INTRODUCTION
Natural foods play an important role in maintaining human health, due to the contribution of compounds associated with functional properties that go beyond nutritional value. Berries are among the fruits with the highest amount of bioactive compounds, including minerals, vitamins, dietary fibers, and notably, phenolic phytochemicals, which have been reported to have various health benefits [1]. Grapes are recognized as one of the most important fruits within this select group of berries, which are considered one of the representative sources of phenolic compounds, and therefore, one of the main functional foods due to their contributions to health. Hence, in recent decades, phenolic compounds from grapes (Vitis) and their derived products have attracted great attention and have been extensively investigated due to their antioxidant properties and the role they play in promoting health, in the prevention of different diseases [2,3], and their potential use in the development of new dietary supplements or chemotherapeutic agents [4–8].
Phenolic compounds are secondary metabolites in plants. Although they are not nutritious, they have multiple bioactive properties and their consumption as part of the diet has protective effects on health. These compounds contain at least one aromatic ring with a hydroxyl group in its structure. There are more than 8,000 phenolic compounds from individual plants, and they show great structural variability [9]. In grapes and derivatives, the study of the total content of polyphenols and the characterization of their antioxidant effect at an in-vitro and in-vivo level has advanced to more specific studies of the scope of the biological effect of the different types of polyphenols, distinguished by the presence of: i) monomeric, dimeric, and oligomeric flavan-3-ols (proanthocyanidins), ii) anthocyanins, iii) flavonols, iv) stilbenesand, and v) phenolic acid or hidroxycinnamic acids and derivates. These studies have been widely documented and reviewed [10–14].
However, in recent years, studies have been published that aim to characterize the biological activity associated with the different parts of this fruit and its various preparations, for example, juices, extracts, and flours [15]. These compounds have been associated with biological functions, for example, antioxidant, antitumor, antisclerotic, and hypoglycemic activity [4,5,16–19].
Several studies have reported that the pulp, seeds, and skin of grapes, as well as the waste left over after grape processing, mainly wine production, contain phenolic components that can help prevent and treat various diseases, for example, cardiovascular disease, neurodegenerative conditions, metabolic syndromes, and cancer [7,8,20–22]. Gupta et al. [23] found that grape seed extracts (GSEs) prevent diseases such as atherosclerosis, reducing inflammation, and inhibiting platelet aggregation. Similarly, González et al. [24] reported the antihypertensive activity of compounds rich in phenols due to their action as suppressors of oxidative stress. Urquiaga et al. [25] demonstrated that the consumption of grape extract has beneficial effects on the health of people suffering from obesity and metabolic disorders. In their study, patients (30–65 years old) with metabolic syndrome were given flour prepared from waste generated from wine production. At the end of the study period, a considerable reduction in blood pressure was observed in the test group.
In addition to the above, the antitumor capacity of various genera and varieties of grapes has been reported in different studies [6,26,27], as has their effect as enhancers of the cytotoxicity of commonly used chemotherapeutic agents [28]. Specifically, the studies show the protection they confer against different types of cancer, such as esophageal, lung, liver, breast, skin, and colon cancer [29]. The protective effect on colorectal cancer (CRC) is of great interest, given the incidence of this type of cancer and its high risk of triggering death. In 2020, CRC was the cancer with the third highest incidence and the second most important cancer as a cause of death worldwide [30]. Although important advances have been made in the prevention of this disease through the adoption of healthy habits, functional foods (such as fruits and vegetables) have a more direct impact on the majority of the population. One of the most studied options for this purpose is the group of succulent berries, for example, grapes, due to their high anthocyanin content. These phenolic compounds have been associated with a biological function that can counteract the degenerative effects of CRC. Furthermore, despite its wide acceptance in current consumption, grapes can be a functional food or ingredient for pharmaceutical or nutritional purposes [31].
Consequently, studies of the protective effect of grapes and their derivatives on cancer, specifically colon cancer, are the main scope of this systematic review, since highly relevant studies in this area considering different forms of grape consumption exist. The purpose of this systematic review of the studies published in the last 10 years in which the content of total phenols and the antioxidant capacity associated with these bioactive compounds present in grapes and their derivatives have been identified and quantified is to differentiate the contribution of the different matrices evaluated and the chemopreventive effect of these antioxidant phenolic compounds in different CRC models. Through this, it is sought to contribute to the identification of the chemical and biological profile of the different functional foods and ingredients available from grapes and their derivatives.
METHODOLOGY
Search strategy-identification
Systematic review of previously published research in the most comprehensive scientific databases in the fields of chemistry and biomedicine: Science Direct, Taylor & Francis, and Wiley, based on the refined search equation in the reference database Scopus, which resulted in the following terms included in the title, keywords, and the abstract: (“Vitis spp” OR “vitis labrusca”) AND (“colorectal cancer” OR “cancer”) AND “phenolic compounds” AND “antioxidant capacity” AND (antiproliferative OR “cytotoxic effect”) AND (“in-vitro” OR “in-vivo”).
These criteria were selected to find studies that reported the content of total polyphenols and the antioxidant capacity in diverse matrices of grapes and their derivatives. The review resulted in a list of research papers related to the evaluation of the chemopreventive effect of grapes and their derivatives on colon cancer reported in in vitro and in vivo studies.
Screening process and exclusion criteria
The first phase of the review consisted of applying a filter from each of the databases consulted, which consisted of restricting the search to only: 1) original articles in English and 2) papers published between 2012 and 2023.
The articles selected in the four databases were analyzed in Rayyan. In the first round of selection, articles were excluded if: 1) they were duplicates, 2) they corresponded to reviews, book chapters, or abstracts of papers, or 3) records with title, abstract, or keyword did not respond to the search equation. In the second round performed from the full article, 1) the exclusion criteria were those articles that were not performed in matrices other than grape, 2) the values of total polyphenols content, antioxidant capacity, and cytotoxic/apoptosis effect in colon cancer lines were not presented, and 3) the results and analysis are far from the objective of this review.
Eligibility and data analysis
The review analyzed studies on grapes and their derivatives, dividing them into two categories: species and variety, based on polyphenol content and antioxidant capacity, and by part of the fruit and derivatives. The second category included results from in vitro, in vivo, and epidemiological studies, considering doses, specimens, and effects.
Finally, the topics selected to address both scopes in depth were chosen for analysis through VOSviewer 1.6.15 (Leiden University, Leiden, The Netherlands) and included the complete records and cited references of publications from 2012 to 2023. These were imported for data analysis, co-occurrence (keyword repeated minimum twice), and visualization. This approach constructed keyword maps and visualized existing connections between publications, thus facilitating literature analysis across disciplines.
Descriptive analysis
The systematic review of studies on chemical and antioxidant characterization of grape matrices was analyzed to identify total polyphenol content values for each type of matrix and its derivatives, differentiated by gender, and grape variety. Data processing was done using R software version 4.1.3 (2022-03-20), by determining nonparametric confidence intervals, in terms of an estimate for a P50 quantile that equals the median, given prior verification of the assumption of normality.
RESULTS AND ANALYSIS
Bibliometric analysis
The bibliometric analysis showed that the main contributors to studies associated with the scope of the chemopreventive effect of grapes and grape derivatives, as well as their composition, are concentrated in countries such as the United States, Italy, Brazil, Germany, and Poland. The compilation of the studies is mainly focused on biochemistry (25.9%), medicine (16%), pharmacology (14.8%), agriculture (12.3%), and chemistry (10.5%), among others. Hence, the studies are led not only by research groups from world-renowned universities, but also by research centers oriented to cancer studies and new sources of functional ingredients. These results have been the subject of scientific production that is mainly disseminated through articles (61.3%), reviews with different approaches (27.5%), book chapters (8.8%), and conference reports (2.5%). It is highlighted that reviews to date have evolved from description of the effect of antioxidants on cancer cells to analysis focused mainly on explaining the effect of polyphenolic compounds by groups (anthocyanins, flavones, flavanols, stilbenes, and phenolic acids) [32,33], on studying individual compounds extracted from specific parts of the grape, or on evaluating different varieties or wines, in in vitro or in vivo studies [34].
The above provides an opportunity to contribute by means of a systematic review that would allow a deeper analysis of original articles from the perspective of the synergistic effect of the total content of polyphenols present in the different parts of the fruit and its derivatives, associating it with the antioxidant capacity (Part 1), where the main aspects extracted were compiled (see item “Phenolic content and antioxidant capacity in different grape matrices”). The analysis of these selected matrices was followed by a presentation of the results of the chemopreventive studies (Part 2), reflected in the cytotoxic and apoptosis effects at the in vitro level, the decrease in tumor growth and the generation of oxidative stress at in vivo level summarized in Table 2. Both analyses made it possible to consolidate the information for the analysis of the last “inclusion” stage of the PRISMA protocol (Fig. 1)
718 keywords related to the selected articles were obtained via the clustering performed by VOSviewer’s network analysis. The result obtained with a co-occurrence of at least 2 keywords left 174 words that met the threshold. This was followed by the filtering of words that were not related to the scope of the review, arriving finally at a consolidated 128 items grouped in 5 clusters of research represented by different colors (Fig. 2).
Each of the clusters identified enabled the definition of the scope and relationship between the studies reported in the period analyzed. Consequently, clusters 1, 4, and 5 could be grouped to explain mainly Part 2 (biological analysis). Meanwhile, clusters 2 and 3 corresponded to the scope of Part 1, where the systematic review concentrated on the studies of the chemical composition in the various reported matrices derived from grapes from some representative varieties worldwide and their evolution over time to the more detailed characterization and relationship with antioxidant capacity, determined by different techniques.
Thus, Cluster 1 (31 items) is represented by the color red and highlights in vitro studies evaluating the antiproliferative and apoptosis effect on colon cancer cells from secondary metabolites (e.g., anthocyanins and some flavonoids) extracted and purified from various phenolic grape species. Cluster 2 (30 items) is in green and has as a differentiator the studies where the antioxidant capacity [2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and ferric reducing antioxidant power (FRAP)] of the isolated and quantified compounds, mainly phenolic acids and flavonoids, were determined by different techniques, making it possible to include in Supplementary Table S1 reports of concentrations of this type of specific phenolic compounds. Cluster 3 (24 items), in blue, includes specific topics on the analysis in different matrices and grape derivatives (seed, grape pomace, and wine), which were subjected to chemical characterization of total phenolic content (TPC) and antioxidant capacity, as well as the evaluation of the bioactivity of these unpurified extracts on cancer cell lines in general. Cluster 4 (23 items), in yellow, is a complement to cluster 1, and consolidates the information and analysis of Part 2 (See item “In vitro studies”) since it is associated with animal experiments associated with studies of controlled murine models (dietary supplements) that show a decrease in cancer risk with antioxidant capacity of the purified extracts used to obtain resveratrol and myricetin, among other substances, from various matrices. Cluster 5 (20 items) is identified with purple and completes the scope of Part 2, since it associates the studies in humans of complete and purified extracts (stilbenes), which are cross-checked with chromatography techniques such as ultra-high performance liquid chromatography with tandem mass spectrometry for more robust identification and quantification.
Therefore, each cluster identified in the systematic review was defined taking into account different aspects of interest that encompass recent advances in different parts of the grape, its various derivatives, and different species and varieties, as will be detailed below.
Phenolic content and antioxidant capacity in different grape matrices
The text discusses the search for natural sources of antioxidants and bioactive compounds for promoting health, with a specific focus on berries from the Vitis genus, known for their role in wine production. Various research efforts have examined the antioxidative potential and TPC of different Vitis berry varieties, and Supplementary Table S1 summarizes these findings. The goal is to provide an overview of how these berries can benefit human health and potentially influence the food and nutraceutical industry.
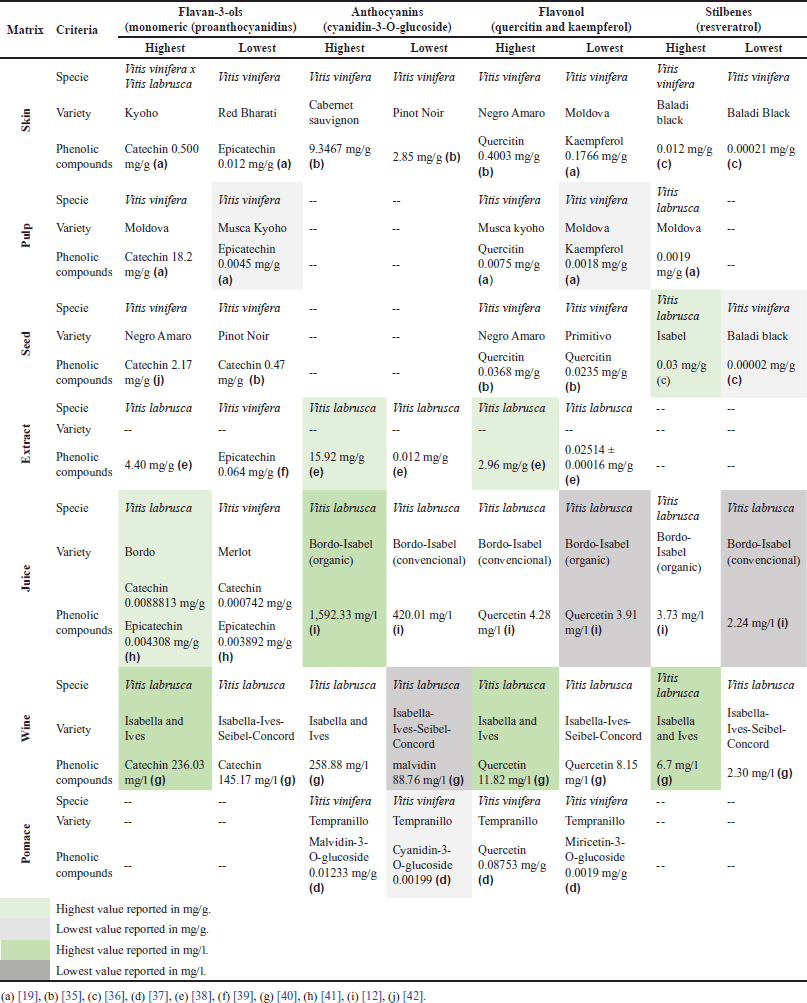 | Table 1. Outstanding values of the main polyphenols present in grapes (skin, pulp and seed), derivatives (juice, wine, extract) and grape pomace. [Click here to view] |
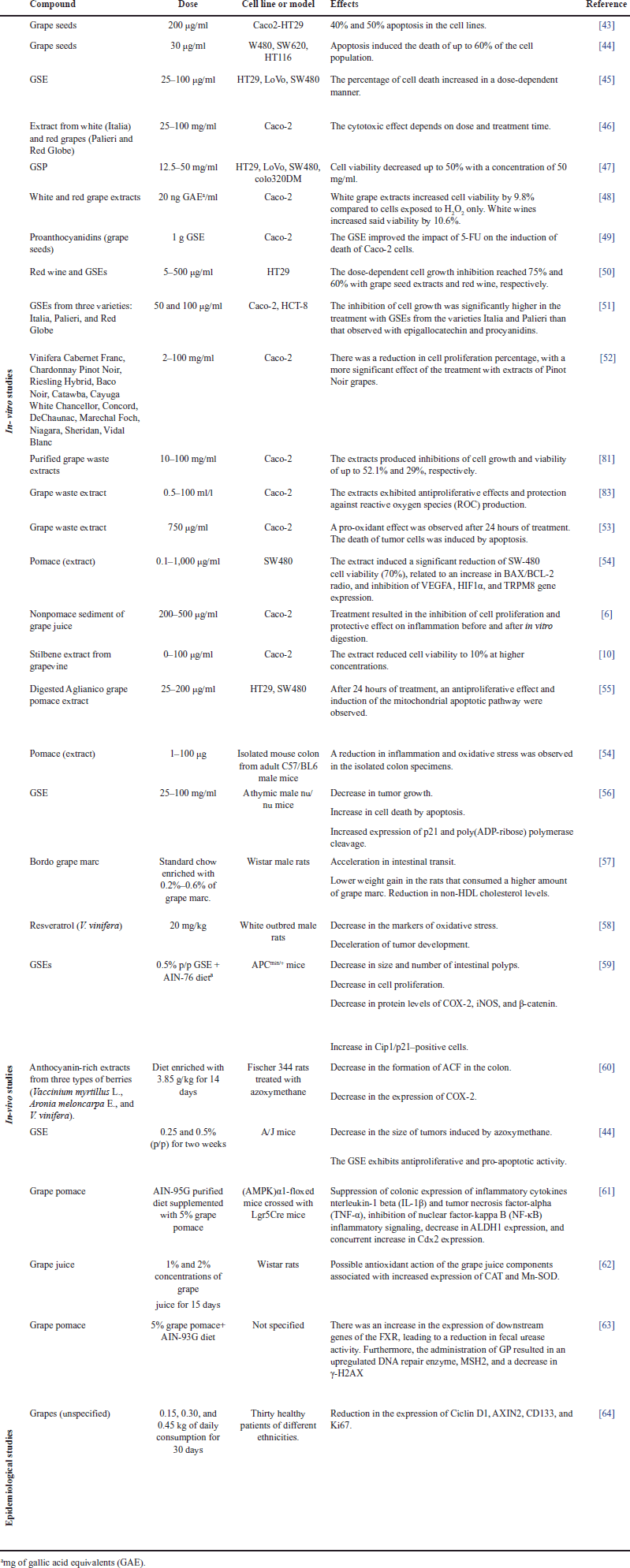 | Table 2. Experimental evidence of the antiproliferative and cytotoxic capacity of phenolic compounds derived from grapes (Vitis). [Click here to view] |
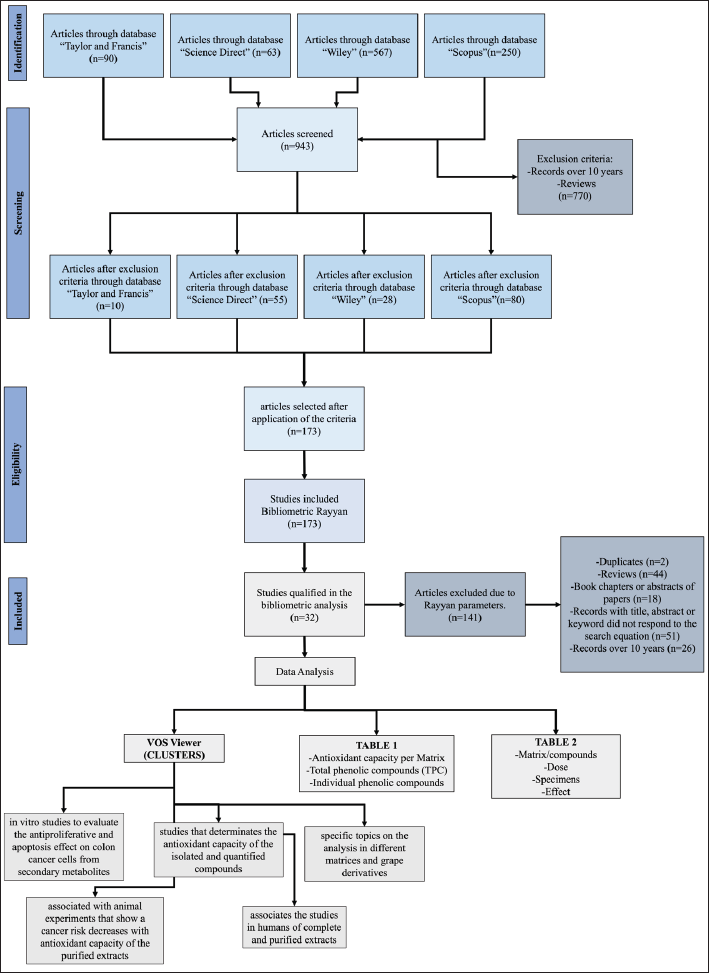 | Figure 1. PRISMA 2020 diagram: methods and filters adopted in bibliographic-searches and selection criteria according to PRISMA 2020 protocol [65]. [Click here to view] |
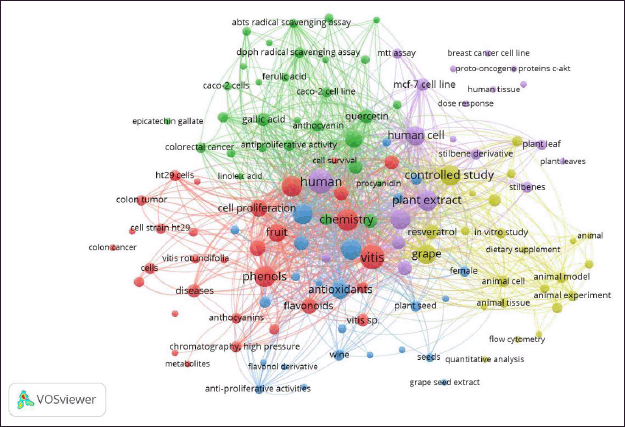 | Figure 2. Analysis of hotspot occurrence was based on bibliographical data for polyphenols and biological properties in different matrices of grape (Vitis) and its derivates from 2012 to 2023. Data analysis used fractional counting for co-occurrence of all keywords (minimum number of occurrences set at two). Visualization via fractionalization used a minimum clustering size of five and a resolution of one. Network visualization with five clusters used different colors. [Click here to view] |
Skin
Fernández López et al. [66] established that the skin of the grape is the part with the second highest phenolic content (after the seeds), which can be confirmed in the studies reviewed in Supplementary Table 1. However, the TPC values observed in the studies are different due to other parameters that also affect seed composition (discussed above). The results show differences in TPC of up to ~50%, which is the case with the Primitivo and Pinot Noir varieties, the latter having the lower TPC [35]. Similar results have been reported by Unterkofler et al. [67], which is evidence of the influence of the variety on the TPC of the fruit.
In addition, skin color intensity is decisive in the functional differentiation of grapes. For instance, Li et al. [19] found that two varieties (Summer Black and Muscat Kyoho) of the species Vitis vinifera x Vitis labrusca presented an 85.4% difference in TPC. This variation was not only associated with the variety of the fruit but also with the color: Muscat Kyoho (red) had a higher TPC than Summer Black (black). Likewise, El-Elimat et al. [36] analyzed the Darawishi Black variety of the species V. vinifera. The researchers discovered that the skin of the specimen had the highest TPC of the varieties studied, at 18 ± 1 mg GAE/g, while the skin of the Harawani, belonging to the same species, displayed the lowest TPC at 5.2 ± 0.4 mg GAE/g. Based on this 71.1% difference in TPC, one could infer that skin color exerts a stronger influence on TPC than grape variety.
Geographical origin is another factor associated with TPC differences. In the study mentioned above, El-Elimat et al. [38] used grapes from different places: Darawishi Black from Jordan and Harawani from Turkey. Similarly, Han et al. [68], Fernández López et al. [66], and Unterkofler et al. [67] have found that factors such as climate, altitude, and planting method cause variabilities in the final phenolic composition of these berries.
The higher the TPC of grape skin, the higher its antioxidant capacity, as seen in Supplementary Table S1. According to Rockenbach et al. [35], there are differences in this respect even among species of the genus Vitis. More specifically, they showed that V. labrusca grapes have a higher concentration of phenolic compounds in their skin—which also means greater antioxidant capacity (measured by DPPH and FRAP assays)—than their V. vinifera counterparts (8.978 ± 0.086 mg CE/g). The latter also presented a lower antioxidant capacity (DPPH: 176.26 ± 4.72; FRAP: 215.62 ± 0.94 mmol Fe/g). Research articles by other authors (e.g., Li et al. [19] and El-Elimat et al. [36]) include graphs that show that, in the skin of grapes, the higher the concentration of phenolic compounds, the greater the antioxidant capacity.
In the big family of phenolic compounds, it has been established that anthocyanins found in grapes present their highest concentration in the skin. Therefore, they are associated with the color of this fruit, and their content is higher in purple, black, and red grapes. Likewise, an association has been found between higher color intensities and higher TPC because anthocyanins (as colored compounds) tend to accumulate during the maturation process of darker grapes. Their accumulation can also be reflected in some cultivars in which the pulp is pigmented as well [69]. Rockenbach et al. [35] conducted a study focused on demonstrating the relationship between anthocyanin content and grape variety. They evaluated five varieties of V. vinifera and Isabella grape (which belongs to V. labrusca), finding that the Cabernet Sauvignon variety exhibited the highest content of anthocyanins (expressed as cyanidin-3-rutinoside equivalents), followed by Primitivo, Isabel, Pinot Noir, Sangiovese, and Negro Amaro. The characterization of TPC—and specifically anthocyanins—in grapes is important because this fruit can be a functional food, as it has been demonstrated, in vitro, that anthocyanins contribute to the promotion of human health [68]. However, grape byproducts should be obtained carefully because anthocyanins are highly thermolabile compounds and are sensitive to light and pH changes, which affects the antioxidant capacity of the final product [70,71].
Seeds
Ordoñez et al. [72] found grapes have the highest total polyphenol content in their seeds and skin, surpassing prickly pear, cocoa, and Amazon tree grapes.
Vitis vinifera has been reported to have, on average, a higher polyphenol content (97.30 ± 0.76 mg CE/g) than Vitis labrusca (21.28 ± 0.19 mg CE/g), also known as Isabella grape [35]. Nevertheless, the latter has been gaining ground because of an increase in its direct consumption (e.g., in juice), which has therefore boosted its production. For instance, in Colombia, the annual production of Isabella grapes is approximately 25,600 tons [73].
Another factor that influences the phenolic content of grape seeds is variety. For instance, Pinot Noir black grapes were reported to have the highest TPC, followed by their Primitivo counterparts, which are widely used for grape-derived products, mainly wine [35]. A different study compared several varieties of V. vinifera grapes cultivated in Jordan, as shown in Supplementary Table S1. In the paper by El-Elimat et al. [36], the variety with the highest phenolic content was Golden Scatt, followed by Red Globe, two types of grapes grown for fruit processing. In turn, varieties such as Baladi exhibited lower phenolic content and are mainly used for commercialization in the fresh market.
The findings discussed above corroborate the special importance of grape seeds due to their properties associated with antioxidant capacity and as sources of anti-inflammatory and antineoplastic compounds, among others [74]. This is particularly relevant at the present time because grape seeds—which are considered waste in the winemaking industry—can have potential in other sectors: pharma, foods, and cosmetics.
Pulp
Although approximately 64% of the overall free phenolic compounds in grapes are found in the seeds, around 30% are in the skin, and the remaining 6% are present in the pulp [19,75,76]. The pulp segment of the market is experiencing growth due to its direct involvement in the preparation of dairy products, making it increasingly popular because of its convenience and associated health benefits [77].
In Supplementary Table S1, the values of total phenolic compounds (TCP) are maintained within a range, which is presented as the lowest among the other mentioned parts (0.33 mg GAE/g) [19]. In addition, the antioxidant capacity is directly proportional to this phenolic content, as demonstrated by Li et al. [19], who reported TCP of the pulp (Species: V. vinifera, Variety: Red bharati) to be 0.701 ± 0.193 mg GAE/g fw, along with antioxidant capacity measured by DPPH and oxygen radical absorbance capacity (ORAC) assays (0.03 mg/ml and 0.008 mmol TE/g, respectively). These values were lower in comparison to the other studied matrices. In contrast to the seeds and skin, this matrix primarily consists of water (comprising 70%–85% of its composition). Moreover, it also exhibits a notable concentration of sugars, potentially exerting an additional influence on the accessibility of the limited polyphenolic content, thereby impacting its antioxidative potential [78,79].
Pomace
During the grape juice production process, grape polyphenols are efficiently transferred into the juice. However, a significant portion remains concentrated in the solid residue known as pomace, which is generated during processing [6]. Pomace, consisting of skins, leftover pulp, seeds, and stalks, contains valuable components such as health-promoting compounds, a substantial fiber content, and polyphenols that endure even after the winemaking process [80,81].
Supplementary Table S1 confirms what was stated by Haas et al. [6] mentioned, since, as reported by Wang et al. [63], pomace of the Tempranillo variety (V. vinifera) has a high phenolic content in comparison with other parts of the Vitis species (TPC 1,967.52 mg GAE/g), which leads to high antioxidant capacity as demonstrated by FRAP methods: 4,020.7.40 ± 0.1025 mg TE/g and ORAC: 19,236 ± 1.9612 mg TE/g DM. On the other hand, the place of origin of the pomace analyzed must also be considered to elucidate these characteristics. This is because, as shown by Recinella et al. [54], the pomace of the Montepulciano variety, which comes from Italy, has a lower TPC than that of Tempranillo, [25.9 ± 0.18 (mg GAE/g)], which comes from China, although the two come from the same species (V. vinifera). This latter region has been considered to have a high impact in terms of production and analysis of this type of berries, which has led to increased modifications that probably increase the phenolic compounds of the grape [82].
Juice, wine, and extract
Supplementary Table S1 displays variations in TPC based on factors such as grape species, variety, and matrix (e.g., seeds, skin, pulp, pomace, extract, wine, or juice). Notably, in the case of juice, there is a TPC difference of up to approximately 38% between V. labrusca (with higher content) and V. vinifera. Among V. labrusca varieties, Bordo has the highest TPC, while Isabel has the lowest. Preliminary treatments to obtain grape juice, as mentioned by Cosme et al. [83], significantly affect polyphenol content, resulting in lower values in juice compared to seeds and skin. The use of pasteurization, especially at temperatures above 80°C to ensure microbiological quality, can largely account for the reduction in TPC, particularly in anthocyanins, which are highly sensitive to heat [70,71,83].
In grape extracts, TPC values vary according to obtention method, species, variety, and cultivation practices, among other factors [83,84]. Supplementary Table S1 shows that Corrales et al. [85] compared extracts from conventional and organic grapes. The former presented a TPC ~73% higher than the latter, which they related to the kind of pesticides used to grow the organic grapes. Such pesticides could significantly reduce the production of phenolic compounds; as a result, the antioxidant capacity of said extracts was reduced by approximately ~77%, which is related to the TPC. The TPC and AC of extracts also depend on the extraction method. Ultrasound-assisted methanolic extraction is more efficient than percolation in an acidic medium [38,85,86].
TPC reference values according to matrix and variety
Based on the values reported in the studies reviewed above, Figure 3 presents the TPC ranges of different grape varieties classified by type of matrix.
The text discusses variations in reporting TPC in different studies. In some cases (Fig. 3A and D), TPC is reported as mg CE/g of the sample, while for juices and wine (Fig. 3B and C), it is reported as mg CE/l. This difference in units results in varying ranges of TPC values, making comparisons and establishing reference points for plant materials challenging. To improve clarity, it is suggested to standardize the unit, preferably using weight on a dry basis to prevent overestimations. However, categorizing data by the type of matrix (e.g., juices and wine) can help identify ranges of polyphenolic content for biological relevance, as discussed further.
In Figure 3A, seeds reached values between 21.28 and 165.18 mg CE/g of sample approximately. This wide range is associated with grape variety or species. In terms of TPC, seeds are followed by the skin (5.15–18.39 mg CE/g) and pulp (0.33–1.57 mg CE/g). In comparison, bananas have TPC values between 0.5 and 1.35 mg GAE/g in their skin and between 0.40 and 1.30 mg GAE/g in their pulp. This means that the consumption, export, and/or production of grapes could grow further [87]. Figure 3A corroborates the results reported in Supplementary Table S1, that is, the highest TPC values in grapes are found in the seeds, followed by the skin and the pulp, in that order.
The results in Figure 3D indicate that grape extracts can have a higher TPC (0.46–43.14 mg GAE/g) than grape skin. A comparison between different juices and wine (Fig. 3B and C, respectively) indicates that the juices obtained from crops treated with different methods varied widely (0.57–3,378.33 mg GAE/l), and the juice with the highest TPC content was that obtained from the whole grape without mixing with water, but subjected to a blender, with previous enzymatic treatment and temperature optimization [12]. The results in Figure 3B and C can be compared to those of other fruits. For instance, Wang et al. [88] reported much lower TPC values in mango (70–97 mg GAE/l), which means that the use, consumption, and production of grapes should be further encouraged because of this advantage as a functional food.
Finally, TPC values in wine are comparable to those found in grape juice macerated with enzymes (830–847 mg GAE/l). However, the TPC results for wine, juices, and extracts, vary depending on the grape variety used. In contrast, there is no significant evidence of wide variations in average TPC values for different grape varieties in skin, pulp, or seeds. The values reported in various studies tend to remain consistent depending on the grape variety. Overall, when it comes to grape pulp and its derivatives (wine, juices, and extract), V. labrusca, either individually or mixed with other Vitis varieties, exhibits the highest TPC. For skin and seeds, Golden Scatt of the V. vinifera species and Pinot Noir stand out [35,36].
The variability in the type and concentration of polyphenolic compounds in different parts of the grape, as well as the various treatments used to produce derivatives such as wines, juices, extracts, and residues, likely accounts for the observed results.
Consequently, for a more effective analysis of the contribution of the most representative phenolic compounds associated with grapes in each matrix evaluated, they were classified in Table 1 according to: i) the highest or lowest concentration present in the pulp, seed, or skin and in the derivatives studied; and ii) the species and variety. The transformation options that can be derived from the grape in its different varieties represent an important opportunity to enhance the concentration of phenolic compounds and their antioxidant activity.
In juices and wines, physical transformations such as maceration or blending facilitate the solubilization of the anthocyanins present mainly in the skin. This is favored by extending the process, which can be corroborated by the results of the evaluation of the juice obtained by maceration with temperature and light control, as these metabolites are thermolabile [12]. The extraction of anthocyanins in wines is added to the previously described stage since maceration before fermentation is contemplated [40]. This action is further favored by biochemical reactions such as exposure to enzymatic pretreatments, which differentiate the juice obtained only by maceration [41] and that which was additionally subjected to hydrolase enzymes. This is because the extraction of polyphenols is favored by promoting mechanisms, including: i) hydrolysis of macromolecules such as sugars, proteins, and pectin, which interfere with the formation of tannin-anthocyanin adducts; and ii) diffusion mechanism from the wall cells both in the skin and in the seed and, to a lesser extent in the pulp of the grape, which increases the availability of both free anthocyanins and proanthocyanidins, mainly concentrated in the seed, hence their release throughout the fermentation of the wine or pretreatment with enzymes.
According to what is described in Table 1, the part of the grape where the highest anthocyanin content is reported is the skin, which is consistent with numerous previous studies. For this review, it was possible to identify from the analysis carried out on V. vinifera that the highest anthocyanin content was found in Cabernet Sauvignon [35], but when the treatments described above are carried out, it is the juice subjected to maceration and enzymatic treatment with heating, from an organic grape of the V. labrusca variety, that has the highest content [12]. In the same sense, it was evidenced that procyanidins stand out in wine and juice in the presence of catechin and epicatechin [40].
.png) | Figure 3. TPC ranges of different grape varieties classified by type of matrix: (A) pulp, seed, and skin; (B) juice; (C) wine; and (D) extract. [Click here to view] |
Flavonols play a crucial role in characterizing the chemical profile of grape varieties. However, only a few of the reviewed studies reported the total flavonoid content, which is a more specific parameter than total polyphenol content and not the focus of this review. Nevertheless, the quantification of reported flavonols aligns with previous findings, indicating that the highest content is typically found in the skin, with quercetin in V. vinifera (Negro amaro) [35] being notable. In addition, extracts and wines from V. labrusca stand out due to their higher flavonol yield with acid extractions. Although their concentrations are lower compared to anthocyanins and proanthocyanins, their potential effects on cancer prevention are of interest, as discussed later in this review.
The main stilbene recognized in grapes is resveratrol and its glycoside, the resveratrol-3-glucoside isomer known as Piced. These are recognized for their antifungal properties, in addition to their preventive and therapeutic effect in the treatment of various types of human cancers, including CRC [58]. These stilbenes are found in low concentrations compared to the other polyphenols. According to the studies evaluated, the matrix where trans-resveratrol and Piced [89] were most reported was in the seed [36] and in wine [40], with V. labrusca being the variety with the greatest contribution, although there are reports on other studies in V. vinifera where comparable values are reported [89]. As it is so sensitive to light, this bioactive compound is subjected to protective treatments such as microencapsulation or liposomal nanoemulsions, among others, for pharmaceutical applications [90]. Hence, the use of seeds as a source of resveratrol is an industrial alternative.
The reported values in defined intervals for various matrices (Fig. 2) and the concentrations of key bioactive compounds linked to different types of polyphenols (Table 1) serve as a valuable reference for identifying methods to obtain functional ingredients or grape-based foods. This compilation of data supports the food, pharmaceutical, and health industries by providing information for assessing the impact of specific bioactive compounds and their synergistic effects. It aids stakeholders in these fields in considering how the presence of these compounds, along with the TPC value and the dose-response relationship, can influence outcomes, as further discussed in item 4.
Antiproliferative and cytotoxic activities of grape-derived phenolic compounds evaluated in CRC
According to the Global Cancer Observatory (GLOBOCAN), in 2020, CRC was the neoplasm with the third highest incidence and the second most significant cause of death by cancer in men and women worldwide [91]. Epidemiological studies show that—in addition to the nonmodifiable causes of this disease, e.g., age, hereditary factors, and inflammatory syndromes—diet and lifestyle can lead to an increase in the development of CRC [92].
Phenolic compounds from grapes have been widely reported as potential chemopreventive agents due to their antiproliferative and cytotoxic effects on different kinds of cancer [62,92,93]. Table 2 summarizes several in vitro, in vivo, and clinical studies that have demonstrated that grape consumption (in different forms) can fight oxidative stress, inhibit the proliferation of malignant cells, induce apoptosis, and modulate signaling pathways involved in the development of CRC.
In vitro studies
In vitro studies that employ cell cultures derived from CRC have demonstrated the biological effect of grapes and their extracts. Ortiz et al. [43] showed that administering a concentration of 200 _g/ml of GSE for 24 hours induced apoptosis close to 50% and 40% in Caco-2 and HT29 cells, respectively. In turn, Derry et al. [44] used GSEs (in low concentrations of up to 30 _g/ml) in SW480, SW620, and HT116 cells. After 12 hours of treatment, they observed that the apoptosis percentage increased in a dose-dependent manner, thus inducing the death of up to 60% of the cell population. Therefore, it can be concluded that, even at low concentrations, grape extracts show an apoptotic effect on CRC-derived cell lines.
Kaur et al. [45] studied the effect of GSE on the apoptosis of human colon carcinoma cells. They treated HT29, LoVo, and SW480 cells with GSE at concentrations of 25, 50, and 100 μg/ml. The results showed an increase in the percentage of apoptotic cells in a dose-dependent manner and a stronger effect on SW480 cells. Dinicola et al. [46] evaluated cell death by apoptosis induced by grape extracts in Caco-2 cells. They used extracts of white (Italia) and red grapes (Palieri and Red Globe) at concentrations of 25, 50, and 100 μg/ml for a treatment time of 24–96 hours. Their results show induction of apoptosis with the activation of caspases in a dose- and time-dependent manner.
Hsu et al. [47] evaluated the mechanism by which grape seed procyanidins (GSPs) induce apoptosis. After treating CRC cells (i.e., SW480, HT-29, LoVo, and Colo 320DM lines) with GSP between 12.5 and 50 mg/ml, they found that GSP has a differential pro-apoptotic effect. GSP had no significant pro-apoptotic effect on the Colo 320DM cell line. In contrast, in the other cell lines (i.e., HT-29, SW-480, and LoVo), GSP inhibited cell proliferation in a dose- and cell line-dependent manner. The treatment with GSP increased the depolarization of the mitochondrial membrane, raised the number of annexin V-positive cells, and revealed increased levels of the apoptosis activation protein, caspase-3, and the cleavage fragment of PARP.
Some studies have employed pure phenolic compounds that had been previously identified in grapes. San Hipólito-Luengo et al. [94] evaluated resveratrol and its role as a modulator of growth and death in CRC-derived cells. Employing the HT-29 and HCT116 cell lines and different resveratrol concentrations, their results show that, at 50–100 μM of polyphenol, the number of cells was reduced, while the percentage of apoptotic or necrotic cells was increased. In addition, they reported that the cytotoxicity induced by resveratrol in HT-29 cells was associated with the activation of NADPH oxidase and an increase in DNA damage (quantified by phosphorylation of histone γH2AX).
Lingua et al. [48] evaluated the percentage of cell viability in the Caco-2 cell line after it was exposed to extracts of white grapes (V. vinifera) and wine. Their study was conducted in a simulated digestion system with H2O2-induced oxidative stress. Their results show that white grape extracts increased cell viability by 9.8% compared to H2O2 only, and wines improved it by 10.6%, without significant differences regarding the variety of grapes that were employed. Cheah et al. [28] evaluated the biological effect of different individual fractions of procyanidins isolated from grape seeds and combined with the chemotherapeutical agent 5-fluorouracil (5-FU). They tested the effects of different isolated fractions (at several concentrations) on the viability of Caco-2 cells. Their results indicate a significant decrease in cell viability, in addition to a boost in the cytotoxic effect of 5-FU on carcinogenic cells.
Another study evaluated the effects of (crude and purified) grape seed and waste extracts from white and red grapes on Caco-2 and HT-29 CRC cells and nonmalignant fibroblasts. Employing concentrations between 75 and 225 _g/ml, the crude and purified extracts of seeds produced the greatest reduction in the viability of CRC-derived cells, as well as a selective effect compared to nontumor cells [43]. These authors also evaluated the fractions rich in anthocyanin and those rich in nonanthocyanin compounds and found a higher cytotoxicity in the latter, with 30%–40% cytotoxicity at all the concentrations under study.
Grape extracts have shown antiproliferative activity as well. Leifert and Abeywardena [49] treated the HT29 cell line with phenolic compounds of red wine and GSEs at concentrations between 5 and 500 _g/ml. They found that said wine and extracts increased the percentage of cell growth inhibition by 60% and 75%, respectively, in a dose-dependent manner. Dinicola et al. [26] evaluated growth inhibition in the Caco-2 and HCT-8 cell lines after being exposed to GSEs from three varieties (Italia, Palieri, and Red Globe) for a period of 0–96 hours. They obtained their most relevant results after 96 hours of treatment with two concentrations (i.e., 50 and 100 μg/ml) of the three extracts in both cell lines. The increase in cell proliferation inhibition was directly proportional to the increase in concentration—reaching inhibition percentages of up to 80%.
Yang et al. [50] compared extracts from 14 different kinds of grapes in terms of their effect on three cell lines: Caco-2 (CRC), HepG2 (liver cancer), and MCF-7 (breast cancer). The treatments for 96 hours at concentrations between 2 and 100 mg/ml of grape extract showed a reduction in proliferation percentage in all the cell lines they employed—obtaining a stronger inhibition (15%) with Pinot Noir extracts. Importantly, all the extracts inhibited the proliferation of CRC cells better than that of their breast and liver cancer counterparts. Similarly, Jara-Palacios et al. [51] determined the antiproliferative effect of purified grape waste extracts on CRC cells (Caco-2). They used concentrations from 10 to 100 _g/ml of the extract and treatment times from 24 to 72 hours. Their results show a dose-dependent effect, with a cell growth inhibition of up to 52.1% at 48 hours with the maximum extract concentration (100 _g/ml).
The byproducts of grapes and the wastes generated in their production have also been studied for their preventive and therapeutical properties against CRC. Signorelli et al. [95] evaluated a lyophilized wine extract called Liofenol™ in HCT116 cells and analyzed its biological activity. They demonstrated that HCT116 cells responded to the Liofenol™ treatment, reducing their proliferation, which was associated with an increase in the p53 and p21 cell cycle regulators. They also observed the induction of the antioxidant response with the activation of the transcription factor Nrf2, which is involved in homeostasis and redox regulation. In addition, they reported the inhibition of the migration and downregulation of E-cadherin, which are related to the epithelial–mesenchymal transition of malignant cells. Lazze et al. [52] reported the biological effect of a grape waste extract on Caco-2 cells. They employed concentrations between 0.5 and 100 ml/l to evaluate the antioxidant and antiproliferative activity of the extract. The results showed that grape extract presents antioxidant activity and protects against ROS production, but it does not protect against lipidic preoxidation in human colorectal adenocarcinoma cells. In addition, their clonogenic assay showed a significant antiproliferative effect.
Another study evaluated the antiproliferative and pro-apoptotic effects of pure trans-resveratrol on HT-29 cells. Its results showed antiproliferative activity with an EC50 value of 78.9 μM, activation of caspase-3, and dose-dependent DNA fragmentation. In addition, mitochondrial ROS production was found after the treatments with polyphenol [96]. Other authors have evaluated the modulation of ROS production in CRC cells treated with grape-derived compounds. Quero et al. [53] quantified ROS levels in Caco-2 cells that were treated with 750 μg/ml of grape stem extract in the presence or absence of H2O2. Their results indicate that the extracts have a prooxidant effect after 24 hours of treatment in the presence or absence of H2O2. This effect is due to the fact that the extract treatments induced a decrease in the antioxidant enzyme TrxR1, which produced an increase in cellular ROS levels, modified the potential of the mitochondrial membrane, and caused the death of tumor cells by apoptosis.
The results from other studies showcase the diverse effects of grape-derived compounds on different cell lines and experimental setups. The nonpomace sediment of grape juice demonstrated inhibitory effects on cell proliferation and anti-inflammatory properties both pre and postdigestion, as assessed in Caco-2 cells [6]. In contrast, the stilbene extract from grapevine exhibited a remarkable reduction in cell viability to 10% at higher concentrations in Caco-2 cells [10]. The digested Aglianico grape pomace extract displayed an antiproliferative impact and initiated the mitochondrial apoptotic pathway in HT29 and SW480 cells after 24 hours of treatment [55]. Finally, a pomace extract exhibited significant anti-cancer potential with a notable reduction in SW-480 cell viability by 70%, correlated with altered BAX/BCL-2 ratio, and suppression of gene expression related to angiogenesis and calcium channel regulation. These results were contrasted in an ex vivo study, where pomace extract showcased its potential to reduce inflammation and oxidative stress in isolated mouse colon specimens [54]. These findings underscore the varied and promising effects of grape-derived compounds in different cellular contexts and models.
In vivo studies
Several in vivo studies have reported the preventive and therapeutic effects of grape extracts. In one of these, Kaur et al. [56] administered HT-29 human colorectal adenocarcinoma cells to athymic male nu/nu mice to induce tumor formation. They observed that parallel feeding with 200 mg/kg of GSE significantly decreased tumor growth. In addition, in the tumors treated with GSE, apoptotic cell death was higher, and there was an increase in the levels of p21 and poly (ADP-ribose) polymerase cleavage. In a recent study, Pertuzatti et al. [57] evaluated the effects of grape marc consumption on healthy Wistar male rats. After 30 days of grape marc ingestion as a dietary supplement, the rats exhibited acceleration of their intestinal transit, which resulted in a lower weight gain in the rats that consumed the highest amounts of grape marc. In addition, the non-HDL cholesterol levels of the rats were reduced after they ingested the grape marc-enriched chow. This demonstrates the benefits of grapes for metabolic and gastrointestinal health and represents a strategy to prevent cancer.
Rytsyk et al. [58] evaluated the effect of pure resveratrol against the development of CRC. For that purpose, they administered a subcutaneous injection with a chemical compound that induces CRC, specifically, 1,2-dimethylhydrazine (DMH), to white outbred male rats. An additional daily dose of 20 mg/kg of resveratrol was administered to the treated group for 30 days. The results indicate that the individuals treated with DMH + resveratrol presented a reduction in the markers of oxidative stress and deceleration of tumor development compared to the control group (which only received the carcinogenic agent).
Velmurugan et al. [59] evaluated the prevention of intestinal tumors in APCmin/+ mice, which are commonly used as animal models of familial adenomatous polyposis and sporadic CRC. In their study, mice in the treated group were administered feed enriched with 0.5% of GSE ad libitum for 6 weeks. The data obtained show that GSE supplementation reduced polyp growth, and the immunohistochemical analyses of small intestinal tissue samples revealed a decrease in cell proliferation and an increase in apoptosis. GSE feeding also showed decreased protein levels of cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and β-catenin but an increase in Cip1/p21-positive cells.
Lala et al. [60] evaluated the chemopreventive activity of anthocyanin-rich extracts from three types of berries: Vaccinium myrtillus L., Aronia meloncarpa E., and V. vinifera. They treated Fischer 344 male rats with azoxymethane, a carcinogen, and fed them (for 14 weeks) with a diet enriched with berry extracts at a concentration of 3.85 g/kg. The results of the supplementation with grape extracts showed a significant decrease in the formation of aberrant crypt foci (ACF) in the colon and a reduction in the expression of COX-2. Derry et al. [93] employed azoxymethane to investigate the efficiency of GSE against colon tumorigenesis in A/J mice. Their results show that feeding the mice with a diet enriched with 0.25% and 0.5% GSE reduced tumor multiplicity in the colon, decreased tumor size, and exhibited antiproliferative and pro-apoptotic activities.
Other findings also highlight the potential health benefits of grape-derived components in various biological contexts. In AMPKα1-floxed mice crossed with Lgr5Cre mice and fed a diet supplemented with 5% grape pomace within the AIN-95G purified diet, noteworthy effects were observed. This regimen led to the suppression of colonic expression of inflammatory cytokines IL-1β and TNF-α, along with the inhibition of NF-κB inflammatory signaling. In addition, a decrease in ALDH1 expression was noted, while an increase in Cdx2 expression was concurrently observed, suggesting a potential role in modulating colonic inflammation and differentiation [61]. In a study involving Wistar rats exposed to grape juice concentrations of 1% and 2% for 15 days, the grape juice components exhibited possible antioxidant effects, as indicated by an increase in the expression of catalase (CAT) and manganese superoxide dismutase (Mn-SOD), thereby suggesting a potential protective mechanism against oxidative stress [62]. Furthermore, the incorporation of 5% grape pomace into the AIN-93G diet demonstrated multifaceted effects. It upregulated downstream genes of the farnesoid X receptor (FXR), leading to a reduction in fecal urease activity, and induced the upregulation of DNA repair enzyme MutS Homolog 2 (MSH2) while decreasing γ-H2AX levels, implying potential roles in regulating gut health and DNA integrity [62]. These diverse outcomes underscore the promising and intricate ways in which grape-related compounds may impact various physiological aspects.
Clinical studies
Some studies have also reported the chemopreventive effect of grape consumption in humans. Holcombe et al. [64] evaluated, in 30 healthy patients, the effect of grape consumption and examined biological biomarkers of proliferation and Wnt signaling in the colonic mucosa. After 2 weeks of daily ingestion of 0.15–0.45 kg of grapes, the results show a reduction in Wnt signaling and mucosal proliferation, which would eventually reduce the risk of mutational events that can facilitate colon carcinogenesis.
CONCLUSIONS AND PERSPECTIVES
This systematic literature review has shown that demonstrating the functional properties of grape matrices is important for science and industry. Nevertheless, the evaluation of these compounds should consider multiple aspects (i.e., material origin, species, variety, agroclimatic conditions, and postharvest and industrialization treatments) because they have a significant influence on the results—as seen above in the case of grapes in the genus Vitis. Each of the studies reviewed here contributes (in the form of incremental innovation) to the chemopreventive profile of berries in the genus Vitis. Although they are commonly cultivated, said berries present TPC values that range widely due to the fact that the agronomic performance of traditional varieties changes from place to place. In addition, this review has established that TPC differences in grapes have a direct influence on their antioxidant capacity.
The phenolic composition of grapes varies across different matrices, species, and varieties. The diversity of flavan-3-ols, anthocyanins, flavonols, and stilbenes underscores the complexity of grape-derived compounds. This diversity is a key factor in harnessing these bioactive components for dietary supplements and functional foods, emphasizing the need for matrix-specific analyses to maximize their potential benefits.
At the biological level, several studies using in vitro models of CRC have reported the protective effect of grape-derived phenolic compounds. Some of the main mechanisms associated with the biological effect of these compounds include apoptosis induction, reduction in cell proliferation, and decrease in the markers of oxidative stress. In turn, in vivo studies have shown the inhibition of tumor growth and the modulation of several signaling pathways involved in the carcinogenic process of CRC. Furthermore, the epidemiological evidence presented aligns with the notion that regular grape consumption may play a role in CRC prevention. The reduction in the expression of key molecules associated with cancer progression among diverse patient groups suggests a potential real-world benefit of grape intake.
The effects observed emphasize the need for comprehensive research to fully uncover the mechanisms through which grape compounds exert their health benefits. This integrated understanding paves the way for the development of novel preventive and therapeutic interventions for CRC, driven by the intricate interactions between grape-derived compounds and their matrices. As we continue to reveal these relationships, the potential to harness grapes as a source of functional ingredients for CRC management becomes increasingly promising.
Future studies in this field should further explore particular species. For instance, V. labrusca is one of the most widely consumed species in South America, and its high content of phenolic compounds has been identified in multiple research articles. However, not enough studies have shown its protective and inhibiting effect against CRC progression.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research was funded by project P20245 of the Instituto Tecnológico Metropolitano, Medellín, Colombia.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER'S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Skrovankova S, Sumczynski D, Mlcek J, Jurikova T, Sochor J. Bioactive compounds and antioxidant activity in different types of berries [Internet]. Int J Mol Ciences. 2015 [cited 2021 Apr 8];16:24673–706. CrossRef
2. Afrin S, Giampieri F, Gasparrini M, Forbes-Hernandez TY, Varela-López A, Quiles JL, et al. Chemopreventive and therapeutic effects of edible berries: a focus on colon cancer prevention and treatment [Internet]. Molecules. 2016 [cited 2020 Sep 27];21 :1–40. CrossRef
3. Brown EM, Latimer C, Allsopp P, Ternan NG, McMullan G, McDougall GJ, et al. In vitro and in vivo models of colorectal cancer: antigenotoxic activity of berries. J Agric Food Chem [Internet]. 2014 May 7 [cited 2020 Sep 27];62(18):3852–66. CrossRef
4. Anantharaju PG, Gowda PC, Vimalambike MG, Madhunapantula SV. An overview on the role of dietary phenolics for the treatment of cancers. Nutr J [Internet]. 2016;15(1):1–16. CrossRef
5. Araújo JR, Gonçalves P, Martel F. Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutr Res [Internet]. 2011;31(2):77–87. CrossRef
6. Haas ICS, Marmitt DJ, Fedrigo IMT, Goettert MI, Bordignon-Luiz MT. Evaluation of antiproliferative and anti-inflammatory effects of non-pomace sediment of red grape juices (Vitis labrusca L.) in healthy and cancer cells after in vitro gastrointestinal simulation. Pharma Nutr [Internet]. 2020;13(June):100204. CrossRef
7. Xia EQ, Deng GF, Guo YJ, Bin LH. Biological activities of polyphenols from grapes. Int J Mol Sci. 2010;11(2):622–46. CrossRef
8. Zhou K, Raffoul JJ. Potential anticancer properties of grape antioxidants. J Oncol. 2012;2012 :1–8. CrossRef
9. de la Rosa LA, Moreno-Escamilla JO, Rodrigo-García J, Alvarez-Parrilla E. Phenolic compounds. In: Postharvest physiology and biochemistry of fruits and vegetables [Internet]. Elsevier Inc.; 2018. pp 253–71. CrossRef
10. Medrano-Padial C, Puerto M, Merchán-Gragero MM, Moreno FJ, Richard T, Cantos-Villar E, et al. Cytotoxicity studies of a stilbene extract and its main components intended to be used as preservative in the wine industry. Food Res Int. 2020 Nov 1;137 :1–10. CrossRef
11. da Silva Haas IC, Toaldo IM, Gomes TM, Luna AS, de Gois JS, Bordignon-Luiz MT. Polyphenolic profile, macro- and microelements in bioaccessible fractions of grape juice sediment using in vitro gastrointestinal simulation. Food Biosci. 2019 Feb 1;27:66–74. CrossRef
12. Toaldo IM, Cruz FA, Alves TDL, De Gois JS, Borges DLG, Cunha HP, et al. Bioactive potential of Vitis labrusca L. grape juices from the Southern Region of Brazil: phenolic and elemental composition and effect on lipid peroxidation in healthy subjects. Food Chem. 2015 Apr 15;173:527–35. CrossRef
13. Martins IM, Macedo GA, Macedo JA. Biotransformed grape pomace as a potential source of anti-inflammatory polyphenolics: effects in Caco-2 cells. Food Biosci. 2020 Jun 1;35 :1–9. CrossRef
14. Wang S, Mateos R, Goya L, Amigo-Benavent M, Sarriá B, Bravo L. A phenolic extract from grape by-products and its main hydroxybenzoic acids protect Caco-2 cells against pro-oxidant induced toxicity. Food Chem Toxicol. 2016 Feb 1;88:65–74. CrossRef
15. Ochoa Medina F, Diaz Garcia AI, Castellanos L, Martinez Diaz J. Obtención de vinagre a partir de uva (Vitis vinifera labrusca L) fruta rica en carbohidratos. Servicio Nacional de Aprendizaje- SENA; 2014.
16. Drosou C, Kyriakopoulou K, Bimpilas A, Tsimogiannis D, Krokida M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind Crops Prod [Internet]. 2015;75:141–9. CrossRef
17. Gonzalez Paramás AM, Esteban Ruano S, Santos Buelga C, Teresa SP, Rivas Gonzalo JC. Flavanol content and antioxidant activity in winery byproducts. Agric Food Chem. 2004;52(2):234–8. CrossRef
18. Kim C, Kim B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: a review. Nutrients. 2018;10(8) :1–29. CrossRef
19. Li FX, Li FH, Yang YX, Yin R, Ming J. Comparison of phenolic profiles and antioxidant activities in skins and pulps of eleven grape cultivars (Vitis vinifera L.). J Integr Agric. 2019 May 1;18(5):1148–58. CrossRef
20. Li AN, Li S, Zhang YJ, Xu XR, Chen YM, Li H Bin. Resources and biological activities of natural polyphenols. Nutrients. 2014;6(12):6020–47. CrossRef
21. Majewska M, Lewandowska U. The chemopreventive and anticancer potential against colorectal cancer of polyphenol-rich fruit extracts. Food Rev Int. 2018;34(4):390–409. CrossRef
22. Unusan N. Proanthocyanidins in grape seeds: an updated review of their health benefits and potential uses in the food industry. J Funct Foods [Internet]. 2020;67(November 2019):103861. CrossRef
23. Gupta M, Dey S, Marbaniang D, Pal P, Ray S, Mazumder B. Grape seed extract: having a potential health benefits. J Food Sci Technol. 2020;57(4):1205–15. CrossRef
24. González J, Valls N, Brito R, Rodrigo R. Essential hypertension and oxidative stress: new insights. World J Cardiol. 2014;6(6):353. CrossRef
25. Urquiaga I, D’Acuña S, Pérez D, Dicenta S, Echeverría G, Rigotti A, et al. Wine grape pomace flour improves blood pressure, fasting glucose and protein damage in humans: a randomized controlled trial. Biol Res. 2015;48:1–10. CrossRef
26. Dinicola S, Cucina A, Pasqualato A, D’Anselmi F, Proietti S, Lisi E, et al. Antiproliferative and apoptotic effects triggered by grape seed extract (GSE) versus epigallocatechin and procyanidins on colon cancer cell lines. Int J Mol Sci. 2012;13(1):651–64. CrossRef
27. Nivelle L, Aires V, Rioult D, Martiny L, Tarpin M, Delmas D. Molecular analysis of differential antiproliferative activity of resveratrol, epsilon viniferin and labruscol on melanoma cells and normal dermal cells. Food Chem Toxicol. 2018;116(April):323–34. CrossRef
28. Cheah KY, Howarth GS, Bindon KA, Kennedy JA, Bastian SEP. Low molecular weight procyanidins from grape seeds enhance the impact of 5-fluorouracil chemotherapy on Caco-2 human colon cancer cells. PLoS One. 2014;9(6) :1–8. CrossRef
29. Yu J, Ahmedna M. Functional components of grape pomace: their composition, biological properties and potential applications. Int J Food Sci Technol [Internet]. 2013 Feb 1 [cited 2021 Mar 28];48(2):221–37. CrossRef
30. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin [Internet]. 2021 Feb 4 [cited 2021 Mar 9]; 71: 1–41. CrossRef
31. Patiño-Márquez IA, Patiño-González E, Hernández-Villa L, Ortíz-Reyes B, Manrique-Moreno M. Identification and evaluation of Galleria mellonella peptides with antileishmanial activity. Anal Biochem [Internet]. 2018 Apr 1 [cited 2020 Sep 27];546:35–42. CrossRef
32. Silva AS, Reboredo-Rodríguez P, Süntar I, Sureda A, Belwal T, Loizzo MR, et al. Evaluation of the status quo of polyphenols analysis: Part I—phytochemistry, bioactivity, interactions, and industrial uses. Compr Rev Food Sci Food Saf. 2020 Nov 1;19(6):3191–218. CrossRef
33. Koyama K, Kamigakiuchi H, Iwashita K, Mochioka R, Goto-Yamamoto N. Polyphenolic diversity and characterization in the red–purple berries of East Asian wild Vitis species. Phytochemistry. 2017 Feb 1;134:78–86. CrossRef
34. Nascimento RP, Machado APF. The preventive and therapeutic effects of anthocyanins on colorectal cancer: a comprehensive review based on up-to-date experimental studies. Food Res Int. 2023;170 :1–18. CrossRef
35. Rockenbach II, Gonzaga LV, Rizelio VM, Gonçalves AE de SS, Genovese MI, Fett R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res Int [Internet]. 2011;44(4):897–901. CrossRef
36. El Elimat T, Jarwan BA, Zayed A, Alhusban A, Syouf M. Biochemical evaluation of selected grape varieties (Vitis vinifera L.) grown in Jordan and in vitro evaluation of grape seed extract on human prostate cancer cells. Food Biosci [Internet]. 2018;24:103–10. CrossRef
37. Wang S, Amigo-Benavent M, Mateos R, Bravo L, Sarriá B. Effects of in vitro digestion and storage on the phenolic content and antioxidant capacity of a red grape pomace. Int J Food Sci Nutr. 2017 Feb 17;68(2):188–200. CrossRef
38. Sequeda Castañeda LG, Barrera Bugallo AR, Celis C, Iglesias J, Morales L. Evaluation of antioxidant and cytotoxic activity of extracts from fruits in fibroblastoma HT1080 cell lines: four fruits with commercial potential in Colombia. Emir J Food Agric [Internet]. 2016 [cited 2020 Sep 27];28(2):143–51. CrossRef
39. Habib HM, El-Fakharany EM, Kheadr E, Ibrahim WH. Grape seed proanthocyanidin extract inhibits DNA and protein damage and labile iron, enzyme, and cancer cell activities. Sci Rep [Internet]. 2022 [cited 2023 Jul 28];12(12393):1–14. CrossRef
40. Arcanjo NMO, Neri Numa IA, Bezerra TKA, da Silva FLH, Pastore GM, Madruga MS. Quality evaluation of red wines produced from the Isabella and Ives cultivar (Vitis labrusca): physicochemical parameters, phenolic composition and antioxidant activity. Food Sci Technol [Internet]. 2017 May 29 [cited 2021 May 27];37(2):184–92. CrossRef
41. Burin VM, Ferreira Lima NE, Panceri CP, Bordignon Luiz MT. Bioactive compounds and antioxidant activity of Vitis vinifera and Vitis labrusca grapes: evaluation of different extraction methods. Microchem J. 2014;114:155–63. CrossRef
42. Pérez-Navarro J, Hermosín-Gutiérrez I, Gómez-Alonso S, Kurt-Celebi A, Colak N, Akpinar E, et al. Vitis vinifera Turkish novel table grape ‘Karaerik’. Part II: Non-anthocyanin phenolic composition and antioxidant capacity. J Sci Food Agric. 2022 Jan 30;102(2):813–22. CrossRef
43. Ortiz JMP, Alonso SG, Alguacil LF, Salas E, Hermosín I. extracts on colorectal cancer cell lines antiproliferative and cytotoxic effects of grape pomace and grape seed extracts on colorectal cancer cell lines. Food Sci Nutr. 2019; 7 (August):2948–57. CrossRef
44. Derry M, Raina K, Agarwal R, Agarwal C. Differential effects of grape seed extract against human colorectal cancer cell lines: the intricate role of death receptors and mitochondria. Cancer Lett [Internet]. 2013;334(1):69–78. CrossRef
45. Kaur M, Mandair R, Agarwal R, Agarwal C. Grape seed extract induces cell cycle arrest and apoptosis in human colon carcinoma cells. Nutr Cancer. 2008;60(SUPPL. 1):2–11. CrossRef
46. Dinicola S, Cucina A, Pasqualato A, Proietti S, D’Anselmi F, Pasqua G, et al. Apoptosis-inducing factor and caspase-dependent apoptotic pathways triggered by different grape seed extracts on human colon cancer cell line Caco-2. Br J Nutr. 2010;104(6):824–32. CrossRef
47. Hsu CP, Lin YH, Chou CC, Zhou SP, Hsu YC, Liu CL, et al. Mechanisms of grape seed procyanidin-induced apoptosis in colorectal carcinoma cells. Anticancer Res. 2009;29(1):283–9.
48. Lingua MS, Theumer MG, Kruzynski P, Wunderlin DA, Baroni M V. Bioaccessibility of polyphenols and antioxidant properties of the white grape by simulated digestion and Caco-2 cell assays: comparative study with its winemaking product. Food Res Int [Internet]. 2019;122(May):496–505. CrossRef
49. Leifert WR, Abeywardena MY. Grape seed and red wine polyphenol extracts inhibit cellular cholesterol uptake, cell proliferation, and 5-lipoxygenase activity. Nutr Res [Internet]. 2008;28(12):842–50. CrossRef
50. Yang J, Martinson TE, Liu RH. Phytochemical profiles and antioxidant activities of wine grapes. Food Chem [Internet]. 2009;116(1):332–9. CrossRef
51. Jara-Palacios MJ, Hernanz D, Cifuentes-Gomez T, Escudero-Gilete ML, Heredia FJ, Spencer JPE. Assessment of white grape pomace from winemaking as source of bioactive compounds, and its antiproliferative activity. Food Chem. 2015;183:78–82. CrossRef
52. Lazze MC, Pizzala R, Pecharromán G, Garnica PFJG, Antolín Rodríguez JM, Fabris N, et al. Grape waste extract obtained by supercritical fluid extraction contains bioactive antioxidant molecules and induces antiproliferative effects in human colon adenocarcinoma cells. J Med Food. 2009;12(3):561–8. CrossRef
53. Quero J, Jiménez-Moreno N, Esparza I, Osada J, Cerrada E, Ancín-Azpilicueta C, et al. Grape stem extracts with potential anticancer and antioxidant properties. Antioxidants. 2021;10(2):1–17. CrossRef
54. Recinella L, Chiavaroli A, Veschi S, Cama A, Acquaviva A, Loreta Libero M, et al. A grape (Vitis vinifera L.) pomace water extract modulates inflammatory and immune response in SW-480 cells and isolated mouse colon. Phytother Res [Internet]. 2022 Jul 7 [cited 2023 Feb 21];36:4620–30. CrossRef
55. Caponio GR, Cofano M, Lippolis T, Gigante I, De Nunzio V, Difonzo G, et al. Anti-proliferative and pro-apoptotic effects of digested aglianico grape pomace extract in human colorectal cancer cells. molecules [Internet]. 2022 Oct 1 [cited 2023 Apr 3];27(20):6791. CrossRef
56. Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin Cancer Res. 2006;12(20 PART 1):6194–202. CrossRef
57. Pertuzatti PB, Mendonça SC, Alcoléa M, Guedes CT, Amorim F da E, Beckmann APS, et al. Bordo grape marc (Vitis labrusca): evaluation of bioactive compounds in vitro and in vivo. Food Sci Technol [Internet]. 2020;129(January):109625. CrossRef
58. Rytsyk O, Soroka Y, Shepet I, Vivchar Z, Andriichuk I, Lykhatskyi P, et al. Experimental evaluation of the effectiveness of resveratrol as an antioxidant in colon cancer prevention. Nat Prod Commun. 2020;15(6):1–10. CrossRef
59. Velmurugan B, Singh RP, Kaul N, Agarwal R, Agarwal C. Dietary feeding of grape seed extract prevents intestinal tumorigenesis in APCmin/+ mice. Neoplasia [Internet]. 2010;12(1):95–102. CrossRef
60. Lala G, Malik M, Zhao C, He J, Kwon Y, Giusti MM, et al. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr Cancer. 2006;54(1):84–93. CrossRef
61. Tian Q, Xu Z, Sun X, Deavila J, Du M, Zhu M. Grape pomace inhibits colon carcinogenesis by suppressing cell proliferation and inducing epigenetic modifications. J Nutr Biochem. 2020 Oct 1;84. CrossRef
62. Dias Ribeiro CCD, Mendes Silva R, Pazine Campanholo VM de L, Ribeiro DA, RibeiroPaiotti AP, Forones NM. Effects of grape juice in superoxide dismutase and catalase in colorectal cancer carcinogenesis induced by azoxymethane. Asian Pac J Cancer Prev. 2018;19(10):2839–44.
63. Wang H, Tian Q, Xu Z, Du M, Zhu MJ. Metabolomic profiling for the preventive effects of dietary grape pomace against colorectal cancer. J Nutr Biochem. 2023 Jun 1;116 :1–8. CrossRef
64. Holcombe RF, Martinez M, Planutis K, Planutiene M. Effects of a grape-supplemented diet on proliferation and Wnt signaling in the colonic mucosa are greatest for those over age 50 and with high arginine consumption. Nutr J [Internet]. 2015;14(1):1–8. CrossRef
65. Moher D, Liberati A, Tetzlaff J, Altman DG, Antes G, Atkins D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 :1–14. CrossRef
66. Fernández-López JA, Almela L, Muñóz JA, Hidalgo V, Carreno J. Dependence between colour and individual anthocyanin content in ripening grapes. Food Res Int [Internet]. 1998;31(9):667–72. Available from: www.elsevier.com/locate/foodres CrossRef
67. Unterkofler J, Muhlack RA, Jeffery DW. Processes and purposes of extraction of grape components during winemaking: current state and perspectives. Appl Microbiol Biotechnol. 2020;104:4737–55. CrossRef
68. Han F, Yang P, Wang H, Fernandes I, Mateus N, Liu Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci Technol. 2019;83:211–24. CrossRef
69. Mucalo A, Maleti´c EM, Zduni´c GZ. Extended harvest date alter flavonoid composition and chromatic characteristics of plavac mali (Vitis vinifera L.) grape berries. Foods [Internet]. 2020;9(1155):1–25. Available from: www.mdpi.com/journal/foods CrossRef
70. Castañeda Vázquez BI. Inducción de antocianinas y capacidad antioxidante por oligogalacturónidos en uvas de mesa cv. ‘Flame Seedless’ [Theses]. Hermosillo, Mexico: Centro de Investigación en Alimentación y Desarrollo, A. C.; 2010.
71. Mikeš O, Vrchotová N, Tríska J, Kyseláková M, Šmidrkal J. Distribution of major polyphenolic compounds in vine grapes of different cultivars growing in South Moravian Vineyards. Czech J Food Sci. 2008;26(3):182–9. CrossRef
72. Ordoñez ES, Leon-Arevalo A, Rivera-Rojas H, Vargas E. Quantification of total polyphenols and antioxidant capacity in skins and seeds from cacao (Theobroma cacao L.), tuna (Opuntia ficus indica Mill), grape (Vitis Vinífera) and uvilla (Pourouma cecropiifolia). Sci Agr. 2019;10(2):175–83. CrossRef
73. Ruales-Salcedo AV, Rojas-González AF, Cardona-Alzate CA. Obtencion de compuestos fenolicos a partir de residuos de uva isabella (Vitis labrusca). Biotecnol Sector Agr Agroind. 2017;2:72–9. CrossRef
74. Chartier LC, Howarth GS, Trinder D, Mashtoub S. Emu oil and grape seed extract reduce tumour burden and disease parameters in murine colitis-associated colorectal cancer. Carcinogenesis. 2021 Feb 1;42(2):202–9. CrossRef
75. Jacob JK, Hakimuddin F, Paliyath G, Fisher H. Antioxidant and antiproliferative activity of polyphenols in novel high-polyphenol grape lines. Food Res Int. 2008;41(4):419–28. CrossRef
76. Fang YL, Zhang A, Wang H, Li H, Zhang ZW, Chen SX, et al. Health risk assessment of trace elements in Chinese raisins produced in Xinjiang province. Food Control. 2010 May;21(5):732–9. CrossRef
77. Zielinski AAF, Ávila S, Ito V, Nogueira A, Wosiacki G, Haminiuk CWI. The Association between chromaticity, phenolics, carotenoids, and in vitro antioxidant activity of frozen fruit pulp in Brazil: an application of chemometrics. J Food Sci. 2014;79(4) :510–6. CrossRef
78. Jakobek L, Seruga M, Novak lvana, Medvidovi-Kosan M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Deutsche Lebensmittel Rund. 2007;103 :369–78.
79. Vinson JA, Su X, Zubik L, Bose P. Phenol antioxidant quantity and quality in foods: fruits. J Agric Food Chem. 2001;49(11):5315–21. CrossRef
80. Spissu Y, Gil KA, Dore A, Sanna G, Palmieri G, Sanna A, et al. Anti- and pro-oxidant activity of polyphenols extracts of Syrah and Chardonnay grapevine pomaces on melanoma cancer cells. Antioxidants [Internet]. 2022 Dec 29 [cited 2023 Mar 5];12(1):80. CrossRef
81. Lu Y, Foo LY. The polyphenol constituents of grape pomace. Food Chem. 1999;65:1–8. CrossRef
82. Wan Y, Schwaninger H, Li D, Simon CJ, Wang Y, Zhang C. A review of taxonomic research on Chinese wild grapes. Vitis. 2008;47(2):81–8.
83. Cosme F, Pinto T, Vilela A. Phenolic Compounds and antioxidant activity in grape juices: a chemical and sensory view. Beverages. 2018;4(1):22. CrossRef
84. Dani C, Oliboni LS, Vanderlinde R, Bonatto D, Salvador M, Henriques JAP. Phenolic content and antioxidant activities of white and purple juices manufactured with organically- or conventionally-produced grapes. Food Chem Toxicol. 2007 Dec;45(12):2574–80. CrossRef
85. Corrales M, Fernandez A, Vizoso Pinto MG, Butz P, Franz CMAP, Schuele E, et al. Characterization of phenolic content, in vitro biological activity, and pesticide loads of extracts from white grape skins from organic and conventional cultivars. Food Chem Toxicol [Internet]. 2010;48(12):3471–6. CrossRef
86. Coelho MC, Pereira RN, Rodrigues AS, Teixeira JA, Pintado ME. The use of emergent technologies to extract added value compounds from grape by-products. Trends Food Sci Technol. 2020;106:182–97. CrossRef
87. Bashmil YM, Ali A, Bk A, Dunshea FR, Suleria HAR. Screening and characterization of phenolic compounds from australian grown bananas and their antioxidant capacity. Antioxidants. 2021 Oct 1;10(10):1–20. CrossRef
88. Wang J, Xie B, Sun Z. Quality parameters and bioactive compound bioaccessibility changes in probiotics fermented mango juice using ultraviolet-assisted ultrasonic pre-treatment during cold storage. LWT. 2021 Feb 1;137. CrossRef
89. Pérez-Navarro J, Izquierdo-Cañas PM, Mena-Morales A, Martínez-Gascueña J, Chacón-Vozmediano JL, García-Romero E, et al. Phenolic compounds profile of different berry parts from novel Vitis vinifera L. red grape genotypes and Tempranillo using HPLC-DAD-ESI-MS/MS: a varietal differentiation tool. Food Chem. 2019 Oct 15;295:350–60. CrossRef
90. Munin A, Edwards-Lévy F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics. 2011;3:793–829. CrossRef
91. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I BF. International Agency for Research on Cancer 2020. [Internet], Global Cancer Observatory: Cancer Today; 2020. Vol. 419. Available from: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf
92. Owczarek K, Lewandowska U. The impact of dietary polyphenols on COX-2 expression in colorectal cancer. Nutr Cancer [Internet]. 2017;69(8):1105–18. CrossRef
93. Derry MM, Raina K, Balaiya V, Jain AK, Shrotriya S, Huber KM, et al. Grape seed extract efficacy against azoxymethane-induced colon tumorigenesis in A/J mice: interlinking miRNA with cytokine signaling and inflammation. Cancer Prev Res. 2013;6(7):625–33. CrossRef
94. San Hipólito Luengo Á, Alcaide A, Ramos González M, Cercas E, Vallejo S, Romero A, et al. Dual effects of resveratrol on cell death and proliferation of colon cancer cells. Nutr Cancer. 2017 Oct 3;69(7):1019–27. CrossRef
95. Signorelli P, Fabiani C, Brizzolari A, Paroni R, Casas J, Fabriàs G, et al. Natural grape extracts regulate colon cancer cells malignancy. Nutr Cancer. 2015;67(3):494–503. CrossRef
96. Juan ME, Wenzel U, Daniel H, Planas JM. Resveratrol induces apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells. J Agric Food Chem. 2008;56(12):4813–8. CrossRef
SUPPLEMENTARY MATERIAL
Supplementary data can be downloaded from the journal’s website