as the mean of the data obtained in each replicate after each analysis was carried out in triplicate.
RESULTS AND DISCUSSION
Antifungal activity
It can be clearly seen that the antifungal effect of EOs increased with their increasing concentration until it reached the maximum diameter of the inhibition zone. Table 3 outlines the antifungal activities of EOs on plant pathogenic fungi. Thyme EO inhibited all the pathogenic fungi at different concentrations (100% inhibition), followed by cinnamon at 500 and 1,000 μl/l concentration (100% inhibition). Clove bud EO also showed an inhibitory effect against understudied pathogens fungi at 1,000 μl/l (100% inhibition). The findings also showed that the sage EO has a low inhibitory effect at all concentrations, with an inhibitory effect that is less than 40% at 300 μl/l. These results are supported by Hu et al. [21], who also found that cinnamon EO has the strongest antifungal activity against plant pathogenic fungi. Numerous studies demonstrate that thyme EO has antifungal effects [22–25]. The antimicrobial activity of EOs depends on their chemical constituents. The antimicrobial activity of thyme EO is related to the presence of phenolic compounds such as thymol and terpene hydrocarbons (γ-terpinene) because they are lead compounds. Due to their lipophilic nature and low molecular weight, these compounds can cause structural and functional damage in the cells of organisms by disrupting the membrane permeability and osmotic balance of the cell, inhibiting the activity of certain enzymes, and interfering with ergosterol biosynthesis [26].
Results displayed that F. graminearum is more resistant to most of the EOs (39%), followed F. oxysporum (26%). Collectotrichum gleosporoides is the most sensitive or least resistant plant pathogenic fungi (14%), followed by Botrytis cinearea (21%). These findings are against the results of Perczak et al. [19] where they found that F. graminearum is more susceptible to oregano and cinnamon EOs. Inhibitors of deoxynivalenol production by F. graminearum are useful for protecting crops from deoxynivalenol contamination [27]. These results were similar to Hong et al. [28] findings where the C. gleosporoides was more sensitive to cinnamon EO. Krzy?ko-?upicka et al. [26] identified horizontal gene transfer (HGT) process within Fusarium. HGT is an important mechanism of eukaryotic genome evolution, particularly in unicellular organisms.
Table 4 shows the results on the antibacterial activity of EOs against pathogenic bacteria. Lemongrass was the most active EO against all pathogenic bacteria except for E. faecium. It had the highest zone of inhibition (22 mm) against M. haemolytica. Tea tree was the second active EO against all pathogenic bacteria except for E. faecium. It had the second-highest zone of inhibition (21 mm) against L. monocytogenes. Thyme was the most sensitive EO against all pathogenic bacteria except for L. monocytogenes (ATCC 19115) and S. enterica, as shown in Table 1. These findings are supported by Naik et al. [29] who also observed that lemongrass was the most active EO against all test organisms except for Pseudomonas aeruginosa. The inhibition effect of lemongrass may be due to its components such as phenols and flavonoids. Phenolic compounds have been found to inhibit pathogenic microorganisms. Active compounds in lemongrass are myrcene, limonene, citral, geraniol, citronellol, geranyl acetate, neral, and nerol. Citral and geraniol serve as an antibacterial agent [30]. EOs are known to produce secondary metabolites, which are chemically bioactive compounds. Most of these bioactive compounds exhibit antimicrobial activity as initial sources of chemical defense in stressful conditions. The results of this study showed that M. haemolytica and E. faecalis (ATCC 29212) were the most resistant pathogens having the greatest numbers of no activity from different EOs. However, L. monocytogenes (ATCC 19115) was the most sensitive pathogen. In a study by Amat et al. [31], they found that the EOs ajowan, thyme, and fennel inhibited M. haemolytica which is contradictory to our findings. Mannheimia haemolytica has been reported to be a major cause of bovine pneumonia, and antibiotics are used in large amounts to control the pneumonia [32].
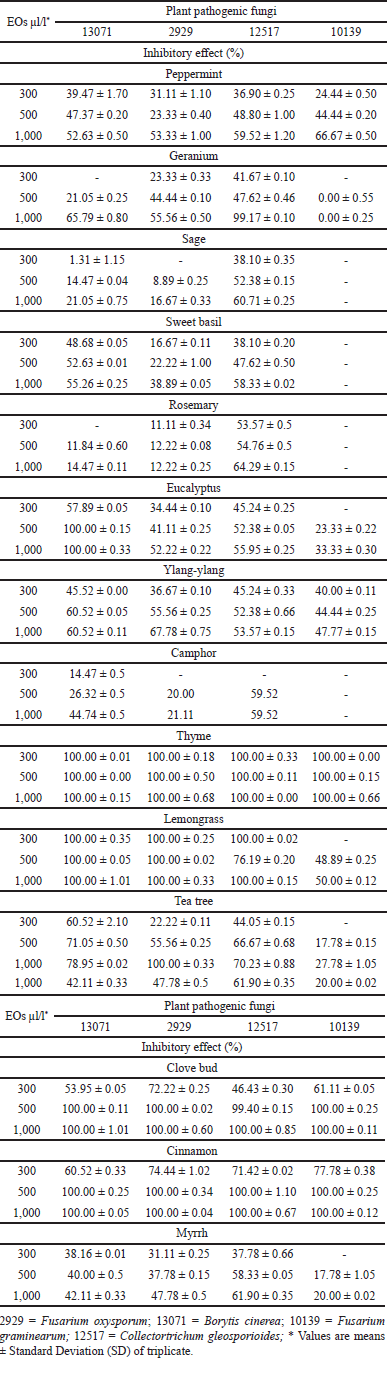 | Table 3. Inhibitory effect (in %) of all selected EOs on plant pathogenic fungi. [Click here to view] |
In addition, another study by Benbelaïd et al. [33] observed that EOs showed good antimicrobial activity and high ability in E. faecalis biofilm eradication. These findings are supported by Mazzarrino et al. [34] who also observed that EOs had a good activity on L. monocytogenes. A study conducted by Dobre et al. [35] found that B. cereus was sensitive against selected EOs and that was also observed in this current study. Most EOs tested showed some inhibition, but only three (lemongrass, tea tree, and cinnamon) showed large inhibition zones against multiple pathogenic strains (Table 4). Lemongrass, thyme, cinnamon, and tea tree were very effective against both Gram-positive and Gram-negative bacteria, while clove bud only inhibited Gram-positive organisms. Previous studies have also shown that cinnamon, clove, and rosemary were inhibitors of bacteria [36]. The least active EOs were thyme and ylang ylang showing the greatest numbers of no-inhibition activity against most pathogenic bacteria that were used. Figure 2 displays the inhibitory activity against bacteria (Fig. 1A and B) and against fungi (Fig. 1C and D).
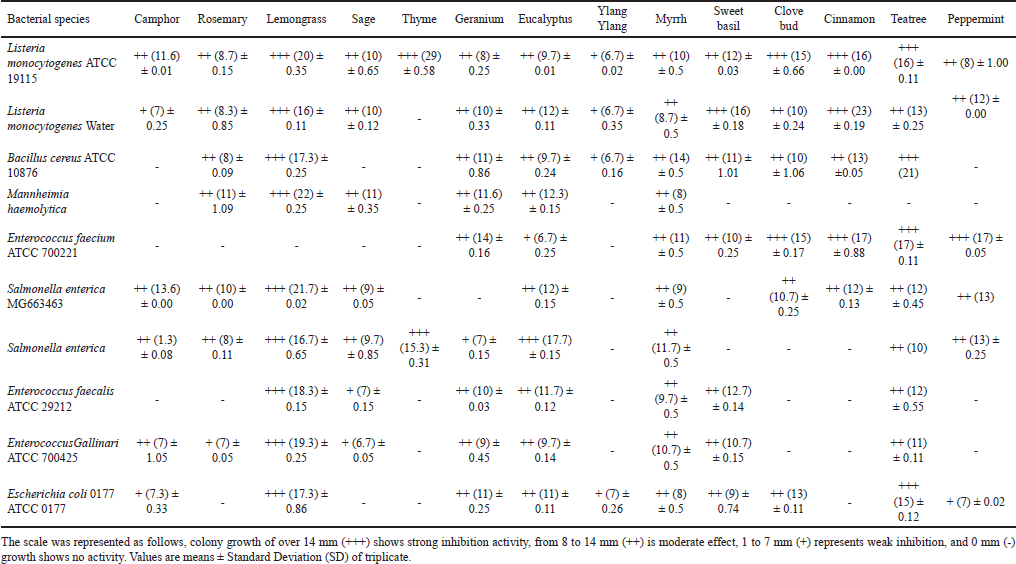 | Table 4. Antibacterial activity of EOs against pathogenic bacterial species. [Click here to view] |
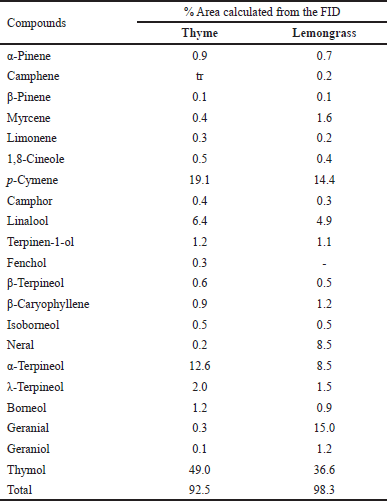 | Table 5. Percentage area of EO analysis of lemongrass and thyme oils. [Click here to view] |
Gas chromatography data reveals that the active EOs comprise one or more high-concentration compounds. The thyme EO was richer in thymol (49%), p-Cymene (19.1%), and α- terpineol (12.6%), and this was in agreement with studies conducted by Santurio et al. [37] and Ahmed et al. [38]. In addition, thymol (36.6%), geraniol (15%), and p-Cymene (14.4%) were the main biocompounds of lemongrass EO. Table 5 shows the breakdown of all the constituents in thyme and lemongrass EOs. Drawing from the results, thymol and p-Cymene are in both EOs at high concentrations, hence we might attribute the activities to these EOs. As anticipated, the EO of lemongrass was found to comprise high levels of thymol and possess some antimicrobial as well as preservation action [39] and insecticidal potential [40].
CONCLUSION
In this study, we established that EOs possess excellent antimicrobial properties against pathogenic-resistant microorganisms. Aromatic plants that produce EOs are rich sources of natural bio compounds exhibiting antibacterial, antifungal, antiviral, insecticidal, and antioxidant activities. Drawing from this, EOs may act as an excellent candidate to decrease the resistance upward trend. Moreover, the utilization of EOs will decrease the minimum effective dose of the drugs, thus reducing their possible adverse effects and the costs of treatment. Subsequently, isolating beneficial compounds and exploring the synergistic effects may lead to toxicity investigation for product development. In conclusion, these EOs have the potential to be used as natural antimicrobial agents in the medical, pharmaceutical, and agricultural industries. This suggests that the EOs tested in this study could be considered as potential alternatives for synthetic antimicrobial agents with modification as their structures could lead to the development of new classes of antibacterial and antifungal compounds.
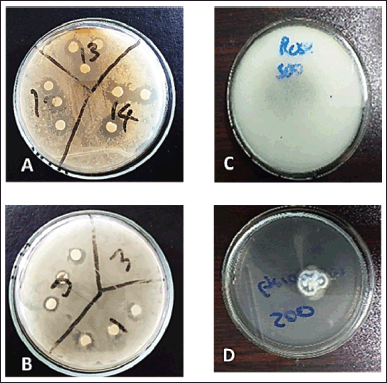 | Figure 2. Zone of inhibition of (A) thyme oil and (B) lemongrass oil against resistant bacteria; inhibitory effects (C) control without oil (D) treated with thyme oil against C. gleosporoides. [Click here to view] |
ACKNOWLEDGMENTS
All authors would like to appreciation to their respective institutes.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJEs) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Akthar MS, Degaga B, Azam T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: a review. GSC Biol Pharm Sci. 2014;2350:1588.
2. Hüsnü K, Ba?er C, Demirci F. Chemistry of essential oils. In: Berger RG, editor. Flavours and fragrances: chemistry, bioprocessing and sustainability. New York, NY: Springer; 2007. pp. 43–86. CrossRef
3. Elyemni M, Louaste B, Nechad I, Elkamli T, Bouia A, Taleb M, et al. Extraction of essential oils of Rosmarinus officinalis L. by two different methods: hydrodistillation and microwave assisted hydrodistillation. Sci World J. 2019;2019. CrossRef
4. Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogenic bacteria. Pharm. 2013;6(12):1451–74. CrossRef
5. Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils–a review. Food Chem Toxicol. 2008;46(2):446–75. CrossRef
6. Lambert RJW, Skandamis PN, Coote P, Nychas GJE. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol. 2001;91:453–62. CrossRef
7. Yuce E, Yildirim N, Yildirim NC, Paksoy MY, Bagci E. Essential oil composition, antioxidant and antifungal activities of Salvia sclarea L. from Munzur Valley in Tunceli, Turkey. Cell Mol Bio. 2014;60(2):1–5.
8. Dorman HD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88(2):308–16. CrossRef
9. Dadalioglu I, Evrendilek GA. Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L), and fennel (Foeniculum vulgare) on common foodborne pathogens. J Agric Food Chem. 2004;52:8255–60. CrossRef
10. De Martino L, De Feo V, Nazzaro F. Chemical composition and in vitro antimicrobial and mutagenic activities of seven lamiaceae essential oils. Molecules. 2009;14:4213–30. CrossRef
11. Álvarez-Martínez FJ, Barrajón-Catalán E, Herranz-López M, Micol V. Antibacterial plant compounds, extracts and essential oils: an updated review on their effects and putative mechanisms of action. Phytomedicine. 2021;90:153626. CrossRef
12. Dawood MA, El Basuini MF, Zaineldin AI, Yilmaz S, Hasan MT, Ahmadifar E, et al. Antiparasitic and antibacterial functionality of essential oils: an alternative approach for sustainable aquaculture. Pathogens. 2021;10(2):185. CrossRef
13. Chouhan S, Sharma K, Guleria S. Antimicrobial activity of some essential oils—present status and future perspectives. Medicines. 2017;4(3):58. CrossRef
14. Yu Z, Tang J, Khare T, Kumar V. The alarming antimicrobial resistance in ESKAPEE pathogens: can essential oils come to the rescue? Fitoterapia. 2020;140:104433. CrossRef
15. Ons L, Bylemans D, Thevissen K, Cammue BP. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms. 2020;8(12):1930. CrossRef
16. Arora H, Sharma A, Sharma S. Thyme essential oil fostering the efficacy of aqueous extract of licorice against fungal phytopathogens of Capsicum annuum L. J Biosci Bioeng. 2023;135(6):466–73. CrossRef
17. Oulkheir S, Aghrouch M, El Mourabit F, Dalha F, Graich H, Amouch F, et al. Antibacterial activity of essential oils extracts from cinnamon, thyme, clove and Geranium against a Gram negative and Gram positive pathogenic bacteria. J Dis Med Plants. 2017;3:1–5.
18. Regnier T, Combrinck S, Du Plooy W, Botha B. Evaluation of Lippia scaberrima essential oil and some pure terpenoid constituents as postharvest mycobiocides for avocado fruit. Postharvest Biol Technol. 2010;57(3):176–82. CrossRef
19. Perczak A, Gwiazdowska D, Marchwi?ska K, Ju? K, Gwiazdowski R, Wa?kiewicz A. Antifungal activity of selected essential oils against Fusarium culmorum and F. graminearum and their secondary metabolites in wheat seeds. Arch Microbiol. 2019;201(8):1085–97. CrossRef
20. Leigh-De Rapper S, Tankeu SY, Kamatou G, Viljoen A, Van Vuuren S. The use of chemometric modelling to determine chemical composition-antimicrobial activity relationships of essential oils used in respiratory tract infections. Fitoterapia. 2021;154:105024. CrossRef
21. Hu F, Tu XF, Thakur K, Hu F, Li XL, Zhang YS, et al. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem Toxicol. 2019;134:110821. CrossRef
22. Manganyi MC. Antimicrobial activity of essentail oils against Fusarium oxysporum isolates and their biofilms [master’s dissertation]. Pretoria, South Africa: Tshwane University of Technology; 2013.
23. Cai J, Yan R, Shi J, Chen J, Long M, Wu W, et al. Antifungal and mycotoxin detoxification ability of essential oils: a review. Phytother Res. 2022;36(1):62–72. CrossRef
24. Moazeni M, Davari A, Shabanzadeh S, Akhtari J, Saeedi M, Mortyeza-Semnani K, et al. In vitro antifungal activity of Thymus vulgaris essential oil nanoemulsion. J Herbal Med. 2021;28:100452. CrossRef
25. Rajkovic K, Pekmezovic M, Barac A, Nikodinovic-Runic J, Arsenijevi? VA. Inhibitory effect of thyme and cinnamon essential oils on Aspergillus flavus: optimization and activity prediction model development. Ind Crops Prod. 2015;65:7–13. CrossRef
26. Krzy?ko-?upicka T, Sokó? S, Sporek M, Piekarska-Stachowiak A, Walkowiak-Lubczyk W, Sudo? A. Effectiveness of the influence of selected essential oils on the growth of parasitic Fusarium isolated from wheat kernels from central Europe. Molecules. 2021;26(21):6488. CrossRef
27. Yaguchi A, Yoshinari T, Tsuyuki R, Takahashi H, Nakajima T, Sugita-Konishi Y, et al. Isolation and identification of precocenes and piperitone from essential oils as specific inhibitors of trichothecene production by Fusarium graminearum. J Agric Food Chem. 2009;57(3):846–51. CrossRef
28. Hong JK, Yang HJ, Jung H, Yoon DJ, Sang MK, Jeun YC. Application of volatile antifungal plant essential oils for controlling pepper fruit anthracnose by Colletotrichum gloeosporioides. Plant Pathol J. 2015;31(3):269. CrossRef
29. Naik MI, Fomda BA, Jaykumar E, Bhat JA. Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacteria. Asian Pac J Trop Med. 2010;3(7):535–8. CrossRef
30. Shendurse AM, Sangwan RB, Kumar A, Ramesh V, Patel AC, Gopikrishna G, et al. Phytochemical screening and antibacterial activity of lemongrass (Cymbopogon citratus) leaves essential oil. J Pharmacogn Phytochem. 2021;10(2):445–9.
31. Amat S, Baines D, Timsit E, Hallewell J, Alexander TW. Essential oils inhibit the bovine respiratory pathogens Mannheimia haemolytica, Pasteurella multocida and Histophilus somni and have limited effects on commensal bacteria and turbinate cells in vitro. J Appl Microbiol. 2019;126(6):1668–82. CrossRef
32. Vulikh K, Bassel LL, Sergejewich L, Kaufman EI, Hewson J, Macinnes JI, et al. Effect of tracheal antimicrobial peptide on the development of Mannheimia haemolytica pneumonia in cattle. PLoS One. 2019;14(11):e0225533. CrossRef
33. Benbelaïd F, Khadir A, Abdoune MA, Bendahou M, Muselli A, Costa J. Antimicrobial activity of some essential oils against oral multidrug–resistant Enterococcus faecalis in both planktonic and biofilm state. Asian Pac J Trop Biomed. 2014;4(6):463–72. CrossRef
34. Mazzarrino G, Paparella A, Chaves-López C, Faberi A, Sergi M, Sigismondi C, et al. Salmonella enterica and Listeria monocytogenes inactivation dynamics after treatment with selected essential oils. Food Control. 2015;50:794–803. CrossRef
35. Dobre A, Gagiu V, Niculi?? P. Preliminary studies on the antimicrobial activity of essential oils against food borne bacteria and toxigenic fungi. Ann Univ Dunarea Jos Galati Fascicle VI-Food Technol. 2011;35(2):16–26.
36. Devi VD, Kalpana G, Saranraj P. Antibacterial activity of essential oils against human pathogenic bacteria. Adv Biol Res. 2017;11(6):357–64.
37. Santurio DF, De Jesus FPK, Zanette RA, Schlemmer KB, Fraton A, Fries LLM. Antimicrobial activity of the essential oil of thyme and of thymol against Escherichia coli strains. Acta Sci Vet. 2014;42(1):1–4.
38. Ahmed OM, Galaly SR, Raslan M, Mostafa MAM. Thyme oil and thymol abrogate doxorubicin-induced nephrotoxicity and cardiotoxicity in Wistar rats via repression of oxidative stress and enhancement of antioxidant defense mechanisms. Biocell. 2020;44:41. CrossRef
39. El-Kased RF, El-Kersh DM. GC–MS profiling of naturally extracted essential oils: antimicrobial and beverage preservative actions. Life. 2022;12(10):1587. CrossRef
40. Ngongang MDT, Eke P, Sameza ML, Djiéto CL, Boyom FF. GC/MS profiling and insecticidal potential of essential oils from Thymus vulgaris (Lamiaceae) and Cymbopogon citratus (Poaceae) against tomato borer, Tuta absoluta. Preprints. 2019;90121. CrossRef