INTRODUCTION
The clinical manifestation (signs and symptoms) and progression of atherosclerosis and coronary heart disease are closely and significantly influenced by hyperlipidemia [1]. In 2019, about 18 million people died from cardiovascular disease (CVD) globally [2]. The major concern related to hyperlipidemia besides that it is not discovered until it reached threatening levels that it is an asymptomatic disorder of lipid metabolism with elevation in low-density lipoprotein cholesterol (LDL-C), serum total blood cholesterol [total cholesterol (TC)] and very LDL-C levels.
Hypercholesterolemia and hypertriglyceridemia (hyperlipidemic-associated lipid disorders) are considered to cause atherosclerotic CVD via implying abnormally increased levels of all lipids in the bloodstream [3]. Atherosclerosis is defined as the building up of waxy plaque on the inner sides of the blood vessels [4,5]. Atherosclerosis-associated diseases such as peripheral vascular, coronary, and cerebrovascular diseases are augmented in patients with hyperlipidemia [6].
Triton WR-1339 (a nonionic surfactant) so far, is commonly utilized to produce “acute” hyperlipidiemic models in animals (in-vivo) to study synthetic and natural compounds [7]. Triton WR-1339 can cause 48-hour elevations in the levels of plasma lipids by blocking the absorption of lipoprotein from the circulatory system by extrahepatic tissues, leading to a boost in the peaks of circulatory lipoproteins [8,9].
Despite that, there are a lot of different classes that act as antihyperlipidemic agents already exist. The most used class as an antihyperlipidemic globally are fibrates and their derivatives including bezafibrate. They act via the reduction of serum lipids, including a significant lowering in triglyceride (TG) levels with a slight decrease in TC, LDL-C, and elevation in the levels of high-density lipoprotein cholesterol (HDL-C) [10,11]. Fibrates act by lowering the production of apoC-III and enhancing the action of lipoprotein lipase. While increased risk of atherosclerosis can be traced by triggering these markers [12,13].
Recently developing novel pharmacologically active lipid-lowering drugs have gained much interest in order to overcome the side effects and current medications and enhance the outcomes. Many studies on synthetic agents with pyrrole nucleus revealed encouraging pharmacological effects as anti-inflammatory [14,15], antitumor [16], antiviral [17], and antioxidative [18]. In addition, regarding compounds containing pyrrole (ring) many researchers, which include our previous work, pointed out that these compounds compared to the others showed a very promising antihyperlipidemic effect [19–21].
Therefore, finding new possible agents that allow us to treat hyperlipidemia is highly important to lessen the threats of developing heart and blood vessel disease, the current research using a novel series of N-(4-benzoylphenyl) pyrrole-2-carboxamides aims to assess their ability to lower the lipid profiles (lipid-lowering-effect) in hyperlipidemic rats model using Triton WR-1339.
EXPERIMENTAL
Materials and methods
All chemical agents were used without any further purifications and bought from (Sigma-Aldrich; St. Louis, MO, and Acros; Belgium). Melting point determination was done utilizing Stuart Scientific Electrothermal (melting point apparatus). Shimadzu IR Affinity-1 spectrophotometer was used to record infrared (IR) spectras. All samples were prepared as potassium bromide (Acros, Belgium) discs. Bruker DRX 400 MHz-NMR spectrophotometer operating at 400.13 (1H) and 100.61 MHz (C-13) using tetramethylsilane as an internal reference.
Synthesis of the targeted compounds
N-(4-Benzoylphenyl)-4-bromo-1H-pyrrole-2 carboxamide (3)
Ethyl-4-bromopyrrole-2-carboxylate (1, 0.5 g, 2.29 mmol) was dissolved in 20 ml dimethylformamide (DMF), followed by adding of 4-aminobenzophenone (2, 1.35 g, 6.88 mmol). The mixture was mixed and refluxed for 5 days at 150°C. Removal of DMF was done via column chromatography using CHCl3:CH3OH (99:1) as eluent for purification under reduced pressure during the evaporation. Then the product was washed by diethyl ether to give the pure compound (3; Fig. 1). Brown pale powder (0.151 g, 18%): Rf = 0.86 (chloroform, methanol, 99:1); mp = 85°C–87°C; 1H-NMR (300 Hz, d6-MeOD): δ = 8.90 (d, J = 12 Hz, 1H, Ar-H), 8.72 (d, J = 8 Hz, 1H, Ar-H), 8.43 (s, 1H, pyrrole-H), 7.86 (s, 1H, pyrrole-H), 7.83 (m, 1H, Ar-H), 7.78 (d, J = 8.0 Hz, 2H, Ar-H), 7.71 (d, J = 8.0 Hz 1H, Ar-H) 7.60 (t, J = 8.0 Hz, 1H, Ar-H), 7.48 (d, J = 8.0 Hz, 1H, Ar-H), 7.21 (d, J = 8.0 Hz, 1H, Ar-H) ppm; 13C-NMR (d6-CDCl3): δ = 196.26 (1C), 162.42 (1C), 159.77 (1C), 141.36 (1C), 138.6 (1C), 133.86 (1C), 132.96 (1C), 132.85 (1C), 132.70 (1C), 132.06 (1C), 130.31 (2C), 130.26 (1C), 128.83 (2C), 128.77 (1C), 119.55 (1C), 117.57 (1C) ppm; IR (KBr disc): ν = 3,116.97 (NH-amide), 1,735.93 (CO-ketone), 1,643.35 (CO-amide) cm−1.
4-Amino-N-(4-benzoylphenyl)-1-methyl-1H-pyrrole-2-carboxamide (5)
Methyl-4-amino-1-methyl-1H-pyrrole-2-carboxylate hydrochloride (4a, 1.0 g, 5.25 mmol) was dissolved in 10 ml DMF, in an ice bath of 1.1 ml of triethylamine (TEA) was added to the mixture to give methyl-4-amino-1-methyl-1H-pyrrole-2-carboxylate (4b). 4-Aminobenzophenone (2, 3.1 g, 0.0157 mol) was added after 10 minutes while stirring the reaction mixture and refluxed for 5 days at 150°C. Removal of DMF was done via column chromatography using CHCl3:CH3OH: ethyl acetate (98:1:1) as eluent for purification (remaining’s) under reduced pressure during the evaporation. Followed up by washing the product by diethyl ether to give the pure compound (5; Fig. 2). Pale brown powder (0.389 g, 25.2%); Rf = 0.45 (chloroform, methanol, 99:1); mp = 80°C–83°C; 1H-NMR (300 Hz, d6-CDCl3): δ = 8.88 (d, J = 12.0 Hz, 1H, Ar-H), 8.67 (s, 1H, Ar-H), 8.42 (s, 1H, NH-amide), 8.18 (s, 1H, H-pyrrole), 7.82 (m, 2H, Ar-H), 7.77 (d, J = 8.0 Hz, 2H, Ar-H), 7.58 (s, 1H, Ar-H), 7.48 (d, J = 8.0 Hz, 2H, Ar-H), 7.20 (d, J = 8.0 Hz, 1H, Ar-H), 3.48 (s, 2.0, 2H, NH2), 1.90 (s, 3H, CH3) ppm; 13C-NMR (d6-DMSO): δ = 196.08 (1C), 162.20 (1C), 159.62 (1C), 141.25 (1C), 137.99 (1C), 133.77 (1C), 133.18 (1C), 132.82 (1C), 132.70 (1C), 131.93 (2C), 130.18 (2C), 128.64 (2C), 119.44 (2C), 117.47 (1C), 15.56 (1C) ppm; IR (KBr disc): ν = 3,101.54 (NH-amide), 1,697.36 (CO-ketone), 1,651.07 (CO-amide) cm−1.
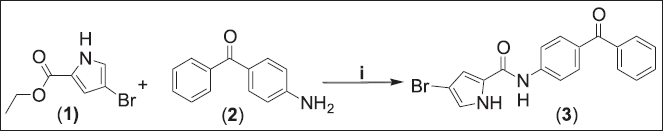 | Figure 1. Synthesis of N-(4-Benzoylphenyl)-4-bromo-1H-pyrrole-2 carboxamide (3). (i) DMF, 150°C, 5 days. [Click here to view] |
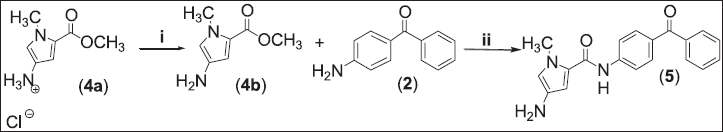 | Figure 2. Synthesis of 4-Amino-N-(4-benzoylphenyl)-1-methyl-1H-pyrrole-2-carboxamide (5). (i) 1) DMF, 0°C, TEA and (ii) DMF, 150°C, 5 days. [Click here to view] |
 | Figure 3. General mechanism of amide formation. [Click here to view] |
Animals experiment
The experiment was conducted on 2 months old male Wister rats, weighing 180–200 g, the study was done in agreement with the general guide for the care and use of laboratory animals in the Animal Care Center of the Faculty of Pharmacy, Al-Zaytoonah University of Jordan, Amman following Federation of European Laboratory Animal Science Associations guidelines [22]. Rats were provided during the experimental duration (24 hours) with ad libitum access only to tap water. Rats were kept under a controlled 12-hour light/dark cycle under controlled humidity and (22°C ± 2°C). All testing was approved by the local Ethical Committee of Animal Welfare with ref no. 21/111/2021-2022; date:10/08/2022
Triton model of hyperlipidemia
Induction of acute hyperlipidemic animals was done via injecting Triton WR-1339 (Sigma-Aldrich, St. Louis, MO) 300 mg/kg body weight intraperitoneally (IP) to the rats [23].
Pharmacological experimental design
Forty overnight fasted rats were divided randomly into five groups each containing eight rats.
The first group got an IP administration of (normal) saline only and served as the control group (NCG); the second group received an IP injection of (Triton WR-1339) dissolved in distilled water representing the hyperlipidemic control group (HCG). Third and fourth group rats were IP injected with Triton, followed by an intragastric administration of (15 mg/kg body weight) of compounds 3 and 5, respectively. The last group received intragastric injection of bezafibrate (100 mg/kg body weight) [24].
Blood samples were collected after 18 hours of treatment followed by immediate centrifugation at (3,000 rpm for 10 minutes). Enzymatic methods along with an automated analyzer (Model Erba XL-300, Germany, Mannheim) were used for the lipid analysis of the obtained serums.
Statistical analysis
The obtained data showed mean values ± standard error of the mean. The results were evaluated using Student’s t-test, and a statistical probability of p < 0.05 was considered to be significant
RESULTS
Chemistry
Novel N-(benzoylphenyl)-pyrrole-2-carboxamide derivatives (3 and 5) were synthesized using substituted pyrrole ester and amino benzophenones as shown in Figures 1 and 2. Ethyl-substituted pyrrole-2-carboxylate (1 and 4b) was dissolved in DMF, followed by adding of 4-aminobenzophenone (2, 3M excess). The mixture was mixed and refluxed for 5 days at 150°C. A nucleophilic attack takes place by the free electrons of the N atom of the amine against the carbon of the carbonyl moiety of the ester derivative. Rearrangement of intermediate compound results in clicking out of an alcohol compound and amide bond formation as shown in Figure 3.
Amide formation was attempted under different conditions including free pyrrole acid with amino benzophenone, dicyclohexylcarbodiimide, and other amide coupling agents. Unfortunately, most of these conditions gave either lower yields or many side products that needed tedious chromatographic separation. Harsh conditions and heating at higher temperatures were major limitations since they led to hydrolysis of the starting materials and the formed products 3 and 5.
Before establishing the reaction condition for compounds 5, a neutralization procedure is needed as shown in Figure 2. The presence of HCl weakens the progress of the reaction. And it might affect the nucleophilicity of aminobenzophenone. TMA as the weaker base was used for the liberation of the free amine since these conditions do not hydrolyze the pyrrole ester.
It’s worth noting that due to the weakness of the aromatic amines; 4-amino-aminobenzophenone as nucleophiles, and the presence of the amine group on the para position of the carbonyl moiety of benzophenone decreases the nucleophilicity of the amine moiety. And, thus in turn affects the progress of the reaction for almost 5 days upon completion.
Pharmacology
Induction of hyperlipidemia by Triton WR-1339
As we mentioned previously (Triton WR-1339) is used to induce acute hyperlipidemia in rats via inhibiting lipoprotein lipase enzyme which leads to blocking the clearance of TG-rich lipoproteins (TLRs). Triton WR-1339 was used in many studies to evaluate the efficacy of several antihyperlipidemic agents. It has been published before that one shot of (Triton WR-1339) IP at a dose of 300 mg/kg to adult rats is enough to produce acute hyperlipidemia and the (peak) plasma TG, LDL-C, and TC levels were reached at 18 hours [25].
Plasma levels of TC, TG, LDL-C, and HDL-C of the NCG and the HCG treated for 18 hours are shown in Table 1.
One shot of IP injection of Triton WR-1339 leads to a significant elevation in plasma TC, TG, and LDL-C (p < 0.0001) levels and a significant reduction in HDL-C (p < 0.0001) in HCG 18 hours after Triton injection compared to the NCG. The plasma TC levels in the HCG were markedly increased by 458%, after 18 hours as compared to the NCG. The increase of the TG levels in the HCG was 3911% in comparison with the NCG, which is about 40 times more than the TG levels in the NCG after 18 hours. LDL-C level in HCG was also raised by 612% after 18 hours as compared to the NCG at the same time, on the other hand, the HDL-C level was significantly reduced by 29% 18 hours after Triton WR-1339 injection.
Effect of compounds 3, 5, and bezafibrate on plasma lipid profile
Figures 4–7 show the effect of novel pyrrole carboxamide derivatives 3, 5, and BF on plasma TC, TG, HDL-C, and LDL-C levels in treated rats after 18 hours. Interestingly, the higher plasma TG levels produced by the single injection of Triton WR-1339 were remarkably (p < 0.001) decreased by 95% and 80% in compounds 3 and BF respectively, and by 23% in compound 5 (p < 0.001) after 18 hours, with respect to HCG treated with Triton (Fig. 4).
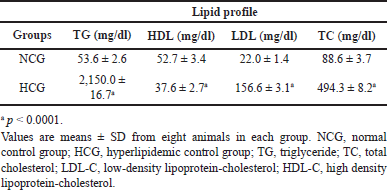 | Table 1. Effect of Triton WR-1339 on plasma lipid levels after 18 hours. [Click here to view] |
Moreover, in compound 3 TC levels were significantly (p < 0.001) decreased by 75% and in compound 5 by 26% (p < 0.01) after 18 hours compared to HCG. Meanwhile, no significant changes were observed in TC levels after 18 hours in comparison to HCG in BF treated group (Fig. 5). After 18 hours of treatment, LDL-cholesterol levels dropped by (77%, p < 0.001) in compound 3 and (22%, p < 0.05) in compound 5. No noticeable changes in LDL-C levels were detected in bezafibrate (Fig. 6).
The HDL-C levels were noticeably improved after 18 hours by 22%, and 51% (p < 0.001) in compound 3 and BF respectively, and by 4.5% in compound 5 (p < 0.05) after 18 hours, compared to Triton treated HCG (Fig. 7).
DISCUSSION
The current study outcomes showed promising antihyperlipidemic impacts of novel N-(4-Benzoylphenyl)-4-bromo-1H-pyrrole-2 carboxamide (3) and 4-Amino-N-(4-benzoylphenyl)-1-methyl-1H-pyrrole-2-carboxamide (5). In hyperlipidemic rats using Triton WR-1339 an inducer that is widely used as a model for testing agents with antihyperlipidemic properties.
In fact, compounds 3 and 5 significantly decreased TG, TC, and LDL-cholesterol levels. In addition, compounds 3 and 5 elevated serum HDL-C, and decreased the risk of atherosclerosis and cardiovascular disorders, after 18 hours of Triton administration.
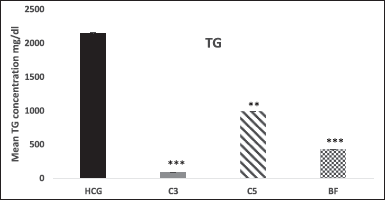 | Figure 4. Effect of novel compounds on TGs (mg/dl) after 18 hours. Values are means ± SD from eight animals in each group. HCG: hyperlipidemic control group; C3: Compound 3; C5: Compound 5; BF: bezafibrate; TG, triglyceride; C3, C5 and BF are compared with HCG. * p < 0.01; ** p < 0.01; *** p < 0.001. [Click here to view] |
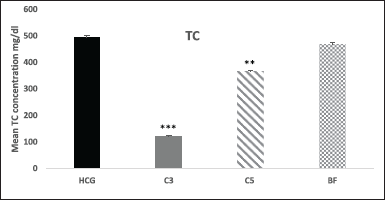 | Figure 5. Effect of novel compounds on TC (mg/dl) after 18 hours. Values are means ± SD from eight animals in each group. HCG: hyperlipidemic control group; C3: Compound 3; C5: Compound 5; BF: bezafibrate; TG, triglyceride; C3, C5 and BF are compared with HCG. * p < 0.05; ** p < 0.01; *** p < 0.001. [Click here to view] |
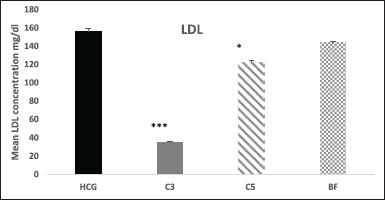 | Figure 6. Effect of novel compounds on LDL-C (mg/dl) after 18 hours. Values are means ± SD from eight animals in each group. HCG: hyperlipidemic control group; C3: Compound 3; C5: Compound 5; BF: bezafibrate; TG, triglyceride; C3, C5 and BF are compared with HCG. * p < 0.05; ** p < 0.01; *** p < 0.001. [Click here to view] |
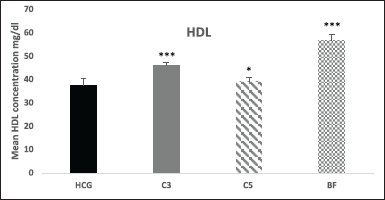 | Figure 7. Effect of novel compounds on HDL-C (mg/dl) after 18 hours. Values are means ± SD from eight animals in each group. HCG: hyperlipidemic control group; C3: Compound 3; C5: Compound 5; BF: bezafibrate; TG, triglyceride; C3, C5 and BF are compared with HCG. * p < 0.05; ** p < 0.01; *** p < 0.001. [Click here to view] |
The rise in plasma TG, TC, and LDL-cholesterol levels seen after a single injection of Triton is mainly due to Apoc3, Hmgcs1, Apob, and Apoa1 gene expression upregulation [26,27]. Moreover, the large reduction in plasma HDL levels in Triton treated rats results mostly from the progressive movement of apolipoprotein A-1 from the HDL surface without loss of lipids [28].
Therefore, taking into consideration that ApoC3 is strongly linked to hypertriglyceridemia with an atherogenic effect and leads to hypertriglyceridemia by blocking the catabolism and the removal of TLRs [29,30], that’s why it is not amazement that the hypolipidemic activity of compounds 3 and 5 was significantly higher for TGs than for cholesterol. These observations along with our previously published data on similar hypolipidemic agents suggest that our novel compounds 3 and 5 contribute by downregulating Apoc3 gene expression in acute hyperlipidemic rat model leading to decreased TG levels [31].
In comparison to bezafibrate at a dose of 100 mg/kg body weight, which in the current study used as a standard reference lipid-lowering drug, compounds 3 and 5 at a dose of 15 mg/kg body weight 18 hours after Triton injection showed the same potential in decreasing TG levels and in elevating HDL-C levels.
The lipid-lowering activity of compounds 3 and 5 adds to the importance of the existence of the three structural components (heterocyclic aromatic ring able of hydrogen bond formation, large lipophilic moiety, and carboxamide linkage) for the anti-hyperlipidemic effects. These findings are compatible with our previously published data [32–35].
CONCLUSION
In the current study, novel N-(4-benzoylphenyl) pyrrole-2-carboxamide derivatives were successfully synthesized and distinguished using IR and NMR. The lipid-lowering action was evaluated using hyperlipidemic rats induced by Triton WR-1339. N-(4-benzoylphenyl) pyrrole-2-carboxamide derivatives; compounds 3 and 5 were shown great potential in enhancing many lipid abnormalities such as hypercholesterolemia and hypertriglyceridemia, and then elevated HDL levels in Triton induced rats. These results are compatible with our previous published data, which approves that agents containing pyrrole-2-carboxamide nucleus do have a lipid-lowering effect [20].
In sum, our obtained result is highly encouraging with great potential, but more studies are required to explain the exact mechanisms of action and how the profile of these novel agents is secured and safe as (lipid-lowering) compounds.
ACKNOWLEDGMENTS
The authors wish to express their honest gratitude to Al-Zaytoonah Private University of Jordan for their technical support.
AUTHOR CONTRIBUTIONS
Al-Qirim, Al-Hiari, and Shattat created and planned the experiments. Shattat took the main role in writing the manuscript. All authors performed the experiments contributed to the explanation of the results and provided important feedback and helped shape the manuscript.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the institutional animal ethical committee of AL-Zaytoonah university of Jordan. Ref no. 21/111/2021-2022 Date:10/08/2022
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Sampathkumar MT, Kasetti RB, Nabi SA, Sudarshan PR, Swapna S, Apparao C. Antihyperlipidemic and antiatherogenic activities of Terminaliapallida Linn. fruits in high fat diet-induced hyperlipidemic rats. J Pharm Bioallied Sci. 2011;3(3):449–52. CrossRef
2. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. CrossRef
3. Mishra PR, Panda PK, Apanna KC, Panigrahi S. Evaluation of acute hypolipidemic activity of different plant extracts in Triton WR-1339 induced hyperlipidemia in albino rats. Pharmacologyonline. 2011;3:925–34. Available from: https://pharmacologyonline.silae.it/files/archives/2011/vol3/098.mishra.pdf
4. Al Gadban M, German J, Truman JP, Soodavar F, Riemer E, Twal W, et al. Lack of nitric oxide synthases increases lipoprotein immune complex deposition in the aorta and elevates plasma sphingolipid levels in lupus. Cell Immunol. 2012;276:42–51. CrossRef
5. Cassar A, Holmes DR, Rihal CS, Gersh BJ. Chronic coronary artery disease: diagnosis and management. Mayo Clin Proc. 2009;84(12):1130–46. CrossRef
6. Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. 2014;5(8):927–46. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4258672/
7. Schurr PE, Schultz JR, Parkinson TM. Triton-induced hyperlipidemia in rats as an animal model for screening hypolipidemic drugs. Lipids. 1972;7:69–74. CrossRef
8. da Rocha JT, Speranca A, Nogueira CW, Zeni G. Hypolipidaemic activity of orally administered diphenyl diselenide in Triton WR-1339-induced hyperlipidaemia in mice. J Pharm Pharmacol. 2009;61:1673–9. CrossRef
9. Kellner A, Correll JW, Ladd AT. Sustained hyperlipemia induced in rabbits by means of intravenously injected surface-active agents. J Exp Med. 1951;93(4):373–84. CrossRef
10. Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, et al. Helsinki heart study: primary-prevention trial with gemfibrozil in middle aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317(20):1237–45. CrossRef
11. Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341(6):410–18. CrossRef
12. Koh KK, Quon MJ, Rosenson RS, Chung WJ, Han SH. Vascular and metabolic effects of treatment of combined hyperlipidemia: focus on statins and fibrates. Int J Cardiol. 2008;124(2):149–59. CrossRef
13. Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res. 1996;37(5):907–25. CrossRef
14. Lessigiarska I, Nankov A, Bocheva A, Pajeva I, Bijev A. 3D-QSAR and preliminary evaluation of anti-inflammatory activity of series of N-pyrrolylcarboxylic acids. Farmaco. 2005;60(3):209–18. CrossRef
15. Ushiyama S, Yamada T, Murakami Y, Kumakura S, Inoue S, Suzuki K, et al. Preclinical pharmacology profile of CS-706, a novel cyclooxygenase-2 selective inhibitor, with potent antinociceptive and anti-inflammatory effects. Eur J Pharmacol. 2008;578(1):76–86. CrossRef
16. Fensome A, Bender R, Chopra R, Cohen J, Collins MA, Hudak V, et al. Synthesis and structure-activity relationship of novel 6- aryl-1,4-dihydrobenzo[d][1,3]oxazine-2-thiones as progesterone receptor modulators leading to the potent and selective nonsteroidal progesterone receptor agonist tanaproget. J Med Chem. 2005;48(16):5092–5. CrossRef
17. Starcevic K, Kralj M, Ester K, Sabol I, Grce M, Pavelic K, et al. Synthesis, antiviral and antitumor activity of 2-substituted-5-amidino-benzimidazoles. Bioorganic Med Chem Lett. 2007;15(13): 4419–26. CrossRef
18. Lehuede J, Fauconneau B, Barrier L, Ourakow M, Piriou A, Vierfond J. Synthesis and antioxidant activity of new tetraarylpyrroles. Eur J Med Chem. 1999;34(11):991–6. CrossRef
19. Kale M, Patwardhan K. Synthesis of heterocyclic scaffolds with anti-hyperlipidemic potential: a review. Der Pharma Chem. 2013;5(5):213–22. Available from: https://www.derpharmachemica.com/pharma-chemica/synthesis-of-heterocyclic-scaffolds-with-antihyperlipidemic-potential-a-review.pdf
20. Shattat G, Sheikha GA, Al-Qirim T, Huwaitat R, El-Huneidi W, Abu Khalaf R, et al. Novel pyrrole derivatives as potent lipid-lowering agents in Triton-WR-1339-induced hyperlipidemic rats. Lat Am J Pharm. 2015;34(6):1258–64. Available from: http://www.latamjpharm.org/resumenes/34/6/LAJOP_34_6_1_31.pdf
21. Tsunemi A, Ueno T, Fukuda N, Watanabe T, Tahira K, Haketa A, et al. A novel gene regulator, pyrroleimidazole polyamide targeting ABCA1 gene increases cholesterol efflux from macrophages and plasma HDL concentration. J Mol Med. 2014;92(5):509–21. CrossRef
22. Guillen J. FELASA guidelines and recommendations. J Am Assoc Lab Anim Sci. 2012;51(3):311–21. Available from: https://pubmed.ncbi.nlm.nih.gov/22776188/
23. Shattat G, Al-Qirim T, Sweidan K, Shahwan M, El-Huneidi W, Al-Hiari Y. The hypolipidemic activity of novel benzofuran-2-carboxamide derivatives in Triton WR-1339-induced hyperlipidemic rats: a comparison with bezafibrate. J Enzyme Inhib Med Chem. 2010;25(6):751–5. CrossRef
24. Mori Y, Tokutate Y, Oana F, Matsuzawa A, Akahane S, Tajima N. Bezafibrate-induced changes over time in the expression of uncoupling protein (UCP) mRNA in the tissues: a study in spontaneously type 2 diabetic rats with visceral obesity. J Atheroscler Thromb. 2004;11(4):224–31. CrossRef
25. Harnafi H, Bouanani N, Aziz M, Caid H, Ghalim N, Amrani S. The hypolipidaemic activity of aqueous Erica multiflora flowers extract in Triton WR-1339 induced hyperlipidaemic rats: a comparison with fenofibrate. J Ethnopharmacol. 2007;109(1):156–60. CrossRef
26. Sahebkar A, Watts GF. New therapies targeting apoB metabolism for high-risk patients with inherited dyslipidaemias: what can the clinician expect? Cardiovasc Drugs Ther. 2013;27(6):559–67. CrossRef
27. Yang Z, Cappello T, Wang L. Emerging role of micro-RNAs in lipid metabolism. Acta Pharm Sin B. 2015;5(2):145–50. CrossRef
28. Ishikawa T, Fidge N. Changes in the concentration of plasma lipoproteins and apoproteins following the administration of Triton WR 1339 to rats. J Lipid Res. 1979;20:254–64. CrossRef
29. Kawakami A, Yoshida M. Apolipoprotein CIII links dyslipidemia with atherosclerosis. J Atheroscler Thromb. 2009;16(1):6–11. CrossRef
30. Zheng C. Updates on apolipoprotein CIII: fulfilling promise as a therapeutic target for hypertriglyceridemia and cardiovascular disease. Curr Opin Lipidol. 2014;25(1):35–9. CrossRef
31. Hamadneh L, Al-Essa L, Hikmat S, Al-Qirim T, Abu Sheikha G, Al-Hiari Y, et al. N-(3-Benzoylphenyl)-1H-indole-2-carboxamide decreases triglyceride levels by downregulation of Apoc3 gene expression in acute hyperlipidemic rat model. Mol Cell Biochem. 2017;431:133–8. CrossRef
32. Al-Qirim T, Shahwan M, Shattat G, Al-Hiari Y, Abu Sheikha G, Zaidi S. Pharmacological evaluation of novel indole-2-carboxamides as potent lipid-lowering agents in Triton-WR-1339-induced hyperlipidemic rats. Z Naturforsch C. 2009;64:619–25. CrossRef
33. Al-Qirim T, Shattat G, Sweidan K, El-Huneidi W, Sheikha GA, Khalaf RA, et al. In vivo antihyperlipidemic activity of a new series of N (Benzoylphenyl) and N-(Acetylphenyl)- 1-benzofuran-2-carboxamides in rats. Arch Pharm. 2012;345(5):401–6. CrossRef
34. Shahwan M, Shattat G, Al-Qirim T, Sheikha GA, Al-Hiari Y, El-Huneidi W, et al. Synthesis and pharmacological evaluation of novel substituted and unsubstituted N-(benzoylphenyl)-1H-indole-2-carboxamides as potent antihypertriglyceridemic agents. Z Naturforsch C. 2010;65:309–16. CrossRef
35. Shattat G, Al-Qirim T, Sheikha GA, Al-Hiari Y, Sweidan K, Al-Qirim R, et al. The pharmacological effects of novel 5-fluoro-N-(9,10-dihydro- 9,10-dioxoanthracen-8-yl)-1H-indole-2-carboxamide derivatives on plasma lipid profile of Triton-WR-1339-induced Wistar rats. J Enzyme Inhib Med Chem. 2013;28(4):863–69. CrossRef