INTRODUCTION
Breast cancer has the highest prevalence in the world for women. Mostly for East Asia and African countries, there were 27/100,000 incidents [1]. Several government policies related to the handling of this cancer include the socialization of healthy living, early detection, improvement of health facilities, and the availability of drugs and vaccines. Currently, the availability of imported cancer drugs is very limited at prices that are not affordable for the lower middle class. This is a very crucial issue. The development of cancer drugs, both herbal and modern drugs for chemotherapy, is a priority. Developing cancer therapy from natural resources needed several steps as follows: basic screening to evaluate the cytotoxic effect on the targeted cancer cells, then a preclinical trial using an animal model, and finally, the full clinical test [2]. Cancer drug development research focuses primarily on terrestrial bioresources such as plants and microorganisms, with little attention paid to marine resources. The study of anticancer drugs in marine invertebrates differs from that of plants in that some plants can be used as herbal medicine, whereas studies of marine invertebrates are primarily interested in finding active lead compounds. Investigating natural products for cancer therapy is very important since 60% of cancer drugs were first found in nature [2]. Researchers studying natural products have discovered that marine organisms can produce active compounds. Between 1985 and 2012, 16,617 new compounds were discovered in marine organisms. Around 4,196 or 25.25% were active compounds, with 56% being anti-cancer [3]. Until 2016, 1,277 new compounds were isolated from marine organisms, 430 of which were from Indonesian sponges and belonged to 40 different genera [4].
Agelas nakamurai is a powerful sponge that belongs to the Agelasidae family and the genus Agelas. Several species in this genus are reported to contain brominated 2-pyrrole carboxylic acid derivatives and unique terpenoid derivatives, such as the sesquiterpene derivatives hypotaurocyamines and adenine derivatives [5]. Several compounds have been identified in A. nakamurai, including 4-(4,5-dibromo-1-methyl-1H pyrrole-2-carboxamido) butanoic acid, agelasin A-D, midpacamide, methyl-4,5-dibromo1-methyl-1H-pyrrole2-carboxylate, dibromohydroxyphakellin HCl, dibromophakellin HCl, mucanadin C, agelasine D-oxime, hymenidine, adenosine, 9-methyladenin, ageloxime D, longamide C [6], 2-guanidinoethanesulfonyl sesquiterpene, cyclohexylagelasidine A [7], elasidines B and C [8], nakamurols A–D [9], 2-oxoagelasiines A and F, 10-hydro-9-hydroxyagelasine F [10], and agelasines E and F [11]. Some of these compounds showed anti-cancer activity, such as agelasin-C, which demonstrated moderate activity against rat lymphoma cells (L5178Y), and cytotoxic activity against lung adenocarcinoma cells A549 with an IC50 of 16.7 μg/mL [12]. Interestingly the semisynthetic of ageladine A derivative by replacing the pyridine with azepine ring showed activity against DU145 prostate, A2058 melanoma, and MDA-MB-435 breast cancer cell lines. [13]. Slagenin B and C were bromopyrrole alkaloids isolated from the Okinawan A. nakamurai that showed cytotoxic activity against L1210 cells lines with IC50 20.94 and 19.55 μM, respectively [14].
So far, there is no information on the activity of the substance isolated from the sponge A. nakamurai against MDA-MB-231 and MCF-7 breast cancer cells. This study will investigate potential anti-cancer substances derived from A. nakamurai against MDA-MB-231 and MCF-7. The methods used in this study were chromatographic separation, in vitro bioassay of the cytotoxic activity of A. nakamurai, and preliminary metabolic profiling using the liquid chromatography mass spectrometry/mass spectrometry (LC-MS/MS). In this study, the preliminary assessment of anti-cancers using cytotoxic tests was applied as the basic screening evaluation to decide further investigation, such as detailed structural determination of lead compounds, mechanism of action, optimizing compounds, and several preclinical and clinical tests.
MATERIALS AND METHODS
Sample characterization
The sponge samples used in this study were collected from the Biak area in 2020. The samples were collected by scuba diving from 5 to 10 m in depth. The fresh samples were immediately sent to the laboratory in cool boxes, where they were stored in Genomic Laboratory BRIN, Bogor, Indonesia, at freezer −20°C until they could be characterized and extracted. The sample with the code Spg-An-2020-Biak was prepared for spicule profiling by macerating the sponge tissue using sodium hypochlorite. After 15 minutes, the tissue was inverted in the Euromax microscope with 10× magnification. The characterization of the sample was done using the taxonomic method by referring to [15] and [16].
Extraction
The sponge samples were cut into cubes and freeze-dried for 24 hours. Extraction was done by maceration using methanol, followed by partitioning using ethyl acetate (EtOAc), butanol, and water as solvents. Following rotary evaporation, an anti-cancer screening was performed.
Separation and fractionation of anti-cancer active compounds
The following steps are taken to separate potential fractions: The fractions were separated using open column chromatography with a hexane-dichloromethane-ethyl acetate-methanol gradient system. The fractions were trapped in each band, removing the solvent and allowing the anti-cancer assay to proceed. In each fraction, the activity of MDA-MB-231 cells was evaluated.
Cytotoxic 3-45-dimethylthiazol-2-yl-25-diphenyltetrazolium (MTT) assay
Against HEK-293 cells line
The cytotoxic test of fractions and subfractions was carried out by the MTT method as proposed by Mosmann [17]. This test was based on the ability of the mitochondrial dehydrogenase enzyme in living cells to cleave the tetrazolium ring of the pale yellow MTT into purple formazan crystals.
The cells were counted and diluted as needed with culture media DMEM (Dulbecco’s Modified Eagle Medium). Cells were then transferred into wells, 100 μL each, and incubated overnight. The media was discarded, the cells were washed with phosphate buffer saline (PBS), and then 100 μL of new media was added to the wells containing 25 μg/mL of A. nakamurai subfractions and incubated for 24 hours. After incubation, the media was removed, cells were washed with PBS, then MTT reagent was added in DMEM (5 μg/mL) at 100 μL/well and incubated for 4 hours. After that, a 10% sodium dodecyl sulfate stopper reagent was added in 0.01 N HCl, and then it was read with a microplate reader 595 nm and obtained an absorption indicating the absorbance of live 4T1 cells.
Against MDA-MB-231 and MCF-7 cell lines
The proliferation test of open column fractions was carried out with the following method.
To evaluate the antiproliferative effect of the fractions, the viability of the cells treated with A. nakamurai fractions was assessed for 24 hours using PrestoBlue™ cells viability reagent (Thermo Fischer Scientific, Waltham, MA). As much as 50 μL of cell suspension (about 1 × 104 cells) was seeded in 96 well plates (Thermo Fischer Scientific, Waltham, MA) and incubated overnight at 37°C. Fifty microliters of Dimethyl sulfoxide with various concentrations of A. nakamurai fractions were added to each well and incubated at 37°C for 24 hours. To measure the viability of the cells, 10 μL of the cells counting solution was added to each well and incubated at 37°C for 3 hours. Infinite M200 PRO microplate reader measured the absorbance at 535 nm with the reference wavelength at 560 nm (Tecan, Männedorf, Switzerland) 2 hours after the PrestoBlue™ cells viability reagent solution was given. The result was derived from triplicate experiments, and the relative cell proliferation inhibition (CPI) rate was calculated as a percentage by the following formula:
CPI rate (%) = (1–absorbance of the treated cells/absorbance of the untreated cells) × 100.
LC-MS/MS analysis
LC-MS/MS Waters were used for analyzing active fractions. LC-MS/MS was carried out under the following conditions.
LC conditions: Solvent A: H2O + 0.1% formic acid (FA), Solvent B: Acetonitrile + 0.1 FA, gradient system (95% A–100% B, flow rate 0.3 mL/minute, volume injection 1 μL, column: ACQUITY UPLC® BEH C8 1.7 μm 2.1 × 100 mm.
Mass conditions
Ionization type: ESI polarity: positive acquisition start time: 0.00 minute acquisition end time: 16.00 minute start mass: 50.00 m/z end mass: 1,200.00 m/z scan time: 0.100 seconds low CE: 6.00 eV high CE ramp start: 10.00 eV high CE ramp end: 40.00 eV cone mode: method settings cone voltage: 30 V collision mode: specific collision energy: 6.00 eV
RESULTS AND DISCUSSION
Characterization of sponge
The sponge (Spg-An-2020-Biak) is irregularly massive with a red color. The surface is smooth with uneven contour. The small osculum appears along the surface (Fig. 1). The texture is relatively compressible, especially when it is dry. The sponge consists of a network of fibers which are cored by spicules. The spicules are megascleres only, which are acanthostyle (around 200 × 10 μm) attached to the fibers. Based on the sponge-guided taxonomy handbook, the sponge is determined as A. nakamurai.
The evaluation of fractions against MDA-MB-231 cells line
Agelas nakamurai was macerated in MeOH to gain a wider compound group. Partition using semipolar solvent was applied, such as EtOAc, n-butanol, and water. This separation generated fractions that evaluated the activity against MDA-MB-231 (Table 1).
The result of evaluating the activity of fractions generated from the liquid–liquid partitions against the MDA-MB-231 cells line (Table 1) showed that semipolar solvent (EtOAc) fractions showed the strongest cytotoxicity. The results showed that the EtOAc fractions had IC50 values of 13.33 μg/mL. The previous study reported that the EtOAc fraction of A. nakamurai collected from Menjangan Island, Bali, Indonesia, did not show promising cytotoxic and protein kinase inhibitor activity. However, some of the isolated compounds agelasins A–D show active cytotoxicity against mouse lymphoma cells L5178Y with the IC50 less than 10.677 μg/mL [16]. Another screening test of Agelas extract from the Micronesian Ocean revealed mild activity against MDA-MB-231 breast cancer cells [18].
Separation of EtOAc fraction and its bioactivity profiling test
The EtOAc fraction was purified on a normal-phased open-column chromatography to give seven subfractions. Each subfraction was then tested in activity against MDA-MB-231 (Table 2).
The EtOAc fraction was then purified on normal-phased open-column chromatography to give seven subfractions. The extrapolation graphic data determined that IC50 of fraction 7 was 10.677 μg/mL (R2: 0.988), and the EtOAc extract was 13.326 μg/mL (R2: 0.977). When the cytotoxic activity of subfraction F7 against the MDA-MB-231 cells line was compared with EtOAc fractions, the open column subfractions F7 were stronger than the partition. This data demonstrated that the active ingredient’s work was not synergistic. The single compound will contribute to the cytotoxicity.
In addition, the evaluation of subfraction F7 against the MCF-7 cells line exhibited IC50 values of 15.154 μg/mL. This IC50 value was stronger than the positive control cisplatin with IC50 81.052 μg/mL.
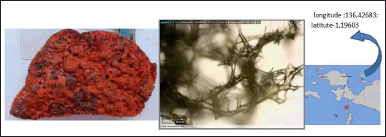 | Figure 1. The profile of A. nakamurai sample and its spicule. [Click here to view] |
 | Table 1. The fractions activity against breast cancer cells MDA-MB-231. [Click here to view] |
The profiling of the proliferation test described that fraction 7 inhibited the MDA-MB-231 cells’ growth until the lowest concentration at 7.81 μg/mL, with the inhibition of 45.389% (Figure 2). Even though this value was higher than IC50 doxorubicin (0.58 μg/mL) [19], this subfraction could be further investigated.
To know the cytotoxic activity against normal cells, all of the subfractions were evaluated against the human embryonic kidney HEK-293 cell line. The result is shown in Table 3.
Data in Table 3 showed that fraction 7 was cytotoxic against HEK-293 with the % viability value at the concentration of 25 μg/mL was 6% ± 1.8%. The noncytotoxic effect of the HEK-293 cells line is indicated by 90%–95% cell viability at the concentration of 100 μg/mL sample [20].
The cytotoxicity of subfraction F7 was abroad to the breast cancer MDA-MB-231 and MCF-7 as well as normal human embryonic kidney cell lines HEK-293. Further study to enhance the toxicity is recommended to find the selective cytotoxic against breast cancer cell lines such as MDA-MB-231 and MCF-7 cells.
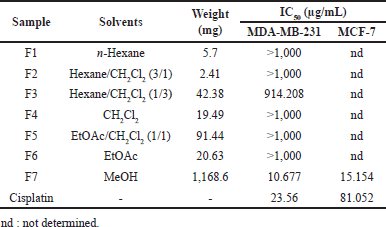 | Table 2. The result of EtoAC fractionation and the cytotoxic assay against MDA-MB-231 and MCF-7 cell lines. [Click here to view] |
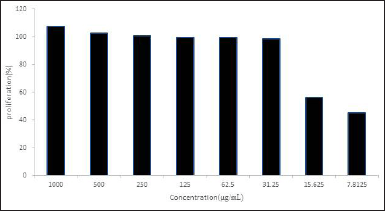 | Figure 2. Growth-inhibition of MDA-MB-231 treated with fraction 7 in several concentration. [Click here to view] |
LC-MS/MS analysis
The LC-MS/MS data of EtOAc fraction and subfraction F7 are described in Table 4.
The active compound detected in EtOAc fraction at a retention time of 8.60 with an exact mass of 436.309 Da was predicted to be similar to the halogenated pyrrole compound midpacamide C13H16Br2N4O3 with a calculated molecular weight of 436.300 Da [6]. The electron spray ionization mass spectroscopy (ESIMS) data showed that midpacamide had the ion-molecule at m/z 437.309 [M + H]+. Previous research reported that midpacamide, with a molecular weight of 436.1 and an exact mass of 435.956 Da, was isolated from A. nakamurai collected from Menjangan Island, Bali. Midpacamide was found to have moderate activity against L5178Y, with IC50 values of 10 μg/mL [6]. Another peak of EtOAc fraction was detected at retention 9.45 minutes and was identified as agelasin-D with molecular weight of 421.31 Da. The unknown compound was also detected at a retention time of 1.6643 minutes. The separation of EtOAc fraction generated active subfraction F7. The profiling of compounds using LC-MS/MS is described in Figure 3.
There were two intense peaks appearing in the subfraction F7 at retention times of 1.2 and 0.46 minutes. The detailed analysis of ESIMS, at the retention time of 1.2 minutes, elicited molecular ion at m/z 422.3274 Da [M + H]+, which was later identified as agelasin-D. The previous researcher also reported that agelasine D has been found in A. nakamurai [17] and Agelas mauritania [8]. The agelasines group was the chemical marker of Agelas sponges that was found in several species of Agelas and microbes associated with this sponge, including agelasin A-I isolated from A. nakamurai and Agelasin B found in the fungus Agelas cf. mauritiana [6,21–23]. In addition, agelasine D was reported as an anti-dormant-mycobacterial [21].
The other compound at retention 0.46 detected in subfraction 7 was ageloxime-D. Previous research reported that ageloxime-D is active against L5178Y mouse lymphoma cells [6] and antimicrobial [8]. The oxime group in ageloxime D was reported to increase the biofilm activity but reduce the toxicity to bacteria [24].
Ageloxime-D in the LC-MS chromatogram peak showed a double peak because of the oxime group’s tautomerism. The tautomerism of the oxime group in ageloxime-D also gives the splitting peak in the UV spectrum and duplication resonance in the nuclear magnetic resonance spectrum and appearing ion molecule m/z at 440.3 and 422.3 [6].
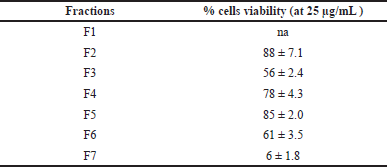 | Table 3. The result of the cytotoxic assay against the HEK-293 cell line. [Click here to view] |
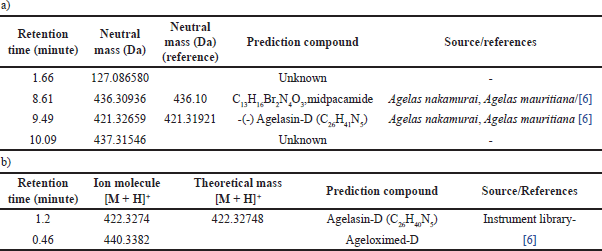 | Table 4. LC-MS data of EtOAc fraction (a) and subfraction F7 (b). [Click here to view] |
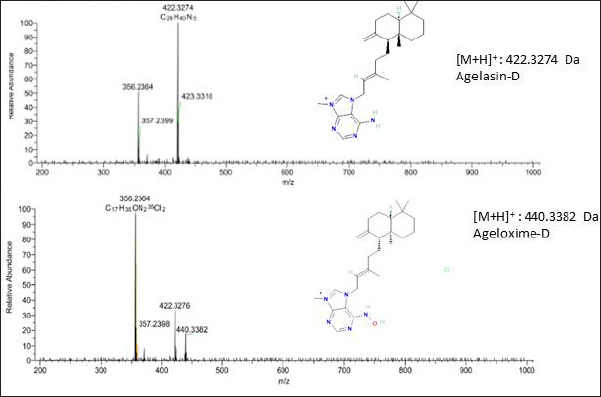 | Figure 3. The profile of MS/MS chromatogram of active subfraction F7. [Click here to view] |
CONCLUSION
Considering the data obtained in this study, the A. nakamurai was the potential source of compounds that were strongly active against MDA-MB-231 (IC50: 10.677 μg/mL) and MCF-7 (IC50:15.154 μg/mL ) cell lines. Active subfraction F7 contained the agelasin-D and ageloximed-D. Even though the subfraction F7 showed cytotoxicity against HEK-293, further structural optimization was needed to decrease cytotoxicity against normal cell lines and increase in the targeted cancer cells.
ACKNOWLEDGMENTS
The authors are grateful to The National Research and Innovation Agency Republic of Indonesia for the financial support of the project DIPA OR Kesehatan 2022–2023. The authors would like to thank Ms. Hanny Nugrahani, M.Pharm. (Laboratory of Pharmaceutical Biology, Universitas Padjadjaran), Lailatul Qodria, and Olga Galih Rakha Siwi for their excellent technical support and assistance.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took Fart in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Sancho-Garnier H, Colonna M. Breast cancer epidemiology [Épidémiologie des cancers du sein]. Presse Med. 2019;48(10):1076–84. Available from: https://tinyurl.com/3uxnttx9
2. Heinrich M, Barnes J, Gibbens S, William EM. Fundamental of pharmacoqnosy and phytotherapy. 2nd ed. London, UK: Elsevier Chruchill Livingstone; 2012. 129–33 pp.
3. Hu Y, Chen J, Hu G, Yu J, Zhu X, Lin Y, et al. Statistical research on the bioactivity of new marine natural products discovered during the 28 years from 1985 to 2012. Mar Drugs. 2015;13(1):202–21. CrossRef
4. Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Marine natural products. Nat Prod Rep. 2015;32(20):116–211. CrossRef
5. Braekman JC, Daloze D, Stoller C, Van Soesti RWM. Chemotaxonomy of age~as (Porifera • Demospongiae). Biochem Syst Ecol. 1992;20(5):417–31.
6. Hertiani T. Isolation and structure elucidation of bioactive secondary metabolites from Indonesian marine sponges [dissertation]. Düsseldorf, Germany: Heinrich Heine University Düsseldorf; 2007.
7. Fu CW, Lin YC, Chiou SF, Huang TY, Yang YJ, Wu SH, et al. 2-guanidinoethanesulfonyl sesquiterpenes from the marine sponge Agelas nakamurai. Tetrahedron Lett. 2022;103:153964. CrossRef
8. Tsuda M, Uemoto H, Kobayashi J. Slagenins A ~ C, novel bromopyrrole alkaloids from marine sponge Agelas nakamurai. Tetrahedron Lett. 1999;40:5709–12.
9. An L, Song WJ, Tang XL, de Voogd NJ, Wang Q, Chu MJ, et al. Alkaloids and polyketides from the South China Sea sponge Agelas aff. nemoechinata. RSC Adv. 2017;7:14323–9.
10. Fujita M, Nakao Y, Matsunaga S, Seiki M, Itoh Y, Yamashita J, et al. Ageladine A: an antiangiogenic matrixmetalloproteinase inhibitor from the marine sponge Agelas nakamurai. J Am Chem Soc. 2003;125:15700–1.
11. Wu H, Nakamura H, Kobayashi J, Ohizumi Y, Hirata Y. Agelasine-E and -F, novel monocyclic diterpenoids with 9-methyladeninium unit possessing inhibitory effects on Na,K-ATPase isolated from the Okinawan sea sponge Agelas nakamurai Hoshino. Tetrahedron Lett. 1984;25(34):3719–22. CrossRef
12. Hertiani T, Edrada-Ebel R, Ortlepp S, Soest RWM, Voogd NJ, Wray V, et al. From anti-fouling to biofilm inhibition: new cytotoxic secondary metabolites from two Indonesian Agelas sponges. Bioorg Med Chem. 2010;18:1297–311.
13. Magoulas GE. Ageladine A, a bromopyrrole alkaloid from the marine sponge Agelas nakamurai. Compounds. 2023;3:107–21. CrossRef
14. Mahamed S, Motal R, Govender T, Dlamini N, Khuboni K, Hadeb Z, et al. A concise review on marine bromopyrrolealkaloids as anticanceragents. Bioorg Med Chem Lett. 2023;8:129102. CrossRef
15. Hoshino T. Description of two new species in the genus Agelas (Demospongia) from Zamami Island, the Ryukyus, Japan. Proceedings of the Japanese Society of Systematic Zoology; 2014. Hiroshima, Japan: Japanese Society of Systematic Zoology; 2014. vol. 30, pp 1–10.
16. de Voogd NJ, Alvarez B, Boury-Esnault N, Carballo JL, Cárdenas P, Díaz MC, et al. World porifera database. 2023 [cited 2023 June 04]. Available from: https://www.marinespecies.org/porifera; CrossRef
17. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63.
18. Choi C, Son A, Lee HS, Lee YJ, Park HC. Radiosensitization by marine sponge Agelas sp. Extracts in hepatocellsular carcinoma cellss with autophagy induction. Sci Rep. 2018;8(1):1–11. CrossRef
19. Maharjan S, Lee MG, Kim SY, Lee KS, Nam KS. Morin sensitizes MDA-MB-231 triple-negative breast cancer cells to doxorubicin cytotoxicity by suppressing FOXM1 and attenuating EGFR/STAT3 signaling pathways. Pharmaceuticals. 2023;16:672. CrossRef
20. Uribe AR, Acosta AM, Diaz MD. Viability of HEK 293 cellss on poly-β-hydroxybutyrate (PHB) biosynthesized from a mutant Azotobacter vinelandii strain. Cast film and electrospun scaffolds. Mater Sci Eng C. 2017;81:236–46. CrossRef
21. Arai M, Yamano Y, Setiawan A, Kobayashi M. Identification of the target protein of agelasine D, a marine sponge diterpene alkaloid, as an anti-dormant mycobacterial substance. ChemBioChem. 2014;15(1):117–23. CrossRef
22. Ogurtsova EK, Makarieva TN, Dmitrenok PS, Denisenko VA, Krasokhin VB, Kuz’mich AS, et al. Isolation of agelasin B from the marine fungus Agelas cf. mauritiana. Chem Nat Compounds. 2015;51(1):189–91. CrossRef
23. Lin YC, Chao CH, Fu CW, Chiou SF, Huang TY, Yang YJ, et al. Computationally assisted structure elucidation of new 2-guanidinoethanesulfonyl sesquiterpenoid alkaloids: agelasidines G–I from the marine sponge Agelas nakamurai. Tetrahedron. 2022;126:133077. CrossRef
24. Paulsen B, Gundersen L. The first synthesis of (–)-Agelasine F; an antimycobacterial natural product found in marine sponges in the Agelas genus. Eur JOC. 2020;15:2244–50. CrossRef