INTRODUCTION
The discovery of endophytic microbial that associated with Neesia altissima, we reported that three endophytic bacterial strains, namely Pseudomonas aeruginosa strains University of Indonesia Culture Collection (UICC) B-40 and UICC B-93, and Pseudomonas azotoformans strain UICC B-91, were discovered [1]. Previous research had identified and isolated endophytic P. azotoformans from stem bark tissue of the N. altissima plant in Halimun forest [1]. Research has been carried out on P. azotoformans UICC B-91 which is proven to be able to inhibit several pathogenic bacteria, such as Escherichia coli American Type Culture Collection (ATCC) 8739, Salmonella typhimurium ATCC 25241, Staphylococcus aureus ATCC 6583, S. aureus ATCC 25923, Bacillus cereus ATCC 10876, Bacillus subtilis ATCC 19659, P. aeruginosa ATCC 15442, and Candida albicans ATCC 10231, with a notable result on anticandidal activity [2]. In addition, other studies have reported that the supernatant from the fermentation medium of P. azotoformans UICC B-91 has anticandidal activity with the largest average value of the inhibition zone produced is 8.00 ± 0.00 mm at a concentration of 50 mg/ml [2].
Candida albicans is a fungal pathogen that can be found in the healthy human microbiome but can also cause mucosal infections or invasive candidiasis. Invasive candidiasis is a deadly disease with a high mortality rate. It has a mortality rate ranging from 20% to 50%. Candida albicans were included in the critical group of three priority groups based on numerical scores, as well as consensus discussions among the World Health Organization Advisory Group on the Fungal Priority Pathogens List [3]. Recent studies indicating higher rates of azole resistance, particularly in low-and middle-income countries, raise concerns that resistance is on the rise. Resistance to antifungals is uncommon. Despite this, resistance rates, particularly in nonsterile site isolates, appear to be increasing, highlighting the need for more effective and efficient therapy. This research was conducted to determine the mechanism of inhibition of the metabolite of P. azotoformans UICC B-91 from the medium on the morphological activity of C. albicans ATCC 10231.
MATERIALS AND METHODS
Microorganisms
The bacterium used is a collection of the UICC, is P. azotoformans UICC B-91, and the yeast used is a collection of the ATCC, is C. albicans ATCC 10231.
Isolation and extraction of anticandidal active compounds
The starter was made by inoculating one loop of P. azotoformans UICC B-91 colonies from the slant nutrient agar into 20 ml of rehydration-fluid bacteria (RB) medium. Then incubated in an incubator shaker at 30ºC for 24 hours at 100 rpm. The 1% inoculum was then transferred into 200 ml RB medium. Fermentation was carried out for 96 hours at a temperature of 30ºC in an incubator shaker at a speed of 100 rpm. The calculation of the number of total plate count (TPC) is carried out to determine the number of colony forming units per milliliter (CFU/ml) with an interval of 24 hours. The fermented culture of P. azotoformans UICC B-91 was then centrifuged at 2,000 g for 20 minutes. The supernatant was separated from the pellet and then macerated with ethyl acetate with a volume of 1:1 (v/v) overnight. The medium layer was then macerated again with ethyl acetate and the same steps were carried out up to two times. The ethyl acetate layer was evaporated using a rotary evaporator [2].
Anticandidal activity test
Suspension of C. albicans ATCC 10231 was prepared and inoculated using cotton and then smeared over the entire surface of sterile saboraud dextrose agar (SDA) medium with side-to-side movements. Smearing is done by rotating the petri dish at an angle of 60° twice. The anticandidal activity test was carried out in two batches. Each testing batch was done in triplicate. Each SDA medium was made into five quadrants in a petri dish. Each quadrant was placed on a disc that had been dripped with 50 μl P. azotoformans UICC B-91 with a concentration of 100, 80, 60, 0.1 mg/ml ketoconazole as a positive control, and 0.5% dimethyl sulfoxide (DMSO) as a negative control. SDA medium was incubated at 30ºC for 24–48 hours. The clear zone formed was measured with a calliper [4].
Determination of minimum inhibitory concentration (MIC) of P. azotoformans UICC B-91
A total of three sets of sterile test tubes with each set containing eight tubes were prepared. Tubes one through six are prepared for treatment. Tubes seven were prepared as a growth control, and tubeseight as a control medium without growth. A total of 3 ml of Potato Dextrose Broth was poured into tubes two to eight using a micropipette in each set. 4.5 ml of 60 mg/ml concentration medium was poured into tube one using a sterile micropipette in each set. Serial dilutions were performed. A total of 1.5 ml from tube one was transferred to tube two using a sterile micropipette and then tubes two was vortexed. The same procedure was repeated from tubes two to three to six in each set. The remaining 1.5 ml in tube six was discarded. After that, 0.15 ml of yeast suspension was poured into tubes two to seven in each set. Tubes eight as a control medium without growth were not poured with yeast suspension. The entire set of tubes was incubated for 12 to 18 hours at 30ºC [5].
Microscopic observation of C. albicans ATCC 10231 after addition of P. azotoformans UICC B-91 with light microscope
The MIC test tubes with a concentration of 60 mg/ml and the growth control which had been incubated were vortexed again for 30 seconds. A total of two slides were prepared and one drop of 10% potassium hydroxide (KOH) was added. The entire suspension was dripped onto a 10% KOH solution on each slide of 20 μl and covered with a cover slip. The preparations were fixed and observed under a microscope up to 1,000× magnification [6].
Microscopic observation of C. albicans ATCC 10231 after addition of P. azotoformans UICC B-91 with scanning electron microscope (SEM)
Suspension of MIC at a concentration of 60 mg/ml and growth control was transferred into 1.5 ml Eppendorf tubes of 200 μl each. Each suspension was immersed in 200 μl of 2.5% glutaraldehyde overnight. Then the suspension was centrifuged at 1,000 rpm for 10 minutes and the supernatant was discarded. The pellets were soaked in 200 μl of 2% tannin acid for 2 hours, then centrifuged and the supernatant was discarded. Pellets were immersed in caccodylate buffer for 10 minutes two times, then centrifuged and the supernatant was discarded. The pellets were immersed in 200 μl of 1% osmium tetraoxide solution for 1 hour. Then it was centrifuged again and the supernatant was discarded. The pellets were washed with 50% ethanol, left for 10 minutes, centrifuged, and then the supernatant was discarded. The pellets were washed again with 50%, 70%, 80%, and 95% ethanol for 10 minutes, respectively. Then washed with absolute ethanol for 10 minutes two times, after that, it was centrifuged and the supernatant was discarded. Pellets were added with tert-butanol for 10 minutes and performed two times. A small amount of tert-butanol was added to the cell precipitate and the cell smear was smeared on a cover slip. The washed cover slips were then smeared with cells, coated with gold under vacuum, and observed under SEM at Zoology Characterization Laboratories-BRIN at 5,000×, 10,000×, and 15,000× magnification [7].
RESULTS
Isolation and extraction of anticandidal active compounds
The production of anticandidal compounds was produced through a fermentation process with 200 ml RB medium. The total volume of RB medium used for the production of anticandidal compounds was 1,600 ml. The starter was made by inoculating one loop of P. azotoformans UICC B-91 colonies into 20 ml RB medium. The medium color changed from transparent yellow to cloudy greenish–yellow after incubated in an incubator shaker at 100 rpm at 30ºC for 24 hours. As much as 1% (v/v) inoculum was transferred into 200 ml RB medium and fermented in an incubator shaker at 100 rpm at 30ºC.
Based on observations every 24 hours, the number of TPC P. azotoformans UICC B-91 at 0 hours had an average of 2.34 × 105 CFU/ml. After fermentation for 24 hours, the amount of TPC increased by an average of 1.80 × 106 CFU/ml. The number of TPC at the 48th hour still increased with an average of 1.62 × 108 CFU/ml. The highest number of TPC occurred at the 72th hour with an average of 1.11 × 1010 CFU/ml. The amount of TPC at 96th hour was 5.10 × 109 CFU/ml and the amount of TPC at the 120th hour decreased to 1.80 × 108 CFU/ml. The results of the TPC calculation were visualized by making a growth curve of P. azotoformans UICC B-91 (Fig . 1).
Fermentation of P. azotoformans UICC B-91 for 96 hours showed a change in the color of the medium from transparent brownish yellow to cloudy greenish-yellow. The fermented medium aged 96 hours was centrifuged and then macerated with ethyl acetate as solvent. The wet weight of the medium obtained from evaporation is 1.229 g. The liquid medium was re-evaporated using an oven at 40ºC until the medium thickened. The constant weight of the medium obtained was 0.86 g. A total of 0.86 g of thick medium P. azotoformans UICC B-91 was dissolved in 8.6 ml of 0.5% DMSO to make a medium solution with a concentration of 100 mg/ml. Serial dilutions were carried out to obtain a medium solution with a concentration of 80 and 60 mg/ml.
Anticandidal activity test
The results of observations after incubation for 24 hours were the zone of inhibition of several treatments and controls. The inhibition zone observed after the addition of the medium concentration of 100 mg/ml averaged 18.82 ± 0.54 mm, the inhibition zone at a concentration of 80 mg/ml averaged 13.75 ± 0.53 mm, the inhibition zone at a concentration of 60 mg/ml mean 9.49 ± 0.43 mm. The zone of inhibition observed in the positive control was 0.1 mg/ml with an average of 7.17 ± 0.26 mm. The zone of inhibition observed in the negative control at 0.5% concentration was not detected at all (Fig. 2) (Table 1).
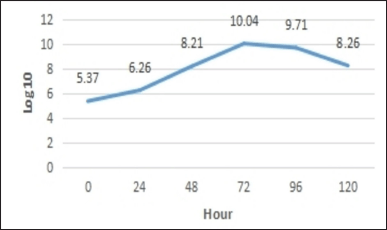 | Figure 1. Growth curve of P. azotoformans UICC B-91. [Click here to view] |
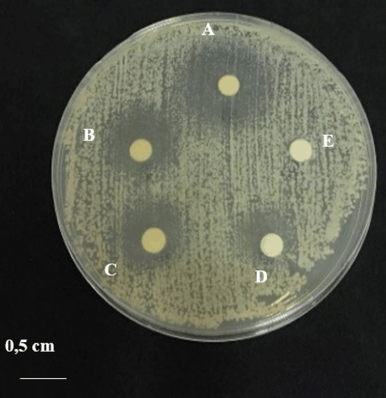 | Figure 2. Anticandidal activity test of C. albicans ATCC 10231: (A) addition of 100 mg/ml concentration medium solution; (B) concentration 80 mg/ml; (C) concentration 60 mg/ml; (D) addition of 0.1 mg/ml ketoconazole; and (E) addition of 0.5% dimethyl sulfoxide (DMSO). [Click here to view] |
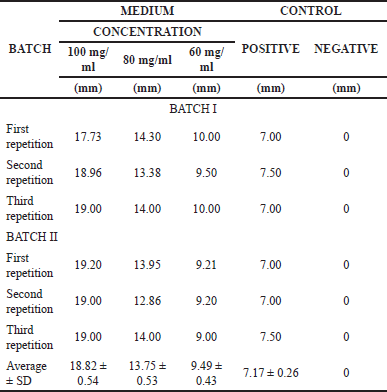 | Table 1. Calculation results of the anticandidal activity of P. azotoformans UICC B-91. [Click here to view] |
Determination of MIC medium solution of P. azotoformans UICC B-91 against C. albicans ATCC 10231
The results of the optical density (OD) measurement can be seen in Appendix 1. Based on the results obtained, the medium concentrations of 60.00, 30.00, 15.00, and 7.50 mg/ml had decreased OD values after incubation, while the concentrations of 3.75 and 1.87 mg/ml increased in OD values after incubation. The growth control OD value after incubation increased. The OD value of the control medium after incubation did not increase or decrease (fixed). The measurement results showed that the medium solution of P. azotoformans UICC B-91 with a concentration of 7.50 mg/ml was determined as the MIC value for C. albicans ATCC 10231 because it did not provide additional OD values at low concentrations (Fig. 3.).
Microscopic observation of C. albicans ATCC 10231 after addition of P. azotoformans UICC B-91 medium with light microscope
Microscopic changes of C. albicans ATCC 10231 after the addition of P. azotoformans UICC B-91 medium solution were compared with the growth control observed under a light microscope. The results of the growth control observations showed that yeast cells were oval in shape, pseudohyphae were found, and the distribution of cells tended to be clustered. The results of observations after the addition of a solution of P. azotoformans UICC B-91 at a concentration of 60 mg/ml were dominated by yeast cells with a less uniform shape because some cells were lysed, pseudohyphae were not found, and the distribution of cells tended to be scattered (Fig. 4).
Microscopic observation of C. albicans ATCC 10231 after addition of P. azotoformans UICC B-91 medium with SEM
Microscopic changes in C. albicans ATCC 10231 after the addition of P. azotoformans UICC B-91 medium compared to growth controls were observed under SEM. The results of the growth control observations showed that yeast cells were oval in shape with a length of 2.54–3.71 μm, width 2.05–3.40 μm, flat cell surface, normal yeast cell shape, and pseudohyphae were found. The addition of P. azotoformans UICC B-91 with a concentration of 60 mg/ml showed that yeast cells were oval in shape with a length of 2.48–3.66 μm, a width of 2.47–3.20 μm, the yeast cell surface was uneven and many cells was broken (ruptured) (Fig. 5).
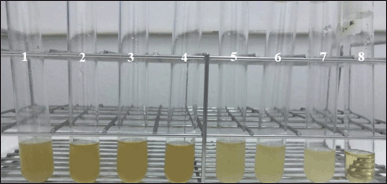 | Figure 3. MIC test of P. azotoformans UICC B-91 medium solution against C. albicans ATCC 10231 (from left to right): (1) medium concentration 60.00 mg/ml; (2) 30.00 mg/ml; (3) 15.00 mg/ml; (4) 7.50 mg/ml; (5) 3.75 mg/ml; (6) 1.88 mg/ml; (7) growth control; and (8) control medium. [Click here to view] |
DISCUSSION
Isolation and extraction of anticandidal active compounds
RB medium was used as the fermentation medium for P. azotoformans. There were 2 g of peptone, 0.4 g of yeast extract, and 0.2 g of MgSO4.7H2O in 200 ml of RB medium. Peptone and yeast extract in the fermentation medium serve as nitrogen and carbon sources for the formation of secondary metabolites, respectively, while MgSO4.7H2O aids in the formation of pigments [8]. The starter is designed to adapt to the substrate that will be used during the fermentation process [9].
The growth curve consists of four phases: the lag phase, the exponential phase, the stationary phase, and the death phase. The lag phase is the phase when bacteria adapt to new conditions, that is a fresh medium. Based on the observations, the lag phase is not observed. According to Madigan et al. [10], the lag phase was not observed because the inoculum inoculated into the new medium came from the same type of medium and conditions. The exponential phase is the phase when the cells enter a period of rapid growth. The exponential phase starts to appear at 0 to 72 hours. The cells in the population divide into two cells in these phases, the stationary phase is the phase when bacteria stop replicating due to depleted nutrients or product accumulation. The stationary phase in the observations occurred after 72 hours. The balance between the number of living and dead cells occurs in this phase. Finally, the death phase occurs when the number of cells that experience death exceeds the number of living cells. The death phase has begun to be seen at the 96th hour. Based on the results of TPC and growth curves obtained, the isolation of bioactive compounds was carried out when the P. azotoformans UICC B-91 culture was 96 hours old. Here, in the final stationary phase, there are limited nutrients remaining in microorganisms and sufficient accumulation of metabolites [11].
Anticandidal activity test
The inhibitory power can be said to be weak if the average value of the inhibition zone produced is <5 mm, moderate if the average value of the inhibition zone is 5–10 mm, strong if the average value of the inhibition zone is 5–10 mm [12]. The average inhibition zone produced is 10–20 mm, very strong if the average value of the inhibition zone is >20 mm. Based on observations, medium P. azotoformans UICC B-91 concentration of 100 and 80 mg/ml had a strong inhibitory effect on C. albicans ATCC 10231 and a concentration of 60 mg/ml had moderate inhibition.
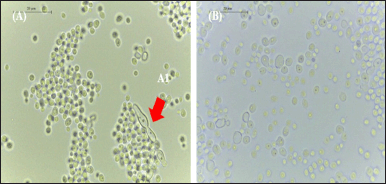 | Figure 4. Morphology of C. albicans ATCC 10231 under a light microscope at 1,000× magnification: (A) in growth control; (A1) pseudohyphae; and (B) with the addition of 150 μl medium concentration 60 mg/ml. [Click here to view] |
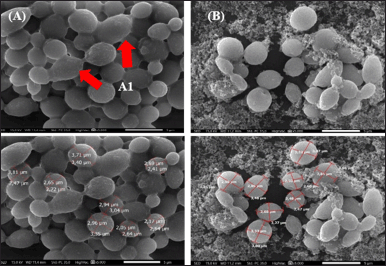 | Figure 5. Yeast cell morphology of C. albicans ATCC 10231 under 5,000× magnification SEM: (A) in growth control; (A1) pseudohyphae; and (B) with the addition of 150 μl of medium concentration of 60 mg/ml. [Click here to view] |
The anticandidal activity test has also been carried out by Oktarina et al. [2]. Testing of P. azotoformans UICC B-91 medium against C. albicans ATCC 10231 was carried out using the disc diffusion method. Medium with concentrations of 0.1, 1, and 5 mg/ml had no zone of inhibition against C. albicans ATCC 10231. Increasing concentrations at 10 and 50 mg/ml had an average zone of inhibition of 4.25 ± 0.25 and 8.00 ± 0.00 mm, respectively.
Determination of MIC medium solution of P. azotoformans UICC B-91 against C. albicans ATCC 10231
Cell suspensions can scatter light and the amount of light scattered is proportional to the number of cells present. The more cells, the more light is scattered, and the cloudier the suspension will look. The decrease in OD value of C. albicans ATCC 10231 at concentrations of 60.00, 30.00, 15.00, and 7.50 mg/ml was due to the higher the concentration of the medium, the greater the amount of bioactive compounds contained so that more and more antifungal compounds were absorbed by C. albicans ATCC 10231 and caused the growth of C. albicans ATCC 10231 to be inhibited and the measured OD value decreased [10].
The OD values in the medium of C. albicans ATCC 10231 with a concentration of 3.75 and 1.87 mg/ml after incubation were increased. The addition of OD values occurred due to an increase in the number of cells after incubation [13]. Furthermore, the addition of the OD value to the growth control occurred because no additional medium was added. The control medium did not increase or decrease the OD value because suspension and medium were not added.
Changes in yeast morphology confirmed with microscopy suggests P. azotoformans disrupts the cell membrane
According to Kabir et al. [14], C. albicans ATCC 10231 has dimorphism ability by forming pseudohyphae. The accumulation of yeast and pseudohyphae forms a biofilm. Candida albicans biofilm structure can cause antimicrobial resistance [15]. Changes in yeast cell morphology after being given the medium occurred due to the bioactive compounds contained in it. According to Pratiwi et al. [16] reported that the medium solution of P. azotoformans UICC B-91 contained alkaloids. According to Priya et al. [17], alkaloid compounds are able to downregulate several adhesion and hyphal-specific genes, such as the Unscheduled Meiotic gene Expression 6 (UME6), Agglutinin-Like Sequence 1 and 3 (ALS1 and ALS3) genes, and Hyphal Wall Protein 1 genes (HWP1) in various strains of C. albicans. The UME6 gene regulates the transition of the yeast form to the hyphal form. The ALS1 and ALS3 genes are involved in the process of adhesion to host cells and biofilm formation in both in vitro and in vivo conditions. The HWP1 gene is a hyphal wall regulator and plays a role in hyphae development, mating, and attachment to host cells. The hwp1 mutant C. albicans was shown to inhibit hyphal germination and pathogenicity to host cells in vivo [18].
Changes in yeast cell morphology after being given the medium occurred due to the bioactive compounds contained in it. Pratiwi et al. [16] reported that P. azotoformans UICC B-91 medium contained alkaloids. According to Tsuchimori et al. [18], the mechanism of action of the alkaloids is thought to be by disrupting cell membranes or the transition of yeast to hyphae. Martins De Andrade et al. [19] reported that alkaloids were able to disrupt cell membranes as evidenced by irregular yeast cell surface changes, and the release of cell components was also known. In addition, the mechanism of action also occurs through the process of inhibiting the transition of yeast to hyphae as reported by Ma et al. [20] that alkaloid compounds significantly inhibit the morphological transition of yeast to hyphae. Inhibition of morphological transition from yeast to hyphae contributes to the inhibition of virulence and pathogenicity that can penetrate host cells. This process makes the alkaloids potential to inhibit the pathogenicity of C. albicans ATCC 10231 [18].
CONCLUSION
The zone of inhibition produced by the medium solution of Pseudomonas azotoformans UICC B-91 against C. albicans ATCC 10231 at a concentration of 80 and 100 mg/ml had a strong inhibitory effect. The mechanism of inhibition of metabolites from P. azotoformans UICC B-91 medium against C. albicans ATCC 10231 is occur through diffusion that disrupts cell membrane function or inhibits the transition from the yeast form to the hyphal form. Endophytic P. azotoformans from N. altissima plant stem bark tissue has pharmaceutical potential because it produces anticandidal compounds, and the medicinal properties of this plant may be due to the ability of its endophytic microorganisms to produce biologically active secondary metabolites. More research is needed to evaluate the spectral composition produced so that new anticandidal drugs can be developed.
ACKNOWLEDGMENTS
This research was funded by Competitive Research Grant 2021-2022 from the Ministry of Education, Research and Technology with contract number 1867/E4/AK.04/2021 and number 018/E5/PG.02.00/2022, awarded to RHP. The authors gratefully acknowledge the facilities, scientific, and technical assistance provided by the Zoology Characterization Laboratories of the National Research and Innovation Agency via E- Layanan Sains.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Pratiwi RH, Hidayat I, Hanafi M, Mangunwardoyo W. Identification and characterization of three endophytic bacteria from Neesia altissima (Malvaceae) antagonistic to diarrhea-causing bacteria. Malays J Microbiol. 2016;12(4):300–7. doi: https://dx.doi.org/10.21161/mjm.81515
2. Oktarina E, Pratiwi RH, Mangunwardoyo W, Hidayat I, Saepudin E. In vitro antimicrobial activities of several extracts endophytic Pseudomonas azotoformans UICC B-91. IOP Conf Ser Earth Environ Sci. 2021;94812(68):1–10. doi: https://dx.doi.org/10.1088/1755-1315/948/1/012068
3. WHO. WHO fungal priority pathogens list to guide research, development and public health action. Geneva, Switzerland: World Health Organization; 2022.
4. Hudzicki J. Kirby-bauer disk diffusion susceptibility test protocol. Washington, DC: American Society for Microbiology; 2009.
5. Cappuccino JG, Sherman N. Microbiology: a laboratory manual. 10th ed. New York, NY: Pearson Education; 2014.
6. Meingassner JG, Sleytr U, Petranyi G. Morphological changes induced by naftifine, a new antifungal agent, in Trichophyton mentagrophytes. J Invest Dermatol. 1981;77(6):444–51. doi: https://doi.org/10.1111/1523-1747.ep12495814
7. Murtey MD, Ramasamy P. Sample preparations for scanning electron microscopy–life sciences. In: Janecek M, Kral R, editors. Modern electron microscopy in physical and life sciences. Rijeka, Croatia: IntechOpen; 2016. pp 161–86. doi: https://doi.org/10.5772/61720.
8. Qazi MA, Malik ZA, Qureshi GD, Hameed A, Ahmed S. Yeast extract as the most preferable substrate for optimized biosurfactant production by rhlB gene positive Pseudomonas putida SOL-10 isolate. J Bioremed Biodeg. 2013;4(7):1–10. doi: https://dx.doi.org/10.4172/2155-6199.1000204
9. Méndez-Vilas A. Industrial, medical and environmental applications of microorganisms: current status and trends. Wageningen, The Netherlands: Wageningen Academic Publishers; 2014.
10. Madigan MT, Bender KS, Buckley DH, Sattley WM, Stahl DA. Brock biology of microorganisms. 16th ed. Seattle, WA: Pearson Education; 2021.
11. Purwestri YA, Kartikasari N, Putri SG, Wilson W, Sembiring L. Metabolic profiling of endophytic bacteria from Purwoceng (Pimpinella pruatjan Molkend) root and antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa. AIP Conf Proc. 2016;1744(1):020063-1–020063-7. doi: https://doi.org/10.1063/1.4953537
12. Ouchari L, Boukeskasse A, Bouizgarne B, Ouhdouch Y. Antimicrobial potential of actinomycetes isolated from the unexplored hot Merzouga Desert and their taxonomic diversity. Biol Open. 2019;8(2):1–7. doi: https://doi.org/10.1242/bio.035410
13. Ghasemi M, Turnbull T, Sebastian S, Kempson I. The MTT assay: utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int J Mol Sci. 2021;22(12827):1–30. doi: https://doi.org/10.3390/ijms222312827
14. Kabir MA, Hussain MA, Ahmad Z. Candida albicans: a model organism for studying fungal pathogens. ISRN Microbiol. 2012;15(1):1–15. doi: https://doi.org/10.5402/2012/538694
15. Jabra-Rizk MA, Falkler WA, Meiller TF. Fungal biofilms and drug resistance. Emerg Infect Dis. 2004;10(1):14–9. doi: https://doi.org/10.3201/eid1001.030119
16. Pratiwi RH, Oktarina E, Mangunwardoyo W, Hidayat I, Saepudin E. Antimicrobial compound from endophytic Pseudomonas azotoformans UICC B-91 of Neesia altissima (Malvaceae). Pharmacogn J. 2022;14(1):172–81. doi: https://doi.org/10.5530/pj.2022.14.23
17. Priya A, Pandian SK. Piperine impedes biofilm formation and hyphal morphogenesis of Candida albicans. Front Microbiol. 2020;11(756):1–18. doi: https://doi.org/10.3389/fmicb.2020.00756
18. Tsuchimori N, Sharkey LL, Fonzi WA, French SW, Edwards JE Jr, Filler SG. Reduced virulence of HWP1-deficient mutants of Candida albicans and their interactions with host cells. Infect Immun. 2000;68(4):1997–2002. doi: https://doi.org/10.1128/iai.68.4.1997-2002.2000
19. Martins de Andrade V, Bardají E, Heras M, Ramu VG, Junqueira JC, Diane Dos Santos J, et al. Antifungal and anti-biofilm activity of designed derivatives from kyotorphin. Fungal Biol. 2020;124(5):316–26. doi: https://doi.org/10.1016/j.funbio.2019.12.002
20. Ma C, Du F, Yan L, He G, He J, Wang C, et al. Potent activities of roemerine against Candida albicans and the underlying mechanisms. Molecules. 2015;20(10):17913–28. doi: https://doi.org/10.3390/molecules201017913
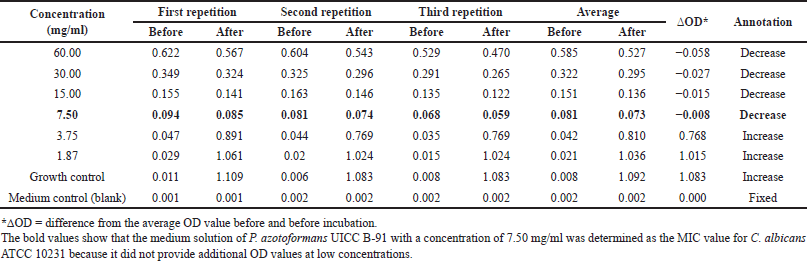 | Appendix 1. MIC test results from P. azotoformans UICC B-91 medium solution towards C. albicans ATCC 10231. [Click here to view] |