INTRODUCTION
Erectile function is dependent on a balance between relaxing and contracting factors acting on cavernous smooth muscle, which is regulated by neural and local control mechanisms. Therefore, changes in these mechanisms may lead to erectile dysfunction (ED) [1]. This clinical condition has been defined by the United States National Institutes of Health as the inability to achieve or maintain a penile erection that would enable satisfactory sexual activity [2].
ED is closely associated with chronic diseases like diabetes and systemic arterial hypertension [3]. Several studies describe a higher incidence of ED in hypertensive men when they occur in normotensive individuals [4–6]. In addition, studies have identified ED as a predictive factor for the development of hypertension [7]. In both situations, ED is triggered by the deregulation of endothelial factors and increased smooth muscle contraction, which consequently causes an increase in vascular pressure, poor cavernous perfusion, and inadequate intumescence [7].
ED treatment is based on the use of phosphodiesterase type 5 inhibitors (iPDE-5), such as sildenafil, vardenafil, and tadalafil [8]. However, the number of studies seeking to develop new strategies for the treatment of ED has been increasing [9] since treatment with iPDE-5 is less effective in patients with an impaired nitric oxide (NO) pathway, especially in individuals with vascular diseases [10]. Antioxidant compounds have been gaining prominence in these studies as they reduce oxidative damage, improve NO bioavailability and have a protective effect on erectile function [11–14].
5-Isopropyl-2-methylphenol is an aromatic monoterpene found in essential oils from various plants, such as Origanum vulgarem (oregano), Thymus vulgaris (thyme), and Citrus aurantium bergamia (bergamot) [15], and has been shown to have potent antioxidant activity [16,17] in addition to antifungal [18], antibacterial [19], antiviral [20], anti-inflammatory [21], and anticarcinogenic [22] activities.
Some studies highlighted the free-radical scavenging activity induced by 5-isopropyl-2-methylphenol, with the compound enhancing antioxidant enzyme activity and non-enzymatic antioxidant activity [15]. In addition, 5-isopropyl-2-methylphenol plays a role in the cardiovascular system, acting as a vasodilator through the interaction with voltage-gated calcium and transient receptor potential (TRP) channels, which also contribute to a hypotensive effect [23,24]. Based on the range of pharmacological activities already described for 5-isopropyl-2-methylphenol and especially its antioxidant activity, this study aimed to evaluate the effect of 5-isopropyl-2-methylphenol on the ED of spontaneously hypertensive rats (SHR).
MATERIALS AND METHODS
Animals
All protocols were carried out using 12-week-old Wistar Kyoto (WKY) and SHR from the IPeFarM Animal Production Unit of the Federal University of Paraiba, where the rats were held under a controlled temperature (21°C ± 1°C) in a 12-hour light/12-hour dark cycle with water and food ad libitum. All experimental protocols were approved by the Animal Research Ethics Committee of the Federal University of Paraiba under certificate no 132/2017.
Study design
The rats were randomly divided into five experimental groups: normotensive control (WKY-CTL; n = 8); hypertensive control (SHR-CTL; n = 8); hypertensive rats treated with 50 mg/kg/day 5-Isopropyl-2-methylphenol (SHR-C50; n = 8) or 100 mg/kg/day 5-Isopropyl-2-methylphenol (SHR-C100; n = 8); and hypertensive rats treated with 1.5 mg/kg/day sildenafil (SHR-S1.5, n = 8). All animals received their treatments intragastrically for 4 weeks.
Reagents
To carry out the experiments, the following substances were used: sodium heparin, L(-)-phenylephrine (Phe) hydrochloride, acetylcholine (ACh) hydrochloride, sodium nitroprusside (SNP), dihydroethidium (DHE), 4-hydroxy-TEMPO (tempol) and Cremophor®, all of which were obtained from Sigma-Aldrich Brazil Ltda. (São Paulo-SP, Brazil); xylazine hydrochloride and ketamine hydrochloride, which were both obtained from Syntec (Santana de Parnaíba—SP, Brazil); and sildenafil citrate, which was obtained from the Sigma-Aldrich Brazil Ltda. (São Paulo-SP, Brazil). 5-Isopropyl-2-methylphenol (carvacrol) was obtained from Sigma-Aldrich Brazil Ltda at 98% purity, with the reference 28219710G, batch #STBH1940, molecular formula C10H14O, molecular mass 150.217 g/mol, and density 0.98 g/ml. The stock solutions of 5-Isopropyl-2-methylphenol were prepared from the solubilization of this substance with Cremophor®. The desired concentrations were obtained by diluting it in saline solution. The concentrations of Cremophor® in the solution did not exceed 0.03%.
Systolic blood pressure (SBP) measurement
The rats’ SBP was evaluated weekly using the tail-cuff method (Panlab, Harvard Apparatus, Spain). To measure the blood pressure, the rats were kept in a heated acrylic container (28°C–30°C) for 10 minutes before the measurement to make the caudal artery pulsation more readily detectable. At least three successive measurements were recorded in the data acquisition system (LabChart® software, version 7.1; ADInstruments, Colorado Springs, CO) to obtain the mean SBP.
Intracavernosal pressure (ICP) measurement
Under anesthesia, a small incision was made in the cervical region for dissection and exteriorization of the right common carotid artery, where a polyethylene (PE) catheter was implanted (PE-10 tube soldered to a PE-50 catheter) to access the thoracic aorta and perform continuous measurements, mean arterial pressure (MAP). Then, the cavernous nerve was identified and stimulated using a bipolar bronze electrode (Animal Nerve Stimulating Electrode, MLA0320, ADInstruments) connected to a stimulus generator (Stimulus generators contained in PowerLab®, ADInstruments). Consecutive electrical stimulation with 1 ms and 6 V pulses at different frequencies (0.2, 0.4, 0.6, 1, 2, 4, 8, and 12 Hz) and lasting 45 seconds were induced in the ganglion to measure the ICP and to construct a frequency response curve. ICP was measured by introducing a 30 G needle connected to a catheter in the crura region of the left corpora cavernosa. MAP and ICP variations were measured with pressure transducers (Disposable BP Transducer, MLT0699, ADInstruments) coupled to the PowerLab® data acquisition system (LabChart® software, version 8.1; ADInstruments, Colorado Springs, CO, USA).
Functional studies in strips of rat corpora cavernosa
For dissection and removal of the rat corpora cavernosa, the penis was removed at the level of the attachment to the ischium and immersed in Krebs solution with the following composition (mM): 118.0 NaCl; 4.7 KCl; 1.56 CaCl2 2H2O; 1.2 KH2PO4; 1.17 MgSO4; 25.0 NaHCO3 and 5.5 glucose. Strips of corpus cavernosum were suspended by metal rods connected to a force transducer and inserted into tanks for isolated organ baths (Panlab Multi Chamber Organ Baths, ADInstruments) containing 10 ml of solution at 37°C that was continuously bubbled with a mixture of 95% O2 and 5% CO2 (pH 7.4). The strips were maintained under a basal tension of 0.5 g for a stabilization period of 60 minutes. The tension changes were measured using isometric transducers (MLT020, ADInstruments, Australia) and recorded in the PowerLab® system (ML870/P, LabChart version 7.0, ADInstruments, Australia).
The contractility of the rat corpora cavernosa was evaluated by increasing the cumulative concentration of Phe (10 nM–300 μM) and via electrical field stimulation (EFS). The EFS was performed using 50 V electric pulses having a duration of 1 ms at different frequencies (1, 2, 4, 8, and 16 Hz) with a duration of 10 seconds for each frequency. The contractile response of the corpora cavernosa to Phe was also evaluated by preincubation for 30 minutes with tempol (1 mM), which is a superoxide dismutase (SOD) mimetic. The relaxation response of the corpora cavernosa in the different treatment groups was assessed by increasing the cumulative concentration to ACh (0.1 nM–100 μM), which is an endothelial muscarinic agonist, or to SNP (10 nM–300 μM), which is a spontaneous NO donor.
Evaluation of superoxide anion production
Reactive oxygen species (ROS) generation in the rat corpus cavernosum was detected with the fluorescent dye DHE. The rat corpora cavernosa were isolated, embedded into the tissue tek optimal cutting temperature compound (OCT) embedding medium, and frozen in liquid nitrogen. Subsequently, sections of corpora cavernosa (10 μM) were incubated with 5 μM DHE at 37°C for 30 minutes in a humid chamber and protected from light. The intensity of the fluorescence emitted by DHE was used to measure the superoxide anion production in the different groups. The digital images were captured using a fluorescence microscope (NIKON Eclipse Ti-E, NIKON, Japan) for further analysis.
Statistical analysis
The data are expressed as the mean ± SEM. For statistical analysis, one-way or two-way ANOVA was used, followed by the Bonferroni post hoc test. The differences between the means were considered significant when p < 0.05. The data were analyzed and plotted in the statistical software GraphPad Prism 7.0®. The maximum relaxation corresponded to the maximum effect (Emax) for the highest concentration used. Pharmacological potency was determined using the EC50 (concentration that induces a response halfway between the baseline and maximum).
RESULTS
5-Isopropyl-2-methylphenol induces an antihypertensive effect in SHR
Treatment with 5-Isopropyl-2-methylphenol at doses of 50 and 100 mg/kg and sildenafil at a dose of 1.5 mg/kg decreased blood pressure in SHR animals compared to the SHR-CTL group after the second week of treatment. However, none of the experimental groups could revert the SBP value to the level of the WKY-CTL group (Fig. 1; Table 1).
5-Isopropyl-2-methyl phenol improves the ICP/MAP ratio in SHR
The representative records of the ICP/MAP ratio in the different experimental groups are shown in Figure 2a. The ICP/MAP ratio was significantly lower in the SHR-CTL group when compared to that in the WKY-CTL group (Fig. 2b and c). Treatment with 5-Isopropyl-2-methylphenol at a dose of 100 mg/kg and with sildenafil at a dose of 1.5 mg/kg significantly improved hypertensive animals’ ICP/MAP ratio. Meanwhile, the SHR-C50 group showed a significant increase in the ICP/MAP ratio only at the 12 Hz frequency compared to the SHR-CTL group.
5-Isopropyl-2-methyl phenol improves relaxation of the corpora cavernosa when exposed to ACh and SNP in SHR
ACh (0.1 nM–100 μM) induced significantly lower relaxation in the corpora cavernosa of the SHR-CTL group when compared to that in the corpora cavernosa of the WKY-CTL group (p < 0.05) (Fig. 3a). The treatments of the SHR-C100 and SHR-S1.5 groups improved the relaxation response induced by ACh in hypertensive rats, presenting a reply similar to that observed in the WKY-CTL group, thus reversing the endothelial dysfunction associated with hypertension. However, treatment of the SHR-C50 group was not able to improve the inadequate relaxant response in hypertension (Fig. 3a).
SNP (10 nM–300 μM) induced significantly lower relaxation in the corpora cavernosa of the SHR-CTL group when compared to that in the corpora cavernosa of the WKY-CTL group (p < 0.05) (Fig. 3b). However, in the SHR-C50, SHR-C100, and SHR-S1.5 groups, the SNP-induced relaxation response in the corpora cavernosa was restored (p < 0.05) (Fig. 3b).
5-Isopropyl-2-methyl phenol reduces hypercontractility of the corpora cavernosa in SHR
Phe (10 nM–300 μM) induced significantly greater contraction in the corpora cavernosa of the SHR-CTL group when compared to the corpora cavernosa of the WKY-CTL group (p < 0.05) (Fig. 4a). In the SHR-C50 and SHR-C100 groups (Fig. 4a), the hypercontractility response to Phe observed in the SHR-CTL group was reduced (p < 0.05). On the other hand, the SHR-S1.5 group did not change the Phe hypercontractility observed in the SHR-CTL group (Fig. 4a).
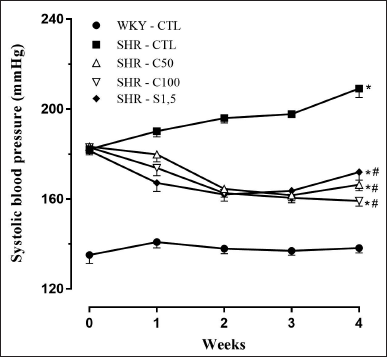 | Figure 1. SBP (mmHg) weekly changes measured by tail cuff. Groups: WKY-CTL (n = 6; ?); SHR-CTL (n = 5; ?); SHR-C50 (n = 6; Δ); SHR-C100 (n = 6; ∇); SHR-S1.5 (n = 6; ♦). Results are expressed as mean ± SEM. *p < 0.05 versus. WKY-CTL; #p < 0.05 versus SHR-CTL. [Click here to view] |
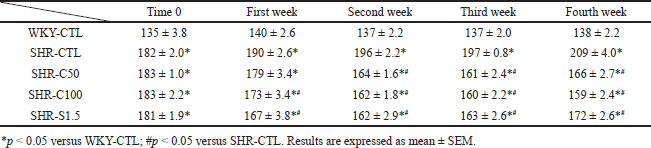 | Table 1. SBP measurement of the different groups over the 4 weeks of treatment. [Click here to view] |
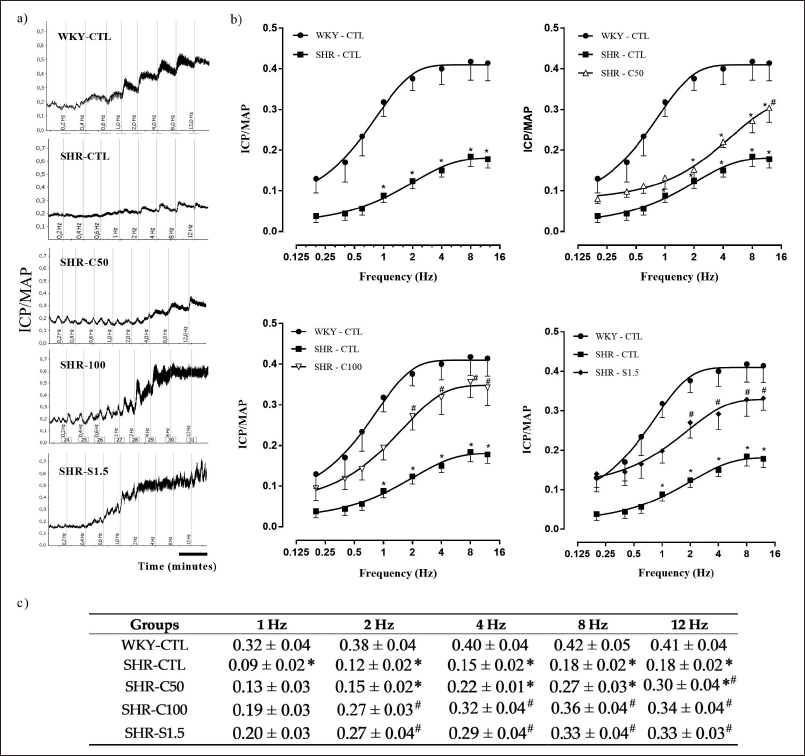 | Figure 2. Effect of carvacrol on ICP/MAP ratio in response to pelvic ganglion stimulations (0.2–12 Hz). a) original tracings of ICP/MAP ratio. b) ICP/MAP ratio curves. c) ICP/MAP values as a function of the different frequencies (Hz) of stimulation in the different groups of animals treated for 4 weeks. Groups: WKY-CTL (n = 5; ?); SHR-CTL (n = 5; ?); SHR-C50 (n = 5; Δ); SHR-C100 (n = 5; ∇); SHR-S1.5 (n = 6; ♦). Results are expressed as mean ± SEM. *p < 0.05 versus. WKY-CTL; #p < 0.05 versus. SHR-CTL. [Click here to view] |
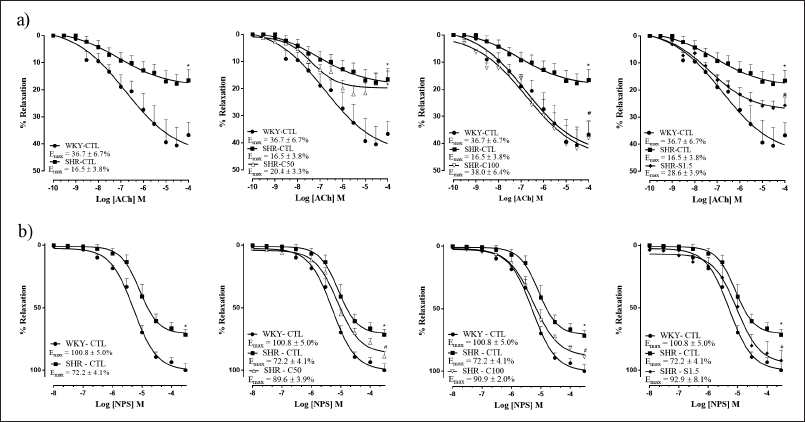 | Figure 3. Vascular reactivity to ACh (a) and SNP (b) in rat corpora cavernosa. Groups: WKY-CTL (n = 5 and 7, respectively; ?); SHR-CTL (n = 6 and 5, respectively; ?); SHR-C50 (n = 5 and 7, respectively; Δ); SHR-C100 (n = 5 and 6, respectively; ∇); SHR-S1.5 (n = 5 and 7, respectively; ♦). Results are expressed as mean ± SEM. *p < 0.05 versus. WKY-CTL; #p < 0.05 versus. SHR-CTL. [Click here to view] |
The representative records of the EFS in the different experimental groups are shown in Figure 4c. Similarly observed with Phe, the SHR-C50 and SHR-C100 groups attenuated the hypercontractility response in the corpora cavernosa to EFS compared to that in the SHR-CTL group (Fig. 4b). The SHR-S1.5 group did not alter the hypercontractility to EFS induced by hypertension compared to the SHR-CTL group (Fig. 4b).
5-Isopropyl-2-methyl phenol reduces hypercontractility in a similar way to tempol of the corpora cavernosa in SHR
The contractile response induced by Phe in the SHR-CTL group was significantly reduced by preincubation of tempol in corpora cavernosa compared to the respective group without this antioxidant (Fig. 5a and e). A similar effect was observed in the SHR-S1.5 group (Fig. 5d and e). In contrast, the contractile response remained unchanged in the SHR-C50 and SHR-C100 groups compared to their respective groups in the absence of tempol (Fig. 5b and c, respectively).
5-Isopropyl-2-methyl phenol reduces ROS in the corpus cavernosum of SHR
The DHE probe emitted baseline fluorescence in the rat corpora cavernosa sections of all experimental groups. The SHR-CTL showed an increase in DHE fluorescence intensity (226.0% ± 13.1%; n = 5) when compared to the DHE fluorescence intensity of the WKY-CTL group (103.3% ± 12.0%; n = 5) (Fig. 6a and b). The corpora cavernosa sections of the SHR-C50 (101.3% ± 10.1%; n = 5), SHR-C100 (103.4% ± 5.0%; n = 6), and SHR-S1.5 (142.5% ± 6.1%; n = 6) groups showed a significant reduction in the fluorescence intensity emitted by the DHE probe when compared to that emitted by the DHE probe in the SHR-CTL group.
DISCUSSION
This study revealed that 5-Isopropyl-2-methylphenol improves the erectile function of SHR, likely through a mechanism involving restoring endothelial dysfunction and reducing rat corpus cavernosum hypercontractility.
First-line therapy for the treatment of ED consists of the administration of iPDE-5. However, their efficacy is reduced when NO bioavailability is decreased. Thus, endothelial dysfunction may limit the efficacy of this therapy [25,26]. Therefore, it is necessary to develop new therapeutic options that may attenuate the progression of the problem in patients with this condition [27].
Plant products have been an important alternative and the subject of several studies that demonstrate a significant effect in the treatment of ED [12,28,13]. In this context, monoterpenes, particularly the isomers carvacrol, and thymol stand out in the literature due to their important activity on the cardiovascular system [29,23,24]. In general, these compounds have antioxidant activity [16] and may, thus, be indicated for protecting cellular constituents susceptible to chronic oxidative damage [17]. Therefore, our group sought to assess whether 5-Isopropyl-2-methylphenol would benefit ED in hypertensive animals.
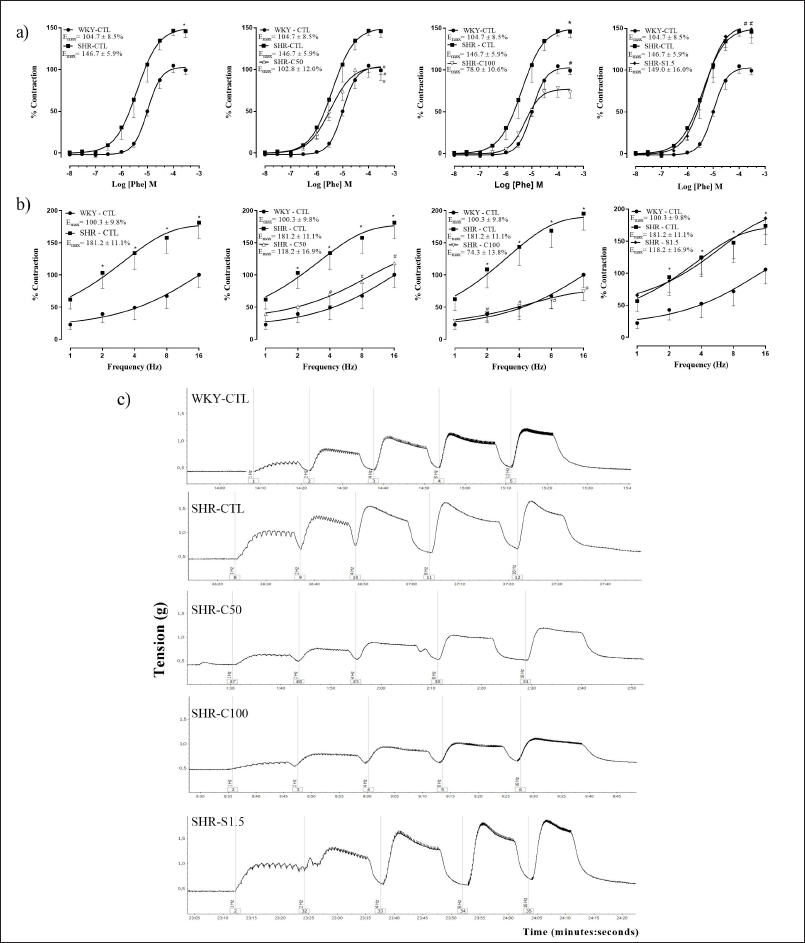 | Figure 4. Concentration-response curves to Phe (10 nM–300 μM) (a) and EFS (1–16 Hz) (b) in rat corpora cavernosa. (c) Original representative tracings showing the contractile frequency-response curves in rat corpora cavernosa. Groups: WKY-CTL (n = 5 and 7, respectively; ?); SHR-CTL (n = 5 and 6; ?); SHR-C50 (n = 6 and 5, respectively; Δ); SHR-C100 (n = 5 and 6, respectively; ∇); SHR-S1.5 (n = 6; ♦). Results are expressed as mean ± SEM. *p < 0.05 versus. WKY-CTL: #p < 0.05 versus. SHR-CTL. [Click here to view] |
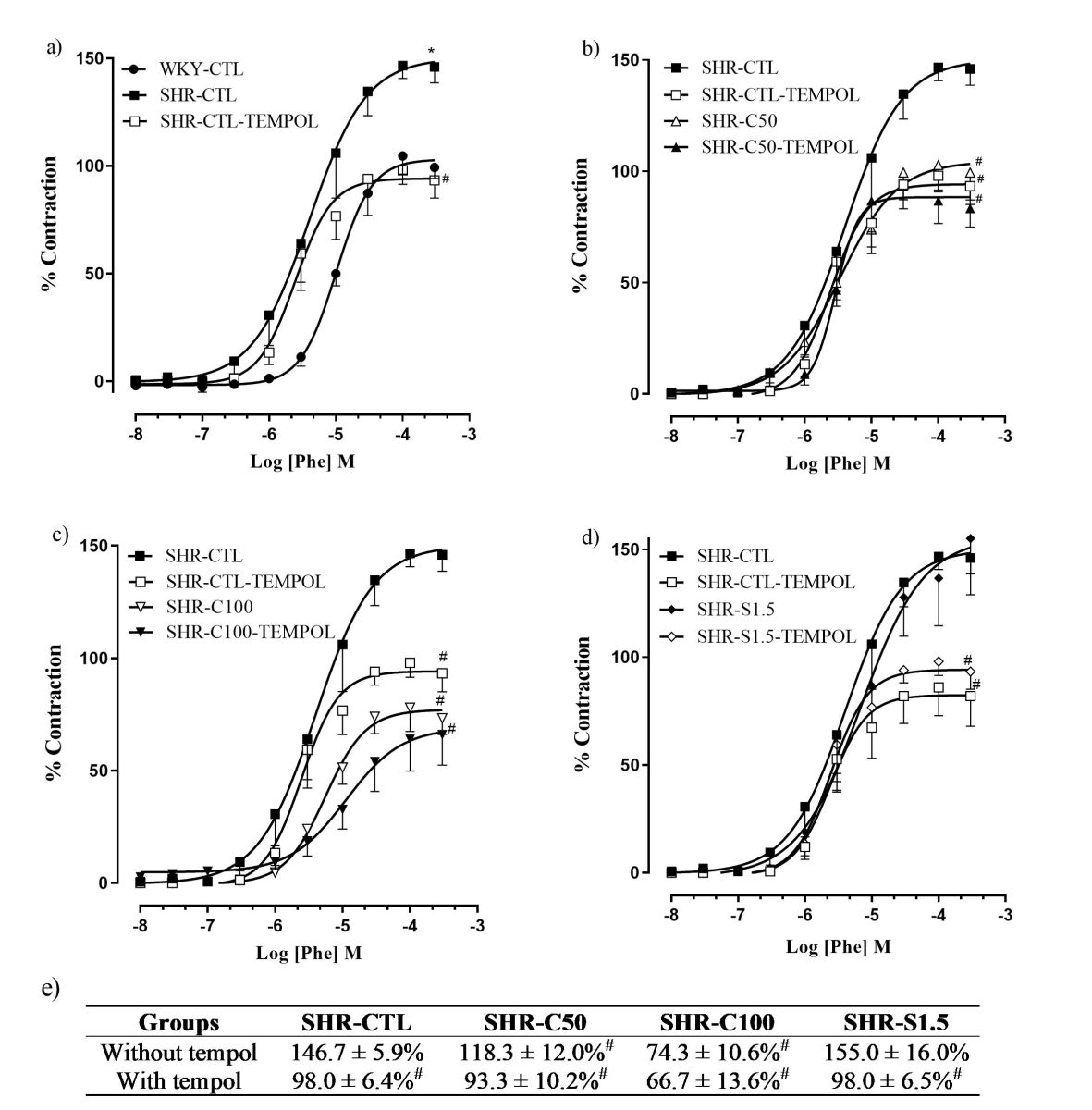 | Figure 5. Effect of carvacrol on concentration-response curves to Phe (10 nM–300 μM), in the presence of tempol (1 mM), in rat corpora in different groups (a–d). Percentage of the maximum effect of the Phe-induced contractile response in the presence and absence of tempol (e). Groups: WKY-CTL (n = 5; ?); SHR-CTL (n = 5; ?); SHR-CTL-TEMPOL (n = 5; ?); SHR-C50 (n = 6; Δ); SHR-C50-TEMPOL (n = 5; ?); SHR–C100 (n = 5; ?); SHR-C100-TEMPOL (n = 6;∇); SHR–S1.5 (n = 6; ◊); SHR-S1.5-TEMPOL (n = 5; ♦). Results are expressed as mean ± SEM. *p < 0.05 versus. WKY-CTL; #p < 0.05 versus. SHR-CTL. [Click here to view] |
Our study confirmed the antihypertensive activity of 5-Isopropyl-2-methylphenol at both doses, corroborating previous studies carried out with SHR animals [30]. The SHR-S1.5 group also showed reduced blood pressure levels, which aligns with literature data showing a reduction in SBP after 2 months of treatment [31]. Interestingly, treatments with 5-Isopropyl-2-methylphenol or sildenafil reduced SBP almost to the same level.
Erectile function was assessed by the ICP/MAP ratio. The ICP/MAP ratio in the SHR-CTL group was significantly reduced compared to that in the WKY-CTL group, confirming the development of ED in SHRs, as previously observed in the literature [32]. In contrast, the SHR-C100 group exhibited a restored erectile function in most stimulation frequencies tested compared to the SHR-CTL group.
These results demonstrate that 5-Isopropyl-2-methylphenol, in addition to having an antihypertensive effect, which may contribute to improving or delaying the development of ED, also influences erectile function, capable of reversing hypertension-associated ED. This statement is in agreement with the results showing that the reduction in pressure levels alone is not effective in improving ED, as demonstrated by the effects induced by some antihypertensive drugs, such as beta-blockers and thiazide diuretics [33,34]. In addition, the SHR-S1.5 group presented an improved erectile function, corroborating with reports in the literature [35–37].
Since NO is considered the primary peripheral pro-erectile physiological regulator and changes in its synthesis or bioavailability may lead to the development of ED [38,39], the activity of ACh was assessed to determine whether there is any impairment of endothelial function. Thus, in the present study, we confirmed the dysfunction of ACh-induced relaxation in SHR, as previously described [40,41].
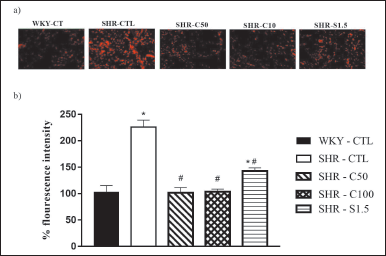 | Figure 6. Effect of carvacrol on ROS in rat corpora cavernosa. Basal fluorescence intensity of DHE in transverse sections of rat corpora cavernosa. Groups: WKY-CTL (n = 5); SHR-CTL (n = 5); SHR-C50 (n = 5); SHR-C100 (n = 6); SHR-S1.5 (n = 6). a) Basal fluorescence intensity emitted by the DHE in transverse sections of rat corpora cavernosa (objective 10×). b) Measurement of basal fluorescence intensity of DHE (%) in transverse sections of corpora cavernosa. The data are expressed as mean of percentage of fluorescence in relation to the control ± SEM. *p < 0.05 versus. WKY-CTL, #p < 0.05 versus. SHR-CTL. [Click here to view] |
5-Isopropyl-2-methylphenol at a dose of 100 mg/kg wholly restored endothelial function in corpora cavernosa SHR, which may suggest the involvement of this mechanism in reversing ED. This effect corroborates data from the literature, demonstrating that different antioxidant substances also improve endothelial function in rat corpora cavernosa [42,43,13].
SNP was used to assess endothelium-independent relaxation. Our study showed that hypertension decreases the response to components of the NO-mediated signaling cascade in smooth muscle cells of cavernous corpora of rats, corroborating data already presented in the literature [44].
5-Isopropyl-2-methylphenol and sildenafil treatments improved the SNP-mediated response, similar to the results observed for the WKY-CTL group. These data suggest that the abovementioned treatments improved the NO-mediated signaling cascade. This result is quite significant compared to data in the literature that showed that treatment with other antioxidants, such as α-tocopherol, could not change this parameter [13].
In hypertension, some pathophysiological mechanisms involved in developing ED are related to cavernous smooth muscle hypertrophy, inducing hypercontractility. In our study, we demonstrated that SHR-CTL animals showed an increase in erectile tissue tone, which may contribute to a reduction in blood flow and ICP, as identified in previous studies [41,45,46]. Differing results were observed by Toblli [41], who showed reduced contractility to Phe in strips of cavernous bodies of SHR at 24 weeks of age.
Treatment with 5-Isopropyl-2-methylphenol at both doses (50 or 100 mg/kg) reduced hypercontractility for Phe and EFS in SHR. These results suggest that the improvement in the contractile response induced appears to involve a common pathway mediated by a reduction in the sensitivity of smooth muscle machinery to contractile substances, which contributed to an improvement in erectile function. However, treatment with sildenafil could not reduce hypercontractility, similar to that demonstrated by Toblli [41], suggesting that the improvement of erectile function does not involve the modulation of contractile muscle response.
Oxidative stress appears to significantly modulate the neurovascular alteration involved in the development of ED. Antioxidant agents act by limiting the activity of ROS-generating enzymes, reversing oxidative damage [47,48].
Therefore, the modulation by O2?- of Phe-induced contraction in corpus cavernosum strips of rats pretreated with tempol, a SOD mimetic, was evaluated. The present study demonstrated that the reduced levels of O2?-, evoked by pre-incubation with tempol, negatively modulate the Phe-induced contraction in the corpora cavernosa of SHR-CTL rats.
In contrast, preincubation with tempol in SHR-C50 and SHR-C100 rats had no additive effect in reducing Phe-induced contraction compared to the effect in the absence of this scavenger. Therefore, treatment with 5-Isopropyl-2-methylphenol at both doses probably reduces contractility through a mechanism similar to that induced by tempol. Conversely, pre-incubation with tempol in the SHR-S1.5 group reduced the Phe-mediated contractile response compared to the SHR-CTL group. This suggests that the treatment with sildenafil seems to improve erectile function mainly by other mechanisms.
To confirm the modulating effect of oxidative stress, we evaluated whether treatment with 5-Isopropyl-2-methylphenol was able to modify baseline ROS levels in corpus cavernosum sections of SHR by measuring the DHE fluorescence intensity.
Our results corroborate data in the literature which show an increase in oxidative stress in the cavernous bodies of SHR [44]. Treatment with 5-Isopropyl-2-methylphenol at the two doses studied (50 and 100 mg/kg) was able to reverse the oxidative damage of hypertension. Animals from the SHR-S1.5 group also showed a reduction in the DHE fluorescence intensity; however, this response was not reversed to the baseline levels of the WKY-CTL group. This result suggests that the treatment with sildenafil reduces oxidative stress; however, this does not appear to be the primary mechanism by which erectile function is improved.
The increase in superoxide anion production in hypertension-associated ED is related to increased nicotinamide adenine dinucleotide phosphate oxidase (NADPH) activity [47]. Studies have shown that 5-Isopropyl-2-methylphenol reduces ROS formation through the modulation of NADPH expression, suggesting that 5-Isopropyl-2-methylphenol can prevent the formation of pathological lesions of atherosclerosis [49].
Finally, TRPC3 and TRPC4 channels positively modulate oxidative stress. The TRPC3 channel amplifies the formation of ROS through interaction with NADPH oxidase (Nox2), which causes the stabilization of this protein complex and consequently increases the production of ROS, leading to functional alteration of cells that culminates in tissue damage [50,51]. Considering the wide action of 5-Isopropyl-2-methylphenol on the blockade of TRP receptors, we can hypothesize a possible involvement of the monoterpene studied in the inhibition of these receptors for a more pronounced reduction of oxidative stress, which needs future investigations.
CONCLUSION
In conclusion, our results demonstrate that treatment with 5-Isopropyl-2-methylphenol improves hypertension-associated ED in SHR, at least by a mechanism involving endothelial function restoration and reduction of rat corpora cavernosa hypercontractility.
ACKNOWLEDGMENTS
The authors wish to sincerely thank José Crispim Duarte and Mirian Graciela da Silva Stiebbe Salvadori for their technical assistance.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Grant no. 1803735) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Grant no. 311711/2018-9).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
All experimental protocols were approved by the Animal Research Ethics Committee of the Federal University of Paraiba under certificate no 132/2017.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. de Souza ILL, Ferreira EDS, Vasconcelos LHC, Cavalcante FA, da Silva BA. Erectile dysfunction: key role of cavernous smooth muscle cells. Front Pharmacol. 2022;13:895044.
2. Consensus N. Development panel on impotence. NIH consensus conference. Impotence. JAMA. 1993;270:83–90.
3. Hernandez-Cerda J, Bertomeu-Gonzalez V, Zuazola P, Cordero A. Understanding erectile dysfunction in hypertensive patients: the need for good patient management. Vasc Health Risk Manag. 2020;16:231–9.
4. Doumas M, Tsakiris A, Douma S, Grigorakis A, Papadopoulos A, Hounta A, et al. Factors affecting the increased prevalence of erectile dysfunction in Greek hypertensive compared with normotensive subjects. J Androl. 2006;27(3):469–77.
5. Moreira, ED Jr, Bestane WJ, Bartolo EB, Fittipaldi JA. Prevalence and determinants of erectile dysfunction in Santos, southeastern Brazil. Sao Paulo Med J. 2002;120(2):49–54.
6. Seftel AD, Sun P, Swindle R. The prevalence of hypertension, hyperlipidemia, diabetes mellitus and depression in men with erectile dysfunction. J Urol. 2004;171(6 Pt 1):2341–5.
7. Wang TD, Lee CK, Chia YC, Tsoi K, Buranakitjaroen P, Chen CH, et al. Hypertension and erectile dysfunction: the role of endovascular therapy in Asia. J Clin Hypertens (Greenwich). 2021;23(3):481–8.
8. Ganapathy AA, Priya VH, Kumaran A. Medicinal plants as a potential source of phosphodiesterase-5 inhibitors: a review. 2021;267:113536.
9. Decaluwe K, Pauwels B, Boydens C, Van de Voorde J. Treatment of erectile dysfunction: new targets and strategies from recent research. Pharmacol Biochem Behav. 2014;121:146–57.
10. Albersen M, Shindel AW, Mwamukonda KB, Lue TF. The future is today: emerging drugs for the treatment of erectile dysfunction. Expert Opin Emerg Drugs. 2010;15(3):467–80.
11. Akomolafe SA, Oyeleye SI, Olasehinde TA, Oboh G. Phenolic characterization, antioxidant activities, and inhibitory effects of Physalis angulata and Newbouldia laevis on enzymes linked to erectile dysfunction. Int J Food Prop. 2018;21(1):645–54.
12. Cevik O, Cadirci S, Sener TE, Tinay I, Akbal C, Tavukcu HH, et al. Quercetin treatment against ischemia/reperfusion injury in rat corpus cavernosum tissue: a role on apoptosis and oxidative stress. Free Radic Res. 2013;47(9):683–91.
13. Ushiyama M, Kuramochi T, Yagi S, Katayama S. Antioxidant treatment with alpha-tocopherol improves erectile function in hypertensive rats. Hypertens Res. 2008;31(5):1007–13.
14. Yu W, Wan Z, Qiu XF, Chen Y, Dai YT. Resveratrol, an activator of SIRT1, restores erectile function in streptozotocin-induced diabetic rats. Asian J Androl. 2013;15(5):646–51.
15. Sharifi-Rad M, Varoni EM, Iriti M, Martorell M, Setzer WN, Del Mar Contreras M, et al. Carvacrol and human health: a comprehensive review. Phytother Res. 2018;32(9):1675–87.
16. El-Sayed EM, Abd-Allah AR, Mansour AM, El-Arabey AA. Thymol and carvacrol prevent cisplatin-induced nephrotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. JBMT. 2015;29(4):165–72.
17. Samarghandian S, Farkhondeh T, Samini F, Borji A. Protective effects of carvacrol against oxidative stress induced by chronic stress in rat’s brain, liver, and kidney. Biochem Res Int. 2016;2016:2645237.
18. Chavan PS, Tupe SG. Antifungal activity and mechanism of action of carvacrol and thymol against vineyard and wine spoilage yeasts. Food Control. 2014;46:115–20.
19. Nostro A, Sudano Roccaro A, Bisignano G, Marino A, Cannatelli MA, Pizzimenti FC, et al. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Med Microbiol. 2007;56(Pt 4):519–23.
20. Gilling DH, Kitajima M, Torrey JR, Bright KR. Antiviral efficacy and mechanisms of action of oregano essential oil and its primary component carvacrol against murine norovirus. J Appl Microbiol. 2014;116(5):1149–63.
21. Fachini-Queiroz FC, Kummer R, Estevao-Silva CF, Carvalho MD, Cunha JM, Grespan R, et al. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evid Based Complement Alternat Med. 2012;2012:657026.
22. Yin QH, Yan FX, Zu XY, Wu YH, Wu XP, Liao MC, et al. Anti-proliferative and pro-apoptotic effect of carvacrol on human hepatocellular carcinoma cell line HepG-2. Cytotechnology. 2012;64(1):43–51.
23. Dantas BP, Alves QL, de Assis KS, Ribeiro TP, de Almeida MM, de Vasconcelos AP, et al. Participation of the TRP channel in the cardiovascular effects induced by carvacrol in normotensive rat. Vascul Pharmacol. 2015;67–69:48–58.
24. Peixoto-Neves D, Silva-Alves K, Gomes M, Lima F, Lahlou S, Magalhães P, et al. Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fundam Clin Pharmacol. 2010;24(3):341–50.
25. Kedia GT, Uckert S, Tsikas D, Becker AJ, Kuczyk MA, Bannowsky A. The use of vasoactive drugs in the treatment of male erectile dysfunction: current concepts. J Clin Med. 2020;9(9):2987.
26. Toque HA, Caldwell RW. New approaches to the design and discovery of therapies to prevent erectile dysfunction. Expert Opin Drug Discov. 2014;9(12):1447–69.
27. Decaluwe K, Pauwels B, Verpoest S, Van de Voorde J. New therapeutic targets for the treatment of erectile dysfunction. J Sex Med. 2011;8(12):3271–90.
28. Li Y, Jiang J, He Y, Jiang R, Liu J, Fan Z, et al. Icariin combined with breviscapine improves the erectile function of spontaneously hypertensive rats. J Sex Med. 2014;11(9):2143–52.
29. Aydin Y, Kutlay O, Ari S, Duman S, Uzuner K, Aydin S. Hypotensive effects of carvacrol on the blood pressure of normotensive rats. Planta Med. 2007;73(13):1365–71.
30. Costa HA, Dias CJM, Martins VA, de Araujo SA, da Silva DP, Mendes VS, et al. Effect of treatment with carvacrol and aerobic training on cardiovascular function in spontaneously hypertensive rats. Exp Physiol. 2021;106(4):891–901.
31. Yaguas K, Bautista R, Quiroz Y, Ferrebuz A, Pons H, Franco M, et al. Chronic sildenafil treatment corrects endothelial dysfunction and improves hypertension. Am J Nephrol. 2010;31(4):283–91.
32. He W, Liu J, Liu D, Hu J, Jiang Y, Li M, et al. Alterations in the phosphodiesterase type 5 pathway and oxidative stress correlate with erectile function in spontaneously hypertensive rats. J Cell Mol Med. 2020;24(24):14280–92.
33. Cordero A, Bertomeu-Martinez V, Mazon P, Facila L, Bertomeu-Gonzalez V,Conthe P, et al. Erectile dysfunction in high-risk hypertensive patients treated with beta-blockade agents. Cardiovasc Ther. 2010;28(1):15–22.
34. Sharp RP, Gales BJ. Nebivolol versus other beta blockers in patients with hypertension and erectile dysfunction. Ther Adv Urol. 2017;9(2):59–63.
35. da Silva CN, Nunes KP, Almeida FDM, Costa FLS, Borges PV, Lacativa P, et al. PnPP-19 peptide restores erectile function in hypertensive and diabetic animals through intravenous and topical administration. J Sex Med. 2019;16(3):365–74.
36. Liu G, Sun X, Dai Y, Zheng F, Wang D, Huang Y, et al. Chronic administration of sildenafil modified the impaired VEGF system and improved the erectile function in rats with diabetic erectile dysfunction. J Sex Med. 2010;7(12):3868–78.
37. Sung HH, Kang SJ, Chae MR, Kim HK, Park JK, Kim CY, et al. Effect of BKCa channel opener LDD175 on erectile function in an in vivo diabetic rat model. J Sex Med. 2017;14(1):59–68.
38. Alves-Lopes RU, Neves KB, Silva MA, Olivon VC, Ruginsk SG, Antunes-Rodrigues J, et al. Functional and structural changes in internal pudendal arteries underlie erectile dysfunction induced by androgen deprivation. Asian J Androl. 2017;19(5):526–32.
39. Sangiorgi G, Cereda A, Benedetto D, Bonanni M, Chiricolo G, Cota L, et al. Anatomy, pathophysiology, molecular mechanisms, and clinical management of erectile dysfunction in patients affected by coronary artery disease: a review. Biomedicines. 2021;9(4):432.
40. Behr-Roussel D, Gorny D, Mevel K, Compagnie S, Kern P, Sivan V, et al. Erectile dysfunction: an early marker for hypertension? A longitudinal study in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R276–83.
41. Toblli JE, Cao G, Lombrana A, Rivero M. Functional and morphological improvement in erectile tissue of hypertensive rats by long-term combined therapy with phosphodiesterase type 5 inhibitor and losartan. J Sex Med. 2007;4(5):1291–303.
42. Long H, Jiang J, Xia J, Jiang R. Icariin improves SHR erectile function via inhibiting eNOS uncoupling. Andrologia. 2018;50(9):e13084.
43. Murat N, Korhan P, Kizer O, Evcim S, Kefi A, Demir O, et al. Resveratrol protects and restores endothelium-dependent relaxation in hypercholesterolemic rabbit corpus cavernosum. J Sex Med. 2016;13(1):12–21.
44. Ushiyama M, Morita T, Kuramochi T, Yagi S, Katayama S. Erectile dysfunction in hypertensive rats results from impairment of the relaxation evoked by neurogenic carbon monoxide and nitric oxide. Hypertens Res. 2004;27(4):253–61.
45. Toblli JE, Stella I, Inserra F, Ferder L, Zeller F, Mazza ON. Morphological changes in cavernous tissue in spontaneously hypertensive rats. Am J Hypertens. 2000;13(6 Pt 1):686–92.
46. Wang XY, Huang W, Zhang Y. Relation between hypertension and erectile dysfunction: a meta-analysisof cross-section studies. Int J Impot Res. 2018;30(3):141–6.
47. Agarwal A, Nandipati KC, Sharma RK, Zippe CD, Raina R. Role of oxidative stress in the pathophysiological mechanism of erectile dysfunction. J Androl. 2006;27(3):335–47.
48. de Oliveira AA, Nunes KP. Hypertension and erectile dysfunction: breaking down the challenges. Am J Hypertens. 2021;34(2):134–42.
49. Lee KP, Sudjarwo GW, Jung SH, Lee D, Lee DY, Lee GB, et al. Carvacrol inhibits atherosclerotic neointima formation by downregulating reactive oxygen species production in vascular smooth muscle cells. Atherosclerosis. 2015;240(2):367–73.
50. Kitajima N, Numaga-Tomita T, Watanabe M, Kuroda T, Nishimura A, Miyano K, et al. TRPC3 positively regulates reactive oxygen species driving maladaptive cardiac remodeling. Sci Rep. 2016;6:37001.
51. Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, et al. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J Biol Chem. 2006;281(19):13588–95.