INTRODUCTION
Immune cell infiltration in the gingiva, which causes connective tissue destruction, resorption of the alveolar bone, and clinical attachment loss, is the hallmark of the chronic inflammatory illness known as periodontitis. A person’s quality of life will be impacted if periodontitis causes an increase in attachment loss. Because it may lead to a chance of worsening masticatory dysfunction by resulting in tooth loss, tooth extrusion, tooth migration, and dental hypermobility. Over the past few decades, there has been a surge in collagen-based biomaterial utilization in tissue engineering applications [1].
Collagen is used as a barrier material to prevent gingival connective tissue from touching the gum surface, create space, and guide periodontal tissue or bone regeneration because of its many benefits, such as biocompatibility, biodegradability, appropriate mechanical strength, flexibility, and the capacity to absorb bodily fluids for nutrient transfer. Collagen is a multipurpose material commonly employed in pharmacology, medicine, and dentistry [1]. Collagen is a prominent protein in various animal bodies and accounts for approximately 30% of these organisms’ protein compounds [2]. Furthermore, it is predominantly concentrated in the bones and skin of animals, including pigs [3]. Several studies have shown that the primary source of commercial collagen includes beef and pork bones [4].
The utilization of beef bone as a source of collagen has raised concerns among producers due to the emergence of bovine spongiform encephalopathy, transmissible spongiform encephalopathy, and foot and mouth disease [3,4]. Therefore, it is necessary to consider using alternative raw materials apart from bovine and pigs. One of the alternatives is the use of marine animals, which contain collagen in their bones, skin, scales, and fins [5,6].
Fish processing generates significant by-products, such as heads, skin, bones, scales, and entrails, typically accounting for 50%–70% of the raw material. However, improper management of these materials without a sewage system can cause environmental pollution [5–8]. The optimization of by-products as raw materials for collagen can be used to increase their added value and reduce environmental pollution [9].
Several studies have been carried out on extracting and characterizing collagen from fish processing by-products. For example, Li et al. [6] examined acid-soluble collagen and pepsin from Spanish mackerel skin and bones. Wu et al. [7] studied deriving the protein from skin fish and grass carp swim bladders (Ctenopharyngodon idella). Chuaychan et al. [8] explored collagen derived from seabass fish scales, and Tylingo and Mania [9] on the collagen extraction of African catfish, salmon, and Baltic cod. Danila et al. [10] studied collagen from Cyprinus carpio L. fish for application in the food, cosmetic, and pharmaceutical industries. Chen et al. [11] studied collagen membranes from fish scales for tissue and bone regeneration. Ampitiya et al. [12] studied collagen from yellowfin tuna (Thunnus albacares), seabass (Scomberomorus commerson), and sea bass (Lates calcarifer) for food, pharmaceutical, and biomedical industries. Last, Wang et al. [13] and Kaewdang et al. [14] studied cold-water fish protein extraction.
Based on previous findings, more studies need to be done on extracting fish collagen from freshwater fish in Indonesia. Common carp, with the Latin name C. carpio L., is a freshwater fish that lives in tropical waters and is widely distributed across public waters [15]. Among the various species cultivated in Indonesia, carp is particularly important and commonly reared in floating ponds in West Java cage net ecosystems [15]. Common carp scales have not been extensively studied as a collagen source in dentistry, making this study unique in its focus on exploring the extraction and characterization of collagen from carp scales. Utilizing this species as a processed product often leads to significant waste generation, especially scales, which account for 30%–40% of whole fish. The abundance of waste from industrial fish processing presents an opportunity for scientists to investigate alternative sources of collagen, such as bones, skin, scales, and fins [16]. Thus, the purpose of this study is to describe and extract collagen from C. carpio L. scales to make use of waste products. The results are expected to provide an alternative source of collagen apart from the body of mammalians.
MATERIALS AND METHODS
Sample preparation
This study used fresh common carp scales obtained from collectors in Kajojo, Antapani-Bandung, Indonesia, as the study materials. Common carp specimens were cultivated in breeder ponds in Subang, West Java, Indonesia. The common carp scales were isolated using the acid extraction method [6], and the dried samples (200 g) were soaked in 0.1 M NaOH solution with a ratio of 1:10 for 6 hours at room temperature. Subsequently, the marinade was filtered, and the solution was discarded. The soaked fish scales were then rinsed with distilled water until the pH reached 7, followed by soaking in 0.5 M CH3COOH at a 1:10 ratio for 3 days at room temperature. The samples were filtered and rinsed with distilled water until a pH of 4.6 was achieved. They were then extracted using a water bath at temperature of ±40°C for 3 hours with a ratio of 1:2, followed by filtration of the solution and removal of scales. The collagen solution was dried using the spray drying technique at 110°C with 250 maltodextrins added as a coating to form collagen powder.
The yield of collagen on a dry basis was determined by calculating the percentage ratio of the dry weight of collagen to the wet weight of raw common carp scales before extraction, following the guidelines provided by AOAC [8,17,18]. Furthermore, the yield was calculated using the formula below:
The characteristic test was carried out to determine the properties of collagen, including yield, chemical, and physical features. The chemical characteristics included moisture and ash content, while the physical properties were measured using an fourier transform infrared spectroscopy (FTIR) spectrophotometer and a pH meter.
Collagen powder samples were immersed in 50 ml of water at room temperature for 24 hours. The soaked samples were placed on filter paper to remove water above the surface of the membrane and then weighed [18]. Water absorption was calculated using the formula below:
A total of 10 g of common carp scales collagen powder was placed in an empty cup and put in a furnace (brand Vulcan 3–550) with a temperature of 600°C for 4 hours or until the ash turned white. Subsequently, the cup was cooled in a desiccator until it reached room temperature, and it was weighed [15,18]. The ash content was calculated using the formula below:
The acidity (pH) degree was measured using a Mettler Toledo Seven Compact. The tool was connected to a power source and turned on by pressing the on/off button, allowing some time for texts to appear on the screen. Furthermore, it was calibrated using pH 7, pH 4, and pH 10 buffers to ensure accuracy. Collagen samples were prepared by mixing with distilled water at a 1:100 (w/v) ratio, followed by homogenization. The pH of the samples was then measured using a pH meter until a stable value was obtained [18].
Statistical analysis
Pellets were prepared by putting 200 mg of KBr into the mortar, then adding 2 mg of the sample and mixing until the mixture was homogeneous. The mixture was then modified into the final form using a pellet maker and stored in a dry place [19]. Measurements were made using a Bruker type Tensor 37, MIR light source, DTGS detector, 4 cm−1 resolution, 32 scans at wave numbers 650–4,000/cm. Furthermore, collagen functional groups were determined based on the absorption peak wavenumber detected with the absorption region for the protein functional groups. FTIR analysis was carried out based on the method proposed by Yu et al. [19]. The data were analyzed using IBM SPSS version 27 and presented in the form of the average of three water content replications and two ash content replications with SD.
RESULTS AND DISCUSSION
The raw material for fish scales was obtained from freshwater common carp fish (Cyprinus common carpio L.) in Subang, West Java, Indonesia, as shown in Figure 1. Common carp scales were washed in cold water, drained, and dried in the sun for 8 hours. Furthermore, dry samples were packaged in plastic and stored at −20°C until ready for use, as shown in Figure 2.
Dry fish scales were soaked in 0.1 M NaOH for 6 hours at room temperature. The saturated solution was discarded, and scale dregs were rinsed with distilled water to a pH of 7. Subsequently, the dregs were soaked in 0.5M CH3COOH for 3 days at room temperature, and the soaking solution was discarded. The samples were then rinsed with distilled water to a pH of 4.6. Waterbath extraction was carried out at 40°C for 3 hours with a ratio of scales to distilled water of 1:2. The dregs of scales were removed to facilitate the production of collagen solution. Drying was carried out using the spray drying technique at 110°C with the addition of 250 g of maltodextrin as a coating until collagen powder was produced.
Yield refers to the amount of collagen produced from the initial raw material. It provided information on the portion of the raw material that might be used and served as a crucial factor in estimating the product’s economic worth and efficacy. This study showed that common carp scales collagen produced a yield of 11%.
The analysis results of water content, ash content, and pH of common carp scales collagen and collagen standards based on Indonesian National Standard (SNI) 8076:2014 are presented in Table 1 [20]. The water content was 3.66%, lower than the standard of 12%. Furthermore, the ash content was 0.32% lower than the SNI 8076:2014 requirements, i.e., a maximum of 1. The results showed that the degree of acidity expressed in pH (6.57–7.38) was still within the range of SNI 8076:2014 quality requirements between 6.5 and 8. The spectrum of FTIR test results for common carp scales collagen (C. carpio L.) is presented in Figure 3.
 | Figure 1. Cyprinus carpio L. [Click here to view] |
.jpg) | Figure 2. Common carp (C. carpio L.) scales. [Click here to view] |
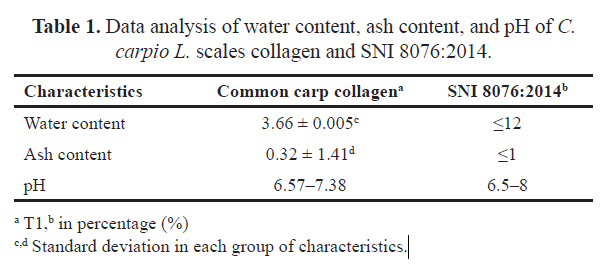 | Table 1. Data analysis of water content, ash content, and pH of C. carpio L. scales collagen and SNI 8076:2014. [Click here to view] |
Based on Figure 3 and Table 2, the FTIR spectrum of common carp scales collagen started at 3,394.10 cm−1 and decreased to a lower frequency of 1,240.50 cm−1. The absorption areas of amide A, B, I, and III were at 3,394.10 cm−1, 2,927.76 cm−1, 1,647.66 cm−1, and 1,240.50 cm−1, while amide II was not identified.
This study aims to use waste materials to extract and characterize collagen from common carp (C. carpio L.) scales. The variation in collagen yield was caused by the extraction method and the concentration of the solution used to remove non-collagen protein, temperature, and length of production [21]. Furthermore, the yield obtained was an essential parameter in assessing the effectiveness of the production process through several stages, such as cutting fish skin, demineralization, hydrolysis, extraction, and drying [22]. The amount of collagen obtained from acetic acid extraction using spray drying was 11%. The yield was relatively small due to a large amount of product wasted during the washing process and the imperfect hydrolysis and extraction processes [22]. According to Putra et al. [23], the yield, character, and composition of collagen molecules were strongly influenced by species, habitat, and treatment differences in the extraction process.
.jpg) | Figure 3. FTIR spectrum of common carp (C. carpio L. L.) scales. [Click here to view] |
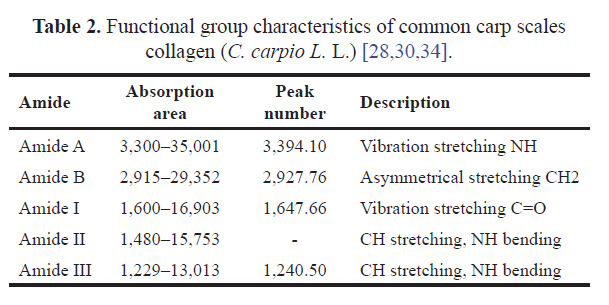 | Table 2. Functional group characteristics of common carp scales collagen (C. carpio L. L.) [28,30,34]. [Click here to view] |
Common carp scales collagen contained 3.66% and 0.32% moisture and ash content, respectively. The high ash content using the spray drying technique was caused by the composition of calcium hydroxyapatite (Ca5(PO4)3 OH) in fish scales, and this was in line with the results of Liu et al. [24]. Furthermore, the low water content showed that the extraction method effectively removes water when extracting collagen. This finding was consistent with Pamungkas et al. [25] regarding the water content in the scales of snakehead fish, and a significant amount of water was lost during the freeze-drying procedure. The low ash content indicated the influence of certain materials on the demineralization process. The results are also in line with Wang et al. [26] who reported that approximately 98% of inorganic components could be removed from cocaine croaker scales after demineralization using 0.5 M EDTA-2Na (pH 7.4).
The presence of amide A collagen at 3,394.10 cm−1 showed that NH groups were involved in hydrogen bonding. The amide A absorption area occurred due to the NH stretching of the amide group with hydrogen bonds and the amino acid hydroxyproline [22,27]. Amide I, II, and III absorption sites were known to be directly related to the shape of the polypeptide. The results showed that the N-H bond occurred in the 3,300–3,500 cm−1 range and then decreased to a lower frequency. Furthermore, amide A form collagen of carp scales decreased amplitude at 3,300 cm−1. The absorption peak of amide B was detected at wave numbers in the range of 2,927.76 and 2,937.21 cm−1, indicating the presence of a typical collagen group. According to Coates [28], wave numbers indicating amide B absorption were formed from the CH2 group asymmetrical stretch.
The absorption peaks of amide I collagen from common carp scales showed values of 1,647.66 cm−1 and 1,655.13 cm−1. The low value of amide I was due to the large non-helical portion of the telopeptide [25]. This affected the intramolecular hydrogen bonds between C=O in the peptide and the lower hydrogen donor linkages [22,29]. Furthermore, it was associated with stretching the carbonyl group C=O bonds along the polypeptide chain, which involved the participation of NH bonds and CN stretching and served as an important factor in determining the secondary structure of proteins [20,24].
Amide I consists of four components, those are α-helix, β-sheet, β -turn, and overlapping random coils [21,22,30,31]. Based on this finding, collagen from tuna skin had a β-sheet structure that had not been denatured into an α-helix characteristic of gelatin [21,30,32]. Collagen physical characteristics based on FTIR analysis showed amide A, B, I, II, and III. The triple helix shape of amide I and III revealed that the molecule generated was collagen [29]. The stability of the triple helix depended on hydrogen bonds [33].
CONCLUSION
Extraction of common carp scale collagen using the acetic acid method and spray drying techniques produced yield, weight, moisture content, ash content, and pH that met the standard collagen composition regarding SNI 8076:2014. FTIR analysis showed a triple helical structure, indicating that the compound produced was collagen and could be used as an alternative in dentistry. However, weaknesses in this study include not conducting scanning electron microscopy (SEM), X-ray diffraction (XRD), and cell viability tests. SEM test needs to be done to observe the morphology of collagen. An XRD test also needs to be done to determine collagen’s structure and particle size. In addition, it is necessary to conduct a cell viability test to determine whether the collagen has a toxic effect. Future research is expected to be able to carry out variations in the type of fish scales and complete tests on collagen such as FTIR, pH meter, SEM, XRD, and cell viability.
AUTHOR CONTRIBUTIONS
Conceptualization, DNC.; methodology, DNC and CS; software, DNC and CS; validation, MHS, BPP and REK; formal analysis, DNC and AS; investigation, DNC; resources, DNC and MHS; data curation, DNC, CS, and REK; writing—original draft, DNC; writing—review and editing, AS and CS; visualization, DNC and CS; supervision, MHS, AS, BPP, CS and REK; project administration, DNC; funding acquisition, DNC. All authors have read and agreed to the published version of the manuscript.
FINANCIAL SUPPORT
This work was supported by the Domestic Postgraduate Education Scholarships (BPPDN: B/67/D.D3/KD.02.00/2019).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Prahasanti C, Arini NL, Wulan KD, Hendro OV, Wijaksana IKE, Ulfah N, et al. The expression of BMP4 and FGF2 in Wistar rats (Rattus norvegicus) post application of gourami fish (Osphronemus goramy) collagen. Dent J (Majalah Kedokt Gigi). 2023 Mar 15;56(2):115–21. CrossRef
2. European Union. Official journal of the European union L54. Luxemburg: European Union; 2011. pp. 1–254.
3. Huang CY, Kuo JM, Wu SJ, Tsai HT. Isolation and characterization of fish scale collagen from tilapia (Oreochromis sp.) by a novel extrusion–hydro-extraction process. Food Chem. 2016 Jan;190:997–1006. CrossRef
4. Silva T, Moreira-Silva J, Marques A, Domingues A, Bayon Y, Reis R. Marine origin collagens and its potential applications. Mar Drugs. 2014 Dec 5;12(12):5881–901. CrossRef
5. Kittiphattanabawon P, Benjakul S, Visessanguan W, Nagai T, Tanaka M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005 Feb;89(3):363–72. CrossRef
6. Li ZR, Wang B, Chi C, Zhang QH, Gong Y, Tang JJ, et al. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius). Food Hydrocoll. 2013 May;31(1):103–13. CrossRef
7. Wu X, Cai L, Cao A, Wang Y, Li T, Li J. Comparative study on acid-soluble and pepsin-soluble collagens from skin and swim bladder of grass carp (Ctenopharyngodon idella). J Sci Food Agric. 2016 Feb;96(3):815–21. CrossRef
8. Chuaychan S, Benjakul S, Kishimura H. Characteristics of acid- and pepsin-soluble collagens from scale of seabass (Lates calcarifer). LWT Food Sci Technol. 2015 Sep;63(1):71–6. CrossRef
9. Tylingo R, Mania S. Isolation and characterization of acid soluble collagen from the skin of African catfish (Clarias gariepinus), salmon (Salmo salar) and baltic cod (Gadus morhua). J Biotechnol Biomater. 2016;6:2. CrossRef
10. Danila E, Stan R, Kaya MA, Voicu G, Marin MM, Morosan A, et al. Valorization of Cyprinus carpio skin for biocompatible collagen hydrolysates with potential application in foods, cosmetics and pharmaceuticals. Waste Biomass Valor. 2022 Feb;13(2):917–28. CrossRef
11. Chen L, Cheng G, Meng S, Ding Y. Collagen membrane derived from fish scales for application in bone tissue engineering. Polymers (Basel). 2022 Jun 21;14(13):2532. CrossRef
12. Ampitiya AGDM, Gonapinuwala ST, Fernando CAN, de Croos MDST. Extraction and characterisation of type I collagen from the skin offcuts generated at the commercial fish processing centres. J Food Sci Technol. 2023 Feb 9;60(2):484–93. CrossRef
13. Wang L, An X, Yang F, Xin Z, Zhao L, Hu Q. Isolation and characterisation of collagens from the skin, scale and bone of deep-sea redfish (Sebastes mentella). Food Chem. 2008 May;108(2):616–23. CrossRef
14. Kaewdang O, Benjakul S, Kaewmanee T, Kishimura H. Characteristics of collagens from the swim bladders of yellowfin tuna (Thunnus albacares). Food Chem. 2014 Jul;155:264–70. CrossRef
15. Carolina C. Resiliency of small-scale common carp (Cyprinus carpio L. L.) farmers in West Pagaden rural area of Subang—West Java (Daya Lenting Pembudidaya Ikan Mas (Cyprinus carpio L. L.) Skala Kecil di Perdesaan Kecamatan Pagaden Barat Subang—Jawa Barat). J Mns dan Lingkung. 2015 Mar 31;22(1):113. CrossRef
16. Sujithra S, Kiruthiga N, Prabhu M, Kumeresan R. Isolation and determination of type I collagen from Tilapia (Oreochromis niloticus) waste. Int J Eng Technol. 2013;5(3):2181–5.
17. Alhana A, Suptijah P, Tarman K. Extraction and characterization of collagen from sea cucumber flesh. J Pengolah Has Perikan Indones. 2015 Nov 23;18(2):150–61. CrossRef
18. AOAC. Method 935.14 and 992.24. 18th ed. Washington, DC: Association of Officiating Analytical Chemists; 2005.
19. Yu D, Chi CF, Wang B, Ding GF, Li ZR. Characterization of acid-and pepsin-soluble collagens from spines and skulls of skipjack tuna (Katsuwonus pelamis). Chin J Nat Med. 2014 Sep;12(9):712–20. CrossRef
20. Standar Nasional Indonesia. Kolagen Kasar dari Sisik Ikan–Syarat Mutu dan Pengolahan. Jakarta, Indonesia: Badan Standarisasi Nasional; 2014. 8076 p.
21. Potaros T, Raksakulthai N, Runglerdkreangkrai J, Worawattanamateekul W. Characteristics of collagen from Nile Tilapia (Oreochromis niloticus) skin isolated by two different methods. Agric Nat Resour. 2009;43(3):584–93.
22. Kolanus JP, Hadinoto S, Idrus S. Karakteristik Kolagen Larut Asam dari Kulit Ikan Tuna (Thunnus albacores.) dengan Metode Hidroekstraksi. J Ris Teknol Ind. 2019 Jul 1;13(1):99. CrossRef
23. Putra ABN, Sahubawa L, Ekantari N. Ekstraksi dan Karakterisasi Kolagen dari Kulit Ikan Nila Hitam (Oreochromis niloticus). J Pascapanen dan Bioteknol Kelaut dan Perikan. 2013 Dec 16;8(2):171. CrossRef
24. Liu W, Li G, Miao Y, Wu X. Preparation and characterization of pepsinsolubilized type I collagen from the scales of snakehead (Opiocephalus argus). J Food Biochem. 2009 Feb;33(1):20–37. CrossRef
25. Pamungkas BF, Supriyadi S, Murdiati A, Indrati R. Extraction and characterization of acid-soluble collagen and pepsin-soluble collagen from the dry scales of the striped snakehead (Channa striatus). J Pengolah Has Perikan Indones. 2018 Dec 28;21(3):513. CrossRef
26. Wang B, Wang YM, Chi CF, Luo HY, Deng SG, Ma JY. Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Mar Drugs. 2013 Nov 21;11(11):4641–61. CrossRef
27. Djailani E, Trilaksani W, Nurhayati T. Extraction optimization and characterization of collagen from yellow pike conger swimbladder with acid-gydro-exctraction method. J Pengolah Has Perikan Indones. 2016;19(2):156–67. CrossRef
28. Coates J. Interpretation of infrared spectra, a practical approach. In: Meyer RA, editor. Encyclopedia of analytical chemistry. Chichester, UK: John Wiley & Sons Ltd; 2000. pp. 10815–37. CrossRef
29. Suptijah P, Indriani D, Wardoyo SE. Isolation and characterization of collagen from the skin of catfish (Pangasius sp.). J Sains Nat. 2018 Jan 30;8(1):8. CrossRef
30. Muyonga JH, Cole CGB, Duodu KG. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus). Food Chem. 2004 Mar;85(1):81–9. CrossRef
31. Singh P, Benjakul S, Maqsood S, Kishimura H. Isolation and characterisation of collagen extracted from the skin of striped catfish (Pangasianodon hypophthalmus). Food Chem. 2011 Jan;124(1):97–105. CrossRef
32. Gadi DS, Trilaksani W, Nurhayati T. The histological, extraction and characterization collagens yellow-pike conger muarenesox talabon. J Ilmu dan Teknol Kelaut Trop. 2017;9(2):665–83. CrossRef
33. Krishnamoorthi J, Ramasamy P, Shanmugam V, Shanmugam A. Isolation and partial characterization of collagen from outer skin of Sepia pharaonis (Ehrenberg, 1831) from Puducherry coast. Biochem Biophys Rep. 2017 Jul;10:39–45. CrossRef
34. Kong J, Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin (Shanghai). 2007 Aug 1;39(8):549–59. CrossRef