INTRODUCTION
Acute inflammation starts after the presence of inducers that eventually triggers the release of cytokines, acute-phase proteins, and chemokines to initiate the migration of neutrophils and macrophages to the site of inflammation [1]. Allergens are categorized as the inducers of inflammation. At the initial stage of inflammation, the cells are activated and liberate proinflammatory proteins. Egg white, which contains approximately 10% of protein [2], among those are allergens [3], could significantly reduce serum immunoglobulin E (IgE) levels, elevate interleukins and tumor necrosis factor-alpha, and activate B lymphocytes in allergy-sensitized mice [3]. The egg white was reported prospective to be used as an inducer for animal models of inflammation [4].
Discovering plant-based anti-inflammatory drugs is challenging yet worth exploring. Studies have been carried out to obtain potential plants [5–9]. Erythrina subumbrans (local name dadap serep) has been traditionally used by the people in Ciamis, West Java, Indonesia, to treat fever and edema. The barks, twigs, and roots of this plant have been reported to contain alkaloids, pterocarpans, flavanones, and triterpenes [10–12]. These metabolites have been explored for their antidiabetic and antimicrobial activity [12]. The leaves have been studied on carrageenan-induced edema in paw rats and revealed that a dose of 400 mg/kg body weight possessed the strongest activity in reducing edema [13]. Although very limited pharmacological descriptions of E. subumbrans are provided, several studies on the leaves and barks of E. variegate, a plant of the same genus, have confirmed its analgesic and anti-inflammatory activity [14–16]. Moreover, flavanones, prenylated flavonoids, prenyl isoflavones, and pterocarpanes isolated from the Erythrina genus have demonstrated anti-inflammatory activity by various mechanisms [17–19]. For that reason, our work aimed to study the effects of E. subumbrans leaves extract (ESE) on the membrane stability of human red blood cells (HRBC) and in egg white-induced edema of male Wistar rats.
MATERIALS AND METHODS
Study area
The in vitro study was carried out in the Biology Pharmacy Laboratory, and the animal study was performed at the Pharmacology Laboratory of Universitas Bhakti Kencana, Bandung, West Java, Indonesia. The duration of the experiment was 8 months (November 2021-July 2022).
Plant materials
The leaves of the plants were collected from Purwajaya Village, Ciamis, West Java, Indonesia, and taxonomically identified by a botanist at the Biology Herbarium Laboratory, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Indonesia. The plant samples were confirmed as E. subumbrans (Hassk.) Merr. (Fabaceae) (Letter No. 21/HB/11/2021). The characteristics of the plant samples matched those described in Naturalis Biodiversity Center (NL)-Botany (https://www.gbif.org/occurrence/2517594190).
Instruments and chemicals
Instruments used were digital analytical balance (Shimadzu), digital rotavapor RV 10 (IKA®), centrifuge (Beckman J2-HS), evaporator glass-ware (Pyrex®), chemical glass-wares (Pyrex®), micropipette (Dragon Lab), water plethysmometer (PanLab), and double-beam UV-visible spectrophotometer (Hitachi U-2900).
Chemicals were ethanol 96% (Brataco-Chemika, Indonesia), egg white 1% (contained albumin, prepared by dissolving 1 ml of freshly taken hen’s egg white in 100 ml of distilled water), sodium diclofenac, sodium carboxymethylcellulose, phosphate buffer solution pH 7.4, Alsever′s solution (A3551 Sigma-Aldrich) to prevent coagulation of blood (which is composed of 2.05% dextrose, 0.8% sodium citrate, 0.055% citric acid, and 0.42% sodium chloride).
Collection of human whole blood
Human whole blood was obtained from the Blood Transfusion Unit of the Indonesian Red Cross Society (https://www.pmikotabandung.org/pendaftaran_online/), postal address at Jl. Aceh 79, Bandung, West Java, Indonesia. The protocol of this study has been approved by Padjadjaran Research Ethics Committee (https://kep.unpad.ac.id/) with document no. 506 /UN6.KEP/EC/2022.
Preparation of E. subumbrans extract (ESE)
The fresh leaves were cleaned from dust and dirt, washed under tap water, and dried in a drying cabinet at room temperature for 5 days until constant weight. The dried leaves (weight 2,045 g) were ground and sieved (mesh 4/18). 250 g of the leaves powder was soaked in 1,500 ml of ethanol 96% for 3 × 24 hours at 25°C–26°C. The extract (313.28 g) was filtered, and the solvent was rotary-evaporated at 50°C–60°C. The viscous extract yielded 15.3 % w/w.
Phytochemical screening
The phytochemical screening was done by following a previous procedure described elsewhere [20] as follows:
Flavonoid assay
0.1 g of ESE was added with 10 ml of distilled water and filtered. The filtrate was added with a small quantity of magnesium powder, a few drops of concentrated hydrochloric acid, and 1 ml of amyl alcohol. The mixture was shaken vigorously. Flavonoids were confirmed present in ESE as indicated by the reddish color in the amyl alcohol layer.
Alkaloid assay
0.1 g of ESE was dissolved in 10 ml of chloroform and added with four drops of ammonium hydroxide to alkaline. The mixture was added with 10 drops of sulfuric acid 2M. The water layer was added with three drops of freshly prepared Mayer reagent (a mixture of 1.36 g of mercuric chloride and 5 g of potassium iodide in 100 ml of water). Alkaloids were present in ESE as indicated by a cream-colored precipitate.
Tannins assay
0.1 g of ESE was added with 10 ml of distilled water and a few drops of ferric chloride 1% solution. Tannins were present in ESE, as indicated by the dark blue color of the solution.
The effect of ESE on the membrane stability of HRBC
The reagents were prepared as described previously [16]. Human whole blood 10 ml was mixed with 10 ml of Alsever’s solution and centrifugated at 27°C for 10 minutes at 3,000 rpm. The supernatant was separated, the packed erythrocyte cells were washed using an isotonic saline solution, and the solution was recentrifugated until a 10% HRBC suspension was obtained [21, 22]. The HRBC (erythrocyte) suspension was incubated at 4°C for further use.
The negative control sample consisted of 0.5 ml HRBC suspension mixed with 1 ml hypotonic saline alone. ESE sample solutions (20, 30, 40, 50, 60, 70, 80, 90, and 100 μg/ml) were prepared in a phosphate buffer saline. 1 ml of sample was mixed with 0.5 ml HRBC suspension and incubated at 37°C for 30 minutes. After incubation, mixtures were centrifuged at 3,000 rpm for 20 minutes, and the supernatant was measured at 560 nm [21]. Diclofenac sodium solutions (20, 30, 40, 50, 60, 70, 80, 90, and 100 μg/ml) were used as the positive control drug.
% inhibition of hemolysis (represents the membrane stability) was calculated by following this formula:
where A1 is the absorbance of phosphate buffer saline and A2 is the absorbance of ESE or sodium diclofenac.
Animals
Twenty-five male Wistar albino rats were purchased from an official animal breeding farm in Majalaya-Bandung, West Java, Indonesia, aged 10–12 weeks and weighing 200–300 g. The rats were acclimatized at 25°C–26°C under a 12 hours light, 12 hours dark cycle, 55% relative humidity, with standard food (composed of carbohydrate 274 g, vegetable protein 706 g, vegetable fat 20 g) and water freely for 1 week. The rats were randomly assigned to five groups. Animal health and behavior were monitored daily during acclimatization week and in vivo study. Animal handling was approved by the Research Ethics Committee, Padjadjaran University, Indonesia (approval document No. 506/UN6.KEP/EC/2022). No animals were found dead during the study.
The effect of ESE in egg white-induced edema rats
The study was conducted by following a previous procedure described elsewhere [23].
The rats were randomly assigned to five groups (5 rats/cage, dimension of the cage, 60 cm length × 40 cm width × 38 cm height): (1) the negative control group (treated with sodium-carboxymethylcellulose 0.5%); (2) the positive control group (treated with diclofenac sodium 4.5 mg /kg body weight); and three test groups treated with (4) ESE 100 mg/kg body weight; (4) ESE 200 mg/kg body weight, and (5) 400 mg/kg body weight, all diluted in distilled water, respectively. The administration of ESE to the rats was done using an oral gavage needle, which directly transfers the extract into the esophagus of the rats [8,24,25]. Diclofenac sodium was chosen as the positive control drug because this drug inhibits the biosynthesis of prostaglandin synthesis [26].
All groups of rats were edema-induced using an intraplantar injection of 0.1 ml egg white 1% on their right limb as described previously with a few modifications [27]. The edema volume was measured every 30 minutes until 180 minutes using a plethysmometer.
Statistical analysis
Data were analyzed using IBM SPSS (International Business Machines Corporation Statistical Product and Service Solutions) Statistics version 20.0 (https://www.ibm.com/spss). The experiments were performed in triplicates, and all values are expressed as mean ± SD. Data obtained from the in vivo study were analyzed by one-way analysis of variance (ANOVA), continued with the least significant difference (LSD) Test to compare between groups and time. The LSD is used when the difference between the population means is significant [28]. Further analysis was done using Tukey and Bonferroni Tests. The mean difference is statistically significant at the p-value < 0.05. The one-way ANOVA and the independent paired-samples t-test were employed for the in vitro study.
RESULTS AND DISCUSSION
The phytochemical screening of ESE confirmed the presence of flavonoids, alkaloids, and tannins. Compared to our results, a previous study reported the presence of alkaloids, flavonoids, and pterocarpans in E. subumbrans barks, twigs, and roots [4–6]. Minor differences in the chemical contents of the leaves compared to those on the bark and roots of another plant (Cassia sieberiana) were also reported [29].
ESE at concentrations of 20–100 μg/ml protected the human erythrocyte membrane against lysis induced by hypotonic solution. The percent hemolysis inhibition diagram for ESE (Fig. 1) revealed a good inhibition of hemolysis in the HRBC membrane. The concentration at which 50% of the blood cells were inhibited from hemolysis (IC50) was determined by plotting concentration against percentage inhibition, keeping the hemolysis produced within the negative control group at 100% (IC50 of ESE = 75.61 ± 0.366 μg/ml); however, compared to that of diclofenac sodium (IC50 = 49.97 ± 0.001 μg/ml), the inhibitory activity of ESE towards hemolysis is weaker.
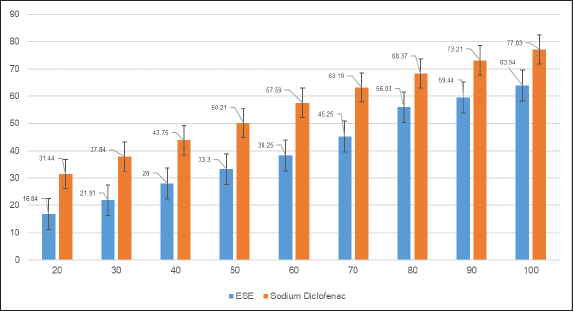 | Figure 1. Diagram of % hemolysis inhibition on HRBC membrane by ESE (blue) and sodium diclofenac (red). IC50 of ESE = 75.61 ± 0.366 μg/ml, IC50 of sodium diclofenac = 49.97 ± 0.001 μg/ml. The x-axis represents the concentration in μg/ml; the y-axis represents the % hemolysis inhibition. Error bars represent the SDs. [Click here to view] |
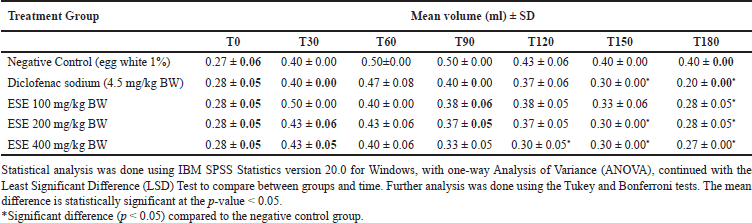 | Table 1. The effect of ESE on the edema volume of egg white-induced male Wistar rats (mean ± SEM) (n = 5). [Click here to view] |
The membrane of the red blood cells comprises a cytoskeleton and a lipid bilayer. Impairments in the membrane may alter the integrity of the cells and lead to cell hemolysis [30]. HRBC membranes are much like lysosomal membranes; thus, hypotonicity-induced HRBC membrane lysis may be adopted to assess the anti-inflammatory activity of drugs [31. A previous study reported that the release of phospholipase A2 (PLA2) can be prevented by stabilizing the erythrocyte membrane. PLA2 is an enzyme that catalyzes the conversion of phospholipid in the cell membrane to arachidonic acid, which is the substrate of cyclooxygenase enzymes. Thus, preventing the release of PLA2 leads to reducing the inflammation process [32].
Inflammation induced by egg white 1% and the anti-inflammatory test resulted in changes in the paw volume of each group (Table 1). In the negative control group, the edema volume increased from T0 to T90 and showed a slightly different trend compared with the other groups due to no inhibition of the inflammatory process. However, the positive control group treated with diclofenac sodium and the ESE groups revealed a diminishing trend starting at T90 (although not significantly compared to the negative control group), which indicated the occurrence of inhibitory activity by the drug and the extracts. Diclofenac sodium significantly reduced the edema volume in rat’s paw at T150 (p < 0.05), similar to that of ESE doses of 100 mg/kg BW and 200 mg/kg BW. Interestingly, the ESE dose of 400 mg/kg BW could significantly reduce the edema at T120. These findings confirmed that ESE could reduce egg white-induced edema. Thus, it is predicted to inhibit inflammation by blocking the liberation of histamine and serotonin, allergenic mediators, which are activated by egg white [33].
Egg white contains proteins, e.g., ovalbumin (OVA), ovotransferrin, ovomucin, lysozyme, and avidin. These proteins reveal antibacterial and immunoprotective activities; in fact, they are also capable of activating unfavorable proinflammatory responses, such as allergens [2–4, 34–37] that are responsible for inducing edema [37, 38] as a result of type 1 hypersensitivity reaction [39]. Type 1 hypersensitivity involves the release of IgE antibodies against the exposure of antigens (allergens). Allergens activate and bind to antigen-presenting cells, eventually releasing peptides. With the aid of T-helper cells, the allergens stimulate B cells to produce IgE antibodies. The IgE then attaches to Fc receptors on the surface of the mast cells and activates the cells, which results in degranulation and the release of histamine and other inflammatory mediators [39]. Inflammation, in this case, edema, often occurs after intraplantar injection of inducers in the hind paw of rats. A previous study reported a strong infiltration of neutrophils and eosinophils in the Wistar rat paws after an inflammation induced by OVA [40].
Our present study discloses the effect of ESE on the inhibition of hemolysis in hypotonicity-induced human red blood cell membranes by the spectrophotometric method and investigates the in vivo effect of ESE on the egg white-induced paw of male Wistar rats. In conclusion, ESE revealed anti-inflammatory activity as confirmed by both the in vitro (IC50 of ESE in inhibiting hemolysis in human red blood cell membranes = 75.61 ± 0.366 μg/ml) and in vivo by reducing the edema volume of rat’s paw at T90. ESE doses of 200 mg/kg BW and 400 mg/kg BW significantly reduced the edema volume in rat’s paw at T150. These findings provide evidence that ESE is the potential to be developed as an anti-inflammatory agent. However, this study still lacks the molecular mechanism of ESE in inhibiting hemolysis in human red blood cell membranes and edema in rats’ paws; therefore, it is challenging to carry out further studies.
AUTHOR’S CONTRIBUTIONS
Sri Adi Sumiwi, Jutti Levita, and Yasmiwar Susilawati were responsible for the conception and design of the study and supervised the project. Elis Susilawati, Widhya Aligita, Marita Kaniawati, and Divi Ade Liani contributed to the extraction process, in vitro study, animal handling, and in vivo study and confirmed the authenticity of all the raw data and statistical analysis. Sri Adi Sumiwi and Jutti Levita contributed equally to the acquisition and interpretation of the reported data. Elis Susilawati and Jutti Levita contributed to the writing and revising of the manuscript. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
All authors declared no potential conflicts of interest.
FUNDING
This work was funded by Universitas Bhakti Kencana via the Center of Research and Community Service. The publication fee is funded by Universitas Padjadjaran via the Directorate of Research and Community Engagement in the scheme of the Academic-Leadership Grant 2022 of Prof. Dr. Jutti Levita.
ETHICS APPROVAL
Animal handling and human whole blood procedures were performed as approved by the Research Ethics Committee (https://kep.unpad.ac.id/; approval document No. 506/UN6.KEP/EC/2022), Universitas Padjadjaran, Indonesia (recognized by Forum of Ethics Review Committee in Asia & Western Pacific Region).
DATA AVAILABILITY
The datasets used and/or analyzed during the present study are available from the first author upon reasonable request.
This journal remains neutral related to the jurisdictional claim of the published institutional affiliation.
REFERENCES
1. Hannoodee S, Nasuruddin DN. Acute Inflammatory Response. [cited 2022 Nov 14]. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556083/
2. Kovacs-Nolan J, Phillips M, Mine Y. Advances in the value of eggs and egg components for human health. J Agric Food Chem. 2005;53:8421–31. doi: https://doi.org/10.1021/jf050964f
3. Kim JH, Song H, Kim HW, Lee WY. Effects of egg white consumption on immune modulation in a mouse model of trimellitic anhydride-induced allergy. Korean J Food Sci Anim Resour. 2015;35(3):398–405.doi: https://doi.org/10.5851/kosfa.2015.35.3.398
4. Barung EN, Dumanauw JM, Duri MF, Kalonio DE. Egg white-induced inflammation models: A study of edema profile and histological change of rat’s paw. J Adv Pharm Technol Res. 2021;12(2):109–12. doi: https://doi.org/ 10.4103/japtr.JAPTR_262_20
5. Wahyuni IS, Sufiawati I, Nittayananta W, Levita J. Anti-Inflammatory activity and wound healing effect of Kaempferia galanga L. rhizome on the chemical-induced oral mucosal ulcer in Wistar rats. J Inflamm Res. 2022;15:2281–94. doi: https://doi.org/10.2147/JIR.S359042
6. Simanjuntak N, Levita J, Subarnas A. Inclusion of biopiperine in the kappa-carrageenan complex might improve its bioaccessibility and in vivo anti-inflammatory activity in edema-induced Wistar rats. J Appl Pharm Sci. 2020;10(06):039–43. doi: http://dx.doi.org/10.7324/JAPS.2020.10606
7. Juwita T, Pakpahan WHP, Puspitasari IM, Saptarini NM, Levita J. Anti-inflammatory activity of etlingera elatior (Jack) R.M. Smith flower on gastric ulceration-induced wistar rats. Pakistan J Biol Sci. 2020;23:1193–200. doi: https://dx.doi.org/10.3923/pjbs.2020.1193.1200
8. Rosdianto AM, Puspitasari IM, Lesmana R, Levita J. Inhibitory activity of boesenbergia rotunda (l.) mansf. rhizome towards the expression of akt and NF-kappaB p65 in acetic acid-induced Wistar rats. Evid-based Complement Alternat Med. 2020;2020:6940313. doi: https://doi.org/10.1155/2020/6940313
9. Levita J, Syafitri DM, Supu RD, Mutakin M, Megantara S, Febrianti M, et al. Pharmacokinetics of 10?gingerol and 6?shogaol in the plasma of healthy subjects treated with red ginger (Zingiber officinale var. Rubrum) suspension. Biomed Rep. 2018;9:474–82. doi: https://doi.org/10.3892/br.2018.1163
10. Rukachaisirikul T, Innok P, Aroonrerk N, Boonamnuaylap W, Limrangsun W, Boonyon C, et al. Antibacterial pterocarpans from Erythrina subumbrans. J Ethnopharmacol. 2007;110:171–5.
11. Rukachaisirikul T, Innok P, Suksamrarn A. Erythrina alkaloids and a pterocarpan from the bark of Erythrina subumbrans. Nat Prod. 2008;71(1):156–8. doi: https://doi.org/10.1021/np070506w
12. Phukhatmuen P, Meesakul P, Suthiphasilp V, Charoensup R, Maneerata T, Cheenpracha S, et al. Antidiabetic and antimicrobial flavonoids from the twigs and roots of Erythrina subumbrans (Hassk.) Merr. Heliyon. 2021;7(4):e06904.
13. Dewi NLKAA, Sintya NL, Sasadara MMV, Cahyaningsih E, Yuda PESK, Santoso P. In vivo anti-inflammatory activity of dadap leaves (Erythrina subumbrans (Hassk.) Merr. Int J Biosci Biotechnol. 2021;9(1):24-–1.
14. Bhagyasri Y, Nagalatha G, Reddy NV, Subramanian NS. Analgesic and anti-inflammatory activity of leaf extracts of Erythrina variegate. Indo Am J Pharm Res. 2017;7:681–92.
15. Haque R, Ali MS, Saha A, Alimuzzaman M. Analgesic activity of methanolic extract of the leaf of erythrina variegata. Dhaka Univ J Pharm Sci. 2006;5:77–9. doi: https://doi.org/10.3329/dujps.v5i1.235.
16. Uddin MM, Emran TB, Mahib MM, Dash R. Molecular docking and analgesic studies of Erythrina variegata?s derived phytochemicals with COX enzymes. Bioinformation. 2014;10(10):630–6. doi: https://doi.org/10.6026/97320630010630.
17. Sokeng SD, Talla E, Jeweldai V, Yaya AJG, Koube J, Dongmo F, et al. Anti-inflammatory effect of abyssinone V-4′-methyl ether on acute and chronic inflammation models. Hygeia J Drugs Med. 2013;5:121–8.
18. Cui L, Thuong PT, Lee HS, Ndinteh DT, Mbafor JT, Fomum ZT, et al. Flavanones from the stem bark of erythrina abyssinica. Bioorg Med Chem. 2008;16(24):10356-62. doi: https://doi.org/10.1016/j.bmc.2008.10.012.
19. Talla E, Njamen D, Mbafor JT, Fomum ZT, Kamanyi A, Mbanya JC, et al. Warangalone, the isoflavonoid anti-inflammatory principle of Erythrina addisoniae stem bark. J Nat Prod. 2003;66(6):891–3. doi: https://doi.org/10.1021/np020599b.
20. Mugiyanto E, Slamet S, Fatmala R. Characterization of dried leaves and extract of dadap serep (Erythrina lithosperma Miq) leaves planted in Pekalongan, Central Java. The 7th University Research Colloqium. 2018. URL: http://repository.urecol.org/index.php/proceeding/article/view/251
21. Saleem TM, Azeem AK, Dilip C, Sankar C, Prasanth NV, Duraisami R. Anti-inflammatory activity of the leaextractsts of Gendarussa vulgaris Nees. Asian Pac J Trop Biomed. 2011;1:147–9.
22. Adnan AZ, Armin F, Sudji IR, Novida MD, Roesma DI, Ali HA, et al. In vitro anti-inflammatory activity test of tinocrisposide and freeze-dried aqueous extract of tinospora crispa stems on human red blood cell by increasing membrane stability experiment. Asian J Pharm Clin Res. 2019;12(5):125–9.
23. Akindele AJ, Oladimeji-Salami JA, Usuwah BA. Antinociceptive and anti-inflammatory activities of Telfairia occidentalis hydroethanolic leaf extract (Cucurbitaceae). J Med Food. 2015;18(10):1157–63. doi: https://doi.org/10.1089/jmf.2014.0146.
24. Abdulaziz Bardi D, Halabi MF, Abdullah NA, Rouhollahi E, Hajrezaie M, Abdulla MA. In vivo evaluation of ethanolic extract of Zingiber officinale rhizomes for its protective effect against liver cirrhosis. Biomed Res Int. 2013;2013:918460. doi: https://doi.org/10.1155%2F2013%2F918460
25. Sini S, Latha PG, Anilkumar TV, Suja SR, Raj G, Rameshkumar KB, et al. Safety assessment of tuberous rhizome of Kaempferia rotunda L. by acute and 28-days repeated dose toxicity studies. Glob J Pharmacol. 2014;8:128–139.
26. Gan TJ. Diclofenac: an update on its mechanism of action and safety profile. Curr Med Res Opin. 2010;26(7):1715–31. doi: https://doi.org/10.1185/03007995.2010.486301.
27. Zhao J, Maitituersun A, Li C, Li Q, Xu F, Liu T. Evaluation on analgesic and anti-inflammatory activities of total flavonoids from Juniperus sabina. Evid Based Complementary Altern Med. 2018;2018:7965306. doi: https://doi.org/10.1155/2018/7965306
28. Least Significant Difference Test. In: Dodge Y editors. The concise encyclopedia of statistics. .New York, NY: Springer; 2008. doi: https://doi.org/10.1007/978-0-387-32833-1_226
29. Kpegba K, Eloh K, Evenamede KS, Afanyibo YG, Elomri A, Simalou O, et al. A comparative study of the chemical composition of the extracts from leaves, stem bark, and root bark of Cassia sieberiana: Antibacterial activities. Orient J Chem. 2019;35(6):1678–89.
30. Aidoo DB, Konja D, Henneh IT, Ekor M. Protective effect of Bergapten against human erythrocyte hemolysis and protein denaturation in vitro. Int J Inflam. 2021;2021:1279359. doi: https://doi.org/10.1155/2021/1279359.
31. Sumathi S, Anuradha R. In vitro anti-inflammatory activity of flower extract of Couroupita guianensis Aubl. Int J Herb Med. 2016;4(5):05–08.
32. Umukoro S, Ashorobi RB. Evaluation of anti-inflammatory and membrane stabilizing property of aqueous leaf extract of momordica charantia in rats. African J Biomed Res. 2006;9(2):119–24.
33. Nwafor PA, Jacks TW, Ekanem AU. Analgesic and anti-inflammatory effects of methanolic extract of Pausinystalia mecroceras stem bark in rodents. J Pharmacol. 2007;3:86–90.
34. Andersen CJ. Bioactive egg components and inflammation. Nutrients. 2015;7(9):7889–913. doi: https://doi.org/10.3390/nu7095372.
35. Yokooji T, Hamura K, Matsuo H. Intestinal absorption of lysozyme, an egg-white allergen, in rats: kinetics and effect of NSAIDs. Biochem Biophys Res Commun. 2013;438(1):61–5. doi: https://doi.org/10.1016/j.bbrc.2013.07.024.
36. Yokooji T, Nouma H, Matsuo H. Characterization of ovalbumin absorption pathways in the rat intestine, including the effects of aspirin. Biol Pharm Bull. 2014;37(8):1359–65. doi: https://doi.org/10.1248/bpb.b14-00290.
37. Enechi OC, Okeke ES, Awoh OE, Okoye CO, Odo CK. Inhibition of phospholipase A2, platelet aggregation and egg albumin induced rat paw oedema as anti-inflammatory effect of Peltophorun pterocarpus stem-bark. Clin Phytosci. 2021;7(75). :1–8. doi: https://doi.org/10.1186/s40816-021-00310-3
38. Akindele AJ, Oladimeji-Salami JA, Usuwah BA. Antinociceptive and anti-inflammatory activities of telfairia occidentalis hydroethanolic leaf extract (Cucurbitaceae). J Med Food. 2015;18(10):1157–63. doi: https://doi.org/10.1089/jmf.2014.0146
39. Abbas M, Moussa M, Akel H. Type I hypersensitivity reaction. StatPearls [Internet]. 2023. Bookshelf
40. Feitosa RFG, Melcõades GB, Assreuy AMS, Rocha MFG, Ribeiro RA, Lima AAM. The pharmacological profile of ovalbumin-induced paw oedema in rats. Med Inflamm. 2002;11:155–63. doi: https://doi.org/10.1080/09622935020138000