INTRODUCTION
Nanotechnology is an emerging technology in the medical field with promising applications in the treatment of inflammatory diseases [1–3]. Nanoparticles are particles with one or more dimensions of 1–100 nm that exhibit unique size-dependent biological, physical, and chemical properties that differ significantly from those of the bulk materials [4,5]. Silver nanoparticles (AgNPs) are one of the manufactured that are currently available for commercial use [6].
AgNPs have been extensively investigated for their anti-inflammatory activity [2,3,7–11]. Fehaid and Taniguchi [2] reported that the mechanism of AgNPs anti-inflammatory activity was related to a reduction in the expression level of TNF receptor-1 (TNFR-1) due to complex formation between AgNPs and intracellular TNFR-1 [2]. In addition, Yang et al. [3] reported that AgNPs could alleviate inflammation through synergistic M1 macrophage apoptosis and the repolarization of M1-to-M2 macrophage inductions. In vivo studies have reported that the presence of AgNPs leads to a lower concentration of pro-inflammatory cytokines [8,12], faster intestinal tissue healing after injury [13], and decreased activity of matrix metalloproteinase 9 [9,12].
For safety purposes, AgNPs can be orally administered while exerting local therapeutic effects on the gastrointestinal (GI) tract [14,15]. The concentration of the drug at the site of undesired action is expected to be minimized, resulting in an increased therapeutic index. Accordingly, rational approaches must be designed to selectively target the colon with an oral drug, owing to the varied physiology of the GI tract [16]. The design of high molecular weight (MW) polar compounds should lead to very low permeability and drug sequestration in the intestinal lumen. A high MW is also correlated with low solubility, thereby further reducing absorption. Care must be taken to ensure that the compound of interest is not a substrate for the apical uptake transporters [15].
Alginates are among the most attractive polysaccharides for ororectal deliveries. Alginate is a linear copolymer chain of β-D-mannuronic acid (M) and α-L-guluronic acid (G) monomers, with MW ranging from 32 to 400 kg/mol [17–19]. From a regulatory perspective, alginates are recognized as a “generally recognized as safe” materials by the US Food and Drug Administration [19]. Alginate is a hydrophilic polymer with a functional group of carboxylate and hydroxyl [20,21]. These properties result in very low permeability and drug sequestration in the intestinal lumen. Moreover, Sandberg et al. [22] reported that alginate is a dietary fiber, and almost no digestion of alginate occurs in the stomach or small intestine. In addition, Mathieu et al. [23] reported alginate lyases found in the human gut bacterial group, making alginate degradation faster.
Previous study [24] has successfully synthesized alginate-stabilized AgNPs via radiolytic reduction using gamma-ray irradiation at a dose of 20 kGy. The AgNPs were small at approximately 10 ± 5 nm, with spherical shapes and smooth edges. Compared to the Fourier transform infrared (FTIR) spectrum of alginate, the alginate-stabilized AgNPs showed only minor changes in the pattern and position of the absorption band. Thus, the physicochemical properties of alginate, which are important for oro-rectal delivery, may not change after irradiation at 20 kGy [24]. Importantly, gamma radiosynthesis offers unique advantages because it avoids the potential toxicity of the residual reducing agents. This method reduces the Ag+ ions to the uncharged state of Ag0 using short-lived (~ 1 μs) reducing radicals produced during water radiolysis [25,26].
It has been reported that the fate and biological activity of AgNPs within living systems vary depending on the cell type, dose, uptake mechanism, and functionalities of the particles [27–29]. Although many studies have been performed on the biological effects of AgNPs upon oral administration, the materials used differ in size and coating, resulting in great difficulty in comparison. Owing to their peculiarity, toxicological evaluation is required for a particular system of colloidal AgNPs.
In the present study, we evaluated the toxicity of alginate-stabilized AgNPs after 14 days of repeated oral administration in healthy mice, as compared with that of the conventional form of silver ions (AgNO3), measured by body weight change, organ index, and hematological parameters. The safety profile of the AgNPs was also assayed by checking the general serum biochemistry in terms of liver [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] and kidney (urea and creatinine) function indicators, as well as general tissue damage [lactate dehydrogenase (LDH)] indicators. Additionally, AgNPs and silver ions, which probably enter the systemic circulation, will be analyzed for their biodistribution in internal organs (the liver, kidney, spleen, heart, testis, lungs, and brain). Finally, histopathological analysis was performed on hematoxylin and eosin (HE)-stained slides of the selected organs (liver and colon) for the potential induction of tissue injury.
MATERIALS AND METHODS
Materials
The primary material in this research was the radiosynthesized alginate-stabilized AgNPs suspensions obtained from the Research Center for Radiation Process Technology, Research Organization for Nuclear Energy, National Research and Innovation Agency, Indonesia, based on a study reported by Perkasa et al. [24]. Briefly, reaction systems consisted of silver ion precursor of 7.78 mM (analytical grade AgNO3, Merck, Germany) and 1.2% (w/v) alginate (molecular biology grade, Himedia, India) were reduced to alginate-stabilized AgNPs using a Co-60 gamma irradiator (Gammacell 220, MDS Nordion, Canada) at a dose of 20 kGy (dose rate of 3.7 kGy/hour) [24].
Prior to the in vivo experiment, the total silver concentration of the nanosuspension was characterized using inductively coupled plasma–optical emission spectroscopy (ICP-OES; Optima 8300, Perkin Elmer, US), which was found at 0.94 mg/ml. In addition, the particle morphology of the nanosuspension was characterized using transmission electron microscopy (TEM; Tecnai G2 20S-Twin, FEI Company, US), as shown in Figure 1.
TEM imaging showed that the alginate-stabilized AgNPs had a spherical shape with smooth edges. Processing of the TEM micrograph revealed that the AgNPs had a diameter size of 7.76 ± 1.94 nm (Adj. R2 = 0.96). However, it can be observed that some particles had a diameter between 15 and 30 nm.
An AgNPs stock solution was prepared by dissolving the nanosuspension in ultrapure water at a concentration of 0.60 mg/ml. The silver ion stock solution was at a concentration of 0.3 mg/ml, which was prepared by dissolving 23.62 mg of AgNO3 salt (silver mass fraction of 107.87:169.88; analytical grade, Merck, Germany) in 50 ml ultrapure water. The stock solutions were vortexed for 15 seconds to ensure uniformity and then stored at 4°C in the dark.
The 14 days repeated dose oral administration in healthy mice
Animal experiments were conducted in accordance with the procedures described in the guide for the care and use of laboratory animals [30]. The protocols were approved by the local ethics committee of the National Research and Innovation Agency of Indonesia (Indonesian: Badan Riset dan Inovasi Nasional, BRIN) (reference number 007/KE.02/SK/6/2022). Twenty-five male mice strain ddY (3–5 weeks old, weighing 15 ± 5 g) were purchased from the Indonesian Food and Drug. Authority (Indonesian: Badan Pengawas Obat dan Makanan, BPOM). Five male mice were housed in standard polypropylene cages containing sterile dust-free wood chips as bedding in ventilated rooms. They were acclimated for a week before starting the experiment in a controlled environment (temperature 24°C ± 2°C, humidity 60% ± 10%, and 12-hour light/dark cycle) with ad libitum access to tap water and pellet diet. The mice were randomly divided into five groups, each containing five male mice, as shown in Figure 2.
Group 1 (control) was healthy mice treated with water; group 2 was mice treated with 5 mg/kg bw silver ion solution; group 3 was the experimental group treated with 2.5 mg/kg bw AgNPs suspension; group 4 was the experimental group treated with 5.0 mg/kg bw AgNPs suspension; and, group 5 is experimental group treated with 10.0 mg/kg bw AgNPs suspension. The dosage was similar with the low doses used in relevant oral [31,32] and intravenous [33] administration studies using AgNPs with size up to 20 nm. Each mouse was exposed to silver ion solution or AgNPs suspensions using a gavage needle below 20 ml/kg body weight daily for 14 days. The appearance and behavior of the mice were observed during the exposure period. On the 14th day after exposure, the mice were anesthetized by intraperitoneal ketamine injection (100 mg/kg body weight).
Determination of body weight change and organ indexes
Each mouse was weighed individually daily during the 14 days of treatment to observe changes in body weight. Body weight change is expressed as the mean body weight of each group at different time points [31,34,35]. At the end of the in vivo experiment, the organs (liver, kidney, spleen, heart, and testis) were carefully collected and weighed using an electronic balance to calculate the organ index. The organ index (in percent) was calculated using Equation (1) based on a recent study [34]:
Determination of hematological parameters
Blood was collected at the end of the in vivo experiment via cardiac puncture via thoracotomy. A portion of the blood samples was collected using a vacutainer containing the anticoagulant EDTA. The vacutainer was then slowly inverted upside-down to prevent blood clotting. The obtained blood samples were stored at 4°C until further use. Hematological parameters of the samples were analyzed using a veterinary hematology analyzer (series VetScan HM5c, Abaxis, US).
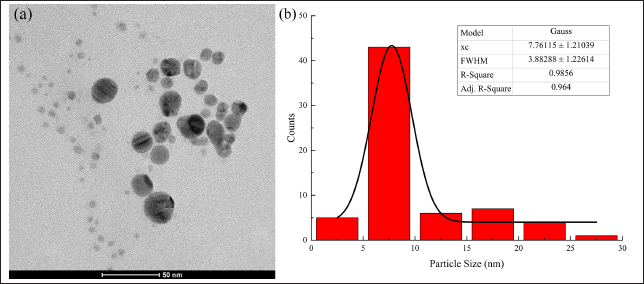 | Figure 1. (a) The TEM micrograph and (b) particle size distribution of the AgNPs suspension. Scale bar 50 nm. AgNPs, silver nanoparticles; TEM, transmission electron microscopy; xc, mean particle size; FWHM, full width at half maximum; R-square, coefficient of determination; Adj. R-square, adjusted R-square. [Click here to view] |
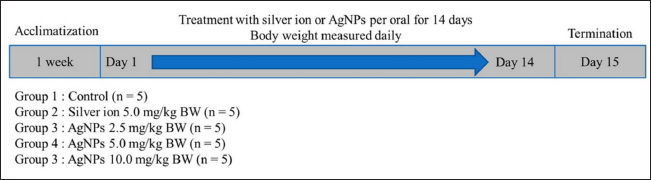 | Figure 2. The timeline of in vivo study. Control, healthy mice; silver ion 5.0 mg/kg BW; silver ion 5.0 mg/kb BW; AgNPs 2.5 mg/kg BW; AgNPs 2.5 mg/kg BW; AgNPs 5.0 mg/kg BW; AgNPs 5.0 mg/kg BW; AgNPs 10.0 mg/kg BW, AgNPs 10.0 mg/kg BW. [Click here to view] |
Determination of biochemical parameters
The serum biochemistry parameters were measured on the basis of the procedure conducted by previous report [36]. The blood samples were centrifuged (3,000 × g) for 15 minutes at 4°C. The serum was harvested and analyzed for liver (ALT and AST), kidney (urea and creatinine), and general tissue damage (LDH) indicators using commercial kits (DiaSys, Indonesia).
Biodistribution of silver in internal organs
Immediately after termination, organs (liver, kidney, spleen, heart, testis, lungs, and brain) were carefully collected and stored at −80°C for silver quantification. Samples were prepared via acid digestion, as following USEPA [37] Method 200.2 with modification. Briefly, a portion of the organ section was homogenized manually to a pasta-like consistency, placed into a digestion vessel, and added with 2.0 ml of concentrated HNO3. The digestion vessel was then gently swirled to mix the acid and the sample. To reduce the sample reactivity during the initial heating, the sample was allowed to equilibrate with the acid mixture at room temperature for 1 hour prior to heating. The sample was then digested for 2 ± 0.25 hours above a hot block at 95°C ± 5°C under atmospheric pressure. Successful digestion was indicated by transformation of the suspension into a transparent solution after digestion.
The samples were then cooled. The extract was diluted quantitatively with ultrapure water to a final volume of 2 ml.The quantification of silver in the biological samples was performed using ICP-OES (series Optima 8300, Perkin Elmer, US) and reported in parts per billion (ppb; 1 ppb ≈ 1 μg/l ≈ 10−3 μg/ml). The silver content was calculated using Equation (2):
Histopathological analysis
Immediately after termination, organs (i.e., colon and liver) were collected and fixed in 10% neutral-buffered formalin (Bio-Optica, Italy). The organs were processed and embedded in paraffin, sectioned at 3–5 μm, and stained with HE dyes for histopathological examination. The scoring scheme for colon inflammation was assessed semi-quantitatively, according to the criteria described by Erben et al. [38]. Liver lesions were assessed according to the criteria described by Neuschwander-Tetri and Caldwell [39]. A blinded pathologist examined the H&E-stained tissue samples. The samples were examined using a light microscope (Optiphot 2, Nikon, Japan) equipped with a camera (series MU900, Amscope, US), in which five field views were examined for each sample.
Statistical analysis
The interval and ratio variables are expressed as mean ± SD, whereas the ordinal variable is expressed as median (minimal-maximal). Experimental data on changes in body weight, organ coefficients, hematological parameters, serum biochemistry, and silver biodistribution were analyzed using one-way ANOVA, followed by post hoc analysis using Tukey’s test. When homogeneity of variance was not assumed, post hoc analysis was performed using Dunnett’s test. The colon and liver histopathology scores were statistically analyzed using the Kruskal-Wallis test followed by post hoc analysis using the Mann Whitney’s pair comparison test. Statistical significance was set at p < 0.05.
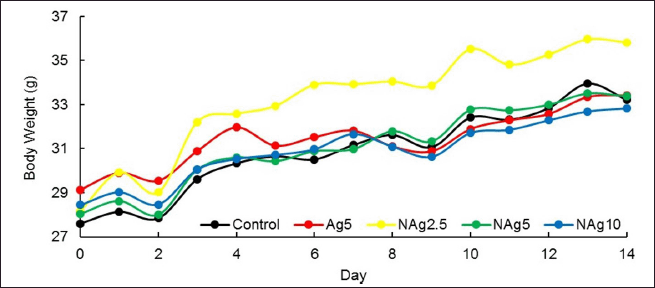 | Figure 3. Dynamic of mean body weight during toxicity study among the groups. The values are presented as means ± SD. Control, healthy mice; Ag5, silver ions 5.0 mg/kb BW; NAg2.5, AgNPs 2.5 mg/kg BW; NAg5, AgNPs 5.0 mg/kg BW; NAg10, AgNPs 10.0 mg/kg BW. [Click here to view] |
RESULT
Body weight and organ index
After 14 days exposure period, no toxicity or mortality was observed in the control or treated groups of mice. All mice were well-groomed and showed no abnormalities in appearance or posture. They maintained normal appetite and activity levels. They also showed a preference for sleeping together in one place. The body weight of all groups increased as the intervention duration increased, and the treatment did not show any significant effect on the body growth pattern of the mice during the 14 days of repeated oral dose administration (Fig. 3). Moreover, there was no significant difference in the final body weight of all animals in the control and treated groups.
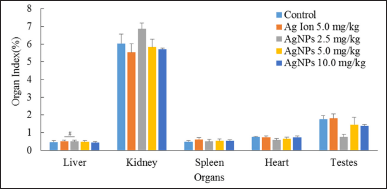 | Figure 4. Percentage of organ indexes among the groups. Values are presented as mean ± SD, #p < 0.05, compared with the silver ion treatment group. Control, healthy mice; Ag ion 5.0 mg/kg, silver ion 5.0 mg/kb BW; AgNPs 2.5 mg/kg, AgNPs 2.5 mg/kg BW; AgNPs 5.0 mg/kg, AgNPs 5.0 mg/kg BW; AgNPs 10.0 mg/kg, AgNPs 10.0 mg/kg BW. [Click here to view] |
Postmortem macroscopic examination of all the studied internal organs (liver, kidney, spleen, heart, and testis) for any possible changes in position, shape, size, and color did not reveal any gross abnormalities. No significant differences were observed in the organ indices of mice in the control and treatment groups, except for the liver, as shown in Figure 4. There was a significant difference in the liver index between the AgNPs 2.5 mg/kg BW treatment group and the silver ion 5.0 mg/kg BW treatment group. However, the differences were not significant when compared with the control group.
Hematological parameters
The effects of 14 days of repeated oral administration of alginate-stabilized AgNPs at various doses (2.5, 5.0, and 10 mg/kg) and silver ions at a dose of 5 mg/kg BW is shown in Table 1. As indicated, there was no significant dose-related change in hematological values related to leukocytes, erythrocytes, and platelets in any of the control and treated groups.
Blood serum biochemistry
Standard biochemical parameters (ALT, AST, LDH, serum urea, and creatinine levels) were examined to investigate the liver and kidney toxicity induced by AgNPs, as shown in Figure 5.
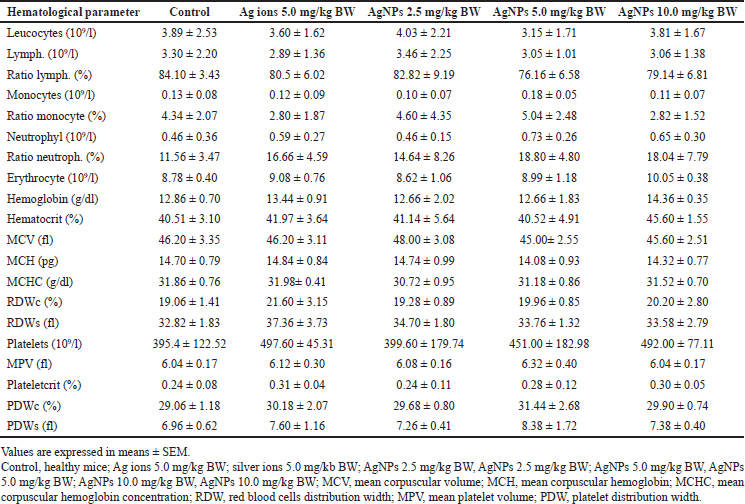 | Table 1. Hematological parameters among the groups. [Click here to view] |
Except for ALT and LDH levels, the biomarkers in the silver ion- and AgNPs-treated groups were not significantly different from those in the control group. Mice treated with silver ions showed a significant elevation in ALT and LDH levels compared with the control. Simultaneously, ALT levels were significantly reduced in the AgNPs-treated groups, which requires further investigation.
Biodistribution of silver
The results showed that the biodistribution of silver after 14 days of repeated dose oral administration of AgNPs followed a dose-dependent manner, as shown in Table 2. For the groups of normal mice and the AgNPs 2.5 mg/kg BW, silver was not detected in various organ samples when measured using ICP-EOS (series Optima 8300, Perkin Elmer, US). The silver level is below the instrument detection limit (7.39 ppb ≈ 7.39 μg/l).
Silver was found in all tested organs after treatment with higher doses of AgNPs and silver ions. The level of total silver was significantly higher in the group treated with 5.0 mg/kg BW silver ions than that in the group treated with AgNPs. Increasing the AgNPs dose from 5.0 to 10.0 mg/kg BW did not significantly increase the total Ag level in the organs.
The silver biodistribution pattern of AgNPs was mainly accumulated in the spleen and liver, with smaller amounts in the kidneys, heart, testis, lungs, and brain. Silver ions mainly accumulate in the liver, spleen, lungs, kidneys, testis, and the brain.
Histopatology analysis of colon and liver
Histopathological examination of the colon was performed to determine the potential inflammation and tissue damage caused by AgNPs exposure (Fig. 6). Microscopic examination of HE-stained longitudinal sections of the colon in the control group showed a normal histological structure (Fig. 6a). At a dose of 2.5 mg/kg BW (Fig. 6c), AgNPs induced mild inflammation, which was observed as the infiltration of inflammatory cells in the colon mucosa. At doses of 5 and 10 mg/kg BW (Fig. 6c and d), the increased penetration of inflammatory cells, loss of surface epithelium, and reduced number of goblet cells were the general conditions. In addition, damage to mucosal architecture has been observed in the form of cryptitis, crypt abscesses, and epithelial hyperplasia. More severe damage was observed in the group treated with silver ions, where granulation tissue was observed, as shown in Figure 6b. It herniated into the submucosal muscle, surrounded by spindle-shaped fibroblasts and inflammatory cells.
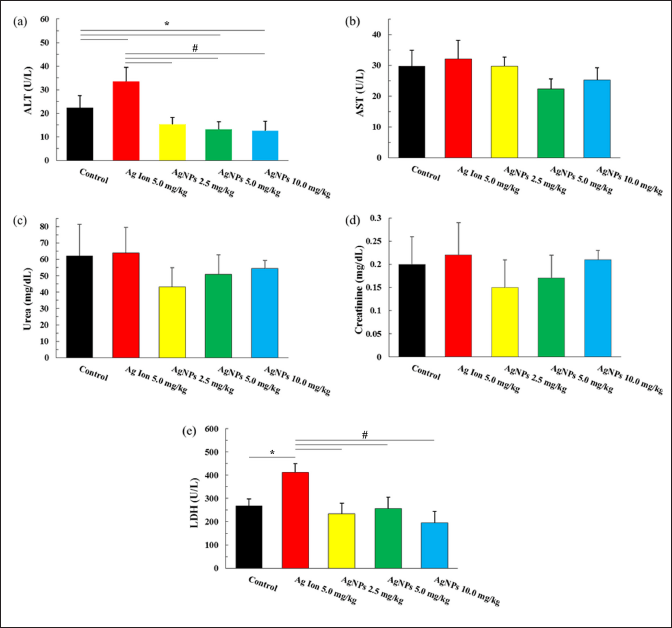 | Figure 5. Serum biochemistry of (a) ALT, (b) AST, (c) urea, (d) creatine, and (e) LDH among the groups. Values are presented as mean ± SD, *p < 0,05 as compared to the control group, #p < 0.05, compared to the silver ion treatment group. Control, healthy mice; Ag ion 5.0 mg/kg, silver ion 5.0 mg/kb BW; AgNPs 2.5 mg/kg, AgNPs 2.5 mg/kg; AgNPs 5.0 mg/kg, AgNPs 5.0 mg/kg BW; AgNPs 10.0 mg/kg, AgNPs 10.0 mg/kg BW; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase. [Click here to view] |
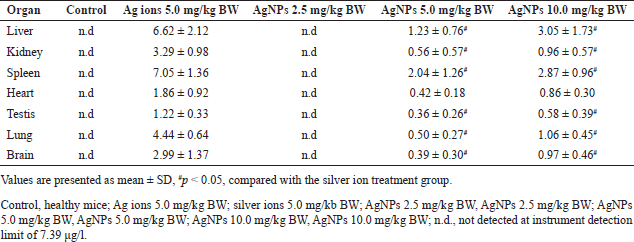 | Table 2. Silver content (μg.g−1) of several mice organs among the groups. [Click here to view] |
The differences in the colon inflammatory score due to silver ion or AgNPs treatment are shown in Table 3. Although mild inflammation was observed, the inflammatory score in the AgNPs 2.5 mg/kg BW group was not significantly different from that in the normal group. While, the increasing AgNPs dose to 5.0 and 10.0 mg/kg BW significantly increased the inflammatory score, but the scores were not significantly different between doses of 5.0 and 10 mg/kg BW doses. These results indicate a dose-dependent effect of AgNPs on the inflammatory response in colon tissue. In addition, the inflammatory score in the 5.0 mg/kg BW silver-ion treatment group was higher than 10.0 mg/kg BW AgNPs group.
Microscopic examination of liver tissue sections from the control group revealed a normal histological structure (Fig. 7a). Simultaneously, mice administered alginate-stabilized AgNPs showed dose-dependent pathological alterations (Fig. 7c–e). As shown in Figure 7c, mice treated with 2.5 mg/kg BW AgNPs showed hepatocellular injury, with remarkable diffuse ballooning and vacuolated cytoplasmic degeneration. Degeneration is reported as reversible cell or tissue damage, and is considered a precursor of necrosis.
Necrotic activity was observed in mice treated with higher AgNPs doses, as shown in Figure 7d and e. Granuloma formation was observed, in which multifocal areas of hepatocellular necrosis were infiltrated by mononuclear inflammatory cells. Congestion of the blood vessel is observed in Figure 7d, while thickened centrilobular veins are observed (Fig. 7e). Compared with Figure 7b, the hepatocellular injury in these groups was similar to that in mice treated with 5.0 mg/kg BW silver ions.
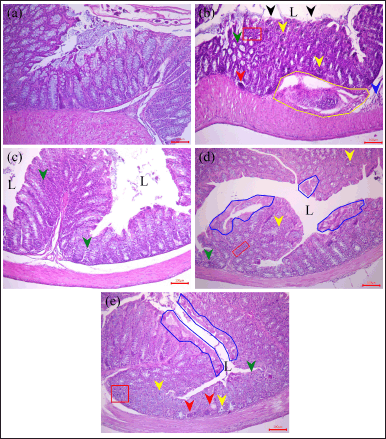 | Figure 6. Representative histological images of HE-stained colon tissue from mice on (a) control, (b) AgNO3 5.0 mg/kg BW, AgNPs (c) 2.5, (d) 5.0, and (e) 10.0 mg/kg BW groups, respectively, at the end of toxicity study. L: lumen; black arrow: epithelial layer erosion; green arrow: inflammatory cells in the mucosa; blue arrow: inflammatory cells in the submucosa; yellow arrow: cryptitis; red arrow: loss of crypt; yellow box: granulation formation area; red box: necrotic area infiltrated with inflammatory cells; blue box: epithelial hyperplasia. Scale bar: 100 μm. Control, healthy mice; Ag ion 5.0 mg/kg BW); silver ion 5.0 mg/kb BW; AgNPs 2.5 mg/kg BW; AgNPs, 2.5 mg/kg BW; AgNPs 5.0 mg/kg BW; AgNPs 5.0 mg/kg BW; AgNPs 10.0 mg/kg BW; AgNPs 10.0 mg/kg BW. [Click here to view] |
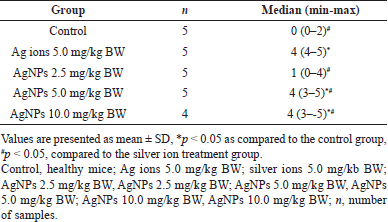 | Table 3. Colon’s inflammatory score among the groups. [Click here to view] |
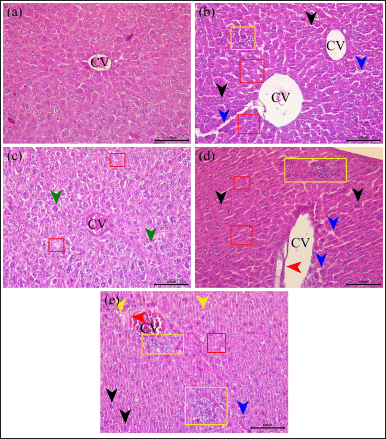 | Figure 7. Representative histological images of HE-stained liver tissue from mice on (a) control, (b) AgNO3 5.0 mg/kg BW, and AgNPs (c) 2.5, (d) 5.0, and (e) 10.0 mg/kg BW groups, respectively, at the end of toxicity study. CV, central vein; green arrow, ballooning hepatocyte with vacuolated degradation; black arrow, bi-nucleate cell; blue arrow, dilated sinusoid; yellow arrow, microvesicle steatosis; red arrow, thickened central vein; yellow box, granulation formation area; red box, necrotic hepatocyte area. Scale bar 100 μm. Control, healthy mice; Ag ion 5.0 mg/kg BW; silver ion 5.0 mg/kb BW; AgNPs 2.5 mg/kg BW; AgNPs, 2.5 mg/kg BW; AgNPs 5.0 mg/kg BW; AgNPs 5.0 mg/kg BW; AgNPs 10.0 mg/kg BW; AgNPs 10.0 mg/kg BW. [Click here to view] |
The difference in the liver lesion score due to silver ion or AgNPs treatment is shown in Table 4. When the mice were treated with AgNPs 2.5 mg/kg BW, the liver lesion score was not significantly different from that of the normal group. However, doses higher than 5 mg/kg BW increased the liver lesion score compared to the normal group, in which the scores were not significantly different between doses of 5.0 and 10 mg/kg BW. These results indicate a dose-dependent effect of AgNPs on the inflammatory response in colon tissue. The liver lesion damage caused by AgNPs was statistically comparable with that of mice treated with silver ion at 5.0 mg/kg BW.
DISCUSSION
The concept of locally acting drugs in the GI tract involves targeting orally administrated drugs to acts specifically in GI tracts for the desired therapeutic action. Thus, systemic uptake of the drug can be minimized, thus minimal drug concentration at non-target tissue, resulting an increased in therapeutic index and lower side effects [14,15]. Previous studies have reported that surface modification is a common method for modifying the properties of AgNPs, such as using polyvinylpyrrolidone (PVP) [31], poly (4-styrenesulfonic acid-co-maleic acid) sodium salt [40], polyethylene glycol [41], sodium alginate [24,36,40], chitosan [42], etc. The type of coating agent determines the toxicity or bioactivity responses [31,40,41]. However, specifically for alginate stabilized AgNPs, the oral toxicity is not fully understood yet. In this study, the research strategies have been designed to avoid the systemic uptake of AgNPs by using alginate as a stabilizing agent.
The biodistribution study showed that the detectable amount of Ag was found in all examined organs of treated mice, except for mice treated with 2.5 mg/kg BW AgNPs. As compared to citrate-stabilized AgNPs at a similar size of about 10 nm, a detectable amount of silver (range 0.001–0.07 μg/g) was found in various mice organs after administered orally at 0.25 and 1.0 mg/kg BW for 4 weeks in which mice were sacrificed 3 days after last administration for recovery [32]. Note that the quantification of Ag content must be affected because AgNPs and Ag ions can be excreted at about 60% and 40%, respectively, since 24 hours after oral administration [43]. These results indicate that high molecular weight stabilizing agent may be effective in preventing systemic uptake as compared to ionic stabilization. Among polymeric stabilizers, alginate showed inferior inhibition as compared to synthetic polymer PVP. At a similar dose of 10 mg/kg BW for 14 days, the highest silver content of 20 nm PVP-stabilized AgNPs was reported in the liver at less than 0.6 μg/ml [31], which is much lower than alginate (3.05 ± 1.73 μg/g).
In all examined organs, the highest Ag content was in silver-ion-treated mice. Prominently, the silver levels were approximately 2–4 times higher for the group treated with 5.0 mg/kg BW silver ions than the two-fold higher AgNPs dose treatment. These results support the evidence that Ag ions show better absorption in the GI tract and biodistribution within the systemic circulation as compared to AgNPs, as previously reported [31,32,44–46].
Among examined organs, the liver had the highest silver levels, indicating that this organ was the main target organ for the toxicity of alginate-stabilized AgNPs. A similar result was observed for AgNPs when stabilized with PVP [31] and amphipilic polymer and carboxylic acid reactive group [34]. The accumulation in this organ is due to uptake by resident phagocytes of the mononuclear phagocytosis system, i.e. Kupffer cells in the liver [31]. Indeed the biodistribution of AgNPs was significantly influenced by its surface chemistry [47]. The highest Ag concentration was detected in the spleen for PEG [47], the lungs for branched polyethylene imine [47], and the brain for citrate anion [31,34].
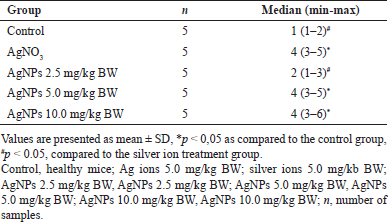 | Table 4. Liver lesion score among the groups. [Click here to view] |
A smaller amount of AgNPs accumulated in the kidneys, heart, testis, lungs, and the brain. The lower silver levels in the kidney than in the spleen and liver indicate that renal excretion is not the primary route of AgNPs elimination. Accordingly, a previous report found that the silver concentration in urine was much lower than in feces [48]. AgNPs are excreted mainly through the biliary pathway from the liver to the bile duct, intestine, and feces [31,48,49]. Specifically of detectable silver content in brain and testis, these results confirm the capability of Ag, whether as AgNPs or Ag ions, to cross the blood-testis barrier and blood-brain barriers [31,32,34,45]. In agreement with a previous studies [32,45], the Ag accumulation in the brain was higher than that in the testis.
As regards to the effects of biodistribution of silver in this study, body weight and clinical behavior are essential non-specific observation parameters in animal experiments that can comprehensively reflect toxicity during animal growth [31,50]. It is note that all treated mice showed no mortality with healthy appearance and behavior. Even more, the mice treated with low-dose (2.5 mg/kg BW) AgNPs had the highest averaged final weight (35.82 ± 2.94 g/mouse) among the five experimental groups (normal group 33.32 ± 0.83 g/mouse), although not statistically significant. The increased body weight similarly to what reported in orally treated mice at 0.25 and 1.0 mg/kg BW for 4 week [32] and rats at 0.2 mg/kg BW for 14 days [51] with citrate coated AgNPs. This results speculating that the low dose of AgNPs had beneficial effect on the growth performance, as reported in pigs treated with AgNPs [52], which could be mediated by antibacterial [26,42,53] or anti-inflammatory [10,11] activity. The insignificant results possibly due to less sensitivity of the body weight parameters to AgNPs administrated orally, as previously reported [31,32,54]. However, the underlying mechanisms and the long-term effects of such a low dose-level administration should be carefully examined.
Upon uptake to systemic circulation, the alginate-stabilized AgNPs up to 10 mg/kg BW for 14 days were not significantly alter the hematological factors in the blood. Similar results were reported in previous studies at lower (0.25–1.0 mg/kg BW of citrate-stabilized AgNPs for 28 days [32]) or similar (10 mg/kg BW of chitosan-stabilized AgNPs for 14 days [33]) dose. Even the hematopoietic organs seem not the main targeted organs for AgNPs toxicity, the alteration in hematology parameters would observed at higher concentration. Hassanen et al. [33] reported reduced leucocyte, erythrocyte, hemoglobin, and platelet were observed in rats treated with chitosan-stabilized AgNPs at 25.0 mg/kg BW and higher.
The effects of alginate-stabilized AgNPs on the main organs of mice examined based on the organ coefficient. Generally, increasing value might be due to hyperemia, edemas, and hypertrophy, while decreasing value indicates that the organ is degenerative and atrophic [31,50,55]. It is found that the organ indexes of the liver, kidney, spleen, heart, and testis of all mice treated with AgNPs were not significantly different observed at the end of treatment compared to that in normal mice. For the liver and spleen with the highest silver content, the silver ion group also showed no significant change in organ indexes.
Further, to observe the effects of AgNPs on the main organ, several standard serum biochemical parameters were examined in terms of liver function (ALT and AST), kidney function (urea and creatinine), and general tissue damage indicator (LDH). The results showed that the oral administration of alginate-stabilized AgNPs was not significantly affect the examined organ functions, except for ALT. The ALT level was significantly reduced at dose 5 mg/kg BW and higher, which required further investigation. For the silver ion group, the elevated level of ALT and LDH were observed. These results provide additional evidence that silver ion is more toxic than AgNPs in biological systems and the liver is the organ target for silver toxicity, as reported by previous studies [31,46,56].
Results from biodistribution and ALT analysis confirmed the potential liver damage, while low absorption of AgNPs and its main excretion route via feces would potentially expose the colon tissue with high concentration AgNPs for a longer period. To confirm the toxicity of AgNPs, histopathological studies on both organs were performed. the liver lesion score was not significantly different from that of the normal group. In regard to no detectable amount of silver in AgNPs treatment at 2.5 mg/kg BW group, the liver and colon histology only showed slight pathological change in which the lesion scorings were not significantly different as compared to normal group. These results are consistent with previous studies reporting no or inconspicuous effects caused by repeated oral administration of naked [57], PVP-coated [58] and citrate-coated AgNPs [32] given at low dose (up to 3.0 mg/kg BW) on host tissues. In contrast, Chen et al. [59] reported the colitis-like symptom in mice that orally administrated with 0.25 mg/kg BW negatively charged AgNPs for 7 days.
The pathological changes in the colon were more obvious at AgNPs dose of 5.0 mg/kg BW and higher. Moderate penetration of inflammatory cells in the mucosa and submucosa, loss of surface epithelium, and reduced goblet cell number were the general conditions. Foci of inflammatory cells were observed in the mucosa during the inflammatory response. Furthermore, damage to the mucosal architecture has been observed in the form of cryptitis, crypt abscesses, and epithelial hyperplasia. Consistently, the silver ion showed significantly higher toxicity even as compared to the twofold dose of AgNPs. Silver ion caused moderate inflammation in the mucosa and submucosa, with epithelial layer erosion at several sites and foci of inflammation. Even more, the herniation of submucosal muscles surrounded by spindle-shaped fibroblasts and inflammatory cells is observed. Either for AgNPs and silver ion, the colon lesion seems initiated upon epithelial damage when in contact with AgNPs in colon’s lumen.
Meanwhile, the hepatocellular injury in AgNPs doses of 5.0 and 10.0 mg/kg BW were similar to that in mice treated with 5.0 mg/kg BW silver ion. The moderate-to-severe hepatic lesions were observed. The hepatocyte injuries manifested as bi-nuclei, ballooning degradation, or as microvesicular steatotic hepatocytes. The granuloma formation was also observed, in which the multifocal areas of hepatocellular necrosis were infiltrated by mononuclear inflammatory cells. Even more, the congestion of the blood vessels and the thickened centrilobular veins were observed.
As compared to polymer-stabilized AgNPs, the alginate-stabilized AgNPs showed the toxic effect on the liver and colon at much lower doses. Wang et al. [60] reported that there was no major macroscopic changes in colon tissue after oral administration of 25 nm PVP-coated AgNPs at 50 mg/kg BW for 10 days. Gan et al. [31] reported that there was no significant pathological in liver tissue after oral administration of 20 nm PVP-coated AgNPs at 10 mg/kg BW for 14 and 28 days. Hassanen et al. [33] reported that there was no significant liver lesion score after intraperitonial administration of 20 nm chitosan-coated AgNPs at 10 mg/kg BW for 14 days.
Overall, the results confirmed that biodistribution and toxicity/biological activity of AgNPs within living systems depend on various factors, including the cell type, dose, uptake mechanism, and functionalities of the particles [27–29]. For the biodistribution study, the steric stabilization using alginate successfully inhibit absorption of orally administrated AgNPs as compared to surface-charge stabilization, in particular using citrate anion. For bioactivity study, the dose of alginate-stabilized AgNPs at 2.5 mg/kg BW can be an upper dose in which no or inconspicuous toxic effect is observed upon repeated oral administration.
In the context of locally acting drugs, the upper dose from alginate (negatively charged biopolymers) is lower as compared to PVP (neutral charged synthetic polymer, at 10 mg/kg BW [31]) and chitosan (positively charged biopolymers, at 10 mg/kg BW [33]). Indeed it has been reported that negatively charge stabilizing agent tend to be more toxic that neutral or positively charged stabilizing agent [40]. However, drug delivery technology I more likely a trade-off rather than fix formula. For example, biopolymer superior for biodegradability, while synthetic polymer excellent in stability and purity; Chitosan is less toxic than alginate, on the other side, alginate can be dissolved in water while chitosan requires an acidic solvent. Owing to anti-inflammation properties of AgNPs [8,10,11,61], alginate can find its application on delivery of locally acting inflammatory agent based on AgNPs which specifically targeting the inflamed mucose in GI tract. It has been reported that the inflammation in the GI tract is associated with the depletion of the mucus layer followed by accumulation of short positively charged bactericidal proteins (such as defensins and antimicrobial anti-proteases) in the lesion site of the mucosal surface [62]. Negatively charged alginate might bind with these proteins via electrostatic interaction, form polyelectrolyte complex, then allow loading of high concentration drugs locally at inflamed mucose [63].
CONCLUSION
In conclusion, there were no signs of toxicity after 14 days of repeated oral administration of alginate-stabilized AgNPs up to a dose of 10 mg/kg BW related to body weight, organ index, hematological parameters, and general serum biochemistry. Signs of toxicity were observed in liver function when mice were treated with silver ions, even at a lower dose. These results suggest that Ag ions are more toxic than alginate-stabilized AgNPs after oral administration.
The toxicity outcomes and biodistribution of alginate-stabilized AgNPs were dose-dependent. Alginate-stabilized AgNPs at a dose of 2.5 mg/kg BW were not detected in the internal organs of mice after 14 days of repeated oral administration. Unfortunately, silver was detected in all tested organs after treatment with a higher dose, including organs with a privileged blood supply, such as the brain and testis. Although the histopathological scores for liver and colon injury were not significantly different, mild injury to these tissues indicated that systemic uptake still occurred at a dose of 2.5 mg/kg BW. Further research should be conducted at doses lower than 2.5 mg/kg BW to determine the highest dose at which signs of toxicity are absent.
ACKNOWLEDGMENT
We thank Muhammad Yasin Yunus Bin Imam Cholil from the Research Center for Radiation Process Technology, Research Organization for Nuclear Energy—National Research and Innovation Agency, for providing alginate-stabilized AgNPs for this research. We also thank Annisa Ulhuriyah from the Department of Pharmacology and Therapeutics, Faculty of Medicine, Universitas Indonesia, for her excellent technical assistance in general serum biochemistry measurements.
AUTHOR CONTRIBUTIONS
DPP, WA, K, and MK designed the experiments, analyzed the data, and wrote the manuscript. DPP, TP, RIL, and AAP performed the experiments on the biodistribution of silver in internal organs. DPP, WA, IS, and DPR performed in vivo experiments. DPP and K performed histopathological analysis. All the authors contributed to the study and approved the final version of the manuscript.
FINANCIAL SUPPORT
This research was supported by grants from the National Research and Innovation Agency, Indonesia, for Program RIIM Gel. I years 2022 (No.: B-2103/III.2/HK.04.03/7/2022) under contract No.: B-811/II.7.5/FR/6/2022.
CONFLICT OF INTEREST
The author reports no conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approval details are given in the Materials and Methods section.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Agarwal H, Nakara A, Shunmugam VK. Anti-in?ammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: a review. Biomed Pharmacother. 2018;109(2019):2561–72. CrossRef
2. Fehaid A, Taniguchi A. Silver nanoparticles reduce the apoptosis induced by tumor necrosis factor alpha. Sci Technol Adv Mater. 2018;19(1):526–34. CrossRef
3. Yang Y, Guo L, Wang Z, Liu P, Liu X, Ding J, et al. Targeted silver nanoparticles for rheumatoid arthritis therapy via macrophage apoptosis and re-polarization. Biomaterials. 2021;264:120390. CrossRef
4. Ghoreishian SM, Kang SM, Raju GSR, Norouzi M, Jang SC, Yun HJ, et al. γ-radiolysis as a highly efficient green approach to the synthesis of metal nanoclusters: a review of mechanisms and applications. Chem Eng J. 2018;360:1390–406. CrossRef
5. ?ubová K, ?uba V. Synthesis of inorganic nanoparticles by ionizing radiation—a review. Radiat Phys Chem Oxf Engl 1993. 2019;158:153–64. CrossRef
6. OECD. List of manufactured nanomaterials and list of endpoints for phase one of the sponsorship programme for the testing of manufactured nanomaterials: revision. Paris, France: OECD; 2010.
7. Singh P, Ahn S, Kang JP, Veronika S, Huo Y, Singh H, et al. In vitro anti-inflammatory activity of spherical silver nanoparticles and monodisperse hexagonal gold nanoparticles by fruit extract of Prunus serrulata: a green synthetic approach. Artif Cells Nanomed Biotechnol. 2017;46(8):2022–32. CrossRef
8. Liu X, Gao P, Du J, Zhao X, Wong KKY. Long-term anti-inflammatory efficacy in intestinal anastomosis in mice using silver nanoparticle-coated suture. J Pediatr Surg. 2017;52(22):2083–7. CrossRef
9. O’Sullivan S, Gilmer JF, Medina C. Matrix metalloproteinases in in?ammatory bowel disease: an update. Mediators Inflamm. 2015;2015:964131. CrossRef
10. Siczek K, Zatorski H, Chmielowiec-Korzeniowska A, Pulit-Prociak J, ?miech M, Kordek R, et al. Synthesis and evaluation of anti-inflammatory properties of silver nanoparticle suspensions in experimental colitis in mice. Chem Biol Drug Des. 2017;89(4):538–47. CrossRef
11. Krajewska JB, D?ugosz O, Sa?aga M, Banach M, Fichna J. Silver nanoparticles based on blackcurrant extract show potent anti-inflammatory effect in vitro and in DSS-induced colitis in mice. Int J Pharm. 2020;585:119549. CrossRef
12. Bhol KC, Schechter PJ. Effects of nanocrystalline silver (NPI 32101) in a rat modelof ulcerative colitis. Dig Dis Sci. 2007;52(10):2732–42. CrossRef
13. Zhang S, Liu X, Wang H, Peng J, Wong K. Silver nanoparticle-coated suture effectively reduces inflammation and improves mechanical strength at intestinal anastomosis in mice. J Pediatr Surg. 2014;49(4):606–13. CrossRef
14. Charmot D. Non-systemic drugs: a critical review. Curr Pharm Des. 2012;18(10):1434–45. CrossRef
15. Filipski KJ, Varma MV, El-Kattan AF, Ambler CM, Ruggeri RB, Goosen TC, et al. Intestinal targeting of drugs: rational design approaches and challenges. Curr Top Med Chem. 2013;13(7):776–802. CrossRef
16. Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Del Rev. 2012;64(6):557–70. CrossRef
17. Agüero L, Zaldivar-Silva D, Peña L, Dias ML. Alginate microparticles as oral colon drug delivery devices: a review. Carbohydr Polym. 2017;168:32–43. CrossRef
18. Sosnik A. Alginate particles as platform for drug delivery by the oral route: state-of-the-art. ISRN Pharm. 2014;2014:926157. CrossRef
19. Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–26. CrossRef
20. Zhao Y, Chen H, Wang Y, Li Q. Effect of sodium alginate and its guluronic acid/mannuronic acid ratio on the physicochemical properties of high?amylose cornstarch. Starch. 2016;68(11–12):1215–23. CrossRef
21. Larsen BE, Bjørnstad J, Pettersen EO, Tønnesen HH, Melvik JE. Rheological characterization of an injectablealginate gel system. BMC Biotechnol. 2015;15:29. CrossRef
22. Sandberg AS, Andersson H, Bosaeus I, Carlsson NG, Hasselblad K, Härröd M. Alginate, small bowel sterol excretion, and absorption of nutrients in ileostomy subjects. Am J Clin Nutr. 1994;60(5):751–6. CrossRef
23. Mathieu S, Touvrey-Loiodice M, Poulet L, Drouillard S, Vincentelli R, Henrissat B, et al. Ancient acquisition of “alginate utilization loci” by human gut microbiota. Sci Rep. 2018;8:8075. CrossRef
24. Perkasa DP, Arozal W, Kusmardi, Syaifudin M. Response surface optimization of gamma irradiation synthesis of alginate-stabilized silver nanoparticles without addition of a hydroxyl radical scavenger. Atom Indones. 2022;48(1):247–57. CrossRef
25. Shirokova LN, Revina AA, Aleksandrova VA, Fenin AA. Radiation-chemical synthesis of silver nanoparticles in aqueous solution of chitin derivative. Inorg Mater Appl Res. 2016;7(5):730–6. CrossRef
26. Hosny AMS, Kashef MT, Rasmy SA, Aboul-Magd DS, El-Bazza ZE. Antimicrobial activity of silver nanoparticles synthesized using honey and gamma radiation against silver-resistant bacteria from wounds and burns. Adv Nat Sci Nanosci Nanotechnol. 2017;8(4):7. CrossRef
27. Swanner J, Fahrenholtz CD, Tenvooren I, Bernish BW, Sears JJ, Hooker A, et al. Silver nanoparticles selectively treat triple-negative breast cancer cells without affecting non-malignant breast epithelial cells in vitro and in vivo. FASEB Bioadv. 2019;1(10):639–60. CrossRef
28. Franková J, Pivodová V, Vágnerová H, Jurá?ová J, Ulrichová J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. J Appl Biomater Funct Mater. 2016;14(2):e137–42. CrossRef
29. Khorrami S, Zarrabi A, Khaleghi M, Danaei M, Mozafari MR. Selective cytotoxicity of green synthesized silver nanoparticles against the McF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int J Nanomed. 2018;2018(13):8013–24. CrossRef
30. NRC. The guide for the care and use of laboratory animals. 8th ed. Washington, DC: The National Academies Press; 2011.
31. Gan J, Sun J, Chang X, Li W, Li J, Niu S, et al. Biodistribution and oxidative damage following 28 days oral administration of nanosilver with/without coating in mice. J Appl Toxicol. 2020;40(6):815–31. CrossRef
32. Recordati C, Maglie MD, Cella C, Argentiere S, Paltrinieri S, Bianchessi S, et al. Repeated oral administration of low doses of silver in mice: tissue distribution and effects on central nervous system. Part Fibre Toxicol. 2021;18:1–8. CrossRef
33. Hassanen EI, Khalaf AA, Tohamy AF, Mohammed ER, Farroh KY. Toxicopathological and immunological studies on different concentrations of chitosan-coated silver nanoparticles in rats. Int J Nanomed. 2019;14:4723–39. CrossRef
34. Yang L, Kuang H, Zhang W, Aguilar ZP, Wei H, Xu H. Comparisons of the biodistribution and toxicological examination after repeated intravenous administration of silver and gold nanoparticles in mice. Sci Rep. 2017;7(1):3303. CrossRef
35. Zhang Y, Ferguson SA, Watanabe F, Jones Y, Xu Y, Biris AS, et al. Silver nanoparticles decrease body weight and locomotor activity in adult male rats. Small. 2013;9(9–10):1715–20. CrossRef
36. Arozal W, Monayo ER, Barinda AJ, Perkasa DP, Soetikno V, Nafrialdi N, et al. Protective effects of silver nanoparticles in isoproterenol-induced myocardial infarction in rats. Front Med. 2022;9:867497. CrossRef
37. USEPA. Method 200.2 Revision 2.8. Sample preparation procedure for spectrochemical determination of total recoverable elements. Washington, DC: United States Environmental Protection Agency; 1994.
38. Erben U, Loddenkemper C, Doerfel K, Spieckermann S, Haller D, Heimesaat MM, et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol. 2014;7(8):4557–76.
39. Neuschwander-Tetri BA, Caldwell SH. Nonalkoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003;37:1202–19. CrossRef
40. Netchareonsirisuk P, Puthong S, Dubas S, Palaga T, Komolpis K. Effect of capping agents on the cytotoxicity of silver nanoparticles in human normal and cancer skin cell lines. J Nanopart Res. 2016;18:1. CrossRef
41. Zhao J, Liu P, Ma J, Li D, Yang H, Chen W, et al. Enhancement of radiosensitization by silver nanoparticles functionalized with polyethylene glycol and aptamer As1411 for glioma irradiation therapy. Int J Nanomed. 2019;2019(14):9483–96. CrossRef
42. Badawy MEI, Lotfy TMR, Shawir SMS. Preparation and antibacterial activity of chitosan-silver nanoparticles for application in preservation of minced meat. Bull Nat Res Cent. 2019;43:1–4. CrossRef
43. Loeschner K, Hadrup N, Qvortrup K, Larsen A, Gao X, Vogel U, et al. Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Part Fibre Toxicol. 2011;6(1):18. CrossRef
44. Zande MVD, Vandebriel RJ, Doren EV, Kramer E, Rivera ZH, Serrano-Rojero CS, et al. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano. 2012;6(8):7427–42. CrossRef
45. Recordati C, Maglie MD, Bianchessi S, Argentiere S, Cella C, Mattiello S, et al. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: nano-specific and size-dependent effects. Part Fibre Toxicol. 2016;13:12. CrossRef
46. Boudreau MD, Imam MS, Paredes AM, Bryant MS, Cunningham CK, Felton RP, et al. Differential effects of silver nanoparticles and silver ions on tissue accumulation, distribution, and toxicity in the Sprague Dawley rat following daily oral gavage administration for 13 weeks. Toxicol Sci. 2016;150(1):131–60. CrossRef
47. Pang C, Brunelli A, Zhu C, Hristozov D, Liu Y, Semenzin E, et al. Demonstrating approaches to chemically modify the surface of Ag nanoparticles in order to influence their cytotoxicity and biodistribution after single dose acute intravenous administration. Nanotoxicology. 2016;10(2):129–39.
48. Park K, Park EJ, Chun IK, Choi K, Lee SH, Yoon J, et al. Bioavailability and toxicokinetics of citrate-coated silver nanoparticles in rats. Arch Pharm Res. 2011;34(1):153–8. CrossRef
49. Xue Y, Zhang S, Huang Y, Zhang T, Liu X, Hu Y, et al. Acute toxic effects and gender-related biokinetics of silver nanoparticles following an intravenous injection in mice. J Appl Toxicol. 2012;2012(32):11. CrossRef
50. Ganguly P, Breen A, Pillai SC. Toxicity of nanomaterials: exposure, pathways, assessment, and recent advances. ACS Biomater Sci Eng. 2018;4(7):2237–75. CrossRef
51. D?browska-Bouta B, Zi?ba M, Orzelska-Górka J, Skalska J, Sulkowski G, Frontczak-Baniewicz M, et al. Influence of a low dose of silver nanoparticles on cerebral myelin and behavior of adult rats. Toxicology. 2016;363:29–36. CrossRef
52. Fondevila M, Herrer R, Casallas MC, Abecia L, Ducha JJ. Silver nanoparticles as a potential antimicrobial additive for weaned pigs. Anim Feed Sci Technol. 2009;150(3–4):259–69. CrossRef
53. Skomorokhova EA, Sankova TP, Orlov IA, Savelev AN, Magazenkova DN, Pliss MG, et al. Size-dependent bioactivity of silver nanoparticles: antibacterial properties, influence on copper status in mice, and whole-body turnover. Nanotechnol Sci Appl. 2020;13:137–57. CrossRef
54. Narciso L, Coppola L, Lori G, Andreoli C, Zjino A, Bocca B, et al. Genotoxicity, biodistribution and toxic effects of silver nanoparticles after in vivo acute oral administration. Nanoimpact. 2020;18:100221. CrossRef
55. Lazic SE, Semenova E, Williams DP. Determining organ weight toxicity with Bayesian causal models: improving on the analysis of relative organ weights. Sci Rep. 2020;10:6625. CrossRef
56. Beer C, Foldbjerg R, Hayashi Y, Sutherland DS, Autrup H. Toxicity of silver nanoparticles—nanoparticle or silver ion? Toxicol Lett. 2012;208(3):286–92. CrossRef
57. Park EJ, Bae E, Yi J, Kim Y, Choi K, Lee SH, et al. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ Toxicol Pharmacol. 2010;30(2):162–8. CrossRef
58. Ansari MA, Khan HM, Khan AA, Alzohairy MA, Waseem M, Ahmad MK, et al. Biochemical, histopathological, and transmission electron microscopic ultrastructural changes in mice after exposure to silver nanoparticles. Environ Toxicol. 2015;31(8):945–56. CrossRef
59. Chen H, Zhao R, Wang B, Cai C, Zheng L, Wang H, et al. The effects of orally administered Ag, TiO2 and SiO2 nanoparticles on gut microbiota composition and colitis induction in mice. NanoImpact. 2017;8:80–8. CrossRef
60. Wang S, Kang X, Alenius H, Wong SH, Karisola P, El-Nezami H. Oral exposure to Ag or TiO2 nanoparticles perturbed gut transcriptome and microbiota in a mouse model of ulcerative colitis. Food Chem Toxicol. 2022;169:113368. CrossRef
61. Moldovan B, David L, Vulcu A, Olenic L, Perde-Schrepler M, Fischer-Fodor E, et al. In vitro and in vivo anti-inflammatory properties of green synthesized silver nanoparticles using Viburnum opulus L. fruits extract. Mater Sci Eng C. 2017;79:720–7. CrossRef
62. Tirosh B, Khatib N, Barenholz Y, Nissan A, Rubinstein A. Transferrin as a luminal target for negatively charged liposomes in the inflamed colonic mucosa. Mol Pharm. 2009;6(4):1083–91. CrossRef
63. Perkasa DP, Arozal W, Suhaeri M. Potentials of alginates as capping agent for oral colon delivery of radiosynthesized silver nanoparticles: a review. Atom Indones. 2022;48(2):125–36. CrossRef