INTRODUCTION
Exposure to long-lasting stress is a precipitating factor for developing major depression and neurocognitive deficits. Depressive disorder is the most common neuropsychiatric condition, with 10%–15% lifetime prevalence being one of the important reasons for disability with enormous personal, medical, and economic costs [1,2]. Major depressive disorder (MDD) is associated with impaired neuronal plasticity and cognitive abilities, including learning and memory [3]. Clinical and preclinical studies demonstrated abnormal processing of cognitive functions in depression. Several stressors are known to alter synaptic plasticity facilitating long-term depression and change in synaptopruning relating to the formation of stress-related memories, developing MDD.Following that, behavioral changes and learned cognition, along with spine and dendrite atrophy, have been seen in many chronic stress models inducing MDD-related phenotype [4–6].
It has been discovered that immobilization stress reduces Glucocorticoid receptor (GluR) levels in the hippocampus and increases the maze passage duration and the number of mistakes in enhanced multibranch maze tests [7]. According to earlier research [8–10], exposure to prolonged immobilization stress decreases hippocampus volume, spatial memory, novel object recognition memory [11,12], and context discrimination [13]. In a recent study, it was shown that animals subjected to chronic immobilization stress (CIS) had poorer spatial acuity and less fast gamma (55–90 Hz) and slow gamma (30–50 Hz) oscillation power in the hippocampal area connected to excitatory input [14].
Neuroimaging studies indicated structural abnormalities in the amygdale, hippocampus, and frontal cortex including the loss of neuronal and glial cells in MDD patients [15–17]. Previously, we showed altered brain derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), glial fibrillary acidic protein (GFAP), and GluR signalling in chronic stress models [9–12, 18,19].
The pathogenesis of depression and the use of antidepressants to treat it are both strongly correlated with impaired serotonergic neurotransmission [20,21]. A previous study demonstrated decreased serotonin levels in the synapses of depressed patients [22]. Also, postmortem and neuroimaging studies indicated reduced serotonin transporter (SERT) density in MDD patients [23,24]. Stress decreased serotonin levels, as demonstrated by a microdialysis study [25]. This will be connected to a reduction in the hippocampus’s SERT expression [26]. Elevated levels of glucocorticoids reduced the serotonin response in the hippocampal neurons [27].
Studies have shown that alteration in BDNF expression leads to depressive-like behavior [28] and anxiety [9]. Many postmortem brain samples of MDD patients had reduced expression of BDNF in serum, plasma, and platelets [29–31]. In addition, postmortem brains of depressed individuals showed downregulation of BDNF and nerve growth factor genes with their TrkB and TrkA receptors, respectively [32–34].
Escitalopram, a selective serotonin reuptale inhibitor (SSRI), is typically the preferred antidepressant medication in the pharmacotherapy of depression [35]. According to Jiang et al. [36] study, escitalopram treatment improved depressive symptoms and normalized BDNF levels in MDD patients. Another study showed that escitalopram treatment for 2 months increased plasma BDNF levels in mild cognitive impairment patients. There is a strong correlation between changes in BDNF levels, significant enhancements in cognitive performance, and reductions in depression and anxiety symptoms [37]. Previously, escitalopram raised levels of synaptophysin, postsynaptic density protein-95, and BDNF in hippocampal neurons [38,39]. It is unknown whether escitalopram affects cognitive functions in the CIS model of depression.
VEGF provides trophic support to neurons and glial cells, thus contributing to neurogenesis and neuroprotection [40]. Previous studies showed that VEGF signaling is crucial for behavioral and cellular responses by different antidepressants [41,42]. Clinical evidence points to VEGF’s involvement in the pathophysiology of depression and antidepressant therapy [43].Reduced GFAP levels have been observed in both clinical [44] and preclinical studies [9–12]. Hence, the current study explored the neuroprotective effects and molecular mechanisms of escitalopram on CIS-induced cognitive deficits.
METHODOLOGY
Animals
Wistar male rats weighing 200–250 g and 2–2.5 months of age were received from the Animal House Facility (CARF), NIMHANS, Bengaluru. Since the estrous cycle intensifies the response to a stressful stimulus, only male rats were employed in the study [45]. Throughout all studies, 3–4 rats were kept in each polypropylene cage with free access to water and normal rat food pellets unless otherwise stated. The humidity (50%–55%) and temperature (25°C ± 2°C) in the animal housing were kept constant. All experiments adhered to the National Institutes of Health Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (The National Academics Press, Washington USA, 2003). The institution’s animal ethical committee gave its approval to the experimentation protocols. The number of rats utilized and their distress has been reduced.
Experimental protocol
Using a randomized block design technique, the animals were allocated into the following five groups at random: group 1: un-stressed rats kept at standard home cage condition (Control); group 2: CIS; group 3: stressed rats administered with the vehicle for 14 days after stress protocol (CIS + Veh); group 4: after the stress procedure, stressed rats were given escitalopram (5 mg/kg body weight each day) for 14 days (CIS + ESC-5); group 5: After the stress procedure, stressed rats were given escitalopram (10 mg/kg body weight each day) for 14 days (CIS + ESC-10). Escitalopram was prepared freshly in normal saline (0.9% w/v) and was intraperitoneally administered to rats for 2 weeks. Different set animals were used for behavioral depression, anxiety, spatial learning and memory, and Western blotting experiments. The number of animals in each group was determined based on earlier studies [9].
Chronic immobilization stress protocol
We have employed repeated immobilization stress [9,10]. Rats were placed in immobilization cones between 10 a.m. and noon each day for 2 hours, where they were exposed to stress and had limited access to water and food. Control animals were kept in their cages and handled very occasionally.
Assessment of anhedonia
Sucrose consumption was used to assess anhedonia in animals. During the test time, the animals were kept in separate housing. Throughout the training period, each animal had access to two bottles in the home cage, one containing 1% w/v sucrose solution and the other with regular drinking water. Water and sugar water consumption was measured daily, and the two bottles’ placements (left and right) were reversed to avoid place preference. After 18 hours of food or water deprivation, a 2-hour test was done (1,600–1,800). Following the test period, each rat’s fluid intake was measured. The amount of sucrose water consumed was multiplied by the total amount of liquid consumed to determine the sucrose preference score [9,46–48].
Forced swim test (FST)
On the first day, rats were kept in a perspex cylinder (60 cm high and 45 cm in diameter) at a constant temperature of 25°C ± 2°C for 15 minutes. The cylinder was filled with tap water to a height of 40 cm. They were then removed from the cylinder, towel-dried, and returned to their respective cages. On day 2, the animals were made to swim for 5 minutes as part of a test, and their behaviour was recorded. Offline analyses were conducted using the coded recorded data by an observer blind to the treatments. The immobility parameter, which is described by a rat floating vertically and only moving slightly to keep its head above the water’s surface, was measured [9,46–48].
Open field test (OFT)
The OFT box is a square wooden box (100 × 100 × 40 cm) with a black-painted inner wall and floor. There are 25 squares (20 × 20 cm) on the floor, with 16 squares on either side of the center and 9 squares in the middle. Only the center of the box was illuminated. Randomly selected rats from each group were placed in an arena corner facing the center. Using the tracking program Noldus Ethovision XT, the behavior was captured for 5 minutes and examined (Noldus, Wageningen, The Netherlands). After each testing session, the arena was cleaned with 70% ethanol, and rats were tested only once. The total distance traveled and the number of squares crossed were counted [9,46,47].
Elevated plus maze (EPM)
The EPM is a reliable ethological test to identify anxiety-like behavior. The EPM (Columbus Instruments, USA) is an apparatus that has a stage that is plus-shaped and has two open arms (50 × 10 cm), two closed arms (50 × 10 × 40 cm), and a center (10 × 10 cm). It is elevated 60 cm off the ground. The EPM was lighted by an overhead light source (60 W bulb). Each rat was given 5 minutes to explore the closed and open arms. Every trial was recorded on video using a camera set on the roof, and the maze was cleaned with 70% alcohol between sessions. The amount of time spent in open and closed arms, open and entire arm entrances, vertical rearing, and head dips were all considered while scoring videos [9,48].
Radial arm maze task
Reference and working memory were tested using a partially baited eight-arm radial maze with eight arms stretching radially from the center. The maze was maintained 40 cm off the ground. Before the experiment, rats were put on a restricted diet to help them stay motivated to find food in the maze and maintain 85% of their free-feeding body weight. The rats were acclimated to the maze for 2 days in a row for 10 minutes each before the acquisition started. Each rat performed two trials each day, separated by 1 hour, during the acquisition phase, lasting 16 consecutive days.The rat has been allowed to 5 minutes to retrieve the bait from the four arms, numbers 2, 3, 6, and 8. When the rat has eaten four baits, the trial is over, or 5 minutes have passed. All four paws crossing from the center into an arm have deemed an entry; an entry was considered the correct decision when the baited arm was first visited in the trial. After each test, the maze was wiped with 70% alcohol to remove odor cluesRe-entry into baited or unbaited arms, reference memory errors (RMEs), working memory errors, and the overall number of arm entries were counted. Two trials were provided to measure memory on the 11th day, 10 days after the completion of acquisition [9,46–48].
Western blot analyses
From Sigma Aldrich, antibodies against BDNF, GFAP, and VEGF were procured (St. Louis, MO). Chemicals of the highest analytical grade were employed in the study. Halothane anesthesia was used to sacrifice rats from all five groups. Dissected samples of the frontal cortex, amygdalar complex, and hippocampus were kept at −80°C until measurement. The frozen tissues were thawed and homogenized in an ice-cold 1× phosphate buffer solution (PBS) solution that contained 10% sucrose, 1 mM EDTA, 20 mM Tris-HCl (pH 7.4), and 1× protease inhibitor cocktail (Sigma-Aldrich, USA) [9]. The tissue was sonicated three times while being placed on ice (each sonication for 10 seconds: QSonica Sonicator model Q125, New York, NY). Before an aliquot was taken to estimate the protein concentration, the homogenized materials were centrifuged at 14,000 rpm for 15 minutes at 4°C in a chilled centrifuge. The ELISA plate reader’s protein estimation procedure was Bradford’s method (TECAN, GmbH, Austria).
30 g of protein and the same volume of 2× Laemmlli buffer [10% w/v SDS, 20% v/v glycerol, 1% -mercaptoethanol, dH2O, 100 mM Tris-HCl (pH 6.8)] were combined for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). This mixture was heated for 10 minutes. After the samples had cooled, the supernatant was centrifuged to remove any insoluble protein pellets. The supernatant was then loaded onto the wells. Unpooled frontal cortex and hippocampus samples were loaded in duplicate (30 µg/lane). The amygdalar complex samples, on the other hand, were pooled and loaded in duplicate along with molecular weight markers in the relevant wells. SDS-PAGE with 10%–12% stacking polyacrylamide gel consists of acrylamide mix 30%; 1.5 M Tris pH 8.8; 1.0 M Tris pH 6.8; SDS 10%; ammonium persulfate 10%; TEMED; distilled water at 100–125 V for 2–2.5 hours was used to separate the samples (Biorad Laboratories Inc., Hercules, CA).
After being separated by SDS PAGE, proteins were electrophoretically transferred from the gel to PVDF membranes using a semi-dry transfer technique (Sree Maruthi Scientific Works, Bangalore, India). After the transfer, the blots were incubated in a blocking solution [Phosphate buffered saline Tween-20 (PBST) containing 5% skimmed milk powder; Nandini Milk Products, Bengaluru, India] for 1–1.5 hours at room temperature or overnight at 4°C to avoid nonspecific binding. The primary BDNF (1:500), VEGF (1:500), GFAP (1:1,000), and anti-ß-actin (1:2,000) antibodies from Abcam in Cambridge, UK, were incubated on the blot for 2 hours at room temperature. After removing any excess primary antibody with PBST (10 minutes × 4), the blot was then incubated for an hour at room temperature with horseradish peroxidase-conjugated secondary antibody (diluted in PBST with a concentration of 1:2,000 for rabbit and mouse) (Bangalore Genei, Bengaluru, India). Membranes were washed with PBST, and the immune response was detected using either the SuperSignalTM West Pico chemiluminescent substrate or developing the membranes in 1× PBS containing 3,3′-diaminobenzidine [1 mg/ml (w/v) and 0.1% H2O2] (Pierce Biotechnology, Illinois, USA).The Gel documentation system captured the images (SYNGENE, Synoptics Model G: Box Chemi XT4, Cambridge, UK). The image was evaluated using Image J program’s densitometric method (Wayne Rasband, Version 1.47, NIH, USA) and each protein band was corrected to be equal to actin [9].
Assessment of volume changes in basolateral amygdale, hippocampus, and dentate gyrus (DG)
On halothane-anesthetized rats, transcardial infusions of ice-cold saline and a 10% v/v formaldehyde solution were administered. The dissected-out brains were postfixed for 2 days. 40 µm thick coronal slices were cut through the whole anterior-posterior range of the hippocampus using a vibratome (Leica, Wetzlar, Germany). The Nissl (cresyl violet) stain was used to stain the serial brain sections. Their contours identified the DG, hippocampus, and basolateral amygdala (BLA) as per Paxinos and Watson [49] rat atlas [9,48]. The volume of the DG, hippocampus, and BLA was calculated using unbiased stereology using the software Stereo Investigator, as described in earlier publications (MBF Bioscience, Microbrightfield, Inc., USA). The procedure was performed in every sixth section, and both hemispheres were examined separately by merging the sections. Cavalieri’s method expressed the overall volume as the left and right hemispheres added together.
Statistical evaluation
The statistical evaluations were performed using Graph Pad Prism 5. A two-way ANOVA and Bonferroni’s post hoc test were used in RAM to examine group differences across different days. One-way ANOVA was used to analyze significant differences between groups for the OFT, EPM, sucrose preference test (SPT), FST, neurotrophic factors, and volume data. Tukey’s post hoc test was then used to compare additional group differences. Statistical significance was defined as a p-value of 0.05 or less.
RESULTS
Behavioral depression and the role of escitalopram treatment
The depressed group’s preference for sucrose was much lower than the control group. In addition, the animals who received escitalopram had significantly greater sucrose preferences (CIS + ESC-5 and CIS + ESC-10; Fig. 1a) than in the CIS group. There was no significant dose-dependent effect seen among two doses of escitalopram. CIS + Veh group continued to show behavioral depressive signs with a reduced preference for sucrose water (F4,51 = 23.37, Fig. 1a; p < 0.001).
Compared to the control group, the CIS group displayed more extended periods of immobility. Interestingly, this behavioral despair was reduced in the FST following 2 weeks of escitalopram treatment. Both doses of escitalopram demonstrated antidepressant effects in FST. The immobility time of CIS animals was unaffected by vehicle administration (F4,51 = 34.01, p < 0.001; Fig. 1b).
Reversal of locomotion and exploration by escitalopram treatment
Locomotory and exploratory behavior was assessed in the open arena (Fig. 2a–e). Open field data indicated significant differences for distance traveled (F4,51 = 9.56, p < 0.001; Fig. 2f) and the number of zone transitions among different groups (F4,51 = 12.00, p < 0.001; Fig. 2g). Concerning distance traveled, and the number of zone changes made, post hoc analysis showed that escitalopram treated animals outperformed CIS and CIS + Veh rats. The difference between the higher and lower escitalopram doses for restoration is statistically significant.
Escitalopram showed an anxiolytic effect on CIS
In the EPM, CIS animals behaved more anxiously than the control group did (Fig. 3a). In Figure 3b, they are shown to spend more time in closed arms (p < 0.001; F4,51 = 10.43) and less duration in open arms (F4,51 = 49.02, p < 0.001; Fig. 3a). Escitalopram showed a dose-dependent effect on anxiety behavior. At 5 mg/kg dose, it did not restore anxiety in CIS animals. On the other hand, at 10 mg/kg dose, escitalopram completely significantly restored anxiety in depressed animals.
Depressed animals had fewer open arm entries (p < 0.001; F4,51 = 12.61, Fig. 3c) and total arm transitions (F4,51 = 19.97, p < 0.001; Fig. 3d) compared with control rats. In CIS animals, the higher dose of escitalopram resulted in an elevated number of open arm and total arm entries (p < 0.001). Lower doses, however, did not impact these behaviors (p > 0.05). About vehicle treatment, neither the overall number of open arms nor total arm entries changed.
The number of vertical rearing was reduced in the CIS group than control (F4,51 = 10.18, p < 0.001; Fig. 3e). Escitalopram (10 mg/kg) partially restored the number of vertical rearing in depressed animals. Depressed animals exhibit decreased head dips (F4,51 = 21.40, p < 0.001; Fig. 3f) in EPM compared to control animals. Escitalopram at a higher dose could completely reverse this behavior.
Escitalopram treatment ameliorated spatial memory and learning ability deficits in depressed animals
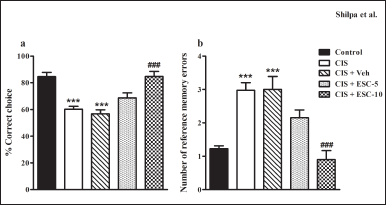
In a RAM task, depressed animals did not perform well. They could not get 80% correct choice even after 16 training days. Animals treated with escitalopram outperformed depressed animals in the RAM task (interaction effect: F28,315 = 4.46, p < 0.001; group effect: F4,315 = 23.20, p < 0.001; Fig. 4a). Escitalopram did not show dose-dependent effect in RAM until the fifth block, but from that point on, the higher dose (CIS + ESC-10) group excelled the lower dose (CIS + ESC-5) group in terms of performance.In depressed rats, a higher dose entirely reversed spatial learning deficiencies, whereas a lower dose only partially restored (7th block: F4,45 = 19.00 and 8th block: F4,45 = 26.40, p < 0.001; Fig. 4b). The CIS + Veh administered group still exhibited learning deficits on the RAM task (p > 0.05).
Unbaited arm entry was regarded as a reference memory deficit. The number of errors in the standard control group decreased over time and reached a lower value on the final acquisition day (0.65 ± 0.14) than in the depressed group (2.75 ± 0.37). Chronic escitalopram administration considerably decreased the number of RMEs produced by depressed animals (Fig. 4c; interaction effect: F28,315 = 2.96; group effect: F4,315 = 15.75). In the depressed group, the 10 mg dose of escitalopram entirely improved reference memory. However, there is no statistical variance between the lower escitalopram dose and the control group for reference memory. In the eighth block, the RME produced by the vehicle group was similar to CIS (2.67 ± 0.25; p > 0.05; Fig. 4d).
Re-entering the baited or unbaited arm was determined to be a sign of working memory impairments. Depression or escitalopram use did not impact working memory in the RAM task (data not shown).
In the retention test, depressed animals still showed memory impairment (% accurate decision: 60.25; RMEs: 2.97 in the CIS group). Only a higher dose of escitalopram was able to improve depressed rats’ memory. In the CIS + ESC-10 group, the RMEs (p < 0.001; F4,45 = 14.01, Fig. 5b) and % correct choice (p < 0.001; F4,45 = 16.62) were comparable to those in the control group.
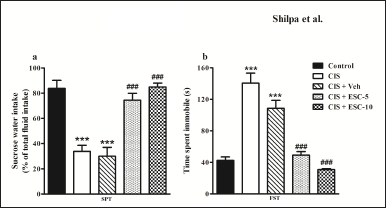 | Figure 1. Escitalopram reversed behavioral depression in stressed rats. CIS rats show decreased sucrose preference and increased immobility in SPT (a) and FST (b). Stressed rats treated with Escitalopram showed more preference to sucrose water in SPT and decreased immobility time in the FST. Data expressed as Mean ± SEM. Control (n = 12): un-stressed rats kept at standard home cage condition; CIS (n = 12): animals subjected to immobilisation stress for 2 hour/day for 10 days; CIS + Veh (n = 12): stressed animals administered with vehicle for 14 days; CIS + ESC-5 (n = 10), CIS + ESC-10 (n = 10): stressed animals treated with Escitalopram 5 mg and 10 mg/kg/day for 14 days intraperitoneally. One-way ANOVA followed by Tukey’s post hoc test; ***n < 0.001 versus control, ###n < 0.001 versus CIS. [Click here to view] |
The frontal cortex and hippocampus’s BDNF, VEGF, and GFAP expression is improved by escitalopram treatment
The hippocampal BDNF levels are decreased in depression compared to the control condition (F3,16 = 32.51, p < 0.001; Fig. 6d). Fronto-cortical BDNF remains unchanged in depressed animals (F3,16 = 0.38, p > 0.05; Fig. 6e). Chronic escitalopram treatment completely normalized BDNF expression in the hippocampus without changing cortical BDNF levels. In CIS animals, VEGF levels are lower in the frontal brain and hippocampal regions. Escitalopram (higher dose) treatment for CIS rats led to full recovery of VEGF expression in the frontal cortex (p < 0.001; F3,16 = 7.13, Fig. 7e) and moderate recovery in the hippocampus (p < 0.001; F3,16 = 12.32, Fig. 7d). In the frontal cortex and hippocampus of depressed animals, the level of the astrocyte marker GFAP was significantly decreased. Escitalopram ultimately increased GFAP levels in the hippocampus after 2 weeks of treatment (p < 0.001; F3,16 = 19.67, Fig. 8d) and only marginally improved it in the frontal cortex (F3,16= 2.53, p > 0.05; Fig. 8e).
Partial restoration of BDNF and GFAP but not VEGF expression in the basolateral amygdalar complex by escitalopram treatment
The amygdalar complex showed increased expression of BDNF (F3,4 = 19.62, p < 0.01; Fig. 6f), VEGF (F3,4 = 17.94, p < 0.01; Fig. 7f), and GFAP (F3,4 = 85.74, p < 0.001; Fig. 8f) in response to depression. Escitalopram only partly improved BDNF and GFAP levels in the amygdala but did not improve VEGF expression.
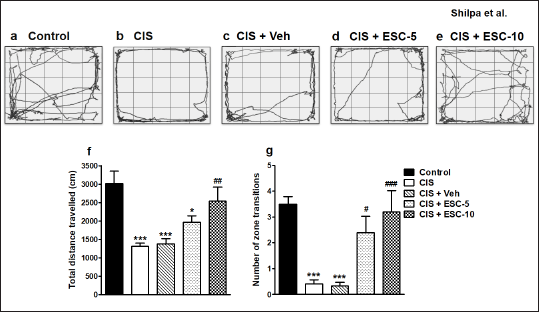 | Figure 2. Restoration of exploratory and locomotor behavior by Escitalopram. Representative path length tracks in open field. Image showing OFT tracks of different groups: (a) Control (n = 12): un-stressed rats kept at standard home cage condition; (b) CIS (n = 12): animals subjected to immobilisation stress for 2 hour/day for 10 days; (c) CIS + Veh (n = 12): stressed animals administered with vehicle for 14 days; (d) CIS + ESC-5 (n = 10), (e) CIS + ESC-10 (n = 10): stressed animals treated with Escitalopram 5 mg and 10 mg/kg/day for 14 days intraperitoneally. Data expressed as Mean ± SEM. One-way ANOVA followed by Tukey’s post hoc test; *p < 0.05, ***p < 0.001 versus control, ###p < 0.001, ##p < 0.01, #p < 0.05 versus CIS. [Click here to view] |
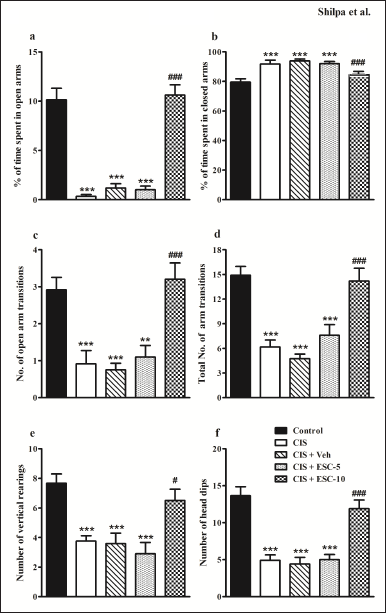 | Figure 3. Chronic escitalopram treatment reduced anxiety-like behavior in depressed rats. (a): % of time spent in open arms, (b): % of time spent in closed arms, (c): number of open arm transitions, (d): total number of arm transitions, (e): number of vertical rearings and (f): number of head dips. Data expressed as Mean ± SEM. Control (n = 12): un-stressed rats kept at standard home cage condition; CIS (n = 12): animals subjected to immobilisation stress for 2 hour/day for 10 days; CIS + Veh (n = 12): stressed animals administered with vehicle for 14 days; CIS + ESC-5 (n = 10), CIS + ESC-10 (n = 10): stressed animals treated with Escitalopram 5 mg and 10 mg/kg/day for 14 days intraperitoneally. One-way ANOVA followed Tukey’s post hoc test; **p < 0.01, ***p < 0.001 versus control. #p < 0.05, ###p < 0.010 versus CIS. [Click here to view] |
Escitalopram completely prevented DG shrinkage and hippocampal hypotrophy and did not affect the size of the amygdala
Depression resulted in the DG and hippocampal volume reduction. On the contrary, the basolateral amygdalar complex showed hypertrophy. Escitalopram effectively reverses the DG and hippocampal volumes at both doses (F4,20 = 23.50 and F4,20 = 30.04, respectively; p < 0.001 in Fig. 9a and b). Unexpectedly, the therapy failed to reverse amygdalar hypertrophy (F4,20 = 9.21, p < 0.001; Fig. 9c).
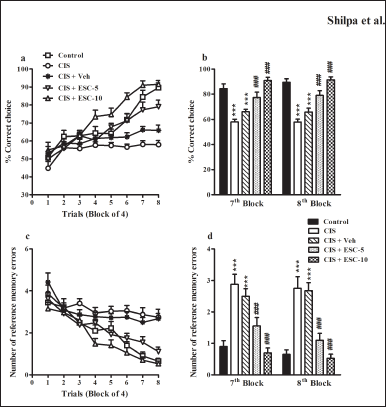 | Figure 4. Escitalopram completely ameliorated spatial learning and memory impairment in depressed rats in a partially baited radial arm maze. (a): % correct choice and (c): Number of RMEs in the acquisition phase of the RAM task across trials. (b): % correct choice and (d): Number of RMEs in blocks 7 and 8. Control (n = 10): un-stressed rats kept at standard home cage condition; CIS (n = 10): animals subjected to immobilisation stress for 2 hour/day for 10 days; CIS + Veh (n = 10): stressed animals administered with vehicle for 14 days; CIS + ESC-5 (n = 10), CIS + ESC-10 (n = 10): stressed animals treated with Escitalopram 5 mg and 10 mg/kg/day for 14 days intraperitoneally. Data expressed as Mean ± SEM. Two-way repeated measure ANOVA followed by Bonferroni’s post hoc test and One-way ANOVA followed by Tukey’s post hoc test; ***p < 0.001 versus control; ###p < 0.001 versus CIS. [Click here to view] |
DISCUSSION
The present study explored the beneficial effects of chronic escitalopram treatment on depressive behavior, heightened anxiety, spatial memory deficits, morphological changes, and molecular markers. CIS causes anxiety and depressive symptoms, poor spatial memory capacity, hippocampus atrophy, and amygdalar hypertrophy. Also, the neurotrophic factors BDNF, VEGF, and astrocytic marker GFAP were reduced. The treatment with escitalopram reversed both depression and anxiety-like behaviors, normalized spatial memory deficits. This behavioral improvement was associated with the restoration of BDNF, VEGF, and GFAP molecular markers. These results imply that escitalopram shows useful effect on cognitive deficiencies associated with CIS.
Previous preclinical studies demonstrated that different chronic stress models alter hippocampal structure and function [9–12,50,51]. We can distinguish between working memory and reference memory components of spatial memory using the partly baited RAM task. Reference memory is the storage of consistent data across trials and is, hence, independent of the trial. On the other hand, working memory is a type of memory that is trial dependent since the information to be retained changes throughout repeated trials [9,52,53]. Working memory and reference memory have been associated with the hippocampus and cerebral cortex, respectively [54–56].
 | Figure 5. Escitalopram restored memory impairment in the retention test. (a): % correct choice and (b): Number of RMEs in the retention test. Control (n = 10): un-stressed rats kept at standard home cage condition; CIS (n = 10): animals subjected to immobilisation stress for 2 hour/day for 10 days; CIS + Veh (n = 10): stressed animals administered with vehicle for 14 days; CIS + ESC-5 (n = 10), CIS + ESC-10 (n = 10): stressed animals treated with Escitalopram 5 mg and 10 mg/kg/day for 14 days intraperitoneally. Data expressed as Mean ± SEM. One-way ANOVA followed by Tukey’s post hoc test; ***p < 0.001 versus control. ###p < 0.001 versus CIS. [Click here to view] |
The hippocampus experiences morphological and functional changes as a result of long-term stress, depression, and the increase in glucocorticoid exposure that follows, including atrophy of the apical dendrites of CA3 pyramidal neurons and decreased synaptic plasticity in the CA1 area [51,57,58]. Prolonged stress, depression, and the resultant increase in glucocorticoid exposure are associated with structural and functional alterations in the hippocampus, including decreased synaptic plasticity in the CA1 area and atrophy of the apical dendrites of CA3 pyramidal neurons area [51,57,58].
In addition, prolonged stress inhibits hippocampal neurogenesis, which may cause hippocampal atrophy [59,60]. Repeated stress exposure decreased the production of BDNF, which is essential for neurogenesis and brain remodeling [9,31,33]. The pathogenesis of MDD is heavily influenced by neurotrophic factor deficits and the subsequent decline in neural plasticity in the hippocampus [9,47,48]. According to postmortem investigations, depression is linked to modifications in the number and structure of both neurons and astrocytes [15,16,61]. Chronic stress decreased the expression of GFAP mRNA in the prefrontal cortex (PFC) and in cells that express GFAP in the hippocampus [9,45].
The serotonergic system plays a major role in behavioral changes associated with stress and depression [22–26,62]. Escitalopram is clinically used to treat anxiety comorbid with depression [62]. Escitalopram effectively treats cognitive deficits in depressed individuals [63] and improves overall cognitive abilities, including verbal and visual memory [64,65]. Earlier studies show the anti-anxiety activity of escitalopram in animals [66,67]. Maternally separated rats treated with escitalopram demonstrate decreased ultrasonic vocalizations [68]. Also, 7 days of escitalopram treatment for MDD patients normalized amygdala hyperactivity in response to negative stimuli [69].
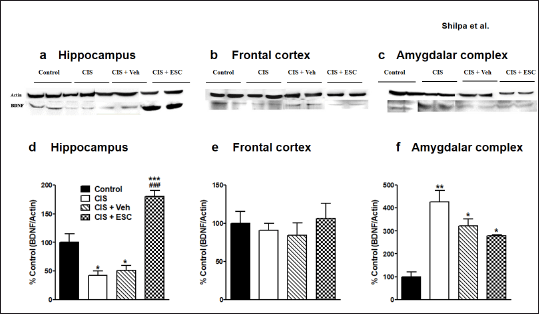 | Figure 6. Effect of chronic escitalopram treatment on stress-induced altered BDNF expression. Representative immunoblots of BDNF and β-actin from the hippocampus (a), Frontal cortex (b) and Amygdalar complex (c). Escitalopram treatment restores BDNF levels in the hippocampus (d). Frontal cortical BDNF expression was not significantly altered in all groups (e). Up-regulated amygdalar BDNF was partially restored in the CIS + ESC-10 group (f). Control (n = 5): un-stressed rats kept at standard home cage condition; CIS (n = 5): animals subjected to immobilisation stress for 2 hour/day for 10 days; CIS + Veh (n = 5): stressed animals administered with vehicle for 14 days; CIS + ESC (n = 5): stressed animals treated with Escitalopram 10 mg/kg/day for 14 days intraperitoneally. Data expressed as Mean ± SEM. One-way ANOVA followed by Tukey’s post hoc test; *p < 0.05, **p < 0.01, ***p < 0.001 versus control. ###p < 0.001 versus CIS. Values were normalised to β-Actin and compared with controls. [Click here to view] |
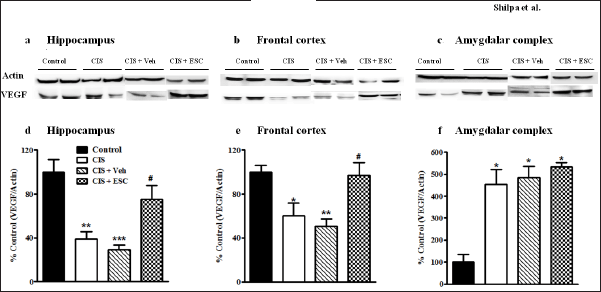 | Figure 7. Escitalopram treatment restored VEGF expression in the hippocampus and frontal cortex. Representative immunoblots of VEGF and β-actin from the hippocampus (a), Frontal cortex (b) and Amygdalar complex (c). Down-regulated VEGF levels in the hippocampus (d) and frontal cortex was restored by escitalopram treatment (e). Up-regulated amygdalar VEGF was not restored in the CIS+ ESC-10 group (f). Control (n = 5): un-stressed rats kept at standard home cage condition; CIS (n = 5): animals subjected to immobilisation stress for 2 hour/day for 10 days; CIS + Veh (n = 5): stressed animals administered with vehicle for 14 days; CIS + ESC (n = 5): stressed animals treated with Escitalopram 10 mg/kg/day for 14 days intraperitoneally. Data expressed as Mean ± SEM. One-way ANOVA followed by Tukey’s post hoc test; *p < 0.05, **p < 0.01, ***p < 0.001 versus control. #p < 0.05 versus CIS. Values were normalised to β-Actin and compared with controls. [Click here to view] |
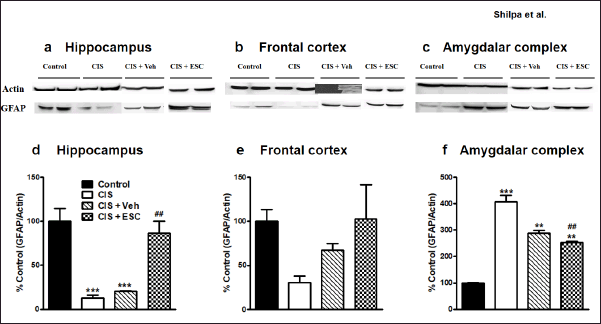 | Figure 8. Escitalopram restored altered GFAP expression in the hippocampus and frontal cortex. Representative immunoblots of GFAP and β-actin from the hippocampus (a), Frontal cortex (b) and Amygdalar complex (c). Treatment with Escitalopram reversed GFAP levels in the hippocampus (d) and frontal cortex (e) and amygdalar complex (f). Control (n = 5): un-stressed rats kept at standard home cage condition; CIS (n = 5): animals subjected to immobilisation stress for 2 hour/day for 10 days; CIS + Veh (n = 5): stressed animals administered with vehicle for 14 days; CIS + ESC (n = 5): stressed animals treated with Escitalopram 10 mg/kg/day for 14 days intraperitoneally. Data expressed as Mean ± SEM. One-way ANOVA followed Tukey’s post hoc test; **p < 0.01, ***p < 0.001 versus control. ##p < 0.01 versus CIS. Values were normalised to β-Actin and compared with controls. [Click here to view] |
The antidepressant treatment restored reduced BDNF levels in MDD patients [36,37]. In line with earlier research [28,70,71], escitalopram in the present investigation augmented BDNF levels in depressed rats. In the current study, escitalopram increased BDNF levels in depressed rats, consistent with previous findings [28,70,71]. Increased BDNF correlates with the restoration of hippocampal atrophy in stressed rats. We observed an overall restoration of GFAP expression, BDNF, and VEGF levels in the hippocampus, followed by escitalopram treatment, contributing to the reversal of hippocampal and DG volumes. In contrast, amygdalar hypertrophy and expression of GFAP, BDNF, and VEGF are not changed after escitalopram treatment. In the previous study, escitalopram partially prevented central amygdala corticotrophin-releasing factor overexpression-induced enhanced anxiety [72]. Previous studies showed that patients with major depression or administered with synthetic glucocorticoids exhibited reduced GFAP in CA1 and CA2 and the left orbitofrontal cortex [45,73]. These studies support the differential effect of escitalopram on amygdala structure and biomarkers in the current study.
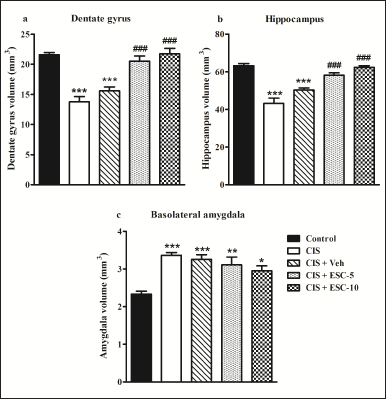 | Figure 9. Chronic escitalopram treatment restores hypotrophy of DG and hippocampus without altering amygdalar hypertrophy. DG (a), hippocampus (b), and BLA (c). Control (n = 5): un-stressed rats kept at standard home cage condition; CIS (n = 5): animals subjected to immobilisation stress for 2 hour/day for 10 days; CIS + Veh (n = 5): stressed animals administered with vehicle for 14 days; CIS + ESC-5 (n = 5), CIS + ESC-10 (n = 5): stressed animals treated with Escitalopram 5 mg and 10 mg/kg/day for 14 days intraperitoneally. Data expressed as Mean ± SEM. One-way ANOVA followed by Tukey’s post hoc test; *p < 0.05, **p < 0.01, ***p < 0.001 versus control. ###p < 0.001 versus CIS. [Click here to view] |
We hypothesize that escitalorpam’s positive impact on chronic immobilizations may be related to how it affects BDNF levels in the hippocampus. The results of our study clearly demonstrated a strong correlation between structural changes and cognitive functions with the expression of neurotrophic factors. It has been observed that the neurotrophic factor levels, BDNF, correlate with the performance in spatial learning. Stress-induced decreased BDNF expression in the hippocampus correlates with impaired hippocampal-dependent learning. Interestingly, chronic escitalopram treatment enhances BDNF in depressed animals, which may be responsible for the amelioration of spatial learning deficits (Fig. 10). Previous studies showed that to 3 weeks of escitalopram treatment restored VEGF, BDNF, and their downstream signaling molecules such as Trk, ERK1/2, mitogen-activated protein kinase, and cyclic AMP-response element binding protein in the hippocampus and PFC in chronic stress models [74–76].
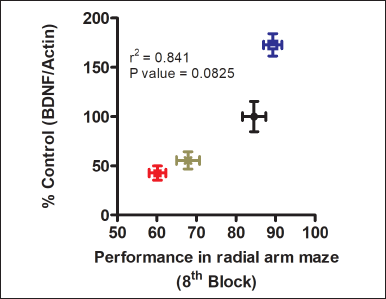 | Figure 10. Correlation between hippocampal BDNF levels with spatial learning in radial arm maze task. Escitalopram treated animals showed enhanced hippocampal BDNF levels, which was associated with better performance in spatial learning in the partially baited radial arm maze test. NC: Normal control; ST: animals subjected to 10 days of chronic immobilization stress; ST + ES-10 and ST + VC: ST rats treated with escitalopram 10 mg/kg b.w., and 0.9% saline i.p., respectively for 14 days. [Click here to view] |
In a previous clinical study, escitalopram treatment increased plasma BDNF levels in patients with mild neurocognitive disorders [37]. Escitalopram restored hippocampal synaptic plasticity in stressful and depressive situations [47,77], which may also be related to increased BDNF and extracellular signal-regulated kinase levels in the hippocampus [77]. Chronic escitalopram administration decreased the endogenous glutamate release triggered by depolarization [78,79], indicating an enhancement in spatial memory ability in depressive-like animals [45,46].
A recent study indicated that escitalopram (15 mg/kg per day) given by oral gavage for 3 weeks restored chronic defeat stress-associated goal-directed motivated behavior [80]. In another study, escitalopram (10 mg/kg i.p.) administered for 28 days in chronic mild unpredictable stress ameliorated cognitive deficits [81]. Coadministration of escitalopram (10 mg/kg, i.p.) during 3 weeks of restraint stress significantly increased mTOR expression in the hippocampus [82]. Escitalopram given during the first 2 weeks in food pellets (0.34 g/kg/day) and 0.41 g/kg/day in the subsequent period for maternally separated rats improved hippocampal-dependent memory [83].
There are some limitations of the present study. As many brain regions are affected in the chronic stress condition other than the hippocampus and amygdala, further studies focusing on an integration of other brain regions such as the PFC and hypothalamus are required. In-vivo long-term potentiation experiments should be considered in the future to correlate with behavior. Also, the neurotransmitter estimation in different brain regions based on the beneficial effect of escitalopram is suggested for further corroboration of current findings.
CONCLUSION
The current research suggests that the SSRI escitalopram attenuates the behavioral effects of depression, including anxiety and poor spatial learning and memory, as a result of regulating the levels of the neurotrophic factors BDNF, VEGF, and GFAP, and reversing hippocampal atrophy.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support provided by the National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India, the Council of Scientific and Industrial Research (CSIR), the Scientific and Engineering Research Board (SERB)—Department of Science and Technology (DST), and the Department of Biotechnology (DBT). Sun Pharmaceuticals, Mumbai, and Apotex Pharmachem, Bengaluru, India, generously donated escitalopram. The researchers acknowledge the technical support provided by G. Harish and Christofer Thomas for the neurochemistry experiments.
AUTHOR CONTRIBUTION
The authors Bhagya and Shankaranarayana Rao developed the study protocol. The experiments and statistical analysis were completed by the authors Shilpa and Bhagya, who also prepared the initial draft of the publication. The final document was co-authored by all writers, and it was approved by all of them.
CONFLICT OF INTEREST
All of the authors have declared that they have no conflicts of interest.
ETHICAL APPROVALS
All experiments adhered to the National Institutes of Health Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (The National Academics Press, Washington USA, 2003). The institution’s animal ethical committee gave its approval (AEC/45/277/N.P) to the experimentation protocols.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Friedrich MJ. Depression is the leading cause of disability around the world. JAMA. 2017;317(15):1517. CrossRef
2. Thom R, Silbersweig DA, Boland RJ. Major depressive disorder in medical illness: a review of assessment, prevalence, and treatment options. Psychosom Med. 2019;81(3):246–55. CrossRef
3. Tartt AN, Mariani MB, Hen R, Mann JJ, Boldrini M. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27(6):2689–99. CrossRef
4. Dong B, Shilpa BM, Shah R, Goyal A, Xie S, Bakalian MJ, et al. Dual pharmacological inhibitor of endocannabinoid degrading enzymes reduces depressive-like behavior in female rats. J Psychiatr Res. 2020;120:103–12. CrossRef
5. Ji Y, Li W, Liu B, Liu J, Ju Y, Wang M, et al. Clinical characteristics of cognitive deficits in major depressive disorder: a 6-month prospective study. Arch Clin Psychiatry. 2020;47(4):101–5. CrossRef
6. Zahra A, Du L, Jia M, Butt MU, Wang Q, Wang Y, et al. Prenatal exposure of citalopram elicits depression-like and anxiety-like behaviors and alteration of morphology and protein expression of medial prefrontal cortex in young adult mice. J Integr Neurosci. 2022;21(2):61. CrossRef
7. Bukia N, Butskhrikidze M, Machavariani L, Svanidze M, Nozadze T. Electric-magnetic stimulation prevents stress-induced deterioration of spatial memory. Georgian Med News. 2023;335:46–53.
8. Rahman MM, Callaghan CK, Kerskens CM, Chattarji S, O’Mara SM. Early hippocampal volume loss as a marker of eventual memory deficits caused by repeated stress. Sci Rep. 2016;6:29127. CrossRef
9. Shilpa BM, Bhagya V, Harish G, Bharath MS, Shankaranarayana Rao BS. Environmental enrichment ameliorates chronic immobilisation stress-induced spatial learning deficits and restores the expression of BDNF, VEGF, GFAP and glucocorticoid receptors. Biol Psychiatry. 2017;76:88–100. CrossRef
10. Tripathi SJ, Chakraborty S, Srikumar BN, Raju TR, Shankaranarayana Rao BS. Inactivation of basolateral amygdala prevents chronic immobilization stress-induced memory impairment and associated changes in corticosterone levels. Neurobiol Learn Mem. 2017;142(Pt B):218–29. CrossRef
11. Tripathi SJ, Chakraborty S, Srikumar BN, Raju TR, Shankaranarayana Rao BS. Prevention of chronic immobilization stress-induced enhanced expression of glucocorticoid receptors in the prefrontal cortex by inactivation of basolateral amygdala. J Chem Neuroanat. 2019a;95:134–45. CrossRef
12. Tripathi SJ, Chakraborty S, Srikumar BN, Raju TR, Shankaranarayana Rao BS. Inactivation of basolateral amygdala prevents stress-induced astroglial loss in the prefrontal cortex. Mol Neurobiol. 2019b;56(1):350–66. CrossRef
13. Tomar A, Polygalov D, Chattarji S, McHugh TJ.The dynamic impact of repeated stress on the hippocampal spatial map. Hippocampus. 2015;25(1):38–50. CrossRef
14. Tomar A, Polygalov D, McHugh TJ.Differential impact of acute and chronic stress on CA1 spatial coding and gamma oscillations. Front Behav Neurosci. 2021;15:710725. CrossRef
15. Kim H, Han KM, Choi KW, Tae WS, Kang W, Kang Y, et al. Volumetric alterations in subregions of the amygdala in adults with major depressive disorder. J Affect Disord. 2021;295:108–15. CrossRef
16. O’Leary LA, Mechawar N. Implication of cerebral astrocytes in major depression: a review of fine neuroanatomical evidence in humans. Glia. 2021;69(9):2077–99. CrossRef
17. Balla A, Dong B, Shilpa BM, Vemuri K, Makriyannis A, Pandey SC, et al. Cannabinoid-1 receptor neutral antagonist reduces binge-like alcohol consumption and alcohol-induced accumbal dopaminergic signaling. Neuropharmacology. 2018;131:200–8. CrossRef
18. Chakraborty S, Tripathi SJ, Raju TR, Shankaranarayana Rao BS. Mechanisms underlying remediation of depression-associated anxiety by chronic N-acetyl cysteine treatment. Psychopharmacology (Berl). 2020a;237(10):2967–81. CrossRef
19. Chakraborty S, Tripathi SJ, Raju TR, Shankaranarayana Rao BS. N-acetyl cysteine ameliorates depression-induced cognitive deficits by restoring the volumes of hippocampal subfields and associated neurochemical changes. Neurochem Int. 2020b;132:104605. CrossRef
20. Borroto-Escuela DO, Ambrogini P, Chruscicka B, Lindskog M, Crespo-Ramirez M, Hernández-Mondragón JC, et al. The role of central serotonin neurons and 5-HT heteroreceptor complexes in the pathophysiology of depression: a historical perspective and future prospects. Int J Mol Sci. 2021;22(4):1927. CrossRef
21. Smith GS, Workman CI, Protas H, Su Y, Savonenko A, Kuwabara H, et al. Positron emission tomography imaging of serotonin degeneration and beta-amyloid deposition in late-life depression evaluated with multi-modal partial least squares. Transl Psychiatry. 2021;11(1):473. CrossRef
22. Yatham LN, Liddle PF, Sossi V, Erez J, Vafai N, Lam RW, et al. Positron emission tomography study of the effects of tryptophan depletion on brain serotonin (2) receptors in subjects recently remitted from major depression. Arch Gen Psychiatry. 2012;69(6):601–9. CrossRef
23. Eggers B, Hermann W, Barthel H, Sabri O, Wagner A, Hesse S. The degree of depression in Hamilton rating scale is correlated with the density of presynaptic serotonin transporters in 23 patients with Wilson’s disease. J Neurol. 2003;250(5):576–80. CrossRef
24. Newberg AB, Amsterdam JD, Wintering N, Shults J. Low brain serotonin transporter binding in major depressive disorder. Psychiatry Res. 2012;202(2):161–7. CrossRef
25. Linthorst AC, Reul JM. Stress and the brain: solving the puzzle using microdialysis. Pharmacol Biochem Behav. 2008;90(2):163–73. CrossRef
26. McKittrick CR, Magariños AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36(2):85–94. CrossRef
27. Karten YJ, Nair SM, Van Essen L, Sibug R, Joels M. Long-term exposure to high corticosterone levels attenuates serotonin responses in rat hippocampal CA1 neurons. Proc Natl Acad Sci U S A. 1999;96(23):13456–61. CrossRef
28. Dionisie V, Ciobanu AM, Toma VA, Manea MC, Baldea I, Olteanu D, et al. Escitalopram targets oxidative stress, caspase-3, BDNF and MeCP2 in the hippocampus and frontal cortex of a rat model of depression induced by chronic unpredictable mild stress. Int J Mol Sci. 2021;22(14):7483. CrossRef
29. Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF-and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17(11):1130–42. CrossRef
30. Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169(11):1194–202. CrossRef
31. Zhao XP, Li H, Dai RP. Neuroimmune crosstalk through brain-derived neurotrophic factor and its precursor pro-BDNF: new insights into mood disorders. World J Psychiatry. 2022;12(3):379–92. CrossRef
32. Banerjee R, Ghosh AK, Ghosh B, Bhattacharyya S, Mondal AC. Decreased mRNA and protein expression of BDNF, NGF, and their receptors in the hippocampus from suicide: an analysis in human postmortem brain. Clin Med Insights Pathol. 2013;6:1–11. CrossRef
33. Castrén E, Monteggia LM. Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol Psychiatry. 2021;90(2):128–36. CrossRef
34. Gonenir Erbay L, Karlidag R, Oruc M, Cigremis Y, Celbis O. Association of BDNF/TrkB and NGF/TrkA levels in postmortem brain with major depression and suicide. Psychiatr Danub. 2021;33(4):491–8. CrossRef
35. Ghaffari Darab M, Hedayati A, Khorasani E, Bayati M, Keshavarz K. Selective serotonin reuptake inhibitors in major depression disorder treatment: an umbrella review on systematic reviews. Int J Psychiatry Clin Pract. 2020;24(4):357–70. CrossRef
36. Jiang H, Chen S, Li C, Lu N, Yue Y, Yin Y, et al.The serum protein levels of the tPA–BDNF pathway are implicated in depression and antidepressant treatment. Transl Psychiatry. 2017;7(4):e1079. CrossRef
37. Levada OA, Cherednichenko NV, Trailin AV, Troyan AS. Plasma brain-derived neurotrophic factor as a biomarker for the main types of mild neurocognitive disorders and treatment efficacy: a preliminary study. Dis Markers. 2016;2016:4095723. CrossRef
38. Furuse K, Ukai W, Hashimoto E, Hashiguchi H, Kigawa Y, Ishii T, et al. Antidepressant activities of Escitalopram and blonanserin on prenatal and adolescent combined stress-induced depression model: possible role of neurotrophic mechanism change in serum and nucleus accumbens. J Affect Disord. 2019;247:97. CrossRef
39. Seo MK, Lee CH, Cho HY, Lee JG, Lee BJ, Kim JE, et al. Effects of antidepressant drugs on synaptic protein levels and dendritic outgrowth in hippocampal neuronal cultures. Neuropharmacology. 2014;79:222–33. CrossRef
40. Joshi R, Salton SR. Neurotrophin crosstalk in the etiology and treatment of neuropsychiatric and neurodegenerative disease. Front Mol Neurosci. 2022;15:932497. CrossRef
41. Shi Y, Luan D, Song R, Zhang Z. Value of peripheral neurotrophin levels for the diagnosis of depression and response to treatment: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2020;41:40–51. CrossRef
42. Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104(11):4647–52. CrossRef
43. Iga JI, Ueno SI, Yamauchi K, Numata S, Tayoshi-Shibuya S, Kinouchi S, et al. Gene expression and association analysis of vascular endothelial growth factor in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):658–63. CrossRef
44. Rajkowska G, Legutko B, Moulana M, Syed M, Romero DG, Stockmeier CA, et al. Astrocyte pathology in the ventral prefrontal white matter in depression. J Psychiatr Res. 2018;102:150–8. CrossRef
45. Lovick TA. Estrous cycle and stress: influence of progesterone on the female brain. Braz J Med Biol Res. 2012;45(4):314–20. CrossRef
46. Bhagya V, Srikumar BN, Raju TR, Shankaranarayana Rao BS. Chronic escitalopram treatment restores spatial learning, monoamine levels, and hippocampal long-term potentiation in an animal model of depression. Psychopharmacology (Berl). 2011;214(2):477–94. CrossRef
47. Bhagya V, Srikumar BN, Raju TR, Shankaranarayana Rao BS. The selective noradrenergic reuptake inhibitor reboxetine restores spatial learning deficits, biochemical changes, and hippocampal synaptic plasticity in an animal model of depression. J Neurosci Res. 2015;93(1):104–20. CrossRef
48. Mahati K, Bhagya V, Christofer T, Sneha A, Shankaranarayana Rao BS. Enriched environment ameliorates depression-induced cognitive deficits and restores abnormal hippocampal synaptic plasticity. Neurobiol Learn Mem. 2016;134 Pt B:379–91. CrossRef
49. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam, The Netherlands: Elsevier Academic Press; 2005.
50. Bhagya V, Christofer T, Shankaranarayana Rao BS. Neuroprotective effect of Celastrus paniculatus on chronic stress-induced cognitive impairment. Indian J Pharmacol. 2016;48(6):687–93. CrossRef
51. Bhagya V, Srikumar BN, Veena J, Shankaranarayana Rao BS. Short-term exposure to enriched environment rescues chronic stress-induced impaired hippocampal synaptic plasticity, anxiety, and memory deficits. J Neurosci Res. 2017;95(8):1602–10. CrossRef
52. Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20(18):7116–21. CrossRef
53. Srikumar BN, Raju TR, Shankaranarayana Rao BS. The involvement of cholinergic and noradrenergic systems in behavioral recovery following oxotremorine treatment to chronically stressed rats. Neuroscience. 2006;143(3):679–88. CrossRef
54. Kesner RP, DiMattia BV, Crutcher KA. Evidence for neocortical involvement in reference memory. Behav Neural Biol. 1987;47(1):40–53. CrossRef
55. Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993;60(1):9–26. CrossRef
56. Durkin TP. Spatial working memory over long retention intervals: dependence on sustained cholinergic activation in the septohippocampal or nucleus basalis magnocellularis-cortical pathways? Neuroscience. 1994;62(3):681–93. CrossRef
57. Bhagya V, Sindhu VK, Mahati K, Shankaranarayana Rao BS. Exposure to an enriched environment promotes dendritic remodelling in hippocampal neurons affected by endogenous depression. Indian J Biochem Biophy. 2022;59(10):998–1005. CrossRef
58. Ramkumar K, Srikumar BN, Shankaranarayana Rao BS, Raju TR. Self-stimulation rewarding experience restores stress-induced CA3 dendritic atrophy, spatial memory deficits and alterations in the levels of neurotransmitters in the hippocampus. Neurochem Res. 2008;33(9):1651–62. CrossRef
59. Veena J, Srikumar BN, Mahati K, Bhagya V, Raju TR, Shankaranarayana Rao BS. Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J Neurosci Res. 2009a;87(4):831–43. CrossRef
60. Veena J, Srikumar BN, Raju TR, Shankaranarayana Rao BS. Exposure to enriched environment restores the survival and differentiation of new born cells in the hippocampus and ameliorates depressive symptoms in chronically stressed rats. Neurosci Lett. 2009b;455(3):178–82. CrossRef
61. Mayegowda SB, Thomas C. Glial pathology in neuropsychiatric disorders: a brief review. J Basic Clin Physiol Pharmacol. 2019;30(4):1–7. CrossRef
62. Burstein O, Franko M, Gale E, Handelsman A, Barak S, Motsan S, et al. Escitalopram and NHT normalized stress-induced anhedonia and molecular neuroadaptations in a mouse model of depression. PLoS One. 2017;12(11):e0188043. CrossRef
63. An H, Choi B, Park KW, Kim DH, Yang DW, Hong CH, et al. The effect of Escitalopram on mood and cognition in depressive Alzheimer’s disease subjects. Alzheimers Dis. 2017;55(2):727–35. CrossRef
64. Lavretsky H, Laird KT, Krause-Sorio B, Heimberg BF, Yeargin J, Grzenda A, et al. A randomized double-blind placebo-controlled trial of combined Escitalopram and memantine for older adults with major depression and subjective memory complaints. Am J Geriatr Psychiatry. 2020;28(2):178–90. CrossRef
65. Reed MB, Vanicek T, Seiger R, Klöbl M, Spurny B, Handschuh P, et al. Neuroplastic effects of a selective serotonin reuptake inhibitor in relearning and retrieval. Neuroimage. 2021;236:118039. CrossRef
66. Muraoka H, Oshibuchi H, Kawano M, Kawano T, Tsutsumi T, Yamada M, et al. Escitalopram attenuates fear stress-induced increase in amygdalar dopamine following methamphetamine-induced sensitisation: implications of fine-tuning action of selective serotonin reuptake inhibitors on emotional processing. Eur J Pharmacol. 2018;834:1–9. CrossRef
67. Wang Q, Dong X, Wang Y, Liu M, Sun A, Li N, et al. Adolescent Escitalopram prevents the effects of maternal separation on depression- and anxiety-like behaviours and regulates the levels of inflammatory cytokines in adult male mice. Int J Dev Neurosci. 2017;62:37–45. CrossRef
68. Fish EW, Faccidomo S, Gupta S, Miczek KA. Anxiolytic-like effects of Escitalopram, citalopram, and R-citalopram in maternally separated mouse pups. J Pharmacol Exp Ther. 2004;308(2):474–80. CrossRef
69. Godlewska BR, Norbury R, Selvaraj S, Cowen PJ, Harmer CJ. Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychol Med. 2012;42(12):2609–17. CrossRef
70. Carboni L, Becchi S, Piubelli C, Mallei A, Giambelli R, Razzoli M, et al. Early-life stress and antidepressants modulate peripheral biomarkers in a gene-environment rat model of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):1037–48. CrossRef
71. Lee CH, Park JH, Yoo KY, Choi JH, Hwang IK, Ryu PD, et al.Pre-and post-treatments with Escitalopram protect against experimental ischemic neuronal damage via regulation of BDNF expression and oxidative stress. Exp Neurol. 2011;229(2):450–9. CrossRef
72. Flandreau EI, Bourke CH, Ressler KJ, Vale WW, Nemeroff CB, Owens MJ. Escitalopram alters gene expression and HPA axis reactivity in rats following chronic over expression of corticotropin-releasing factor from the central amygdala. Psychoneuroendocrinology. 2013;38(8):1349–61. CrossRef
73. Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, et al. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48(8):861–73. CrossRef
74. Alboni S, Benatti C, Capone G, Corsini D, Caggia F, Tascedda F, et al. Time-dependent effects of Escitalopram on brain derived neurotrophic factor (BDNF) and neuroplasticity related targets in the central nervous system of rats. Eur J Pharmacol. 2010;643(2–3):180–7. CrossRef
75. Doron R, Lotan D, Versano Z, Benatav L, Franko M, Armoza S, et al. Escitalopram or novel herbal mixture treatments during or following exposure to stress reduce anxiety-like behavior through corticosterone and BDNF modifications. PLoS One. 2014;9(4):e91455. CrossRef
76. Schulte-Herbrüggen O, Fuchs E, Abumaria N, Ziegler A, Danker-Hopfe H, Hiemke C, et al. Effects of Escitalopram on the regulation of brain-derived neurotrophic factor and nerve growth factor protein levels in a rat model of chronic stress. J Neurosci Res. 2009;87(11):2551–60. CrossRef
77. Musazzi L, Mallei A, Tardito D, Gruber SH, El Khoury A, Racagni G, et al. Early-life stress and antidepressant treatment involve synaptic signaling and Erk kinases in a gene-environment model of depression. J Psychiatr Res. 2010;44(8):511–20. CrossRef
78. Bonanno G, Giambelli R, Raiteri L, Tiraboschi E, Zappettini S, Musazzi L, et al. Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J Neurosci. 2005;25(13):3270–9. CrossRef
79. Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011;13:22–37. CrossRef
80. Yoshida K, Drew MR, Kono A, Mimura M, Takata N, Tanaka KF. Chronic social defeat stress impairs goal-directed behavior through dysregulation of ventral hippocampal activity in male mice. Neuropsychopharmacology. 2021;46(9):1606–16. CrossRef
81. Wu C, Gong WG, Wang YJ, Sun JJ, Zhou H, Zhang ZJ, et al. Escitalopram alleviates stress-induced Alzheimer’s disease-like tau pathologies and cognitive deficits by reducing hypothalamic-pituitary-adrenal axis reactivity and insulin/GSK-3β signal pathway activity. Neurobiol Aging. 2018;67:137–47. CrossRef
82. Seo MK, Choi CM, McIntyre RS, Cho HY, Lee CH, Mansur RB, et al. Effects of Escitalopram and paroxetine on mTORC1 signaling in the rat hippocampus under chronic restraint stress. BMC Neurosci. 2017;18:1–10. CrossRef
83. do Couto FS, Batalha VL, Valadas JS, Data-Franca J, Ribeiro JA, Lopes LV. Escitalopram improves memory deficits induced by maternal separation in the rat. Eur J Pharmacol. 2012;695(1–3):71–5. CrossRef