INTRODUCTION
Colorectal cancer (CRC), also known as colorectal carcinoma, occurs in the large intestine or rectum [1]. The progression of CRC ranges from stage 0 to IV. Stage 0 cancer is limited to the inner layer of the large intestine or rectum wall. The spread to lymph nodes or other organs makes a difference between stages I, II with stages III, IV. The cancer in stage I grow beyond the inner layer of the large intestine or rectum. Whereas in stage II, the cancer has penetrated the wall of the large intestine or rectum and may have spread to surrounding tissues. Stage III of CRC is marked by spreading cancer to the lymph nodes around the large intestine or rectum but has not spread to other organs. On the other hand, stage IV cancer has expanded to other organs, such as the liver, lungs, or bones, through the bloodstream or lymphatic system. This stage is also referred to as metastatic CRC [2–4].
The etiopathology of CRC involves pleiotropic factors that can influence its development. Some known risk factors include genetic factors, a family history of CRC, large intestinal polyps, advanced age, unhealthy dietary patterns (low fiber, high fat), unhealthy lifestyle (lack of physical activity, smoking habits), and certain medical conditions such as inflammatory bowel disease [1].
CRC is one of the most frequently diagnosed types of cancer worldwide. The number of new cases reported yearly is increasing and tends to be more prevalent with the aging population [5]. According to the World Health Organization, CRC is the third most common cancer globally, with an estimated 1.8 million new cases reported in 2018. It is also the second leading cause of cancer-related deaths, with approximately 900,000 deaths worldwide. It is estimated that by the year 2035, the total number of deaths from rectal and colon cancer will increase by 60% and 71.5%, respectively [6,5].
Therefore, with the continuously increasing number of new cases and death each year, the research on the investigation of CRC treatment, especially in the early stage, is crucial to conduct. This strategy aims to reduce the high prevalence of colorectal carcinoma and improve patient survival rates. Through ongoing research, it is hoped that more effective therapies, more sensitive early detection methods, and better prevention efforts can be discovered to address the issue of CRC globally.
Dendrophthoe pentandra (DPT) is a parasitic plant, including the family Loranthaceae that lives on branches or other plant trees. These plants are found in many areas of Asia, including Indonesia, Thailand, Malaysia, and China [7]. Traditionally DPT has been used as a medicine for cough, diabetes, hypertension, cancer, diuretics, smallpox, ulcers, skin infections, and after child-birth treatment. Previous studies have proven that DPT has anticancer effects [8], antioxidants [9], and hypertension [10]. The anticancer effect of DPT is due to the inhibition of cancer cell proliferation and the induction of P53 [11]. Moreover, DPT can also induce apoptosis of cancer cells and disrupt the cell cycle in the G0, G1, S, and G2-M phases [12,13]. However, the biological mechanisms of the active compounds acting as anticancer in DPT and their specific target genes have not been fully discussed.
Herbal medicines contain multicomponent, which is multitarget and multipathway, in providing therapeutic effects, especially by modulating the biological network of the body system [14]. Thus, it is relatively difficult to detect the mechanism of action of herbal medicines solely by conventional experiments [15]. As a result, a suitable new approach is urgently needed to systematically and comprehensively dissect the mechanism of action of herbal medicines [16]. Network pharmacology is a modern and systematic approach that has proven effective in uncovering herbal medicines’ molecular mechanisms and pharmacology. In contrast to the previous reductionist approach of considering “one drug means one target,” network pharmacology recognizes that multiple active compounds can interact with diverse genes or proteins. This perspective allows for a more comprehensive understanding of the holistic mechanisms involved [17,18]. Moreover, pharmacological networks can reflect and clarify the interactive relationships between active compounds, targets, and diseases. In addition, pharmacological networks can visualize their relationships into a network model and describe the action of drugs on the human biological system in a systematic way [19]. Research on the pharmacological network of DPT compound for the identification of potential anticancer compounds, determination of target genes, and molecular mechanisms in CRC has not been reported by researchers before. Therefore, our research aims to identify potential anticancer compounds, predict target genes and their mechanism of action, and determine the molecular mechanism pathways of DPT as an anticancer compound, specifically in CRC.
MATERIALS AND METHODS
Material
The databases used in this study included PubChem, GeneCards, STRING, and DisGeNET. The software used included Masslynx version 4.1 software (Waters, Massachusetts, USA), ChemDraw Office 2010 (PerkinElmer), and Cytoscape 3.7.1.
Plant material
The DPT leaves were taken from Malang, East Java, Indonesia. The DPT has been determined by the Indonesian Institute of Sciences (LIPI) with a specimen number of 074/212/102 20-A/2022. Subsequently, the DPT was dried at a temperature of 50°C for 5–7 days. The resulting powder was stored at room temperature.
Extraction of plant material
50 g of DPT powder was extracted using 500 ml of ethanol 96% with Ultrasound assisted extraction. The obtained extract was then condensed into solid extracts by utilizing a rotary evaporator after being filtered via filter sheets. The solid extract was then kept at a temperature of −4°C before receiving further treatment [20].
Ultra performance liquid chromatography-quadrupole time of flight-mass spectrometry (UPLC-QToF-MS) analysis
The analysis of UPLC-QToF-MS employed UPLC-MS systems with QToF as the analyzer and positive electrospray ionization as the ionization source with the Acquity C18 column 1.8 μm; 2.1 × 150 mm. The applied Eluent was a mixture between (A) Water (HPLC grade)/formic acid (Merck, Darmstadt, Germany) 99,9/0,1 [v/v]; (B) Asetonitril (Merck, Darmstadt, Germany)/formic acid 99,9/0,1 [v/v] and the system of gradient elution. The source temperature was 100°C and the desolvation temperature was 350°C. A 10 mg extract sample was solved in a 10 ml volumetric flask with absolute methanol then, 5 μl volumes were injected into the UPLC-MS system. From chromatogram data, the area was in percentage. Parameters for analysis were set using positive ion mode with spectra acquired over a mass range from m/z 120 to 1,000. Software Masslynx version 4.1 was used to process the chromatogram (Waters, Massachusetts, USA). The component identification was based on the ratio of measured m/z in Masslynx and PubChem (https://pubchem.ncbi.nlm.nih.gov/). A compound’s accuracy for confirmation was determined based on MS/MS fragment matching and inaccuracy of less than 5 parts per million (ppm) [21].
Screening of active constituents
An important pharmacokinetic feature known as oral bioavailability is used to measure the amount of an oral medication that has reached the bloodstream and has pharmacological effects. Important parameters of the identified compounds, such as molecular weight, gastrointestinal absorption, H-bond acceptors (HBA), H-bond donors (HBD), and lipophilicity, were screened using SwissADME (http://www.swissadme.ch/index.php) [22].
Potential CRC target
Prediction of a chemical gene target is a crucial aspect of drug research [23]. Thus, the gene targets of active ingredients based on liquid chromatography-tandem mass spectrometry analysis in DPT were identified from the GeneCards databases. The gene target is selected if the score >0.7. The next step was exploring gene targets associated with lung cancer using DisGeNET’s (https://www.disgenet.org) database. The active ingredient-gene targets and the disease-gene target results were used to make network pharmacology and built using Cytoscape software [24].
Construction of the target protein-protein interaction
The intersection gene targets of active ingredients and disease were selected for further analysis using (STRING) 11.0 platform (https://string-db.org/). The protein-protein interaction (PPI) network was built using common target proteins with the minimum necessary interaction score of 0.400 [25].
Target pathway and enrichment analysis
The PPI network analysis was used to investigate the biological activities by examining the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment of protein and their functions in signal transduction [26].
RESULTS
Metabolite profiling
The mass spectroscopy total ion chromatogram is shown in Figure 1. By integrating with libraries, MassLynx v. 4.1 (Waters Corporation, Milford, MA) processed the chromatogram and produced a list of 23 compounds (Table 1) with their elemental formulae. The observed and computed m/z, molecular formula, error in ppm, retention duration, and MS fragmentation pattern for the identified compounds are shown in the mass spectrometric data below. The proposed names of the compounds are matched within a 25 ppm error tolerance. The TOF-MS method’s high mass accuracy might show a mass inaccuracy of less than 5 ppm.
Our findings identified 23 compounds in DPT leave extract which belong to the flavonoid, phenolic, terpenoid, alkaloid, and coumarin groups. The flavonoids found were quercetin, luteolin, isorhamnetin 3-rutinoside, and kaemferol. The terpenoid group is phylanthusin E, cyclohexanone oxime, abacavir carboxylate, etc. The coumarin group is umbelliferone. The alkaloid group includes piperidine, 2-Hexyl-3,5-dipentylpyridine, and piptamine (Table 1).
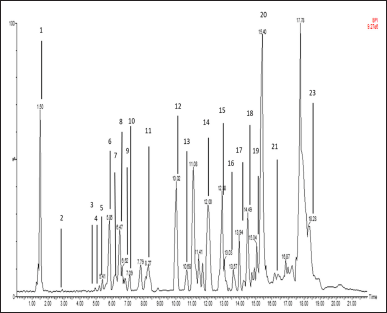 | Figure 1. Chromatogram of DPT leave extract using UPLC-QToFMS/MS method. It was C18 stationary phase; the mobile phase was water/formic acid [99.9/0.1 (v/v)] and acetonitrile/formic acid 99.9/0.1 (v/v). Each chromatogram peak indicated one compound. [Click here to view] |
Screening of physicochemical properties
The physicochemical properties of the compounds identified in DPT were evaluated using Lipinski’s five-law approach (Table 2). This evaluation aimed to predict the absorption ability and permeability of the compound. The parameters of the physicochemical properties are log P = 5, molecular weight = 500, H-bond donor = 5, and H-bond acceptor = 10. As a result of the findings of this study, 14 compounds had passed Lipinski’s 5 law.
Potential CRC target gene
The target genes of 18 compounds of DPT extract were determined using the Gene Cards database. Compounds that passed the screening have a target gene with a similarity score >7. We found four compounds that fulfill the criteria. These compounds were quercetin, phyllanthusiin E, cyclohexanone oxime, and abacavir carboxylate. Quercetin was found to target 25 genes, phyllanthusiin targeted 1 gene, cyclohexanone oxime targeted 2 genes, and abacavir carboxylate targeted 10 genes. The results of screening active compounds in DPT that have target genes with a similarity score >7 (Table 3).
The interaction between the group of compounds and the active compounds, the active compound and the target gene, the target gene, and disease has been developed using Cytoscape. There are 8 compounds with 39 target genes. With an average number of 3,108 neighbors and a characteristic path length of 3,186, this network had a density of 0.049. Analysis results show 65 nodes and 101 edges form a pharmacological network “active component-component-target gene” (Fig. 2).
Our findings demonstrated that two compounds in DPT have gene targets in CRC, namely, quercetin and phyllanthusiin E. Quercetin has eight gene targets, namely, Caspase 3 (CASP3), Jun proto-oncogene (JUN), Mitogen-activated protein kinase 1 (MAPK1), B-cell lymphoma 2 (BCL2), Mitogen-activated protein kinase 8 (MAPK8), Bcl-2-associated X protein (BAX), Mitogen-activated protein kinase 14, Carbonyl reductase 1, and Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma (PIK3CG), while phyllanthusiin E had one gene target, namely, MYC.
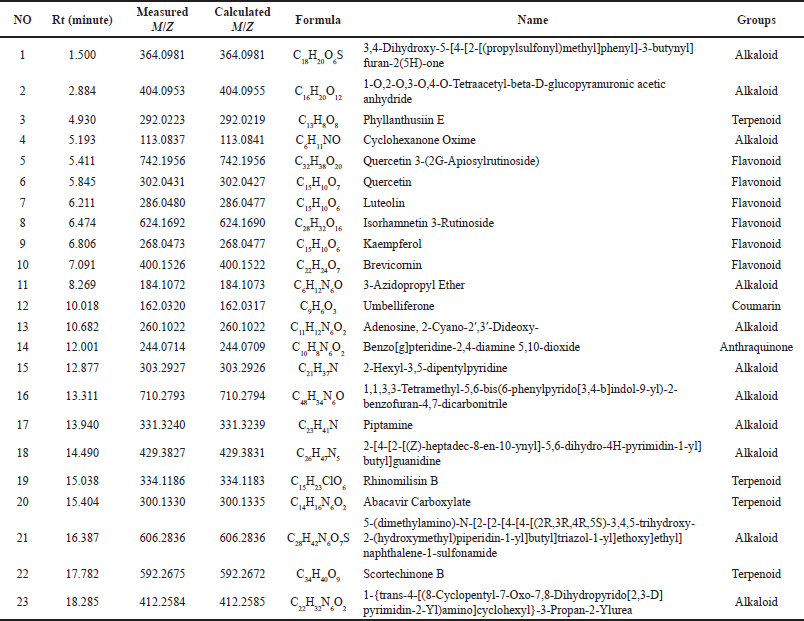 | Table 1. The results of metabolite identification of DPT leave extract using UPLC Qtof MS/MS method. [Click here to view] |
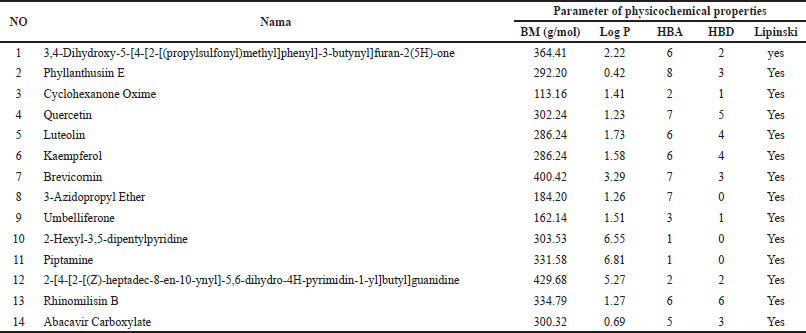 | Table 2. Compounds in DPT that passed the parameter of physicochemical properties. [Click here to view] |
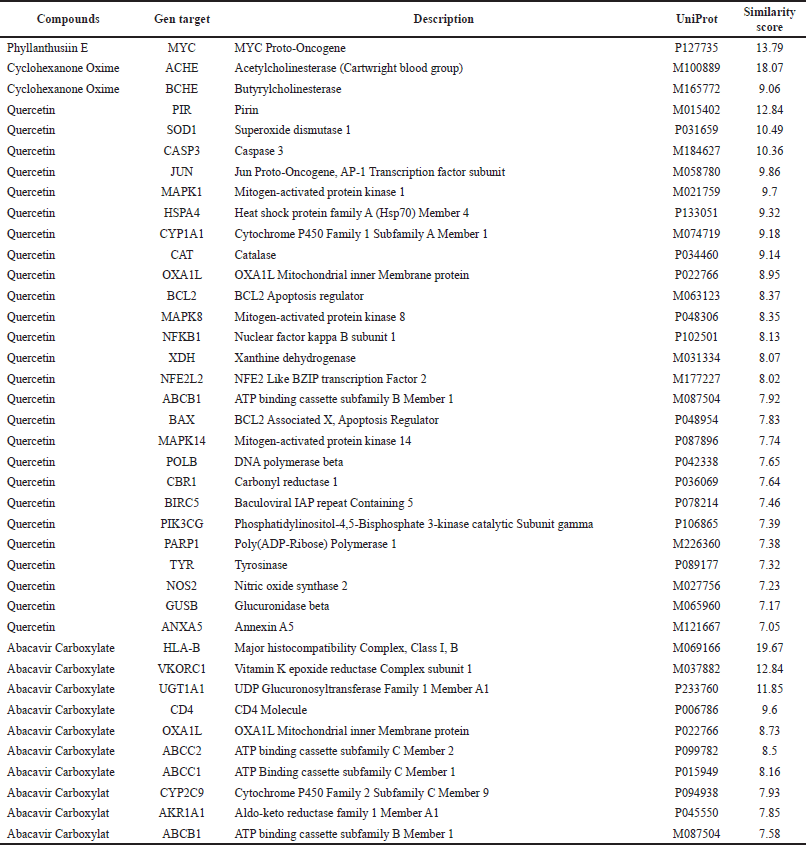 | Table 3. Screening of anti-CRC components in DPT (score>7). [Click here to view] |
Construction of the target protein-protein interaction
Based on the experimental interaction selected from the STRING database interactome, we used the PPI to explain biological processes, cellular components, molecular activities, and pathways of target proteins [27]. The cutoff value was based on p-value = 0.05. The network visualization using all protein targets as input produced a PPI network with a variety of interactions (Fig. 3).
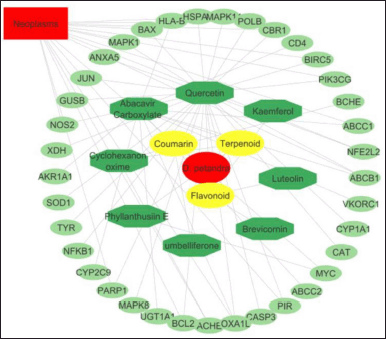 | Figure 2. Interactive network of DPT—compound class—active compound—gene-diseases. The red oval indicates the plant species, the yellow hexagon indicates the compound group, and the active compound is indicated by the green octagon. The target gene was indicated by a green ellipse, while the red rectangle was a disease target. [Click here to view] |
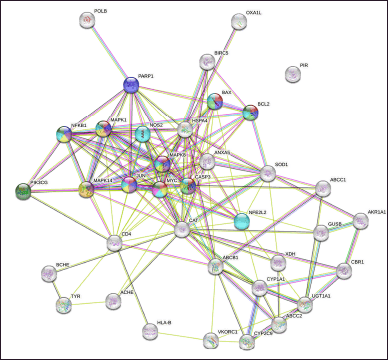 | Figure 3. Network of PPIs between CRC gene targets and chemicals from DPT. The red node was a CRC pathway (hsa05210); the blue node was an apoptosis pathway (hsa04210). [Click here to view] |
In the network, there were 37 viable protein target nodes connected by 159 edges, with an average node degree of 8.59 and an average local clustering coefficient of 0.538. Because the PPI enrichment p value was less than 1.0 e16, it can be concluded that proteins interact with one another more frequently than would be predicted if a random sample of proteins of a given size were randomly selected from the genome. The presence of such a large enrichment suggested that the proteins were at least biologically connected as a group.
Target pathway and enrichment analysis
Our findings showed that the analysis of signaling pathways with KEGG from the DPT compound obtained 147 pathways related to cancer, the immune system, inflammation, and metabolism. Meanwhile, the pathways related to molecular CRC are apoptosis, P53 signaling, Mitogen-activated protein kinase (MAPK) signaling, Wnt signaling, and Phosphatidylinositol 3-kinase-protein kinase B (PI3K-Akt) signaling pathway (Fig. 4, Table 4).
DISCUSSION
Network pharmacology is an approach that integrates information regarding drug interactions, molecular targets, and biological pathways within a complex network [28]. In the context of bioinformatics utilization, network pharmacology utilizes databases that store information about target genes and PPIs to predict drug effects and reveal mechanisms of action within the body. In cancer studies, network pharmacology, with the aid of bioinformatics, can assist in identifying target genes involved in cancer cell proliferation, angiogenesis, or mechanisms of therapy resistance [29,30]. The use of databases and bioinformatics algorithms enables researchers to analyze complex protein networks and identify interactions between target proteins and potential drug compounds. This can aid in the discovery of new drugs or the repurposing of existing drugs for cancer treatment.
Furthermore, in biomarker prediction, network pharmacology utilizes databases containing information about the relationship between gene expression and clinical phenotypes. Using bioinformatics algorithms, researchers can identify potential biomarkers associated with the diagnosis, prognosis, or response to cancer treatment [31]. This approach can assist in developing more accurate diagnostic tests and using biomarkers in patient monitoring [32]. On the other hand, in drug resistance studies, network pharmacology can help identify biological pathways involved in resistance to chemotherapy or targeted therapy [33]. Researchers can uncover resistance mechanisms and seek solutions to overcome this issue by analyzing PPIs within complex networks. Bioinformatics approaches also enable the identification of potential drug combinations to address drug resistance.
In the discovery of plant-based drugs, network pharmacology, and bioinformatics can be used to analyze interactions between natural compounds in medicinal plants and molecular targets related to cancer [34]. Databases and bioinformatics algorithms aid in identifying potential compounds with anti-cancer activity and predicting their mechanisms of action within the body [35]. This can assist in the discovery and development of new plant-based drugs. Therefore, in this study, a network pharmacology approach is employed to identify potential compounds and predict target genes and mechanisms of action of compounds in CRC treatment.
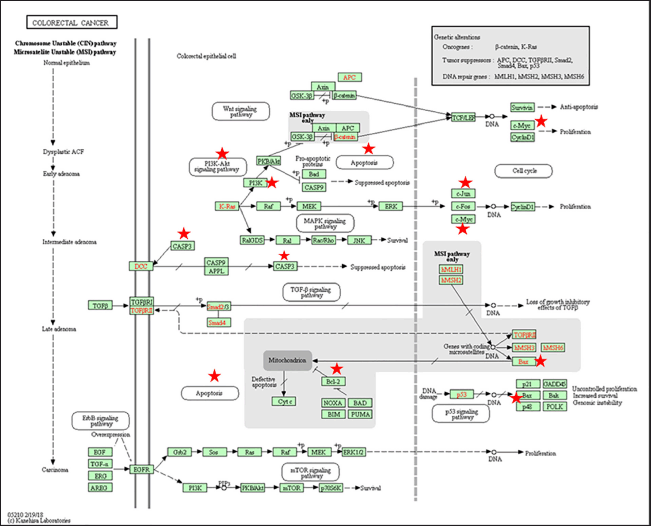 | Figure 4. Prediction of the molecular activity of quercetin in DPT against target genes involved in the inhibition of CRC in the KEGG pathway. The asterisk indicates the target gene for the compound quercetin. [Click here to view] |
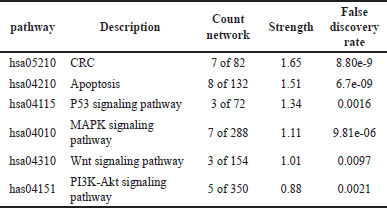 | Table 4. Pathway signaling analysis based on KEGG for active compounds in DPT. [Click here to view] |
This study adopted a combination of the UPLC−Q-TOF/MS/MS method and network pharmacology to explore data in depth and to perform network analysis. Through the interaction between the chemical components of DPT and molecular targets of colon cancer, the mechanism of compounds in DPT against colon cancer can be revealed. Our findings show that DPT contains the compound quercetin, which has a potential gene target in CRC.
The Wnt signaling pathway is a critical mediator of tissue homeostasis and repair and is frequently co-opted during tumor development [36]. In addition, the Wnt/β-catenin pathway contributes to the growth, invasion, and survival of CRC cells. Abnormal activation of the Wnt/β-catenin pathway will trigger excessive proliferation and cause upregulation of c-myc [37]. Therefore, this activation is known to be a trigger for most colon cancers. This study found that phyllantusin E acts on the Wnt/β-catenin pathway (hsa04310) with the Myc target gene. Inhibition of c-myc will lead to increased apoptosis of colon cancer cells.
Phosphatidylinositol-3-kinase (PI3K) is one of the most important intracellular pathways as a master regulator for cancer. CRC might be effectively treated by inhibiting PI3K signaling [38]. A lipid kinase called PI3K, which is encoded by PIK3CA, is essential for activating and controlling the signaling pathways involved in cell growth, migration, survival, apoptosis, and metabolism [39]. Mutations in the PIK3CA gene can start the PI3K/AKT/mTOR pathway’s constitutive activation, which leads to carcinogenesis and tumor growth [40]. Meanwhile, the upregulation of PI3K enhances prostaglandin-endoperoxide synthase two activity and prostaglandin E2 synthesis, which inhibits the apoptosis of CRC cells [41]. In our study, it was found that quercetin in DPT inhibits the PI3K-Akt signaling pathway (has04151) because it has an effect on inhibiting proliferation and increasing apoptosis of colon cancer cells. Fortunately, according to our study, previous studies have proven that quercetin can inhibit protein kinase B (AKT) phosphorylation in CRC cells such as HT-29, CaCo-2, SLJJ, and HCT. In addition, quercetin also inhibits PI3K/AKT in Caco-2 and DLD-1 cells [42].
The MAPK pathway is one of the cellular signaling pathways that plays a crucial role in regulating cell proliferation and differentiation in organisms. In colon cancer, the MAPK pathway can be activated abnormally and continuously, leading to cancer cells’ continued growth and survival. Abnormal activation of the MAPK pathway in colon cancer can occur through several mechanisms, including mutations in genes that regulate the MAPK pathway, activation of receptors that are associated with the MAPK pathway, or increased production of signaling molecules associated with the MAPK pathway [43]. Abnormal activation of the MAPK pathway in colon cancer can cause cellular changes such as uncontrolled proliferation, resistance to cancer therapy, and the ability of cancer cells to spread to tissues and organs in the body (metastasis) [44]. Therefore, the MAPK pathway is one of the important targets in the development of colon cancer therapy.
In our study, it was found that quercetin acts on the MAPK signaling pathway (hsa04010). Specifically, quercetin can target the c-Jun target gene on the MAPK pathway, which plays an important role in the development and progression of CRC. C-Jun is a transcription factor protein produced from the c-jun gene and is one of the downstream targets of the MAPK pathway. Abnormal activation of the MAPK pathway can increase c-jun expression, triggering the proliferation of CRC cells and enhancing their invasive ability [45]. Therefore, inhibiting the activation of the c-Jun target gene is a promising strategy to inhibit the growth of CRC cells. Our study was supported by previous research that revealed quercetin can inhibit the activation of the MAPK pathway, including the K-Ras-c-Jun pathway, in CRC cells. Quercetin can inhibit K-Ras activity by inhibiting its interaction with downstream effectors, such as Raf and Mitogen-activated protein kinase, thus preventing MAPK pathway activation. Quercetin can also inhibit c-Jun expression by inhibiting the activation of the c-Jun N-terminal kinase pathway, which is another downstream target of the MAPK pathway [42].
Our research findings also indicated that quercetin has been shown to play a role in regulating the expression of genes involved in the P53 signaling pathway (hsa04115), including the target genes CASP3, BCL2, and BAX. CASP3 is a key enzyme involved in the process of programmed cell death, or apoptosis, and is activated in response to various stimuli, including DNA damage [46]. Quercetin has been shown to increase the expression of CASP3, thereby promoting apoptosis in colon cancer cells [47]. BCL2 and BAX are members of the BCL2 family of proteins, which play a key role in regulating apoptosis. BCL2 is an anti-apoptotic protein that blocks the activity of pro-apoptotic proteins such as BAX [48]. Quercetin has been shown to decrease the expression of BCL2 while increasing the expression of BAX, thereby shifting the balance toward apoptosis and inhibiting the growth of colon cancer cells [49]. Overall, quercetin appears to exert its anticancer effects in part by regulating the expression of genes involved in the P53 signaling pathway, including CASP3, BCL2, and BAX.Top of Form
This research has several limitations. Metabolite profiling still uses an untargeted approach; therefore, identifying compounds and quantifying the concentration still need to be carried out further. This research is still focused on in silico to reveal the mechanism of action; therefore, it is still predictive.
CONCLUSION
Based on our research findings, we discovered that DPT contains 18 active compounds with 39 potential targets. Pharmacological network analysis revealed that DPT contains quercetin and Phyllanthusiin E compounds that exhibit pharmacological effects on CRC by targeting specific molecules such as MYC, CASP3, JUN, MAPK1, BCL2, MAPK8, BAX, and PIK3CG. Furthermore, KEGG pathway analysis in our study indicated that the quercetin and Phyllanthusiin E compounds in DPT interact with various signaling pathways related to CRC development, including CRC (hsa05210), apoptosis (hsa04210), P53 signaling pathway (hsa04115), MAPK signaling pathway (hsa04010), Wnt signaling pathway (hsa04310), and PI3K-Akt signaling pathway (hsa04151). The findings of this study can serve as a basis for further research. In future studies, it is recommended to conduct in vitro testing on cancer cells and in vivo testing on animal models of CRC using the active anti-cancer compounds found in DPT. Additionally, it is suggested to delve deeper into the molecular mechanisms involved in the pharmacological effects of quercetin and Phyllanthusiin E on CRC. This may involve further analysis of the signaling pathways and the PPIs that influence the effects of these compounds on specific target genes.
LIST OF ABBREVIATIONS
LC-MS/MS, Liquid chromatography-tandem mass spectrometry; PI3K, Phosphatidylinositol-3-kinase; PI3K-Akt, Phosphatidylinositol 3-kinase-protein kinase B; PIK3CG, Phosphatidylinositol-4,5- bisphosphate 3-kinase catalytic subunit gamma.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research was funded by the Competitive Research of the Faculty of Medicine and Health Sciences, UIN Maulana Malik Ibrahim, in the year 2022.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Sawicki T, Ruszkowska M, Danielewicz A, Nied?wiedzka E, Ar?ukowicz T, Przyby?owicz KE. A eeview of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers. 2021;13(9):2025.
2. He Q, Long J, Yin Y, Li Y, Lei X, Li Z, et al. Emerging roles of lncRNAs in the formation and progression of colorectal cancer. Front Oncol. 2020;9:1542.
3. Patel M, Horgan PG, McMillan DC, Edwards J. NF-κB pathways in the development and progression of colorectal cancer. Transl Res. 2018;197:43–56.
4. Wang D, DuBois RN. Pro-inflammatory prostaglandins and progression of colorectal cancer. Cancer Lett. 2008;267(2):197–203.
5. Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14(10):101174.
6. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
7. As NA, Mubarakati NJ. Bioprospeksi Benalu Teh–Benalu Mangga Sekarang dan yang Akan Datang (Terapi Adjuvan terhadap Hipertensi). 1st edition. Malang, Indonesia: Inara Publisher; 2021.
8. Mutiah R. Cytotoxic activities profile of parasite mango (Dendrophthoe Pentandra) from various areas in Indonesia against T47d breast cancer cells and normal vero cell lines. J Islamic Pharm. 2019;4(1):1.
9. Artanti N, Firmansyah H, Darmawan A. Bioactivities evaluation of Indonesian mistletoes (Dendrophthoe pentandra (L.) Miq.) leaves extracts. J Appl Pharm Sci. 2012;2:24–7.
10. Oktaviana NA, Sjakoer NAA, Mubarakati NJ. Effect of mistletoe (Tea and Mango) extract combination on histopathological profile of brain in hypertensive rats treated with deoxycorticosterone acetate (DOCA)-salt. Biota Biologi Dan Pendidikan Biologi. 2021;14(1):21–33.
11. Endharti AT, Wulandari A, Listyana A, Norahmawati E, Permana S. Dendrophthoe pentandra (L.) Miq extract effectively inhibits inflammation, proliferation and induces p53 expression on colitis-associated colon cancer. BMC Complement Altern Med. 2016;16(1):1–8.
12. Mutiah R, Listiyana A, Indradmojo C, Griana TP, Dwi HH, Atmaja RRD. Induction of apoptosis and phase-cell cycle inhibition of G0-G1, S, G2-M of T47D breast cancer cells on treatment with ethyl acetate fraction of jackfruit parasite leaves (Macrosolen cochinensis). J Appl Pharm Sci. 2017;07(10):10.
13. Yee LS, Fauzi NFM, Najihah NN, Daud NM, Sulain M. Study of Dendrophthoe pentandra ethyl acetate extract as potential anticancer candidate on safety and toxicity aspects. J Anal Pharm Res. 2017;6(1):1–11.
14. Tang H, He S, Zhang X, Luo S, Zhang B, Duan X, et al. A network pharmacology approach to uncover the pharmacological mechanism of XuanHuSuo powder on osteoarthritis. Evid-Based Complement Altern Med. 2016;2016:3246946.
15. Tang F, Tang Q, Tian Y, Fan Q, Huang Y, Tan X. Network pharmacology-based prediction of the active ingredients and potential targets of Mahuang Fuzi Xixin decoction for application to allergic rhinitis. J Ethnopharmacol. 2015;176:402–12.
16. Liu H, Zeng L, Yang K, Zhang G. A network pharmacology approach to explore the pharmacological mechanism of xiaoyao powder on anovulatory infertility. Evid-Based Complement Altern Med. 2016;2016:2960372.
17. Zhang S, Shan L, Li Q, Wang X, Li S, Zhang Y, et al. Systematic analysis of the multiple bioactivities of green tea through a network pharmacology approach. Evid-Based Complement Altern Med. 2014;2014:512081.
18. Fang J, Wang L, Wu T, Yang C, Gao L, Cai H, et al. Network pharmacology-based study on the mechanism of action for herbal medicines in Alzheimer treatment. J Ethnopharmacol. 2017;196:281–92.
19. Zeng L, Yang K. Exploring the pharmacological mechanism of Yanghe decoction on HER2-positive breast cancer by a network pharmacology approach. J Ethnopharmacol. 2017;199:68–85.
20. Mutiah R, Sari RA, Firsyaradha WY, Listiyana A, Indrawijaya YYA, Wafi A, et al. Activity and toxicity of Eleutherine palmifolia (L.) Merr. Extract on BALB/c mice colitis-associated colon cancer model. Asian Pac J Cancer Prev APJCP. 2020;21(12):3579–86.
21. Mutiah R, Bhagawan WS, Ma’arif B, Rahmandika JMS. Metabolite fingerprinting Eleutherine palmifolia (L.) Merr. Using UPLC-QTOF-MS/MS. Majalah Obat Tradisional. 2019;24(3):3.
22. Mutiah R, Hariz MF, Indrawijaya YYA, Ma’arif B. In silico prediction of isoliquiritigenin and oxyresveratrol compounds to BCL-2 dan VEGF-2 receptors. Indones J Cancer Chemoprev. 2019;10(2):2.
23. Rask-Andersen M, Almén MS, Schiöth HB. Trends in the exploitation of novel drug targets. Nat Rev Drug Discov. 2011;10(8):8.
24. Liu X, Wu J, Zhang D, Wang K, Duan X, Zhang X. A network pharmacology approach to uncover the multiple mechanisms of Hedyotis diffusa Willd. On colorectal cancer. Evid-Based Complement Altern Med. 2018; 2018:e6517034.
25. Huang S, Zhang Z, Li W, Kong F, Yi P, Huang J, et al. Network pharmacology-based prediction and verification of the active ingredients and potential targets of Zuojinwan for treating colorectal cancer. Drug Des Devel Ther. 2020;14:2725–40.
26. Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57.
27. Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–8.
28. Zhou Z, Chen B, Chen S, Lin M, Chen Y, Jin S, et al. Applications of network pharmacology in traditional Chinese medicine research. Evid-Based Complement Alternat Med. 2020;2020:e1646905.
29. Nogales C, Mamdouh ZM, List M, Kiel C, Casas AI, Schmidt HHHW. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci. 2022;43(2):136–50.
30. Guo L, Shi H, Zhu L. Siteng fang reverses multidrug resistance in gastric cancer: a network pharmacology and molecular docking study. Front Oncol. 2021;11:671382.
31. He Z, Gao K, Dong L, Liu L, Qu X, Zou Z, et al. Drug screening and biomarker gene investigation in cancer therapy through the human transcriptional regulatory network. Comput Struct Biotechnol J. 2023;21:1557–72.
32. Wang X, Wang ZY, Zheng JH, Li S. TCM network pharmacology: a new trend towards combining computational, experimental and clinical approaches. Chin J Nat Med. 2021;19(1):1–11.
33. Chaudhary RK, Khanal P, Mateti UV, Shastry CS, Shetty J. Identification of hub genes involved in cisplatin resistance in head and neck cancer. J Genet Eng Biotechnol. 2023;21(1):9.
34. Gu J, Gui Y, Chen L, Yuan G, Lu HZ, Xu X. Use of natural products as chemical library for drug discovery and network pharmacology. PLoS One. 2013;8(4):e62839.
35. Batool S, Javed MR, Aslam S, Noor F, Javed HMF, Seemab R, et al. Network pharmacology and bioinformatics approach reveal the multi-target pharmacological mechanism of Fumaria indica in the treatment of liver cancer. Pharmaceuticals. 2022;15(6):654.
36. Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36(11):11.
37. Zhang S, Li Y, Wu Y, Shi K, Bing L, Hao J. Wnt/β-catenin signaling pathway upregulates c-Myc expression to promote cell proliferation of P19 teratocarcinoma cells. Anat Rec. 2012;295(12):2104–13.
38. Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18(1):26.
39. Cathomas G. PIK3CA in colorectal cancer. Front Oncol. 2014;4:35.
40. Chong ML, Loh M, Thakkar B, Pang B, Iacopetta B, Soong R. Phosphatidylinositol-3-kinase pathway aberrations in gastric and colorectal cancer: meta-analysis, co-occurrence and ethnic variation. Int J Cancer. 2014;134(5):1232–8.
41. Anselmi Júnior RA, de Souza CM, de Azevedo MLV, Netto MRM, Baldin RKS, Sebastião APM, et al. The role of phosphatidylinositol 3 kinase (PI3K) and cycloxygenase-2 (COX2) in carcinogenesis of colorectal polyps. J Coloproctol. 2018;38(1):1–8.
42. Refolo MG, D’Alessandro R, Malerba N, Laezza C, Bifulco M, Messa C, et al. Anti proliferative and pro apoptotic effects of flavonoid quercetin are mediated by CB1 receptor in human colon cancer cell lines. J Cell Physiol. 2015;230(12):2973–80.
43. Vega P, Valentín F, Cubiella J. Colorectal cancer diagnosis: pitfalls and opportunities. World J Gastrointest Oncol. 2015;7(12):422–33.
44. Al-Sohaily S, Biankin A, Leong R, Kohonen-Corish M, Warusavitarne J. Molecular pathways in colorectal cancer. J Gastroenterol Hepatol. 2012;27(9):1423–31.
45. Lee SJ, Moon GS, Jung KH, Kim WJ, Moon SK. C-Jun N-terminal kinase 1 is required for cordycepin-mediated induction of G2/M cell-cycle arrest via p21WAF1 expression in human colon cancer cells. Food Chem Toxicol. 2010;48(1):277–83.
46. Zhou M, Liu X, Li Z, Huang Q, Li F, Li CY. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int J Cancer. 2018;143(4):921–30.
47. Chien SY, Wu YC, Chung JG, Yang JS, Lu HF, Tsou MF, et al. Quercetin-induced apoptosis acts through mitochondrial- and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum Exp Toxicol. 2009;28(8):493–503.
48. Khodapasand E, Jafarzadeh N, Farrokhi F, Kamalidehghan B, Houshmand M. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran Biomed J. 2015;19(2):69–75.
49. Neamtu AA, Maghiar TA, Alaya A, Olah NK, Turcus V, Pelea D, et al. A comprehensive view on the quercetin impact on colorectal cancer. Molecules. 2022;27(6):6.