INTRODUCTION
The liver is a vital organ that plays a crucial role in maintaining overall health and well-being. It is responsible for a wide range of biochemical reactions and is often referred to as the “metabolic engine room of the body.” The liver is responsible for monitoring, recycling, modifying, and distributing all of the various compounds absorbed from the digestive tract. Additionally, it plays an important role in removing toxic compounds such as pharmaceutical drugs or chemicals. However, due to the non-stop biochemical and xenobiotic action in this organ, it is also a common room for the free radical generation. These free radicals can damage cellular macromolecules and contribute to hepatocellular injury when produced in excess [1].
There are over 200,000 synthetic chemicals in existence that can harm the environment. The liver is the main organ responsible for breaking down these chemicals. Carbon tetrachloride (CCl4) is a commonly used laboratory reagent that is known for its toxicity and ability to cause liver damage and fibrosis. It has been widely used in liver research studies [2]. Lipid peroxyl radicals are created by adding molecular oxygen to carbon-centered radicals (R) during the lipid peroxidation (LPO) process (ROO). Then, under the catalysis of transition metal complexes, these peroxyl radicals disintegrate to produce alkoxyl (RO) or hydroxyl (HO) radicals. Following that, these radicals take part in a series of processes that result in the abstraction of hydrogen and the continuation of the LPO process. The generation of organic hydroperoxides from the creation of peroxyl radicals also results in the removal of hydrogen from other polyunsaturated fatty acids (PUFAs). One initial hit in this reaction can cause the conversion of several PUFAs to lipid hydroperoxides, which is known as propagation. Alkyl, peroxyl, and alkoxyl radicals are used in this procedure in that order. Through rearrangement into a conjugated diene, which keeps more stable byproducts including hydroperoxides, alcohols, aldehydes, and alkanes, the resultant fatty acid radical is stabilised. The first stable byproduct of the LPO reaction is lipid hydroperoxide (ROOH) [3].
Vitamin D is a collection of compounds known as sterols, act like hormones in the body. These compounds bind with proteins called Vitamin D receptors (VDR) inside cells. The VDR complex then communicates with the DNA in the nucleus of the cells, turning on or off certain genes. Vitamin D plays an important role in regulating the levels of calcium and phosphorus in the body, but it also affects the immune system [4], helping the body fight off infections. Studies have shown that vitamin D is involved in the processes of cell division, growth, and differentiation. Additionally, research is ongoing to fully understand the many ways that vitamin D affects the body.
Vitamin D has a vital effect on the immune system and can lower inflammation and fibrosis. Studies have shown that pro-inflammatory indications in liver monocytes and macrophages might regulate the local calciferol production, leading to an increase in the appearance of CYP27B1 and the limited production of 25-hydroxycholecalciferol (25(OH)D), which helps control excessive inflammatory responses. Research suggests that macrophages are found in liver hepatocytes, which means that hepatic creation of 25(OH)D is reduced through inflammatory diseases of the liver [5]. High levels of vitamin D receptor are present in biliary epithelial cells other non-parenchymal cells and macrophages, further emphasizing the important role of vitamin D in the liver.
When the body has enough vitamin D, certain compounds that contribute to cellular oxidative stress are decreased. This is mainly due reduction in the number of receptors on the cell surface that respond to certain molecules. However, when there is not enough vitamin D in the body, the ability to control oxidative stress is compromised, leading to increased cellular damage and a higher rate of cell death. In addition, vitamin D helps to increase the production of an enzyme called glutathione peroxidase, which helps to neutralize harmful molecules known as reactive oxygen species [6].
Liv.52 is a herbal supplement that has been used worldwide since 1955 to support liver health. It works by promoting the breakdown of toxins and protecting the liver from harmful substances found in food and medication. Liv.52 also helps to maintain healthy levels of liver enzymes and markers. In addition, it has been found to protect the liver cells by reducing the damage caused by LPO. Due to its effectiveness, Liv.52 is commonly prescribed for patients with liver disorders in many countries [7].
MATERIAL AND METHODS
Animal care and housing
This study was conducted with the approval of the Institutional Animal Ethical Committee (Meenakshi Medical College Hospital and Research Institute, REG No. 765/03/ca/ Committee for the Purpose of Control and Supervision of Experiments on Animals CPCEA) and in compliance with the CPCEA. Wistar rats with a weight range of 150–200 g were obtained from the Biogen Laboratory Animal Facility, Bangalore, Karnataka. The animals were kept in polypropylene cages and kept in a controlled environment at a temperature of 23°C ± 2°C and a relative humidity of 50%–70% on an alternating 12-hour light/dark cycle. The rats were fed a regular pellet meal from M/s. Hindustan Lever Ltd., Mumbai, India, and had unlimited access to water. The rats were allowed to acclimate to the lab environment for a week before the trial began.
Experimental design
The study involved dividing the animals into six different groups, each containing six animals. The first group, referred to as the control group, did not receive any treatment (Group I). The second group received twice-weekly intraperitoneal injections of 50% CCl4 in olive oil for nine weeks as their CCl4 exposure (Group II). In addition to the CCl4 injections as explained for group II, the third and fourth groups received Liv-52 and vitamin D dissolved in distilled water at doses of 500 IU and 1 ml/kg body weight, respectively. The fifth group received CCl4 injections coupled with a combination of Liv-52 and VD. Group VI, the last group, received the vitamin D and Liv-52 combination without any CCl4 injection.
Samples and parameters
At the end of the experimental period, the blood was collected without EDTA for the separation of serum to determine LPO and various indicators of lipid profiles. The presence of lipid peroxides was determined using the technique developed by Hiroshi et al. [8]. The amount of total cholesterol present was calculated using the method established by Parekh and Jung [9]. The levels of triglycerides (TGLs) and very low-density lipoproteins (VLDL) were measured using the method created by Rice et al. [10]. Lastly, the quantity of high-density lipoproteins (HDL) was determined using the method of Rice et al. [10].
Data analysis
The analysis of data in this study was conducted using the statistical package for social sciences for Windows version 21.0 software. To compare the group means, Duncan’s Multiple Range Test and the one-way ANOVA approach were utilized. A p-value of 0.05 or less was considered statistically significant.
RESULTS
The results in Table 1 show the effects of different treatments on LPO levels in the serum of animals. The hepatotoxic group II animals had significantly higher levels of LPO in the serum compared to the control group I animals (p < 0.001). When compared to the hepatotoxic group II animals, vitamin D treatment (group III) led to a considerable reduction in these levels (p < 0.001). Similar to group II animals treated with CCl4, group IV animals treated with Liv-52 also had a significant decline in LPO levels (p < 0.001). When compared to the hepatotoxic group II animals, the group V animals that received a mixture of vitamin D and Liv-52 showed the largest reduction in LPO levels (p < 0.001). The protective effects of vitamin D alone (group III) were found to be similar to the protection provided by Liv-52 alone (group IV), with no significant differences observed between the two. Furthermore, there was no discernible change in the levels of LPO between the animals in control group I and those in control group VI that received both Liv-52 and vitamin D. In our study, we conducted a series of experiments to validate our findings (see Supplementary Material).
The activity of the serum lipid profile is shown in Table 2. The study found that exposure to CCl4 (group II) resulted in significantly higher levels of total cholesterol, TGL, and VLDL in the rats, while the level of HDL decreased compared to the normal control rats (group I). Treatment with vitamin D (group III) led to a significant decrease in total cholesterol, TGL, and VLDL, while the level of HDL increased compared to the CCl4-treated rats (group II). Similarly, treatment with Liv-52 (group IV) also caused a significant decrease in total cholesterol, TGL, and VLDL, while the level of HDL increased compared to the CCl4-treated rats (group II). However, combining vitamin D and Liv-52 therapy (group V) resulted in a much more significant reduction in total cholesterol, TGL, and VLDL, while the level of HDL increased compared to the CCl4-treated rats (group II). The findings suggest that Liv-52 and vitamin D both protect the liver. The comparison between groups III and IV shows that the protection provided by vitamin D alone is similar to that provided by Liv-52 alone. The combination of vitamin D and Liv-52 did not have any toxic effects on the liver, as evidenced by the lack of a significant difference in total cholesterol, TGL, HDL, and VLDL levels between the control rats treated with just VD (group I) and the control rats treated with just Liv-52 (group VI).
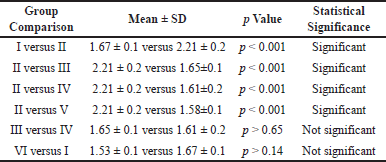 | Table 1. Effect of vitamin D and Liv-52 on LPO level in the serum of control and experimental animals. [Click here to view] |
DISCUSSION
The main causes of CCl4 are damage to the liver caused by LPO, decreased activity of antioxidants, and the creation of free radicals. These effects are due to the presence of high levels of PUFAs and transition metals in biological membranes, which are constantly exposed to various types of damage.
Cells need vitamin D to be protected from damaging oxidative stress and cellular deterioration. It accomplishes this by lowering the blood level of malondialdehyde MDA and raising the body’s total antioxidant capacity. Studies have demonstrated that both in living things and in laboratory settings, a vitamin D shortage can result in an increase in oxidative stress [11]. For instance, in rats, a deficiency in vitamin D has been associated with reduced levels of the antioxidant enzymes catalase and superoxide dismutase (SOD), leading to mild oxidative stress in the liver. On the other hand, when vitamin D is given, the liver’s SOD activity significantly increases and oxidative stress is reduced [11].
Vitamin D has the power to reduce oxidative stress in cells and regulate the generation of free radicals. Binding to the VDR in the nucleus or using the hydrophobic regions of the vitamin D molecule are the two possible mechanisms by which this can occur. According to studies, vitamin D can shield mature red blood cells—even those without a nucleus—from oxidative stress and lipid damage. This shows that vitamin D’s hydrophobic characteristics are what cause its antioxidant action on cell membranes. However, vitamin D inhibits the generation of free radicals in the liver cells of diabetic mice via binding to the VDR in the nucleus [12].
An alternative text for the manuscripts would be: The study by Holick et al. [13] found a correlation between vitamin D deficiency and increased levels of both lipid and protein oxidation. Specifically, individuals with low vitamin D levels had higher concentrations of MDA, a marker of LPO, and carbonyl groups, a marker of protein oxidation, compared to those with sufficient vitamin D levels. This suggests that vitamin D may play a role in regulating oxidative stress, which is linked to various pathological conditions. Baeke et al. [11] conducted a study in 2010 to examine the effects of oral vitamin D and calcium supplementation on diabetic rats’ LPO and antioxidant enzyme activity. The medication reduced LPO levels, which suggests that vitamin D and calcium may help reduce the oxidative stress brought on by diabetes.
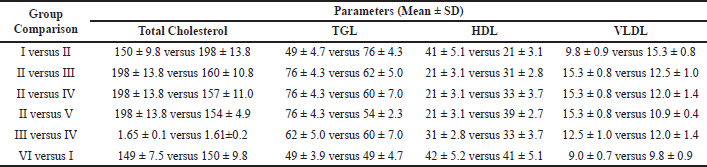 | Table 2. Effect of vitamin D and Liv-52 on lipid profile in the serum of control and experimental animals. [Click here to view] |
Liv.52’s ability to reduce the presence of harmful substances known as peroxides in the body helps protect important components of the liver, such as the lipid membrane and the cytochrome p-450 enzyme system. This, in turn, ensures that the integrity of the cell membrane is maintained, allowing the liver to function at optimal levels. By aiding in the detoxification process and protecting against toxins from food and drugs, additionally, Liv.52 can aid in preserving normal liver enzyme levels. Liv.52 is renowned for boosting the liver’s inherent capacity to break down lipids and keep the body’s metabolism in balance [14].
In this study, it was found that rats treated with CCl4 had elevated levels of LPO in their blood. However, treatment with vitamin D was able to significantly decrease these levels by affecting both the immune system and the expression of certain proteins. Similarly, treatment with Liv-52 was able to decrease LPO by boosting the body’s antioxidant system. When both vitamin D and Liv.52 were used together in rats with liver fibrosis, the levels of LPO were even further reduced. These results suggest that both vitamin D and Liv-52 have a strong ability to decrease LPO.
Damage to intracellular and plasma membranes results from the activated metabolites of CCl4 attacking lipids more readily [15]. The primary cellular effects of CCl4-induced fatty liver include the formation of radicals and lipid oxidation. A significant buildup of lipids in the liver is considered a pathological condition and can lead to fibrotic changes and decreased liver function if left unchecked for a prolonged period. In addition, higher levels of cholesterol, TGLs, and free fatty acids (FFAs) are observed in plasma and tissue samples. CCl4 also accelerates the production of fatty acids and triglycerides from acetate, potentially due to the increased availability of acetate within the liver cells. The production of cholesterol is also increased in CCl4 poisoning [15].
Alternatively, CCl4 has been found to inhibit the breakdown and oxidation of TGLs, leading to a greater availability of fatty acids for storage. Research has also shown that during CCl4 poisoning, there is an increase in the transfer of fatty acids from adipose tissue to the liver, resulting in fat accumulation [16]. Furthermore, CCl4 has been shown to decrease the production of apolipoproteins, which in turn reduces the formation of lipoproteins. In addition, there is evidence that bile acid secretion is also decreased in the presence of CCl4.
Previous studies have shown that after 8 weeks of CCl4 treatment, there is a significant increase in lipid levels, liver enzymes, and markers of oxidative stress, while total protein and HDL levels decrease [17]. Microscopic examination of the treated rats revealed changes in lipid levels and an increase in inflammatory collections, loss of normal liver cells, and the formation of fibrous tissue [18]. The chronic toxicity caused by CCl4 also affects cardiac function, decreasing the activity of antioxidant enzymes and the levels of glutathione, while increasing LPO. In addition, CCl4 treatment causes DNA fragmentation and histopathological abnormalities in rats [19].
Wang, et al. [20] proposed that there is a connection between vitamin D and lipid values. They suggested that when vitamin D levels are high, it can inhibit the production and release of parathyroid hormone (PTH), which in turn can increase the absorption of calcium in the intestines. This increased absorption of calcium can then decrease the absorption of fats due to the formation of insoluble calcium-fatty complexes during digestion. In addition, calcium can promote the synthesis of bile acids from cholesterol, which can lower serum cholesterol levels. When vitamin D levels are not high, PTH levels may not be inhibited, leading to an increase in lipogenesis and a decrease in lipolytic action, ensuing in a higher level of TGLs. However, in the presence of higher vitamin D levels, PTH levels are suppressed, leading to an increase in lipolytic activity and a reduction in TGL levels. By decreasing TGL production and release in the liver and raising the expression of very-low-density lipoprotein (VLDL-C) receptors, vitamin D can also alter lipoprotein metabolism. A high vitamin D level thus causes a drop in TGL and VLDL-C levels.
There is a connection between having low levels of vitamin D and both cardiovascular risk factors and the development of cardiovascular disease. Studies have shown that having abnormal levels of lipids, such as high levels of TGLs, total cholesterol, low-density lipoprotein cholesterol, and low levels of high-density lipoprotein cholesterol, can increase the risk of atherosclerosis and heart disease in adults. It is believed that a deficiency in vitamin D may contribute to this. In addition, studies show that higher vitamin D levels are linked to a healthy lipid profile, whereas lower levels are linked to an increased risk of atherosclerosis [21].
According to a study by Crouse et al. [22], individuals with chronic alcoholism had increased levels of plasma TGLS and cholesterol. The reason for this may be due to a decrease in the removal of TGL-rich lipoproteins and/or an increase in the production of VLDL-TGL. The slowed clearance of chylomicron-TGL could be caused by competition for the removal of excessive VLDL-TGL and by the development of a clearance malfunction. The use of Liv.52 was found to effectively prevent alcohol-induced hypertriglyceridemia, likely due to its impact on lipid levels [22].
According to recent studies, both vitamin D and Liv.52 have been found to have cholesterol-lowering effects. This can be brought on by a reduction in cholesterol absorption or a rise in HDL cholesterol levels. This suggests that excess cholesterol is being moved from other parts of the body to the liver. Furthermore, vitamin D has been shown to activate the enzyme 7a-hydroxylase, which helps in the removal of biliary cholesterol by converting it to bile acids. It is also possible that levels of TGLs and phospholipids may have decreased as a result of vitamin D’s impact on the synthesis of FFAs, which may have suppressed certain FFA-related enzymes [14].
Our research has uncovered strong biochemical evidence that high levels of total cholesterol, TGL, and VLDL were present, while HDL levels were low. When 500 IU/kg of vitamin D was administered alone, there was a significant decrease in the levels of total cholesterol, TGL, and VLDL, and an increase in HDL levels. Similarly, when 1 ml/kg of Liv.52 was administered alone, there was a decrease in the levels of total cholesterol, TGL, and VLDL, and an increase in HDL levels. However, the most significant changes were observed when both vitamin D and Liv-52 were administered together. This combination resulted in a highly significant decrease in the levels of total cholesterol, TGL, and VLDL compared to rats injected with CCl4, and a highly significant increase in HDL levels.
CONCLUSION
Vitamin D supplementation combined with Liv.52 shows promising results in mitigating the effects of CCl4-induced liver disease: The study demonstrated that the administration of vitamin D supplementation along with Liv.52 has a beneficial effect on liver health in Wistar rats exposed to CCl4-induced liver disease. These findings suggest that this combination treatment may have potential therapeutic benefits for liver diseases.
The research indicates that the combined treatment of vitamin D supplementation and Liv.52 helps in reducing LPO in the liver. LPO is a process where free radicals attack and damage lipids, leading to oxidative stress and liver damage. By reducing LPO, the combined treatment can protect liver cells from oxidative damage and maintain their structural integrity.
The study suggests that the combination of vitamin D supplementation and Liv.52 positively influences lipid profiles in Wistar rats with CCl4-induced liver disease. Abnormal lipid profiles, including elevated levels of total cholesterol, TGLs and VLDL, are commonly observed in liver diseases. The combined treatment appears to restore lipid balance, potentially reducing the risk of further liver complications.
Overall, the findings of this research support the potential effectiveness of vitamin D supplementation with Liv.52 in ameliorating liver disease induced by CCl4. The combination treatment shows promise in reducing LPO and improving lipid profiles, indicating its potential as a therapeutic intervention for liver diseases. However, further studies, including human clinical trials, are necessary to validate these findings and establish the optimal dosage and treatment regimen for vitamin D supplementation combined with Liv.52 in the management of liver disease.
LIMITATIONS OF THE STUDY
One potential limitation of the study evaluating the effectiveness of vitamin D supplementation with Liv-52 in Wistar rats on CCl4-induced liver disease, specifically focusing on the effects on LPO and lipid profiles, could be the generalizability of the findings to human subjects. While animal models such as Wistar rats provide valuable insights into disease mechanisms, the response to interventions and the underlying biology can differ between animals and humans. Therefore, caution should be exercised when extrapolating the results to human populations, and further research involving human subjects would be necessary to establish the clinical relevance and applicability of the findings.
ACKNOWLEDGMENT
We wish to thank Meenakshi Medical College Hospital and Research Institute, MAHER (Deemed to be University), for granting us permission and support to carry out the project.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests regarding the publication of this manuscript.
ETHICAL APPROVALS
This study was conducted with the approval of the Institutional Animal Ethical Committee (Meenakshi Medical College Hospital and Research Institute, REG No. 765/03/ca/ Committee for the Purpose of Control and Supervision of Experiments on Animals CPCEA) and in compliance with the CPCEA.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Subramonium A, Pushpangadan P. Development of phytomedicines for liver diseases. Indian J Pharmacol. 1999;31:166175. doi: https://doi.org/313166-1890229_051502
2. Huang Z, Zhai G, Zhang WR, Zhang XR. Study of heteroserum-induced rat liver fibrosis model and its mechanism. World J. Gastroenterol. 1998;4:206–9. doi: https://doi.org/10.3748/wjg.v4.i3.206
3. Cimbaljevi? B, Vasilijevi? A, Cimbaljevi? S, Buzadzi? B, Kora? A, Petrovi? V, et al. Interrelationship of antioxidative status, lipid peroxidation, and lipid profile in insulin-depended and non-insulin depended diabetic patients. Can J Physiol Pharm. 2007;85:997–1003. doi: https://doi.org/10.1139/Y07-088. CCIN:07088
4. White JH.Vitamin D metabolism and signaling in the immune system. Rev Endocr Metab Disord. 2012;13:21–9. doi: https://doi.org/10.1007/s11154-011-9195-z
5. Verway, M, Bouttier M, Wang, TT. Vitamin D induces interleukin-1β expression:paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog. 2013;9:e1003407. doi: https://doi.org/10.1371/journal.ppat.1003407
6. Song L, Papaioannou G, Zhao H. The vitamin D receptor regulates tissue resident macrophage response to injury, Endocrinology. 2016;157:4066–75. doi: https://doi.org/10.1210/en.2016-1474
7. Saini MR, Saini N. Liv. 52 protection against radiation induced lesions in mammalian liver. Radiobiol Radiother (Berl). 1985;26:379–84. doi: https://doi.org/10.1021/ac60294a044
8. Hiroshi O, Nobuko O, Kunio Y. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem.1979;95:351–8. doi: https://doi.org/10.1016/0003-2697(79)90738-3
9. Parekh AC, Jung DH. Cholesterol determi-nation with ferric acetate-uranium acetate and sulfuric acid-ferrous sulfate reagents. Anal Chem. 1970;42:1423. doi: https://doi.org/10.1021/ac60294a044
10. Rice EC, Epstein MB, Witter RF, Platt HA. 1970. Triglycerides in serum: Standard methods of clinical chemistry. Ceds Roberict, P,and Medorald Cambridge, MA: Academic press; 1970. Vol. 6 215–22 pp. doi: https://doi.org/10.1016/B978-0-12-609106-9.50027-0
11. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharma. 2010;10:482–96. doi: https://doi.org/10.1016/j.coph.2010.04.001
12. Bikle DD. Vitamin D receptor, a tumor suppressor in skin. Can J Physiol Pharm. 2014;93:1–6. doi: https://doi.org/10.1139/cjpp-2014-0367. CCIN:0367
13. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: https://doi.org/10.1210/jc.2011-0385
14. Sivakumar J, Ponnazhagan K, Gopalakrishnan T. The protective effect of Vitamin D and combination with liv-52 on lipid profiles in carbon tetrachloride-induced liver disease in Wistar rats. Asian J Med Sci. 2021;12:71–8. doi: https://doi.org/10.3126/ajms.v12i11.39219
15. Saile B, Ramadori G. Inflammation, damage repair and liver fibrosis - Role of cytokines and different cell types. Z Gastroenterol. 2007;45:77–86. doi: https://doi.org/10.1055/s-2006-927395
16. Lieber CS. Alcoholic liver disease: new insights on pathogenesis lead to new treatment. J Hepatol. 2000;32:113–128. doi: https://doi.org/10.1016/s0168-8278(00)80420-1
17. Rubenstein B, Rubinstein D. The effect of carbon tetrachloride on hepatic lipid metabolism. Can J Biochem. 1964;12:1263–73. doi: https://doi.org/10.1139/o64-136. CCIN:64136
18. Dong S, Chen Q, Feng Q, Hu YY, Liu P, Su SB, et al. Study on molecular mechanism of improving rat liver fibrosis with Fuzheng Huayu Formula based on gene profile. CJTCMP. 2015;30:1812–17. doi: https://doi.org/ 201502218422013844
19. Rubinstein D, Kanics L. The conversion of carbon tetrachloride and chloroform to carbon dioxide by rat liver homogenates. Can J biochem. 1964;42:1577–87. doi: https://doi.org/10.1139/o64-169. CCIN:64169
20. Wang L, Nancollas GH, Henneman ZJ, Klein E, Weiner S, et al. Nanosized particles in bone and dissolution insensitivity of bone mineral. Biointerphases. 2006;1:106–11. doi: https://doi.org/10.1116/1.2354575
21. Polkowska A, G?owinska-Olszewska B, Tobiaszewska M, Bossowski A, et al. 2014. Risk factors for cardiovascular disease in children with type 1 diabetes in 2000-2010 in podlasie province. Pediatr. Endocrinol. Diabetes Metab.2014;20:47–54. doi: https://doi.org/10.18544/PEDM-20.02.0002
22. Crouse, JR, Gerson CD, De Casli LM, Lieber CS, et al. Role of acetate in the reduction of plasma free fatty acids produced by ethanol in man. J. Lipid Res. 1968;9:509–12. doi: https://doi.org/10.1016/S0022-2275(20)42731-2
SUPPLEMENTARY MATERIAL
The data used and analyzed in this study has been enclosed in its supplementary material after the reference of this document.