INTRODUCTION
Plants and products derived from them have tremendous pharmacological importance. They produce a number of phytochemicals, many of which have ethnomedicinal applications and have also found a place as lead molecules for discovering new drugs [1,2].
Mangroves are special plants that have been used in folklore medicine for a long time. They have developed unique adaptations and several novel metabolites to tolerate and thrive in harsh, salty waters. Distributed in over 120 countries globally, they form unique and extremely productive communities with other mangroves and associated flora and fauna. They are also of ecological, economical, and medicinal importance. No wonder there exist numerous reports that establish the therapeutic potential of mangroves [3–6].
Sonneratia alba J. E. Smith is a true mangrove species. This evergreen tree is a core mangrove occurring in the Indo-West Pacific region of the globe [7]. Inspite of having numerous applications, a dedicated review on S. alba could not be found. Therefore, an attempt has been made to compile up-to-date information available on this plant to highlight its significance.
MATERIALS AND METHODS
Globally recognized databases such as Google Scholar, Scopus, PubMed, Scilit, ResearchGate, and Science Direct were screened to gather the reported work done on S. alba. The specific keywords included Sonneratia alba, S. alba, phytochemistry, pharmacognosy, antibacterial, mangrove, pedada, perepat, bioactivity, phytomedicine, pharmacological activity, and ethnobotany. While the focus has been on articles dated 2013 to 2023 (the last 10 years), few published before 2013 have been also included to avoid missing out on some important observations on the study plant. Relevant references cited in these articles were also considered to make this narrative review as complete as possible.
RESULTS AND DISCUSSION
Classification and taxonomy
The name of genus Sonneratia has been conferred in honor of a French explorer and botanist, Peirre Sonnerat whereas the name of the species alba was given owing to the presence of peculiar white-colored stamens and petals in this species (Alba meaning white in Latin) [8]. Earlier this species used to be classified into the Sonneratiaceae family but phylogenetic studies using molecular markers have shown that Sonneratiaceae happens to be a sub-set of the family Lythraceae [9]. The Lythraceae family includes more than 30 genera having 620 plus species; the majority of which are widespread in tropical regions and few in temperate regions of the world [10].
Earlier, Sonneratia genus was represented by five main species viz. S. alba Sm., Sonneratia ovata Backer, Sonneratia apetala Banks, Sonneratia griffithii Kurz, and Sonneratia caseolaris (L.) Engler [11,12]. However, it was improved by the inclusion of Sonneratia lanceolata Blume [13], making it a total of six species that can be distinguished from each other on the morphological characters such as size and shape of the fruit and leaf, the color of the flowers, and so on. In the overlapping regions, these species often hybridize naturally, resulting in Sonneratia × hainanensis, Sonneratia × gulngai, and Sonneratia × urama [12]. The hybrids, however, exhibit reduced genetic fitness than their true parent species with a large number of sterile pollens [14,15].
English botanist Sir James Edward Smith in the year 1816 first described S. alba in Cyclopedia with a typified name of Sonneratia acida var. mucronata. Other synonyms for S. alba include S. acida Benth., Sonneratia iriomotensis Masam., Sonneratia mossambicensis Klotzsch ex Peters, and Chiratia leucantha Montr. Sonneratia griffithii Watson is now a synonym of S. alba Sm. [12,15].
This mangrove species is taxonomically classified as follows:
Kingdom: Plantae
Sub-kingdom: Viridiplantae
Infra-kingdom: Streptophyta
Phylum: Tracheophyta
Sub-phylum: Spermatophytina
Class: Magnoliopsida
Super-order: Rosanae
Order: Myrtales
Family: Lythraceae
Genus: Sonneratia
Species: Sonneratia alba
Sonneratia alba is most commonly identified as “mangrove apple” or “sweet-scented mangrove apple” for the apple-like fruits it produces. This mangrove is also known by several region-specific names as mentioned in Table 1 [12,14–22].
Botany
Sonneratia alba (Fig. 1) usually grows to a height of 10–15 m. However, shrub-like specimens of 3 m tall and huge trees growing to 30 m have also been occasionally found. The tree is branched and the creamish/brownish bark is characterized by longitudinal fissures. This mangrove is surrounded by thick, short, blunt pneumatophores having an average size of 25–30 cm. Leaves are glabrous, leathery, stalked, simple, opposite having elliptic to obovate shape. At the apex, they become sub-orbicular obtuse. Leaves are normally 5–12 cm in length having a breadth of 4–6 cm. The base of the leaf is narrowed into a short petiole of just 3–5 mm long. This mangrove produces bisexual, white-colored flowers (3–6 cm) which are usually present singly or at times, in groups of three. The petals are small and white. Six to eight lobes are present inside the calyx tube which resembles a cup. Sepals are long and have a green color outside and a red color inside. Stigma is capitate, style is around 4 cm long whereas the ovary is globose. Every flower has long, attractive, 5–8 cm long, white stamens which are many in number. Flowers are nocturnal; they open in the late evening and last only for that one night. The calyx is filled with large amounts of nectar, attracting insects (such as Hawk moths), birds, and mammals (bats) that facilitate its pollination [14,15]. Flowering season has been observed to vary from country to country having different geographical and climatic conditions. In general, it usually flowers from February to July. Fruits are hard, around 4 cm in diameter, have a smooth surface, and are green in color. Around 150 small-sized, curved seeds are produced inside each fruit. The fruiting season depends upon the region-specific flowering season and in general, the fruiting is observed from August to February [15,23].
Global distribution
The species has a widespread global distribution in tropical and sub-tropical countries ranging from East Africa to northern tropical Australia (passing through India and South East Asia), the west Pacific Islands, and southwest Oceania. Sonneratia alba has been reported in more than 23 countries in Africa, Asia, and Australia continents. Being a quite commonly seen species through most of its global range, it is categorized with the “Least Concern” tag in the IUCN’s Red List Book of Threatened Species [24].
 | Table 1. Common and Vernacular names of S. alba. [Click here to view] |
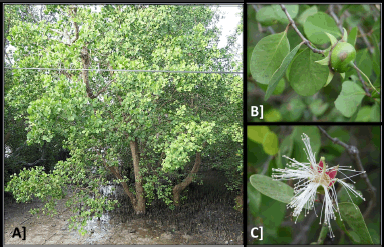 | Figure 1. Sonneratia alba with its important parts. (A) Tree with spike-like pneumatophores. (B) Close-up of the leaves and fruit. (C) White flower (stamens) with pink colored base. [Click here to view] |
Habitat
It is a pioneer species which means that it prefers higher salinities of 18%–30% and is generally present on the seaward side (away from the mainland). It is unable to tolerate much of freshwater. It has been often found thriving in sandy clay habitats or on the recently developed mud flats but at times, has been also seen growing along rocky coastal shores [15,25].
Local and ethno-medicinal uses
Sonneratia alba is well-known by the locals for its edible fruit which is often consumed. In the Goa state of India, ripe fruits of Sonneratia sp. are used for making curries while unripe ones are used in making jellies [25]. They are also used in making pickles and vinegar. Tribals of Bhitarkarnika Wildlife Sanctuary in Odisha state, India have been preparing a vegetable using it [18]. Cakes are also made for eating [26]. The bark is used for the extraction of tannins and as a source of fuel whereas honey is also gathered thanks to its nectar-producing flowers [23]. Wood is utilized for making boxes, as a packing material as well as in the building material. In the Sulawesi province of Indonesia, boats and houses are constructed using the timber of S. alba. There are reports of bridges, cabinets, wharfs, furniture, floorings, and musical instruments being made from wood. Tribal folks from Papua New Guinea have been making use of pneumatophores in making floats and corks [15]. Fishing communities of East African coastal countries have been using S. alba wood for making ribs and keels of large watercraft (dhows) and also in oars, paddles, and masts [20,27]. Bark extract has been regularly used by natives in Indonesia to delay the fermentation and thereby preserve the alcoholic, palm tree-based drink [28].
Traditional healers and tribes have been using this plant for treating health ailments. Extracts or pulp from the fruit are used as a poultice for treating muscular swellings and sprains. A compression is made using the fruit pulp and is applied to stop hemorrhage. It is also used to cure various skin disorders [15]. The plant is believed to have antiseptic properties. Sonneratia alba is commonly used in Indian and Indonesian folklore medicine for treating injuries (sprains and wounds), diarrhea, and fever [18,21,23].
Phytochemistry
Plants produce phytochemicals in the form of primary and secondary metabolites. The former is involved in metabolic activities, while the latter is involved in protecting the plant from the attack of herbivores by playing a crucial role in the defense mechanisms [1]. Members of the genus Sonneratia are rich sources of phytochemicals [29]. Primary metabolites in S. alba include various carbohydrates and sugars, proteins and amino acids, lipids and fatty acids whereas a wide range of secondary metabolites belonging to classes such as triterpenoids, phenolics, steroids, and so on, have also been documented.
Several reports are available involving qualitative as well as quantitative analyses, showing the presence of all major phytochemical classes such as alkaloids, phenolics, flavonoids, saponins, tannins and terpenoids, quinones, and steroids in S. alba leaf, fruit, bark samples indicating it produces a wide range of primary as well as secondary metabolites [30–35].
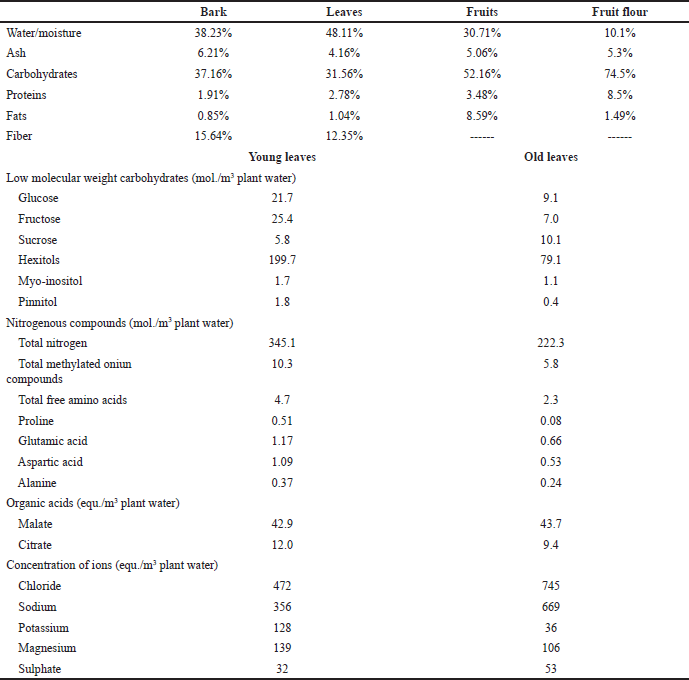
Table 2 depicts the proximate elemental content in different parts of S. alba [36–41]. Values seem to vary in different parts. Analyzing ion concentrations of Na+, Cl−, and K+ is essential in the case of mangroves such as S. alba that grow in a harsh, saline habitat. Stress experienced from such abiotic factors acts as inducers in mangroves leading to the production of novel metabolites [5].
The composition of natural saturated, monounsaturated, and polyunsaturated fatty acids was studied in the freshly collected leaves and stems. Results indicated that S. alba can act as a rich alternative source of natural essential fatty acids (EFAs), predominantly gamma linolenic acid (GLA). Higher percentages (36%) were found in leaf samples whereas stem had 11% GLA. EFAs are required in important metabolic reactions governing normal body functioning and are known to prevent the risk of heart attack and cancer [42].
From the elemental analysis of S. alba fruits and flour, it was evident that content and nutritional value in terms of carbohydrates and proteins increased when the flour was prepared from the fruits. On the contrary, fruits contain relatively higher fat content than those found in flour prepared from the fruits [38]. Owing to the presence of a higher vitamin content (294.26 ppm) and significantly higher antioxidant activity, it is suggested to use S. alba leaf tea as a nutritionally rich, healthier beverage [34]. Tannin content (hydrolysable type) in the bark of S. alba has been reported at 7.6% [43].
The floral scents of mangrove species found on the Iriomote Islands, Japan, were analyzed for chemical characterization [44]. Sonneratia alba floral scent was reported to have the presence of four main compounds viz. trans-β-ocimene (a mono-terpene), 2, 4-dithiapentane (an organo-sulfur), 2-heptanone (a ketone), and methyl-2-methylbutanoate (a fatty acid ester). Detection of 2, 4-dithiapentane in floral scent is attributed to the fact that nocturnal flowers of S. alba are pollinated by bats. Other molecules are believed to help in attracting other pollinating insects and birds.
 | Table 2. Proximate elemental composition in different parts of S. alba. [Click here to view] |
The composition of lipids in S. alba leaves and roots was studied [45]. Wax ester is the largest component comprising 19.3% of the total lipid, followed by sterol ester (16.8%) and polar lipids (11.7%). Similar to other mangroves, triterpenoid alcohols and phytosterols are commonly found in S. alba. Studying triterpenoids is crucial since they are known to play an important role in the adaptation process of several plants such as mangroves to the salt stress [46,47].
Table 3 and Figure 2 show a few important novel compounds and phytochemical classes isolated from the different parts of S. alba. Many of these have been reported to have bioactivities, and their pharmacological importance has been well-documented.
Pharmacological studies
Anti-bacterial
There are numerous reports highlighting the broad range of antibacterial potential of S. alba against both Gram-positive and negative bacteria [61].
Promising activity in vitro was reported by ethanol and aqueous leaf extracts against human bacterial pathogens Staphylococcus aureus, Streptococcus sp., multidrug-resistant Salmonella typhi, Proteus vulgaris, and Proteus mirabilis [62]. The antibacterial activity of crude methanol extract of bark showed promising activity against eleven tested bacteria. Staphylococcus auerus, Salmonella typhimurium, Shigella flexneri, and Vibrio cholera were found to be the most susceptible [63]. Carbon tetrachloride (CTC) fraction of methanol bark extract exhibited moderate inhibition of various Gram-stain-positive bacteria viz. Bacillus cereus, Bacillus subtilis, Sarcina lutea as well as Gram-stain-negative bacteria viz. Pseudomonas aeruginosa and Shigella dysenteriae [64]. Another in vitro study done in Malaysia reported that the methanol leaf extract was found to be effective against Gram-stain-positive S. aureus and B. cereus, and the Gram-stain-negative Escherichia coli bacteria [65]. These bacteria were reported to be sensitive to methanol as well as ethanol extracts prepared from the leaves and bark but tolerant to chloroform extract [66]. Aqueous extracts prepared from leaves, fruits, and bark have been found active against E. coli and S. aureus [67].
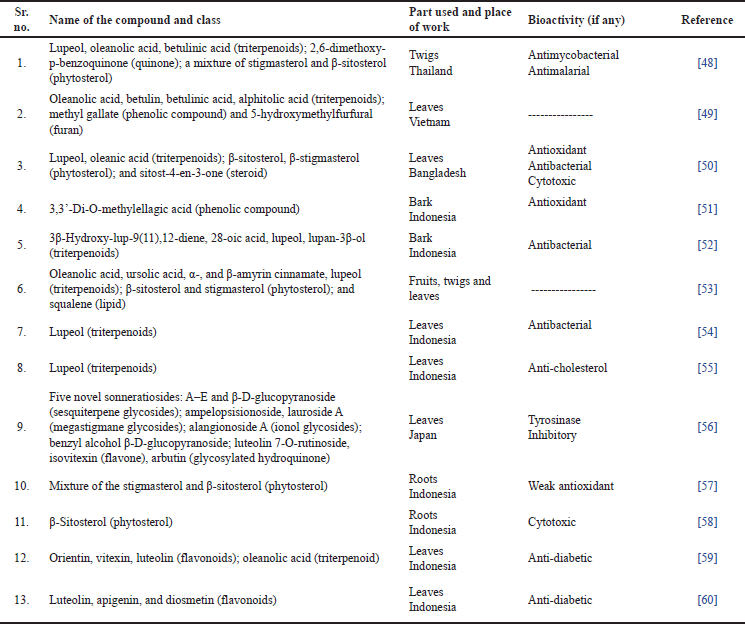 | Table 3. Novel compounds/secondary metabolites isolated/identified from S. alba. [Click here to view] |
Quorum sensing (QS) involves cell-to-cell communication, a life process controlling mechanism in bacteria (such as expression of pathogenicity and production of biofilms). Hence, disturbing QS can kill the bacteria. Sonneratia alba root extract is reported to have anti-QS activity in vitro against the bacteria Chromobacterium violaceum [68].
Flavonoids extracted from the S. alba fruits exhibited activity against pathogenic Vibrio alginolyticus whereas the isolated triterpenoid inhibited pathogenic S. aureus, P. aeruginosa, and E. coli in vitro [54,69]. Three lupane-type triterpenoids isolated from S. alba inhibited Gram-positive bacteria S. aureus ATCC 6538 and Streptococcus mutans ATCC 25175 [52]. Synthesized zinc oxide nanoparticles using S. alba leaf extract have shown excellent in vitro antibacterial activity against Gram +ve as well as −ve bacteria [70].
In an in vivo study, giant tiger prawn post-larvae were fed with Brine shrimps enriched with S. alba fruit extracts and later challenged with their common pathogen Vibrio harveyi. Treated larvae showed a significantly higher survival rate clearly indicating that S. alba extracts can have an important application in inhibiting V. harveyi infection in cultured prawns, ultimately increasing the commercial production [71]. Similar results have been reported of the protection conferred against Saprolegnia sp. [72]. Sonneratia alba ethanol leaf extract was highly antibacterial against a fish pathogen Salmonella arizonae with a zone of inhibitions comparable with those shown by standard antibiotics. Further testing in vivo showed a clear reduction and delay of the onset of goldfish mortality when infected with S. arizonae [73].
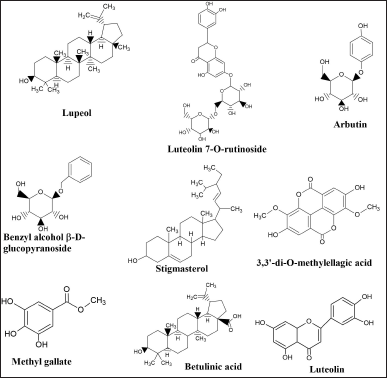 | Figure 2. Representative bioactive compounds identified in S. alba. [Click here to view] |
Anti-fungal
Extracts of S. alba fruits prepared in n-hexane, ethyl acetate, and ethanol exhibited moderate in vitro activity against the fungus Candida albicans whereas methanol fruit extracts inhibited the growth of the fungus Helminthosporium sp. which is known to cause corn leaf blight [74,75]. Methanol leaf extract was found to be effective against the fungus Cryptococcus neoformans [65]. CTC fraction of methanol bark extract showed mild antifungal activity against C. albicans, Aspergillus niger, and Saccharomyces cerevisiae when tested in vitro [64].
Anti-viral
Sonneratia alba leaf-synthesized silver nanoparticles tested in vitro at concentrations of 5–15 μg/ml were able to down-regulate the expression of the gene responsible for the envelope and a crucial protein in the dengue virus (serotype DEN-2) [76].
Insecticidal
In an in vivo study, methanol, ethyl acetate, and hexane extracts of S. alba fruits at 10% concentration exhibited 98%, 44.5%, and 28.4% mortality in Nezara viridula larvae. Nezara viridula commonly known as green stink or shield bug is a polyphagous plant eater and, therefore, needs to be controlled [77]. Silver nanoparticles synthesized using S. alba leaves were found to be lethal against the larvae and pupae of Aedes aegypti mosquitoes (LC50 of 3.15–15.61 ppm) in vivo. When treated with these S. alba-derived nanoparticles, adult guppy fish were found to predate mosquito larvae at an accelerated rate [76].
Antioxidant
Lipid peroxidation is known to be involved in aging, cancer, and so on [78]. Antioxidants are known to protect our body cells from harmful damage caused by free radicals. Therefore, studying antioxidant activity is considered to be important since it can lead to the identification of a bioactive molecule with therapeutic potential [79].
There are numerous in vitro studies that report the antioxidant potential of S. alba. Ethanol and methanol crude extracts of both leaves and bark along with the fractions partitioned in water showed IC50 values in the range of 0.019–0.038 mg/ml clearly indicating the antioxidant ability when compared with the IC50 value of 0.018 mg/ml by the standard vitamin C in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay [66]. Ethyl acetate fraction of methanol extract prepared using sepals was found to be a very strong antioxidant having an EC50 of 2.57 μg/ml and it also showed a strong lipid peroxide formation inhibition activity with IC50 of 0.84 μg/ml [80]. Nufus et al. [37] reported anti-oxidant activities at 9.83, 15.17, and 8.38 ppm for n-hexane, methanol, and ethyl acetate extracts prepared from S. alba fruits and described them as very strong since they were below 50 ppm. The chloroform bark extract showed the highest free radical scavenging activity with an IC50 value of 12 μg/ml in comparison with positive control butylated hydroxyl toluene (10 μg/ml). At the same time, the crude methanolic extract also exhibited strong antioxidant potential having an IC50 value of 14 μg/ml [64]. Methanol extract of stem and leaves showed an IC50 of 62.5 and 87.5 μg/ml whereas ethanol extract of bark and leaves showed an IC50 of 67.2 and 88.7 μg/ml [30,81].
It is well established that the total phenolic contents and antioxidant activities are positively correlated, flavonoids and other phenolic compounds contribute to antioxidant activity [82]. Scopoletin (a coumarin) along with other phenolics detected in the ethanol extract were suggested to be contributing to the activity. Extracts prepared from young leaves using soxhlet (in methanol) and by maceration (in ethanol) reported IC50 of 5.16 and 5.01 μg/ml, respectively, comparatively better than shown by standard ascorbic acid (5.21 μg/ml) indicating potent in vitro antioxidant activity [32]. Aqueous and methanol extract of S. alba bark showed almost double the concentration of total phenolic content than found in green tea and, therefore, exhibited potent in vitro DPPH radical scavenging activity [83]. Sumartini et al. [34] reported very strong in vitro antioxidant activity (average IC50 of 50 ppm) of the tea prepared using S. alba young and old leaves powder. 3,3’-di-O-methylellagic acid, an isolated phenolic compound, methanol bark extract, and ethyl acetate fraction all showed potent in vitro antioxidant activity when compared with L-(+)-ascorbic acid as standard by using DPPH assay. The samples tested exhibited significantly lesser IC50 values around 12 μg/ml than the standard which showed 17.64 μg/ml [51].
Thalassemia patients require antioxidant supplements to prevent oxidative stress. Ethanol leaf extract when tested ex vivo, was found to be a decent antioxidant and could be supplied as a natural antioxidant to anaemic thalassemia patients [84]. Ethyl acetate root extract and the isolated phytosterol, however, showed relatively weaker activity in vitro [57]. Silver nanoparticles synthesized from aqueous stem extracts and zinc oxide nanoparticles synthesized from leaf extract have also exhibited remarkable in vitro antioxidant activities [70,85].
Hypoglycemic/anti-diabetic
A polysaccharide isolated from the leaf extract of S. alba exhibited remarkable blood glucose attenuating activity in vivo as it lowered the blood sugar concentration by 19.2% during the first 6 hours and reduced it further to 66.9% post 12 hours of treatment [86]. In another in vivo study on STZ drug-induced hyperglycemic mice, a promising lowering of blood sugar levels (average being 39.6%) was observed post 6 hours after administration of S. alba nontoxic fraction separated from the leaf extract which further dropped to 56.4% 12 hours after the injection, clearly highlighting the presence of hypoglycemia-inducing principle present in the tannin-containing purified fraction [87]. Using en silico molecular docking studies, phytochemical compounds identified from methanol and ethyl acetate extract of leaves of S. alba were found to be α-glucosidase inhibitors and suggested to have a potential to be developed into anti-diabetic agents [59,60].
Analgesic
Methanol fraction of S. alba leaves extract was tested in vivo for its pain-relieving potential by observing formalin-induced hind foot/paw licking in Swiss albino mice. Mice that were pre-treated with methanol fraction (at 200 and 400 mg/kg) exhibited an encouraging dosage-dependent decrease in the hindfoot licking induced by formalin. Analgesic activity was comparable with the standard painkiller drug diclofenac but at a higher dose of 400 mg/kg suggesting further purification was warranted to enhance the activity [88].
Anti-inflammatory
Sonneratia alba extract at a dose of 150 and 300 mg/kg was found to be effective against inflammation (carrageenan-induced edema in albino mice). Results of this in vivo study, were highly promising when compared to the groups treated with the standard anti-Inflammatory drug Ibuprufen [88]. Silver nanoparticles synthesized from aqueous stem extracts and zinc oxide nanoparticles synthesized from leaf extract have also exhibited remarkable in vitro anti-inflammatory activities when tested for albumin protein denaturation inhibition and human erythrocytes membrane stabilization, respectively [70,85].
Acetyl cholinesterase inhibitory activity
Compounds with this ability can enhance the neuromuscular transmission process by increasing the levels and available time of the neurotransmitter enzyme acetylcholine in the central nervous system (CNS). A positive correlation has been reported between the % inhibitory activity and concentration of the S. alba leaf and bark extract prepared in methanol and dichloromethane when tested in vitro [89].
CNS depressant
Methanol faction of S. alba leaf extracts was tested in vivo to study the locomotor exercise, by employing gap cross and open-up field analysis tests in study mice. Results clearly showed that the samples tested were able to decrease the frequency and the degree of movement. Since the CNS plays a decisive role in regulating locomotor activity, the reduced activity is suggested to result from the sedation caused by the tested plant samples [88].
Tyrosinase inhibitory
Enzyme tyrosinase plays a pivotal role in melanin pigment production in the skin. Hence, anti-tyrosinase agents are being explored since they can help reduce melanin formation thereby skin darkening. Bark extract was found to have the highest anti-tyrosinase activity (82.4%), followed by leaf extract (72.5%). Root extracts exhibited relatively lower activity (40%). Results of this in vitro experiment suggest that purified products from bark and/or leaves could find a place in developing cosmetics [83]. Luteolin 7-O-rutinoside (a flavonoid), and arbutin (a hydroquinone) isolated from the leaves showed in vitro tyrosinase inhibitory activity in terms of IC50 at 387 ± 38.2 and 525 ± 77.4 μM, respectively [56]. Acetone leaf extract showed an IC50 of 0.55 mg/ml indicating potent anti-tyrosinase activity in vitro [90].
Polyphenol oxidase (PPO) inhibitory
It is studied since PPO inhibitors can control/delay the post-harvest browning of vegetables and fruits; increasing the shelf life and sale value. Promising inhibition of 82% of PPO in sweet potato was exhibited by S. alba extract in an in vitro investigation [91].
Anti-atherosclerotic
Atherosclerosis is defined as inflammation in blood vessels, specifically in the medium-sized arteries, it is a significant risk factor for cardiovascular disease. Masdar et al. [92] employed an in vivo rat model for the investigation of the anti-atherosclerotic potential of S. alba fruit extract. Atherosclerosis condition was induced in Wistar rats by administering a high-fat diet. Sonneratia alba fruit methanol extract showed an inhibitory effect on high-fat diet-induced atherosclerosis at the initial stage but did not affect the lipid profile in blood in Wistar rats [92]. In an in vitro study, a triterpenoid compound (lupeol) isolated from leaf methanol extract was shown to reduce cholesterol in a concentration-dependent manner from concentrations of 5 to 80 ppm at 13.7% to 77.0% indicating its potential [55].
Cytotoxic
There are in vivo cytotoxicity studies done against brine shrimps Artemia salina. Sonneratia alba leaf extracts prepared in three different solvents were found to be toxic against A. salina. LC50 values recorded were 3.59, 6.37, and 98.37 ppm for ethyl acetate, ethanol, and n-hexane extracts indicating ethyl acetate extracts to be most cytotoxic [91]. In another study, leaf methanol extract showed LC50 values of 817.5 and 515.8 ppm in the case of acute and chronic treatments with brine shrimps which indicate mild toxicity [86]. Cytotoxicity against A. salina exhibited by the CTC soluble partitionate of methanol bark extract was found to be promising [64]. Phytosterol isolated from the root extract was found to have an IC50 of 10.04 μg/ml clearly indicating its toxic nature and potential to be developed into an anticancer drug [58].
Anticancer
Leaf extract prepared in ethyl acetate was found to have moderate cytotoxicity in vitro against cervical cancer HeLa cells with an IC50 value of 478.63 μg/ml. If the extract is further purified, isolated compounds could exhibit potent anticancer activity [93]. In a recent in vitro study, gold nanoparticles synthesized from the fruits showed promising cytotoxic activity against A549 nonsmall cell lung adenocarcinoma [94].
Anti-malarial
In an in vitro experiment, 2, 6-dimethoxy-p-benzoquinone isolated from S. alba twigs has been reported to be a potent antimalarial compound with an IC50 of 3.08 μg/ml against a multidrug-resistant strain of Plasmodium falciparum [48]. Methanol leaf extract (rich in quinones) was found to be effective in reducing the parasitemia levels in mice erythrocytes infected by protozoan Plasmodium berghei. This ex vivo study reporting anti-plasmodial activity was noteworthy since the activity recorded was even better than the one exhibited by the standard anti-malarial drug pyremethamine [95].
CONCLUSION
Mangrove apple S. alba has a wide range of bioactivities such as antimicrobial, anti-oxidant, anti-diabetic, anti-viral, insecticidal, analgesic, cytotoxic, and so on. Having acetyl cholinesterase inhibitory activity can have numerous medicinal applications in the treatment of conditions such as myasthenia gravis, glaucoma, Alzheimer’s disease, and so on. It is found to be a strong anti-oxidant, in addition to being nutritionally rich, edible, and cholesterol inhibitor, making it a good candidate to be developed as a food additive. Being a tyrosinase inhibitor, it can find applications in cosmetics for manufacturing creams that can control skin darkening. In silico studies have suggested that leaf extract of S. alba would have anti-diabetic potential. These activities are attributed to the presence of various phytochemicals such as terpenoids, phytosterols, flavonoids, coumarins, and so on. It is concluded that this mangrove can be an excellent natural resource for carrying out purification, isolation, and chemical characterization of bioactive principles to make the best of its therapeutic potential.
ACKNOWLEDGMENT
Authors are thankful to Shri. Dinesh Valke, a naturalist from Maharashtra state, India for kindly permitting to use the photograph of S. alba for educational purpose.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Pemmereddy R, Chandrashekar KS, Pai SRK, Pai V, Mathew A, Kamath BV. A review on the phytochemical and pharmacological properties of Syzygium caryophyllatum. Rasayan J Chem. 2022;15(1):01–11. CrossRef
2. Murugan P, Salim A, Chenthamara D, Robert B, Subramaniam S. Therapeutic and pharmacological efficacy of selective Indian medicinal plants—a review. Phytomed Plus. 2021;1(2):100029. CrossRef
3. Kathiresan K. Mangrove forests of India. Curr Sci. 2018;114(5):976–81. CrossRef
4. Bandaranayake WM. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetl Ecol Manag. 2002;10:421–2. CrossRef
5. Manohar SM. A review of the botany, phytochemistry and pharmacology of mangrove Lumnitzera racemosa Willd. Pharmacogn Rev. 2021;15(30):107–16. CrossRef
6. Nabeelah Bibi S, Mahomoodally MF, Zengin G, Jeewon R, Nazurally N, Rengasamy Kannan RR, et al. Ethnopharmacology, phytochemistry, and global distribution of mangroves-a comprehensive review. Mar Drugs. 2019;17(4):231. CrossRef
7. Spalding M, Kainuma M, Collins L. World atlas of mangroves. London, UK: Earthscan; 2010. CrossRef
8. Tomlinson PB. The botany of mangroves. Cambridge, UK: Cambridge University Press; 1986.
9. Shi S, Huang Y, Tan F, He X. Phylogenetic analysis of the Sonneratiaceae and its relationship to Lythraceae based on ITS sequences of nrDNA. J Plant Res. 2000;113:253–8. CrossRef
10. Lee S-K, Lau L-F, Ko W-C, Lo H-S. Lythraceae; Sonneratiaceae; Punicaceae. In: Fang W-P, Chang C-Y, editors. Flora Reipublicae Popularis Sinicae. Beijing, China: Science Press; 1983. Vol. 52, no. 2, pp 67–121.
11. Backer CA, Van Steenis CGGJ. Sonneratiaceae. Flora Malesiana. 1951;4(3):280–9.
12. Dahdouh-Guebas F, editor. World Mangroves database, Sonneratia L.f. [Internet]. World Register of Marine Species.
13. Duke N-C, Jackes BR. A systematic revision of the mangrove genus Sonneratia (Sonneratiaceae) in Australasia. Blumea. 1987;32:277–302.
14. Qin H, Graham SA, Gilbert MG. Sonneratia. Flora China. 2007;13:286.
15. Giesen W, Wulffraat S, Zieren M, Scholten L. Mangrove guidebook for Southeast Asia. FAO Regional Office for Asia and the Pacific, Bangkok, Thailand. Bangkok, Thailand: Wetlands International; 2006.
16. Ray T. Customary use of mangrove tree as a folk medicine among the Sundarban resource collectors. Int J Res Hum Arts Lit. 2014;2(4):43–8.
17. Sathe SS, Lavate RA, Patil SB. Ethnobotanical and medicinal aspects of mangroves from Southern Kokan (Maharashtra). Int J Emerg Trends Pharm Sci. 2014;3(4):12–7.
18. Pattanaik C, Reddy CS, Dhal NK, Das R. Utilisation of mangrove forests in Bhitarkanika wildlife sanctuary, Orissa. Ind J Trad Knowl. 2008;7(4):598–603.
19. Sasidharan N. Flowering plants of Kerala, a checklist CD. Kerala, India: Kerala Forest Research Institute; 2011.
20. Dahdouh-Guebas F, Mathenge C, Kairo JG, Koedam N. Utilization of mangrove wood products around Mida Creek (Kenya) amongst subsistence and commercial users. Econ Bot. 2000;54(4):513–27.
21. Noor YS, Khazali M, Suryadipura INN. Introduction guide of Indonesian mangroves. Bogor, Indonesia: Directorate General of Forest Protection, Ministry of Forest, Indonesia; 2006. CrossRef
22. Ramasubramanian R, Ravishankar T, Sridhar D. Mangroves of Andhra Pradesh identification and conservation manual. Chennai, India: M. S. Swaminathan Research Foundation; 2003.
23. Dhargalkar VK, D’Souza R, Kavlekar DP, Untawale AG. Mangroves of Goa. Goa, India: Forest Department, Government of Goa and Mangrove Society of India; 2014.
24. Kathiresan K, Rajendran N. Mangrove ecosystems of the Indian Ocean region. Indian J Mar Sci. 2005;34(1):104–13.
25. Kothari MJ, Rao KM. Mangroves of Goa. In: Singh NP, Rao PSN, editors. Goa, India: Ministry of Environment and Forests, Botanical Survey of India; 2002.
26. Bandaranayake WM. Traditional and medicinal uses of mangroves. Mang Salt Marsh. 1998;2(3):133–48. CrossRef
27. Baba S, Chan HT, Aksornkoae S. Useful products from mangrove and other coastal plants. ISME Mangrove Educational Book Series No. 3. Yokohama, Japan: International Society for Mangrove Ecosystems (ISME), Okinawa, Japan, and International Tropical Timber Organization (ITTO); 2013.
28. Firdaus, Sinda L. Peranan kulit kayu buli Sonneratia sp, dalam fermentasi nira aren menjadi minuman beralkohol. Marina Chim Akta. 2003;5(1):24–8.
29. Liu B, Wang X, Wang Y, Chen X, Jin X, Luo X. Review of compounds and activities from mangrove Sonneratia genus and their endophytes. J Holistic Integr Pharm. 2023;4:218–27. CrossRef
30. Gawali P, Jadhav BL. Antioxidant activity and antioxidant phytochemical analysis of mangrove species Sonneratia alba and Bruguiera cylindrical. Asian J Microbiol Biotechnol Environ Sci. 2011;13(2):257–61.
31. Mahmiah, Andayani R. Phytochemical and antioxidant activity of mangrove plant Sonneratia sp. In: Proceedings of International Conference on Medicine and Health Sciences, 2016 Aug 31–Sept 1. Jember, Indonesia, 2016, pp 51–4.
32. Dotulong V, Wonggo D, Montolalau, Lita ADY. Phytochemical content, total phenols, and antioxidant activity of mangrove Sonneratia alba young leaf through different extraction methods and solvents. Int J ChemTech Res. 2018;11(11):356–63. CrossRef
33. Rahmaniya N, Herpandi, Rozirwan. Phytochemical test of mangrove Avicennia alba, Rhizophora apiculata and Sonneratia alba from Musi River Estuary, South Sumatra. Biovalentia Biol Res J. 2018;4(2):1–8. CrossRef
34. Sumartini, Ratrinia PW, Hutabarat RF. The effect of mangrove types and leave maturity on the mangrove leaves (Sonneratia alba) and (Rhizophora mucronata) tea powder. IOP Conf Ser Earth Environ Sci. 2022;967:012018. CrossRef
35. Fatminati I, Asikin A, Zuraida I, Irawan I, Mismawati DA. The addition of pedada fruit extract (Sonneratia alba) as a natural antioxidant in making skin lotion. Mismawati J Kelautan Perikanan Terapan. 2022;5(2):143–50. CrossRef
36. Anggraeni R. Karakterisasi ekstrak etanol kulit batang dan daun mangrove Bogem (Sonneratia alba) sebagai antioksidan. [Characterization of ethanol extract of bark and leaf of mangrove Bogem (Sonneratia alba) as an antioxidant] [bachelor’s degree thesis]. Malang, Indonesia: Brawijaya University; 2018.
37. Nufus H, Gazali M, Alaudin, Mursawal A, Wahyuni S, Akla CMN, et al. Bioactive and antioxidants compounds from mangrove Sonneratia alba J.E. Smith from Lhok Bubon village, Samatoga District, West Aceh Regency. J Kelautan Tropis Maret. 2023;26(1):59–70. CrossRef
38. Ardiansyah PR, Wonggo D, Dotulong V, Damongilala LJ, Harikedua SD, Mentang F, et al. Proksimat pada tepung buah mangrove Sonneratia alba (Proximates in Sonneratia alba mangrove fruit flour). Media Teknol Hasil Perikanan. 2020;8(3):82–7. CrossRef
39. Popp M. Chemical composition of Australian mangroves. I. Inorganic ions and organic acid. Z Pflanzenphysiol Bd. 1984;113:395–409. CrossRef
40. Popp M. Chemical composition of Australian mangroves. II. Low molecular weight carbohydrates. Z Pflanzenphysiol Bd. 1984;113:411–21. CrossRef
41. Popp M, Larher F, Weigel P. Chemical composition of Australian mangroves. III. Free amino acids, total methylated onium compounds and total nitrogen. Z Pflanzenphysiol Bd. 1984;114:15–25. CrossRef
42. Patil PD, Chavan NS, Sabale AB. Sonneratia alba J. Smith: a vital source of gamma linolenic acid (GLA). Asian J Pharm Clin Res. 2012;5(Suppl. 1):172–5.
43. Balasooriya SJ, Sotheeswaran S, Balasubramanium S. Economically useful plants of Sri Lanka. Part IV: Screening of Sri Lanka plants for tannins. J Nat Sci Coun Sri Lanka. 1982;10(2):213–9. CrossRef
44. Azuma H, Toyota M, Asakawa Y, Takaso T, Tobe H. Floral scent chemistry of mangrove plants. J Plant Res. 2002;115:47–53. CrossRef
45. Oku H, Baba S, Koga H, Takara K, Iwasaki H. Lipid composition of mangrove and its relevance to salt tolerance. J Plant Res. 2003;116(1):37–45. CrossRef
46. Nishidono Y, Niwa K, Tanaka K. Effect of salt stress on the accumulation of triterpenoid saponins in aseptic cultured Glycyrrhiza uralensis. Plant Growth Regul. 2023;100:25–31. CrossRef
47. Zia M, Ali JS, Hanif S, Sajjad A, Abbasi BH. Lupeol, a plant triterpenoid mitigates salt induced stress: growth and antioxidative response of Brassica nigra under in vitro condition. Plant Cell Tiss Organ Cult. 2023;154:327–35. CrossRef
48. Chaiyadej K, Wongthap H, Vadhanavikit S, Chantrapromma K. Bioactive constituents from the twigs of Sonneratia alba. Walaik J Sci Technol. 2004;1:15–22.
49. Thu NTH, Khánh LP, Duy NT, Chánh NTK, Phung NKP, Hansen PE. Chemical constituents from leaves of Sonneratia alba J.E. Smith (Sonneratiaceae). J Sci Tech Dev. 2011;14(T6):11–7. CrossRef
50. Asad S, Hamiduzzaman Md, Zafrul Azam ATM, Ahsan M, Masud MM. Lupeol, oleanic acid and steroids from Sonneratia alba J.E. Sm. (Sonneratiaceae) and antioxidant, antibacterial and cytotoxic activities of its extracts. Int J Adv Res Pharm Bio Sci. 2013;3(4):1–10.
51. Herawati N, Firdaus. 3,3’-di-O-methylellagic acid, an antioxidant phenolics compound from Sonneratia alba bark. J Nat Indones. 2013;15(1):63–7. CrossRef
52. Harizon, Pujiastuti B, Kurnia D, Sumiarsa D, Shiono Y, Supratman U. Antibacterial triterpenoids from the bark of Sonneratia alba (Lythraceae). Nat Prod Commun. 2015;10(2):277–80. CrossRef
53. Ragasa CY, Ebajo Jr. VD, Reyes MM, Mandia EH, Brkljaca R, Urban S. Triterpenes and sterols from Sonneratia alba. Int J Curr Pharm Rev Res. 2015;6(6):256–61.
54. Musa WJA, Duengo S, Situmeang B. Isolation and characterization triterpenoid compound from leaves mangrove plant (Sonneratia alba) and antibacterial activity test. Int Res J Pharm. 2018;9(3):85–9. CrossRef
55. Musa WJA, Bialangi N, Situmeang B, Silaban S. Triterpenoid compound from metanol extract of mangrove leaves (Sonneratia alba) and anti-cholesterol activity test. J Pendidikan Kimia. 2019;11:18–23. CrossRef
56. Katsutani, Sugimoto S, Yamano Y, Otsuka H, Matsunami K, Mizuta T. Eudesmane-type sesquiterpene glycosides: sonneratiosides A–E and eudesmol β-d-glucopyranoside from the leaves of Sonneratia alba. J Natl Med. 2019;74(1):119–26. CrossRef
57. Latief M, Utami A, Amanda H, Muhaimin, Afifah Z. Antioxidant activity of isolated compound from perepat roots (Sonneratia alba). IOP Conf Ser J Phys. 2019;1282:012088. CrossRef
58. Latief M, Nelson, Amanda H, Tarigan IL, Aisyah S. Potential tracking of cytotoxic activities of mangrove perepate (Sonneratia alba) root extract as an anti-cancer candidate. Pharmacol Clin Pharm Res. 2020;5(2):48–55. CrossRef
59. Puspitasari YE, Hardoko, Sulistiyati TD, Fajrin AN, Tampubolon HO. Phytochemical compound identification of mangrove leaves Sonneratia alba and in silico analysis as antidiabetic. J Perikanan Dan Kelautan. 2022;27(2):241–8. CrossRef
60. Puspitasari YE, Tampubolon HO, Fajrin AN, Sulistiyati TD, Hardoko H. Inhibition of α-glucosidase by flavonoid prepared from ethyl acetate extract of Sonneratia alba leaves as antidiabetes with molecular docking. Saintek Perikanan Indones J Fisheries Sci Technol. 2023;19(1):15–22.
61. Chan EWC, Lim WY, Wong CW, Ng YK. Some notable bioactivities of Rhizophora apiculata and Sonneratia alba. ISME/GLOMIS Electron J. 2022;20(4):23–6.
62. Sahoo G, Mulla NSS, Ansari ZA, Mohandas C. Antibacterial activity of mangrove leaf extracts against human pathogens. Indian J Pharm Sci. 2012;74(4):348–51. CrossRef
63. Raut S, Anthappan PD. Studies on antimicrobial compounds of extract of bark of Sonneratia alba. Trends Biosci. 2013;6(6):831–7.
64. Milon MA, Muhit MA, Goshwami D, Masud MM, Begum B. antioxidant, cytotoxic and antimicrobial activity of Sonneratia alba Bark. Int J Pharm Sci Res. 2012;3(7):2233–7.
65. Saad S, Taher M, Susanti D, Qaralleh H, Awang AFIB. In vitro antimicrobial activity of mangrove plant Sonneratia alba. Asian Pac J Trop Biomed. 2012;2(6):427–9. CrossRef
66. Haq I, Hossain AS, Moneruzzaman M, Amir K, Merican F, Faruq G, et al. Antioxidant and antibacterial activities of different extracts and fractions of a mangrove plant Sonneratia alba. Int J Agric Biol. 2014;16(4):707–14.
67. Gonzalez JB, Fronda CM. Antibacterial potential of aqueous extract of Sonneratia alba from pocket mangrove forest Romblon State University San Agustin Campus. Asia Life Sci. 2023;13(2):1653–66.
68. Chan EWC, Ng SM, Sim BW, Tan HC, Lo ZL. Antioxidant, anti-tyrosinase and anti-quorum sensing activities of four mangrove tree species vs. green tea. J Appl Pharm Sci. 2017;7(7):225–9.
69. Sulistijowati R, Karim Z, Junianto. The inhibition of Vibrio alginolyticus by the flavonoid extract of Sonneratia alba fruit. AACL Bioflux. 2020;13(1):28–35.
70. Khan MS, Dhavan PP, Ratna D, Shimpi NG. Ultrasonic-assisted biosynthesis of ZnO nanoparticles using Sonneratia alba leaf extract and investigation of its photocatalytic and biological activities. J Clust Sci. 2021;33:1007–23.
71. Cahyadi J, Satriani GI, Gusman E, Weliyadi E. Inhibiting Vibrio harveyi infection in Penaeus monodon using enriched Artemia salina with mangrove fruit Sonneratia alba extract. AACL Bioflux. 2020;13(3):1674–81. CrossRef
72. Saptiani G, Asikin AN, Ardhani F. Sonneratia alba extract protects the post larvae of tiger shrimp Penaeus monodon against Vibrio harveyi and Saprolegnia sp. In: Proceedings of the 3rd International Symposium on Marine and Fisheries Research Conference. Yogyakarta, Indonesia, vol. 147; 2020. CrossRef
73. Limbago JS, Sosas J, Gente AA, Maderse P, Rocamora MN, Gomez DK. Antibacterial effects of mangrove ethanolic leaf extract against zoonotic fish pathogen Salmonella arizonae. J Fisheries. 2021;9(2):92205. CrossRef
74. Kurniawan D, Muliawan A, Kuspradini H. Efektivitas ekstrak buah Sonneratia alba terhadap aktivitas bakteri. J Harpodon Borneo. 2017;10(1):1–12.
75. Kusumadewi T, Khotimah S, Yanti AH. Ekstrak metanol buah Sonneratia alba J.E. Sm sebagai penghambat pertumbuhan Helminthosporium sp. yang diisolasi dari Daun Jagung. Protobiont. 2014;3(2):149–54.
76. Murugan K, Dinesh D, Paulpandi M, Subramaniam J, Rakesh R, Amuthavalli P, et al. Mangrove helps: Sonneratia alba-synthesized silver nanoparticles magnify guppy fish predation against Aedes aegypti young instars and down-regulate the expression of envelope (E) gene in dengue virus (Serotype DEN-2). J Clust Sci. 2016;28(1):437–61. CrossRef
77. Siahaya VG, Moniharapon T, Mailoa MN, Leatemia JA. Potential of mangrove apples (Sonneratia alba) as a botanical insecticide. Modern Appl Sci. 2018;12(1):1–8. CrossRef
78. Manohar SM, Yadav UM, Kulkarnii CP, Patil RC. An overview of the phytochemical and pharmacological profile of the spurred mangrove Ceriops tagal (Perr.) C.B. Rob. J Nat Remedies. 2023;23(1):57–72.
79. Kasote DM, Katyare SS, Hegde MV, Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci. 2015;11(8):982–91. CrossRef
80. Bunyapraphatsara N, Jutiviboonsuk A, Sornlek P, Therathanathorn W, Aksornkaew S, Fong HHS, et al. Pharmacological studies of plants in the mangrove forest. Thai J Phytopharm. 2003;10(2):1–12.
81. Budi SB, Dwi ST, Hardoko. Phytochemicals and identification of antioxidant compounds from ethanol extract of Sonneratia alba leaves and bark. Russian J Agri Soc Econ Sci. 2019;11(95):190–6. CrossRef
82. Miller NJ, Begoña Ruiz-Larrea M. Flavonoids and other plant phenols in the diet: their significance as antioxidants. J Nutr Environ Med. 2002;12(1):39–51. CrossRef
83. Suh SS, Hwang J, Park M, Park HS, Lee TK. Phenol content, antioxidant and tyrosinase inhibitory activity of mangrove plants in Micronesia. Asian Pac J Trop Med. 2014;7(7):531–5. CrossRef
84. Latief M, Utami A, Fadhilah N, Bemis R, Amanda H, Heriyanti, et al. Antioxidant activity from perepat plant (Sonneratia alba) ethanol leaf extract with Cap-e methods to overcome oxidative stress in thalassemia. J Pharm Sci Res. 2018;10(9):2160–2.
85. Gawali P, Jadhav BL. Synthesis of Ag/AgCl nanoparticles and their action on human serum albumin: a fluorescence study. Process Biochem. 2018;69:106–22. CrossRef
86. Morada NJ, Metillo EB, Uy MM, Oclarit JM. Anti-diabetic polysaccharide from mangrove plant, Sonneratia alba Sm. In: Proceedings of the International Conference on Asia Agriculture and Animal, 2011 July 2–3. Hong Kong, China; 2011, vol. 13, pp 197–200.
87. Morada NJ, Metillo EB, Uy MM, Oclarit JM. Toxicity and hypoglycemic effect of tannin-containing extract from the mangrove tree Sonneratia alba Sm. Bull Environ Pharmacol Life Sci. 2016;5(6):58–64.
88. Asad S, Nesa L, Deepa KN, Sohel KM. Analgesic, anti-inflammatory and CNS depressant activities of methanolic extract of Sonneratia alba leaves in mice. Natl Prod Chem Res. 2017;5:277–84.
89. Sharudin SZ. Anti-cholinergic and anti-microbial properties of Sonneratia alba (Perepat) [Undergraduate thesis]. Terengganu, Malaysia: Univeristi Malaysia; 2014.
90. Lim WY, Chan EWC, Phan CW, Wong CW. Tyrosinase inhibiting extracts from coastal plants as potential additives in skin whitening formulations. Curr Appl Sci Technol. 2021;21(2):481–94.
91. Lim WY, Cheng YW, Lian LB, Chan EWC, Wong CW. Inhibitory effect of Malaysian coastal plants on banana (Musa acuminata colla Lakatan), ginger (Zingiber officinale Roscoe) and sweet potato (Ipomoea batatas) polyphenol oxidase. J Food Sci Technol. 2021;58(11):4178–84. CrossRef
92. Masdar H, Hamidy MY, Darmawi, Trihardi R, Perwira A, Utari D. Anti-atherosclerotic effects of Sonneratia alba fruit extract in atherosclerotic-induced rats. Int J Appl Pharm. 2020;12(3):41–3. CrossRef
93. Suryaningrum FD, Sasmito BB. The effect of mangrove leaf extract dosage Sonneratia alba on Hela cell viability. J SCRTE. 2021;5(1):30–40. CrossRef
94. Gawali P, Ramteke L, Jadhav BL, Khade BS. Trypsin conjugated Au nanoparticles using Sonneratia alba fruits: interaction and binding studies with antioxidant, anti-inflammatory, and anticancer activities. J Clust Sci. 2023;34:2759–79. CrossRef
95. Muhaimin M, Latief M, Putri RD, Chaerunisaa AY, Aditama AY, Pravitasari NE, et al. Antiplasmodial activity of methanolic leaf extract of mangrove plants against Plasmodium berghei. Pharmacog J. 2019;11(5):929–35. CrossRef