INTRODUCTION
Cancer is among the main global causes of death [1]. Cancer is a malignant tumor caused by abnormal cells in body tissues that grow and develop rapidly and uncontrollably. Breast cancer is one of the most prevalent malignancies in women; an estimated 2.3 million new cases are reported annually worldwide [2]. Several signaling mechanisms, such as the estrogen receptor (ER), Human epidermal growth factor receptor-2 (HER2), and Wnt/β-catenin signaling pathways, which affect stem cell proliferation, cell death, cell differentiation, and cell motility, regulate the normal development of breast and mammary stem cells [3,4]. (HER2 protein receptor is a type of receptor often used as a target for breast cancer. HER2 can provide the invasion of cancer cells due to overexpression. This expression can induce migration and metastasis in cancer cells by directly inducing dimerization, autophosphorylation, and focal adhesion kinase (FAK) activation [5,2]. Administering specialist drugs such as lapatinib has direct and indirect side effects. In addition, the administration of these drugs can also cause death because these drugs act on active cells in the hemopoietic and gastrointestinal tissues [6,7]. Then, there is inflammation that increases the risk of activation of cancer cells. Inflammation predisposes cancer development and promotes all stages of tumorigenesis [8]. Induction of acute inflammatory reactions frequently boosts dendritic cell maturation and antigen presentation, resulting in an antitumor immune response. However, chronic inflammation promotes tumor formation and treatment resistance [9]. One of the receptors that play a role in inflammation is COX-2, with the mechanism of prostaglandin formation, which has an inflammatory effect. Giving synergistic drugs to inhibit HER-2 and COX-2 is one way to inhibit the development of cancer cells and inflammation. So, it is necessary to develop synergistic drug compounds from natural ingredients that have lower side effects and are relatively safer than synthetic drugs. Natural materials that are often used are plant materials [10,11].
The jambu semarang plant (Syzygium samarangense) is one of the potential plants to be developed as a raw material for developing medicinal compounds. Syzygium samarangense is often researched and has many benefits because it has many activities, such as anti-diabetic, antibacterial, and good antioxidants. The flowers of this plant have astringent properties and are used as a fever and diarrhea medicine in Taiwan. The plant also contains tannins; the flowers are reported to have weak antibiotic activity against Staphylococcus aureus, Mycobacterium smegmatis, and Candida albicans and contain desmethoxymatteucinol, 5-O-methyl-4′-desmethoxymatteucinol, oleanolic acid, and β-sitosterol [12–14]. The leaves are rich in phenolic and flavonoid compounds; a study report stated that the leaves had a phenolic content of 66.56 mg GAE/g DW and a flavonoid content of 17.25 mg QE/g DW. In addition, the leaves had an antioxidant IC50 value using a 2,2-diphenyl-1-picrylhydrazyl of 13.66 μg/ml, nitric oxide of 51.57 μg/ml, and 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) of 6.31 μg/ml. The leaves also have the ability to inhibit the alpha-glucosidase enzyme with an IC50 of 0.88 μg/ml, which represents its ability as an antidiabetic [15]. The essential oil from the leaves was found in a study report to have the dominant compound trans-caryophyllene with antibacterial activity against Staphylococcus aureus (MIC = 500 μg/ml); Bacillus cereus (MIC = 500 μg/ml); Listeria monocytogenes (MIC = 500 μg/ ml); Escherichia coli (MIC = 500 μg/ml); Salmonella thypi (MIC = 500 μg/ml) [16]. In human HepG2-C8 cells that have been transfected with the stable antioxidant responsive element (ARE)-luciferase plasmid, its aqueous extract has been shown to promote the Nrf2-ARE pathway at doses of 100 and 250 μg/ml. TPA also successfully stopped the transformation of mouse epidermal JB6 P+ cells, suggesting that the extract might have some therapeutic potential [17,18]. The part of the plant that is rarely developed is the stem bark. The research that has been reported related to the stem bark of the plant is the study of phytochemical screening on the stem bark. Stem bark extract contains reducing sugars, gums, terpenoids, steroids, tannins, saponins, and phenolics [12,19] with high potency as an antioxidant. Studies on the anti-inflammatory effect of ethanol extract showed effective activity in mice [20]. In addition, the extract also provides a practical antiworm effect, such as albendazole, an anthelmintic with good effectiveness [4,14]. Therefore, the plant has the potential to be developed as a raw material for breast cancer and anti-inflammatory drugs; this study aims to describe the content of bioactive compounds and their potential as antibreast cancer and anti-inflammatory from the stem bark of S. samarangense.
METHOD AND MATERIALS
Preparation and extraction of samples
The stem bark of S. samarangense was dried and ground into powder and macerated using methanol p.a. for 1 × 24 hours. The sample is immersed in a volume solvent up to 1 cm above the sample. After that, the filtrate was filtered using a Buchner funnel under a vacuum. The filtrate was then evaporated using a vacuum rotary evaporator to obtain a thick extract.
Identification of bioactive compound by liquid chromatography mass spectrometry (LC-MS) analysis
Determination of the bioactive compounds in S. samarangense extract was conducted using the LC-MS instrument (Shimadzu LCMS-8040). A total of 1 μl of the sample was injected into the LC instrument using a Shim Shim Fuilly controlled-octadecyl silane column (2 × 150 mm, particle size 3 μm) at 35°C. A sample injection volume of 1 μl. Separation was performed at a flow rate of 0.5 ml/minute with an isocratic model. The ion spray needle voltage is 3.5 kV, and the capillary temperature is 400°C. Ionization was carried out using electrospray ionization. Compounds were identified using the National Institute of Standards and Technology and Faculty-Specific Technology Programme-National University of Singapore data libraries from LC-MS.
Molecular docking assay
Preparation of protein target
The proteins used in this study were HER-2 target protein (PDB ID: 3WSQ) as an anti-breast cancer target and COX-2 (PDB ID: 1PXX) as an anti-inflammatory target. X-ray crystals for each protein receptor were obtained from the Research Collaboratory for Structural Bioinformatics webserver, then sterilized using PyMOL to remove water molecules, ligands, and other molecules that were not needed. Then, it is inputted into the PyRx program as a macromolecule.
Preparation of ligand compound
3D conformers of the identified compounds from LC-MS and control drugs (lapatinib and rofecoxib) were obtained from the PubChem web server in .sdf format. Then, minimize it using OpenBabel on the PyRx software to get a flexible conformer and change the compound file format from .sdf to .pdb [21]. Compounds that have been minimized and stored as ligand compounds are ready for docking.
Docking and visualization
The prepared proteins and ligands were then docked using the Vina Wizard in the PyRx program [22]. Docking simulation is carried out at coordinates X: 156.432; Y: 10.486; Z: 52.883 for HER-2 and X: 27.115; Y: 24.090; Z: 14.936 for COX-2. Compounds with lower binding affinity than control drugs were visualized using PyMOL and Discovery Studio to determine the type and position of the interaction between ligand and receptor.
Absorption, distribution, metabolism, excretion, and toxicity (ADMET) pharmacokinetic
Potential compounds were analyzed by ADMET pharmacokinetics. ADME parameters included the Lipinski rule of drug affinity, blood-brain barrier (BBB) permeability, human intestinal absorption (HIA), P-glycoprotein (P-gp), cytochrome P450 (CYP) inhibitory isoenzymes, and skin permeation performed using the SWISSADME webserver [23]. Toxicity prediction was carried out through the ProTox-II webserver [24]. Evaluation of potential bioactivity compounds as anticancer and anti-inflammatory was carried out through the PASS web server; analysis was carried out based on the value of Pa (probability of being active) and Pi (probability of being inactive) based on the existing categories [25].
RESULTS AND DISCUSSION
LCMS analysis
The results of the LC-MS analysis in the form of a chromatogram in Figure 1 showed that there were a total of 60 compounds in the methanol extract of S. samarangense stem bark. A total of 60 identified compounds are reported in Table 1, covering retention times, concentrations, and compound names. The major compound in the methanol extract of the plant is myricetin-3-(3"-galloylrhamnoside). The compounds can be grouped into phenolics, flavonoids, terpenoids, steroids, and esters. Most groups of the compounds are flavonoids and phenolic groups. Flavonoids and phenolic groups are a class of compounds that are well-known for their antioxidant bioactivity. This supports the results of a study by Metasari et al. [26] which revealed that the IC50 value of the methanol fraction of the plant was 31.83 μg/ml, indicating that its antioxidant activity was classified as strong and very active. Most identified flavonoid groups are derivatives of quercetin, myricetin, kaempferol, and chalcone.
Molecular docking analysis
Molecular docking analysis was carried out to evaluate the potential of bioactive compounds in S. samarangense stem bark as anti-breast cancer and anti-inflammatory. The anti-breast cancer target in this study is the HER-2 protein, which directly induces dimerization, autophosphorylation, and activation of FAK [5]. Meanwhile, the anti-inflammatory target is the COX-2 protein which plays a role in the production of prostaglandins as inflammatory mediators [27,28]. The results of the molecular docking analysis in Table 1 showed that three compounds have lower binding affinity values than the control drugs, lapatinib and rofecoxib. The three potential compounds are syzyginin B, kaempferol-7-rhamnoside-4′-glucoside, and casuarinin. The lower the binding affinity, the more stable the complex, and the more stable the complex, the more significant the inhibitory activity [29]. The compound with the lowest binding affinity is syzyginin B as known that syzyginin B has bioactivity as an antiviral for COVID-19 [30]. Studies on casuarinin compounds reported that these compounds have bioactivity as an antibacterial against P. aeruginosa bacteria [31]. These three compounds are classified as rarely explored, so reports on these three compounds are limited.
Visualization of the three compounds: Figure 2 shows the interactions of the three compounds with their respective potential receptors on their active sites. A complex contains a variety of interactions, including hydrogen bonds, hydrophobic bonds, and electrostatic bonds; the interaction formed influences the binding affinity value of the complex [32,33]. In addition, stable complexes are complexes that have few unfavorable bonds [34]. Each compound has a similar amino acid residue position to the drug control compound. This similarity supports validating its activity as anti-breast cancer and anti-inflammatory because these compounds inhibit amino acids in the same position [35]. The 2D visualization of the HER-2 complex is shown in Figure 3 and the COX-2 complex in Figure 4. Kaempferol-7-rhamnoside-4′-glucoside has similarities with the control drug lapatinib on the HER-2 receptor with positions Arg 412, Tyr 281, Val 3, and Leu 291. The similarity of syzyginin B with lapatinib on the HER-2 receptor with positions Ser 441, Tyr 281, Leu 291, and Arg 412. Next, syzyginin B with COX-2 has a similar inhibitory position with the control drug rofecoxib at Gly 2134 and Asp 2157. Casuarinin with COX-2 has similarities with the positions of Gly 2134 and Ala 2156. The more similar the positions of the inhibitors are to the drug compounds, the more similar their inhibitory activities are [36]. Syzyginin B has more similarities than the other two potential compounds; it has a higher potential as an anticancer agent for breast HER-2 inhibitors and anti-inflammatory COX-2 inhibitors. Based on the previous research on this plant, there is potential for the methanol extract and ethyl acetate fraction of the pulp to have anticancer activity against human colonies SW-480 [37]; in the leaves, there is a derivative of dimethyl chalcone (DMC). DMC suppresses colorectal carcinoma cell growth, arrests the G2/M cell cycle, and induces autophagy [38]. Studies on its inflammatory activity reported that its bark has good anti-inflammatory activity induced in mice [20]. The root methanol extract has an effective ability to suppress protein denaturation as an anti-inflammatory [39]. However, research on its potential as an anticancer breast HER2 inhibitor and anti-inflammatory COX inhibitor has not been carried out in vitro. This study provides an overview of the possibility of the stem bark as a natural ingredient for HER2 and COX-2 inhibitors.
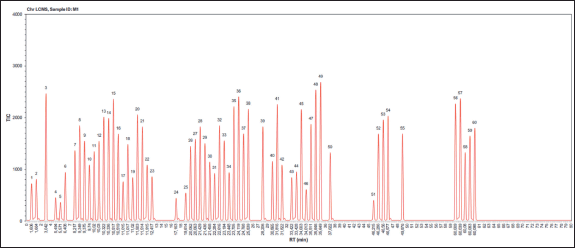 | Figure 1. LC-MS chromatogram of S. samarangense methanolic extract. [Click here to view] |
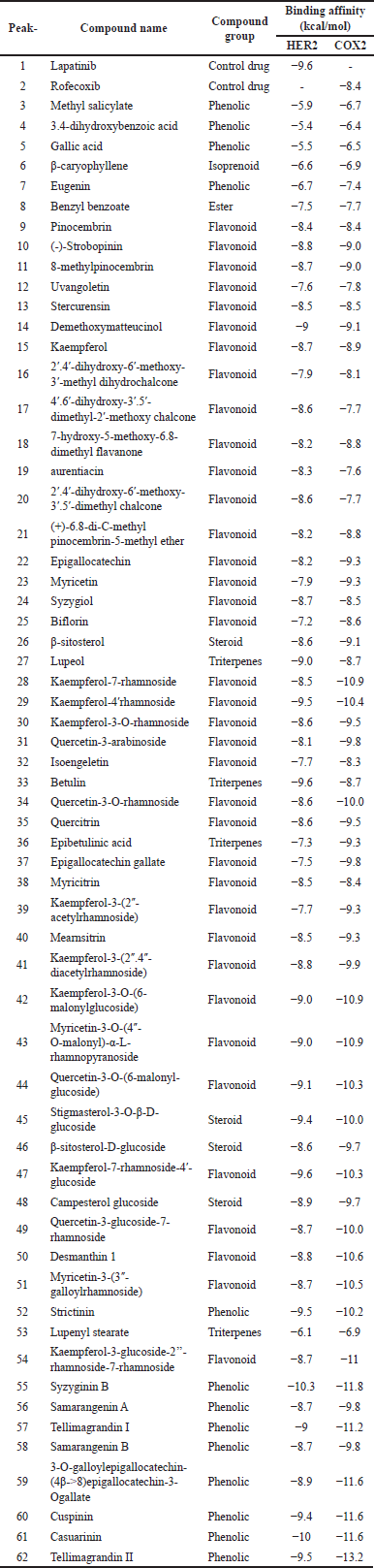 | Table 1. Bioactive compounds of methanolic extract of S. samarangense stem bark identified by LC-MS and molecular docking result analysis. [Click here to view] |
 | Figure 2. Visualization 3D of potential and drug compound in active site. [Click here to view] |
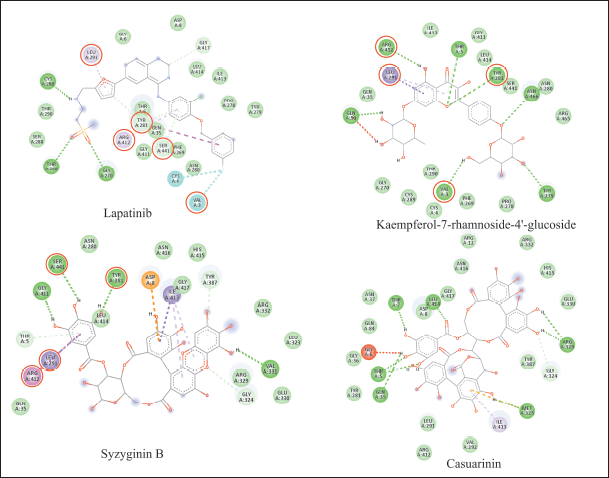 | Figure 3. Visualization 2D of potential and drug compound with HER-2. [Click here to view] |
ADMET pharmacokinetic analysis
ADMET pharmacokinetic analysis, which includes ADMET, was performed to evaluate the profile of possible medicinal agents. The Lipinski rule of druglikeness, BBB permeability, HIA, P-gp substrate, CYP inhibitory isoenzymes, and skin permeation are ADME parameters. The factors predicting toxicity are hepatotoxicity, carcinogenicity, immunotoxicity, mutagenicity, cytotoxicity, and the LD50 dose. In Table 2, the findings of the ADMET pharmacokinetic analysis demonstrated that the pharmacokinetic profiles of the three candidate drugs are comparable. According to Lipinski’s druglikeness rule specifications, the three compounds did not adhere to his five rules (Ro5). Lipinski’s rule of five is a therapeutic rule that determines if a substance can be delivered orally if the following criteria are met: molecular weight <500 g/mol; hydrogen donor-acceptor <10; hydrogen donor bonds <5; log P (lipophilicity) <5; and molar refractivity 40–130 [40]. This condition makes the bioavailability value low (0.17) for the three potential compounds. Thus, these three potential compounds may not be suitable for oral consumption due to their low bioavailability [41].
Absorption parameters showed that the third compound had low gastrointestinal absorption, which supports Lipinski’s druglikeness and low bioavailability value, so it is unsuitable for oral consumption. When gastrointestinal absorption is low, the compound is absorbed in the intestine only slightly; it will not be optimal [42,43]. However, these three compounds do not have the potential to cross the BBB, so they will reduce the adverse impact on the central nervous system. The BBB is a semipermeable barrier of endothelial cells that prevents dissolved substances in the blood from spreading to the central nervous system [44–46]. The third potential compound has the potential as a substrate of P-gp, whereas the third one will be easy to pump out back into the intestinal lumen. P-gp has a role in absorption and excretion; when a compound has a profile as a substrate of P-gp, it is easily pumped out of the cell [47]. However, the P-gp mechanism can be inhibited by P-gp inhibitors which are easy to find even in food so that the drug is not pumped out of the cell [48].
The third potential compound does not have the potential to inhibit all isoforms of the CYP enzyme. This is very beneficial because the body will quickly metabolize and excrete these compounds [49]. CYP450 has a role in drug metabolism, and when there are compounds that inhibit the CYP450 family, these compounds will be inactivated by CYP and secreted out of the body [50]. In toxicity prediction, the three potential compounds have the same profile for their properties. All three compounds are not hepatotoxic, carcinogenic, mutagenic, or cytotoxic. However, they can potentially affect immunology because they have immunotoxic properties. Immunotoxicity is an effect that attacks the immune system. A drug with immunotoxic properties can affect changes in the immune system and result in immunosuppression or excessive immune reactions [51]. The predicted LD50 value showed the same category for the three compounds; they belong to class 5 (possibly dangerous class). Evaluation using the PASS web server in Table 3 showed that the highest probability value is in causarinin for antineoplastic (breast cancer), while syzyginin B is for anti-inflammatory. Both have a probability value above 0.3, so all three have a medium probability; when the probability value is above 0.7. Then, they have a high bioactive probability [25,52].
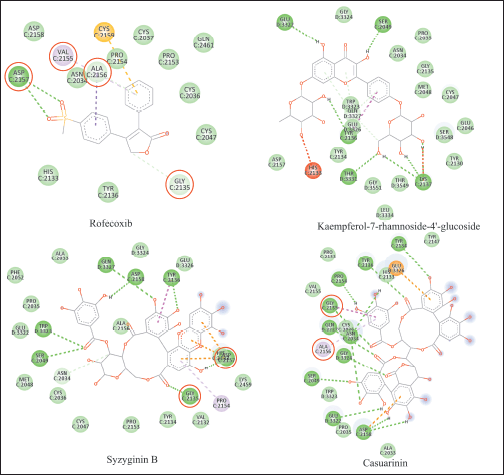 | Figure 4. Visualization 2D of potential and drug compound with COX-2. [Click here to view] |
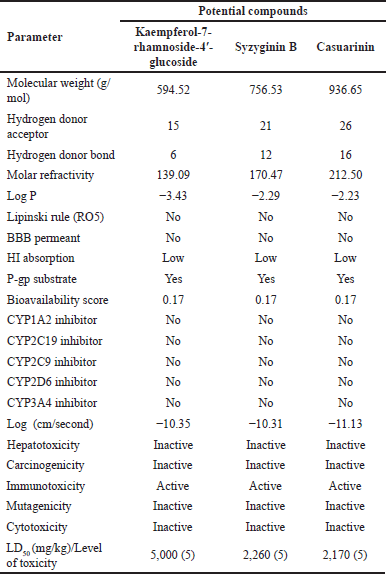 | Table 2. Pharmacokinetic ADMET profile of potential compound. [Click here to view] |
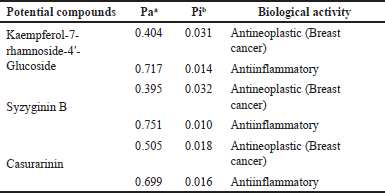 | Table 3. Biological activity prediction PASS of potential compounds. [Click here to view] |
CONCLUSION
Based on the results of this study, 60 bioactive compounds were found in the stem bark of S. samarangense with three potential compounds as anticancer breast HER-2 inhibitors and anti-inflammatory COX-2 inhibitors through molecular docking, among others, syzyginin B, kaempferol-7-rhamnoside-4′-glucosides, and casuarinin. Based on the overall results, it was found that syzyginin B has the potential with a fairly good ADMET profile as an anticancer and anti-inflammatory agent. However, more studies are needed, such as isolation and purification, as well as further in vitro and in vivo tests for further development.
ACKNOWLEDGMENTS
The authors would like to thank Unesa, who has provided funds for this research with Rector Decree through contract number: B/42877/UN38.3/LT.02/2022 on July 1, 2022.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
There are no conflicts of interest declared by the authors.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. WHO. Global tuberkulosis report 2021. Geneva, Switzerland: World Health Organization; 2021.
2. Lukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanislawek A. Breast cancer—epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—an updated review. Cancers (Basel). 2021;13:4287.
3. Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, et al. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5:77–106. doi: https://doi.org/https://doi.org/10.1016/j.gendis.2018.05.001
4. Narod SA. Personalised medicine and population health: breast and ovarian cancer. Hum Genet. 2018;137:769–78.
5. Berdan CA, Ho R, Lehtola HS, To M, Hu X, Huffman TR, et al. Parthenolide covalently targets and inhibits focal adhesion kinase in breast cancer cells. Cell Chem Biol. 2019;26:1027–35.
6. Johnson SC, Dweck CS, Chen FS, Stern HL, Ok SJ, Barth M. At the intersection of social and cognitive development: internal working models of attachment in infancy. Cogn Sci. 2010;34(5):807–25. doi: https://doi.org/10.1111/j.1551-6709.2010.01112.x
7. Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, et al. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36:D901.
8. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. doi: https://doi.org/10.1016/j.immuni.2019.06.025
9. Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6:263. doi: https://doi.org/10.1038/s41392-021-00658-5
10. Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33:1582–614. doi: https://doi.org/https://doi.org/10.1016/j.biotechadv.2015.08.001
11. Lim TK. Edible medicinal and non-medicinal plants. vol. 1. New York, NY: Springer Dordrecht; 2012. doi: https://doi.org/10.1007/978-94-017-7276-1
12. Aung EE, Kristanti AN, Aminah NS, Takaya Y, Ramadhan R. Plant description, phytochemical constituents and bioactivities of Syzygium genus: a review. Open Chem. 2020;18:1256–81. doi: https://doi.org/doi:10.1515/chem-2020-0175
13. Srivastava R, Shaw AK, Kulshreshtha DK. Triterpenoids and chalcone from Syzygium samarangense. Phytochemistry. 1995;38:687–9. doi: https://doi.org/https://doi.org/10.1016/0031-9422(94)00739-G
14. Tarigan C, Pramastya H, Insanu M, Fidrianny I. Syzygium samarangense: review of phytochemical compounds and pharmacological activities. Platin Open Access J. 2021;12(2):2084–107.
15. Idris NS, Khandaker MM, Rashid ZM, Majrashi A, Alenazi MM, Nor ZM, et al. Polyphenolic compounds and biological activities of leaves and fruits of Syzygium samarangense cv. `Giant Green’ at three different maturities. Horticulturae. 2023;9:326. doi: https://doi.org/10.3390/horticulturae9030326
16. Choironi NA, Sunarto S, Utami ED, Fareza MS. GC-MS analysis and antibacterial activity of essential oils of five Syzygium species leaves. ALCHEMY J Penelit Kim. 2023;19(1):61–7. doi: https://doi.org/1020961/Alchemy1916740161-67
17. Abdulrahman MD, Hama HA. Anticancer of genus Syzygium: a systematic review. Explor Target Anti-Tumor Ther. 2023;4:273–93. doi: https://doi.org/10.37349/etat.2023.00134
18. Thampi N, Shalini JV. Anti-proliferative and apoptotic activities of Syzygium samarangense (wax apple) fruits extract against human A549 lung cancer cell lines. Cell. 2015;10:11.
19. Tukiran T, Suyatno S, Safitri FN. Identification of the chemical constituents of the selected fraction of the dichloromethane extract of Syzygium samarangense stem bark using LC-ESI-MS and evaluation its potential as antifungal agent. Indones J Chem. 2021;21:340–9.
20. Mollika S, Islam N, Parvin N, Kabir A, Sayem MW, Luthfunnesa SR. Evaluation of analgesic, anti-inflammatory and CNS activities of the methanolic extract of Syzygium samarangense leave. Glob J Pharmacol. 2014;8:39–s46.
21. Sururi AM, Raihan M, Aisa ER, Safitri FN, Constaty IC. Anti-inflammatory activity of stem bark dichloromethane fraction Syzygium samarangense extract as COX-2 inhibitor: a bioinformatics approach. J Kim Ris. 2022;7:94–100. doi: https://doi.org/10.20473/jkr.v7i2.39662
22. Trott O, Olson AJ. AutoDock vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–61. doi: https://doi.org/10.1002/jcc.21334
23. Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:1–13.
24. Banerjee P, Eckert AO, Schrey AK, Preissner R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018;46:W257–63.
25. Lagunin A, Stepanchikova A, Filimonov D, Poroikov V. PASS: prediction of activity spectra for biologically active substances. Bioinformatics. 2000;16:747–8.
26. Metasari S, Elfita E, Muharni M, Yohandini H. Antioxidant compounds from the stem bark of Syzygium samarangense L. Molekul. 2020;15:175–83.
27. Desai SJ, Prickril B, Rasooly A. Mechanisms of phytonutrient modulation of cyclooxygenase-2 (COX-2) and inflammation related to cancer. Nutr Cancer. 2018;70:350–75.
28. O’Banion MK. Cyclooxygenase-2: molecular biology, pharmacology, and neurobiology. Crit Rev Neurobiol. 1999;13:45 -82. doi: https://doi.org/10.1615/critrevneurobiol.v13.i1.30
29. Musfiroh I, Muchtaridi M, Muhtadi A, Diantini A, Hasanah AN, Udin LZ, et al. Cytotoxicity studies of xanthorrhizol and its mechanism using molecular docking simulation and pharmacophore modelling. J Appl Pharm Sci. 2013;3(6):7–15. doi: https://doi.org/10.7324/JAPS.2013.3602
30. Chandra Manivannan A, Malaisamy AK, Eswaran M, Meyyazhagan A, Arumugam VA, Rengasamy KRR, et al. Evaluation of clove phytochemicals as potential antiviral drug candidate targeting SARS-CoV-2 main protease: an computational docking, molecular dynamics simulation and pharmacokinetic profiling. Front Mol Biosci. 2022;9:1–12.
31. Shimozu Y, Kuroda T, Tsuchiya T, Hatano T. Structures and antibacterial properties of isorugosins H–J, oligomeric ellagitannins from Liquidambar formosana with characteristic bridging groups between sugar moieties. J Nat Prod. 2017;80:2723–33. doi: https://doi.org/10.1021/acs.jnatprod.7b00496
32. Ahmad B, Batool M, Ain QU, Kim MS, Choi S. Exploring the binding mechanism of PF-07321332 SARS-CoV-2 protease inhibitor through molecular dynamics and binding free energy simulations. Int J Mol Sci. 2021;22:9124. doi: https://doi.org/10.3390/ijms22179124
33. Sururi AM, Maharani DK, Wati FA. Potensi senyawa eugenol dari cengkeh (Syzygium aromaticum) sebagai inhibitor protease HIV-1 (PR). Unesa J Chem. 2023;12(1):26–30.
34. Freire E. Do enthalpy and entropy distinguish first in class from best in class? Drug Discov Today. 2008;13:869–74. doi: https://doi.org/https://doi.org/10.1016/j.drudis.2008.07.005
35. Kharisma V, Widyananda M, Nege A, Naw S, Nugraha A. Tea catechin as antiviral agent via apoptosis agonist and triple inhibitor mechanism against HIV-1 infection: a bioinformatics approach. J Pharm Pharmacogn Res. 2021;9:435–45.
36. Shifeng P, Boopathi V, Murugesan M, Mathiyalagan R, Ahn J, Xiaolin C, et al. Molecular docking and dynamics simulation studies of ginsenosides with SARS-CoV-2 host and viral entry protein targets. Nat Prod Commun. 2022;17:1934578X221134331. doi: https://doi.org/10.1177/1934578X221134331
37. Simirgiotis MJ, Adachi S, To S, Yang H, Reynertson KA, Basile MJ, et al. Cytotoxic chalcones and antioxidants from the fruits of a Syzygium samarangense (Wax Jambu). Food Chem. 2008;107:813–9. doi: https://doi.org/10.1016/j.foodchem.2007.08.086
38. Ko H, Kim YJ, Amor EC, Lee JW, Kim HC, Kim HJ, et al. Induction of autophagy by dimethyl cardamonin is associated with proliferative arrest in human colorectal carcinoma HCT116 and LOVO cells. J Cell Biochem. 2011;112:2471–9. doi: https://doi.org/10.1002/jcb.23171
39. Madhavi M, Ram MR. Phytochemical screening and evaluation of biological activity of root extracts of Syzygium samarangense. Int J Res Pharm Chem. 2015;5:753–63.
40. Lipinski CA. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1:337–41. doi: https://doi.org/https://doi.org/10.1016/j.ddtec.2004.11.007
41. Martin YC. A bioavailability score. J Med Chem. 2005;48:3164–70. doi: https://doi.org/10.1021/jm0492002
42. Hoff PM, Saad ED, Ajani JA, Lassere Y, Wenske C, Medgyesy D, et al. Phase I study with pharmacokinetics of S-1 on an oral daily schedule for 28 days in patients with solid tumors. Clin Cancer Res. 2003;9:134–42.
43. Pires DEV, Kaminskas LM, Ascher DB. Prediction and optimization of pharmacokinetic and toxicity properties of the ligand BT --computational drug discovery and design. In: Gore M, Jagtap UB, editors. New York, NY: Springer New York; 2018. pp 271 -84. doi: https://doi.org/10.1007/978-1-4939-7756-7_14
44. Clark DE. Prediction of intestinal absorption and blood-brain barrier penetration by computational methods. Comb Chem High Throughput Screen. 2001;4:496.
45. Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. doi: https://doi.org/10.1101/cshperspect.a020412
46. Lin J, Sahakian DC, De Morais SM, Xu JJ, Polzer RJ, Winter SM. The role of absorption, distribution, metabolism, excretion and toxicity in drug discovery. Curr Top Med Chem. 2003;3:1125–54.
47. Elmeliegy M, Vourvahis M, Guo C, Wang DD. Effect of P-glycoprotein (P-gp) inducers on exposure of P-gp substrates: review of clinical drug–drug interaction studies. Clin Pharmacokinet. 2020;59:699–714.
48. Yu J, Zhou Z, Tay-Sontheimer J, Levy RH, Ragueneau-Majlessi I. Intestinal drug interactions mediated by OATPs: a systematic review of preclinical and clinical findings. J Pharm Sci. 2017;106:2312–25. doi: https://doi.org/10.1016/j.xphs.2017.04.004
49. Guengerich FP. Cytochrome p450 and chemical toxicology. Chem Res Toxicol. 2008;21:70–83. doi: https://doi.org/10.1021/tx700079z
50. Zahno A, Brecht K, Morand R, Maseneni S, Török M, Lindinger PW, et al. The role of CYP3A4 in amiodarone-associated toxicity on HepG2 cells. Biochem Pharmacol. 2011;81:432–41. doi: https://doi.org/10.1016/j.bcp.2010.11.002
51. Gulati K, Ray A. CHAPTER 40—Immunotoxicity. In: Gupta RC, editor. Handbook of toxicology of chemical warfare agents. San Diego, CA: Academic Press; 2009. pp. 595–609. doi: https://doi.org/https://doi.org/10.1016/B978-012374484-5.00040-7
52. Rahmaningsih S, Pujiastutik H. An in vitro and in silico evaluation of the antibacterial activity of the bioactive compounds in Majapahit (Crescentia cujete L.) fruit. Vet World. 2019;12:1959–65. doi: https://doi.org/10.14202/vetworld.2019.1959-1965