INTRODUCTION
Omega-3 polyunsaturated fatty acids (ω-3 PUFAs) are important fatty acids having beneficial roles in human health; therefore, food, nutraceutical, and pharmaceutical products are widely used as health supplements [1]. Among ω-3 PUFAs, eicosapentaenoic acid or eicosapentaenoic acid (C20:5, ω-3) (EPA) and docosahexaenoic acid or docosahexaenoic acid (C22:6, ω-3) (DHA) are reported to be found, especially in marine sources such as fish, microalgae and krill [2]. Some beneficial effects of Omega-3 fatty acids (ω-3 Fas) on human health have been reported [3]. There is a positive relationship between the reduced risk of cardiovascular diseases (CVD) with the high intake of ω-3 FAs of EPA and DHA because of the capability of these ω-3 FAs to modulate some risk factors associated with CVD, including platelet aggregation, blood lipids, blood pressure, and inflammation [4]. In addition, a negative correlation was observed between the association of ω-3 FAs and the risks of ulcerative colitis and Crohn’s disease [5]. The intake of ω-3 FAs could reduce some depression symptoms and exhibit anti-inflammatory effects by forming active metabolites capable of promoting tailored therapy in psychiatric and neurological conditions and involve the brain development in patients with Alzheimer’s disease [6]. These FAs are also reported to have anti-cancer activity in vitro by inducing the apoptotic in cancer cells and immune stimulants [2].
Because of the positive effects of ω-3 FAs on human health, some International Organizations, including Food and Agriculture Organization and World Health Organization, have recommended an intake of ω-3 FAs (EPA + DHA) of 0.25–2 g per day. In addition, to reduce the risk of heart disease and lowering blood pressure, the European Food Safety Authority has set a daily intake of long-chain ω-3 FAs 250 mg in adults [2]. Therefore, the quality controls of ω-3 FAs by determining the components of ω-3 FAs using some analytical methods is very urgent. In addition, EPA and DHA present in fish oils make them excellent nutritional sources. Consequently, fish oils containing ω-3 FAs are targeted to be adulterated with cheaper oils to get economic profits. Therefore, the levels of EPA and DHA could indicate that fish oils have been adulterated [7].
Various analytical approaches have been developed and used for the analysis of ω-3 FAs in fish oils, such as chromatographic-based methods including gas chromatography using a flame ionization detector [8], mass spectrometer detector [9], high performance liquid chromatography (HPLC) with UV detection liquid [10] and chromatography/tandem mass spectrometry (LC-MS/MS) [11]. However, these methods involve sophisticated instruments, require a lot of solvents, and require skillful analysts; therefore, more user-friendly methods must be developed mainly based on molecular spectroscopic methods. In this review, the employment of molecular spectroscopy, including near-infrared (NIR), Fourier transform infrared (FTIR), Raman, and nuclear magnetic resonance (NMR) spectroscopy combined with multivariate calibrations, were employed for analysis of ω-3 FAs.
METHODS
While performing this narrative review, some databases including Scopus, Web of Science, PubMed, and Research Gate were explored and used to search the relevant articles. The keywords used include omega fatty acids or EPA and DHA + FTIR spectroscopy or vibrational spectroscopy or molecular spectroscopy + chemometrics or multivariate data analysis (MDA). Some Boolean functions were also employed for searching the relevant article and abstracts.
Molecular spectroscopy
Molecular spectroscopy techniques, including UV-Vis, fluorescence, infrared (IR), and NMR, have been widely employed for the quality control of medicinal products and diagnostic tools for certain diseases. All spectroscopic measurements were based on the interaction between electromagnetic radiation (EMR) at certain wavelengths, frequencies, or wavenumbers with certain properties of analytes of interest [12]. Depending on EMR regions, spectroscopic methods are named and applied. UV-Visible (UV-Vis) spectroscopy is based on the interaction between EMR in UV and Vis regions corresponding to a wavelength of 200–380/400 nm with analytes to provide electronic transitions from the ground state into singlet excited state, while mid-IR and Raman spectroscopy used wavenumbers of 400–4,000 cm−1 to result in vibrational transitions of functional groups in molecules [13]. The wavelength, transition types, and EMR regions typically applied in spectroscopic methods are depicted in Figure 1.
The molecular spectra obtained could be used as qualitative and quantitative information for certain analytical tasks, including diagnostic tools. Some spectra, especially vibrational spectroscopy (IR, Raman, and NMR), exhibited fingerprinting profiles and are useful for differentiating the samples with different classes (for example, discrimination between healthy individuals and oral cancer patients). Molecular spectra are also useful for quantitative analysis based on Lambert-Beer law, in which the absorbance values of molecular spectra are proportional to the concentrations of objects (samples) [14]. The data obtained during molecular spectroscopic measurement are very large, making it challenging to be extracted into understandable information. Fortunately, special statistics tools called chemometrics could handle this obstacle [15].
Multivariate data analysis
MDA or chemometrics is a special statistical technique for treating chemical data since molecular spectroscopy deals with big data analysis [15]. For example, the analyst could extract hundreds or thousands of responses from single measurements. FTIR spectra scanned at wavenumbers of 4,000–400 cm−1 with a resolution of 2 cm−1 could provide about 1,700 absorbance values. For this reason, several chemometrics techniques were introduced with the main objective of extracting the chemical data into easily understandable information. Chemometrics is complementary to molecular spectra and the most common chemometrics techniques used are spectral preprocessing, classification modeling or pattern recognition, and multivariate regressions [16].
Prior to building the quantitative models using absorbance values of molecular spectra, some preprocessing techniques were typically applied. Before chemometrics analysis, molecular spectra are subjected to preprocessing spectra intended for specific purposes. Preprocessing treatment of molecular spectral data has become an essential part of chemometrics modeling. The preprocessing of spectral data is aimed at removing physical phenomena in the spectra to improve the subsequent chemometrics applications (exploratory analysis, classification model, or multivariate calibrations) [17]. The most preprocessing methods widely employed in molecular spectra can be divided into two groups, namely, (1) scatter correction methods including multiplicative scatter correction (MSC), extended MSC, inverse MSC, extended inverse MSC, de-trending, standard normal variate (SNV), and normalization; (2) spectral derivatives including Norris–Williams and Savitzky–Golay polynomial derivatives. Both preprocessing methods could apply spectral smoothing before calculating the spectral derivative intended to decrease the detrimental effect on the signal-to-noise ratio [18]. Whatever the types of preprocessing, the objective of this action is to enhance a subsequent exploratory analysis or pattern. recognition, to improve a subsequent classification model, and to enhance the capability of multivariate calibrations for quantitative modeling [19].
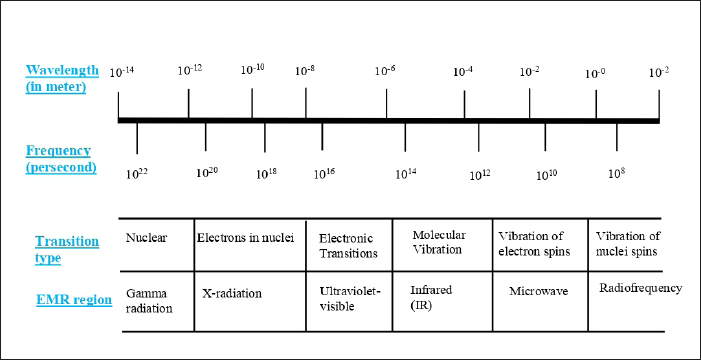 | Figure 1. The wavelength, transition types, and EMR regions typically applied in spectroscopic methods. Adapted from Breuer [13]. [Click here to view] |
Among the chemometrics techniques, multivariate calibrations are the most commonly used in quantitative analysis or prediction. Multivariate calibrations deal with the correlation between chemical responses (such as absorbance values and peak area) and the measured or predicted responses (mostly concentrations of analytes) [20]. Depending on the variables used as predictors or responses, multivariate calibrations are typically classified into two groups: classical and inverse calibrations. In classical calibrations, the variable responses or dependent variables (y-axis) were modeled using the variable of concentrations or independent variables (x-axis), while inverse calibrations modeled concentrations (y-axis) with absorbance values (x-axis) as predictor variables. The most commonly used inverse calibrations included principal component regression, partial least squares regression (PLSR), and SMLR. The performance of multivariate calibrations used for developing the correlation between predictor variables and response variables was evaluated by several statistical parameters, including R2-values for accuracy assessments and error values represented by ratio of prediction to deviation (RPD), root mean square error of calibration (RMSEC), root mean square error of prediction (RMSEP), and bias [21].
The general steps for quantitative modeling involving molecular spectra and multivariate calibrations include the preprocessing spectra, the selection of multivariate calibration types, the selection of wavenumbers of wavelength regions along with a number of factors, and assessing the statistical performances obtained from this optimization step [22]. The selection of wavenumbers or wavelength regions of molecular spectra is a key factor during the building of calibration models, and some chemometrics techniques typically applied for this included iterative partial least squares [23]. In addition, the selection of the optimum number of latent variables was very critical to produce robust, reliable, and unbiased multivariate calibration models, and this task was typically performed based on the stable minimum number of root mean square error of cross-calibration (RMSECV) during cross-validation [24].
Analysis of ω-3 Fas using near-infrared spectroscopy
Near-infrared spectroscopy (NIRS) is a versatile technique for the quality control of food and pharmaceutical products. NIRS combined with PLSR has been developed for the determination of EPA, DHA, and total ω-3 FAs fish oil in some fish oils and supplements claiming to contain EPA and DHA (omega-3 products). During this research, NIR spectra were acquired in transflectance mode at the wavelength region of 1,100–2,500 nm using 2 nm intervals (with 700 data points) at a resolution of 4 cm−1 and a number of scanning of 32 scans. NIR spectra were subjected to Savitzky–Golay first and second derivatives to resolve the overlapping peaks and finally to provide better quantitative modeling between actual values of EPA, DHA, and total ω-3 FAs as determined using GC-FID and NIRS predicted values, assisted by chemometrics of PLSR. Some NIR spectral regions were optimized to provide the best modeling as indicated by the highest R2- values in calibration and cross-validation and by the lowest values of RMSEC and RMSECV. Finally, the whole NIR spectral region at 1,100–2,500 nm could predict EPA, DHA, and ω-3 FAs with comparable values of R2 (higher than 0.969), with calibration errors of 3.43, 3.20, and 2.18 for EPA, DHA, and ω-3 FAs respectively. This region is also capable of predicting EPA, DHA, and ω-3 FAs in cross validations, thus by selecting the appropriate condition, NIRS combined with chemometrics is effective means for analysis of ω-3 FAs with high accuracy and precision [25].
NIRS combined with PLSR has been employed to analyze ω-3 FAs in salmon oil. PLSR was used for the correlation between actual values of ω-3 FAs as determined by gas chromatography-flame ionization detector (GC-FID) and NIRS predicted value. The selection of spectral region used for predicting ω-3 FAs was assisted by interval partial least-squares regression, and finally, the wavenumbers of 10,000–8,000 cm−1 were used for quantitative modeling. The number of partial least-squares (PLS) factors during modeling was based on RMSECV values obtained during cross-validation, and 5 PLS factors were used. The relationship between actual values of ω-3 FAs and NIRS predicted values offered a good relationship with R2-values close to 1.00 in either calibration or prediction models. Furthermore, principal component analysis (PCA) was applied for the representativity of calibration sets, and the results indicated that the first three principal components (score plots) could represent total variances of 96.5% in which PC1, PC2, and PC3 contributed to 71.79, 14.43 and 10.28% for PC1, PC2, and PC3, respectively. PLS-NIR offered acceptable predictive capability with relative RMSEP ≤ 1.8% [26].
The Fourier transform NIR (FT-NIR) has been successfully used to classify fish oils supplement and quantify ω-3 FAs of DHA and EPA in commercial samples of fish oils in soft gels and liquid form. Samples were measured at the wavenumber of 12,500–4,000 cm−1 using the resolution of 8 and number of scanning of 32. PLSR regression was applied to quantify EPA and DHA contained in fish oil supplement samples. Results from FT-NIR and PLSR were comparable with the results obtained from gas chromatography-mass spectrometry (GC-MS) determination of EPA and DHA in the fish oil supplement samples. The results from PLSR using FT-NIR data had a high linear correlation with those obtained from GC-MS as the reference data. The PLSR model was validated by using cross-validation and an independent validation test set [27]. In combination with PLSR, NIR spectroscopy has also been successfully used to predict the concentration of several ω-3 Fas in fish oils, including arachidonic acid, DHA, and EPA, in the nutraceutical industry. The concentration of those three ω-3 Fas used as the references was obtained from gas chromatography measurement. Results demonstrated that the NIR method combined with PLSR was equivalent to gas chromatography for quantification of omega-3 fatty acids in fish oils nutraceutical samples observed by its R2 calibration, R2 prediction, standard error of calibration (SEC) as well as standard error of prediction. In addition, the PLSR model using NIR data provided a good precision model within the prescribed criteria for the quantification of fish oil nutraceuticals. Both cross and independent validation demonstrated the PLSR model validity [28].
Mid-infrared spectroscopy for analysis of ω-3 Fas
Among vibrational spectroscopic techniques, FTIR spectra have been widely applied to analyze EPA, DHA, and total ω-3 FAs in fish oils and commercial omega-3 supplements [29]. FTIR spectroscopy combined with chemometrics of preprocessing spectra (Savitzky–Golay first and second derivatives) and PLSR has been employed to determine EPA, DHA, and total ω-3 FAs. During this study, the optimization is carried out by selecting the FTIR spectral regions offering the best modeling performances in terms of the highest R2-values in calibration and validation data sets, the lowest values of errors in calibration and cross-validation, bias, and a number of PLS factors. The evaluated IR regions for determination of EPA and DHA included the whole FTIR spectra (4,000–650 cm−1), the combined regions of 3,100–2,700 and 1,820–650 cm−1, 3,100–2,700 cm−1, and selected fingerprint IR region of 1,820–650 cm−1. Meanwhile, for determination of total ω-3 FAs, the regions evaluated include 4,000–650 cm−1, the combined regions of 3,090–2,800 and 1,790–650 cm−1, 3,090–2,800 cm−1, and region of 1,790–650 cm−1. The optimization results indicate that absorbance values at the whole region (4,000–650 cm−1) can be preferred for the determination of EPA, DHA, and total ω-3 FAs with high values of R2 in calibration and cross-validation with low prediction errors. The R2 values obtained were higher than 0.97, with standard error of cross-validation (SECV) values for EPA, DHA, and total ω-3 FAs of 3.05%, 3.09%, and 2.75%, respectively. The numbers of factors are 7 PLS factors (for EPA and DHA) and 2 PLS factors (for ω-3 FAs). The use of minimum PLS factors indicated the lower complexity of PLSR modeling. The conclusion extracted from this result is that the combination of FTIR spectroscopy-PLSR at the whole IR region provides accurate and precise analytical methods for determining EPA, DHA, and total ω-3 FAs [25]. By selecting the appropriate wavenumbers region, FTIR spectra are proven to be effective tools for the analysis of ω-3 FAs. Toyoda et al. [30] used the combined regions at 3,050–2,820 and 1,500–1,000 cm−1 for quantitative modeling of ω-3 FAs, assisted with PLSR, and the results reported that this condition could provide acceptable accuracy and precision method. FTIR spectroscopy combined with PLS-1 at the whole IR region was successful for the analysis of EPA, DHA, and EPA + DHA using five, six, and five PLS factors, respectively. The R2 value, SEC, SECV, and RPD for EPA, DHA, and EPA + DHA are acceptable in terms of accuracy and precision of analytical methods [31].
FT-NIR and FTIR spectroscopy combined with the chemometric of multivariate regressions were compared to quantify EPA and DHA. The selected method was based on the capability of two spectroscopic methods to provide high accuracy during the prediction of EPA and DHA in the analyzed samples of marine oil dietary supplements, as indicated by low RMSEP and high R2 (close to 1). The percent accuracy of the support vector regression (SV-R) model to predict the values of EPA and DHA in the certified reference material (CRM) was in the range of 90% to 110%. The SV-R models also provided closer agreement between actual values of EPA and DHA in CRM as determined by GC-MS and predicted values in the reference standard. Thus, the SV-R methods using both FT-NIR and FTIR had superior accuracy and predictive quality for predicting the concentrations of EPA and DHA in the analyzed samples [32].
FTIR spectroscopy and MDA have been developed for rapid determination of EPA and DHA of oils from marine sources. In this study, the spectra data were collected without sample preparation. 300 oil spectra were randomly divided into calibration (two-thirds of spectra) and validations (one-third of spectra) datasets. To build the model, 200 spectra data of the calibrations set were optimized using PLSR with the cross-validation method. The EPA, DHA, PUFAs, and UFAs content from the GC technique was used as the actual value. On the other hand, the validation dataset used 100 spectra combined with 10 spectra from unknown oil. Two spectra ranges (3,050–2,800 and 1,800 and 650 cm−1) were used during the optimization stage. This study revealed the FTIR spectra combined PLSR using normal spectra at region combined 3,050–2,800 and 1,800 and 650 cm−1 suited to the quantitative determination of EPA, DHA, PUFAs, and UFAs based on minimum RMSEC and the coefficient of determination value closest to 1 [33].
Daoud et al. [34] have developed a fast and direct analysis of omega-3 oxidation levels in functional food products (oil-in-water emulsions form). Lipid oxidation leads to product degradation which can reduce consumer acceptance. Thus, monitoring lipid oxidation in oil-in-water emulsions is important. Tuna fish oil from different suppliers was used during this study. Tuna fish oils (20%) were prepared in 10 mM phosphate buffer solution (80%) and cetyltrimethylammonium bromide (0.7%) as an emulsifier. 2,2′-Azobis(2-methylpropionamidine) dihydrochloride, sulfate iron solutions were added for radical initiation, monitoring lipid oxidation by measuring conjugated dienes (CD) levels. Each emulsion sample was measured using FTIR at 4,000–600 cm−1 region for the developed model. The absorbance of FTIR spectra each sample was optimized to be used as predictor variables, while CD level was the actual value by PLSR. The whole FTIR spectra regions, 4,000–600 cm−1, 1,800–1,500 cm−1, and 1,500–900 cm−1 regions, had a good correlation toward CD level. This result confirmed that FTIR spectroscopy is a fast dan simple method for monitoring lipid oxidation, especially omega-3, in functional food without sample preparation.
Similar research was conducted by Daoud et al. [35] in an infant formula sample. The high DHA content in infant formulas is very prone to oxidation which can alter the flavors and nutritional level of the product. In this study, monitoring the iron-inducer oxidation in liquid infant formula using ATR-FTIR (mid and near regions) combined PLSR. The result showed that FTIR detected the unique spectral to lipid oxidation although the complex composition of the milk formula. Thus, FTIR spectroscopy can be applied in dairy industries for quality control.
Application of Raman spectroscopy for analysis of ω-3 Fas
Raman spectra previously subjected to preprocessing of polynomial curve fitting with SNV transformation combined with chemometrics have been optimized to analyze EPA, DHA, and total ω-3 FAs. Raman spectral regions and their combination were optimized to be used as variables for developing the relationship models between actual values of EPA, DHA, and total ω- 3 FAs as determined using GC-FID and FTIR predicted values assisted by PLSR. The peaks at four regions, namely, at (1) the whole region of Raman spectra at wavenumbers of 3,450–769 cm−1, (2) the combined regions of 3,150–2,460 and 1,800–769 cm−1, (3) 3,150–2,460 cm−1, and (4) 1,800–769 cm−1, were assessed, and regions capable of providing the highest accuracy as indicated by the highest R2-values and highest precision as indicated by the lowest values of RMSEC and RMSEP was selected. Based on this criterion, the whole Raman spectra region and the combined regions of 3,150–2,460 + 1,800–769 cm−1 (1) and regions of 1,800–769 cm−1 (4) provide the comparable results during the calibration modeling in terms of R2-values, SECV, and the number of PLS factors. These regions were then used to predict the levels of EPA, DHA, and total ω-3 FAs in cross-validation models, and the results indicated that absorbance values at the combined regions of 3,150–2,460 + 1,800–769 cm−1 (1) and regions of 1,800–769 cm−1 (4) can be employed for predicting the responses (concentrations of EPA, DHA, and total ω-3 FAs) with comparable degrees of accuracy. This result confirmed that the combination of Raman spectra and PLSR could be used for the quantitative analysis of ω-3 FAs with acceptable accuracy and precision performances [25]. Raman spectra combined with chemometrics of PLSR and PCA could be used for quantitative analysis of EPA, DHA, and total ω-3 FAs and classification of fish oils and ω-3 PUFA concentrates in intact soft gel (gelatin) capsules [36].
The handled Raman spectroscopy combined with multivariate calibrations of PLSR using the algorithm of noniterative partial least squares has been employed for analysis of EPA, DHA, and EPA + DHA in the commercial supplement samples of encapsulated omega-3 oils. The absorbance values of Raman spectra at regions of 1,800 to 800 cm−1 were used for this task. Raman spectra were previously subjected to some preprocessing techniques, including the transformations of the Savitzky–Golay second-derivative to remove baseline features and SNV to remove intensity differences coming from the effects of variable focus and path length. The multivariate regression of PLSR was applied for modeling the correlation between actual values of EPA, DHA, and EPA + DHA as determined using GC-MS and Raman spectroscopy predicted values of fifteen commercial samples of fish oils and omega-3 concentrate. There is a good correlation between actual values of EPA, DHA as determined by GC-MS (x-axis) and handled FT-Raman spectroscopy (y-axis) with R2 values of 0.97 either in calibration or in cross-validation with RMSEC of 25 and RMSECV of 26. Furthermore, the errors obtained were also comparable using Raman spectra and GC-MS (38, 24, and 32 mg/g in Raman spectra compared to 32, 22, and 26 mg/g) for EPA, DHA, and EPA + DHA, respectively. From model loadings, the wavenumbers of Raman spectra providing the most contribution for predicting EPA and DHA are at 1,440 and 1,420 cm−1 [37]. This result obtained from the combination of PLSR and Raman spectra is comparable to that obtained using the reference method of GC-MS.
The quantification of EPA and DHA in krill oil samples has been successfully carried out using Raman spectroscopy. Krill oil is known for its content of DHA, EPA, and polyunsaturated fatty acids of long-chain omega 3. It also contains astaxanthin, which protects EPA and DHA from oxidation. Astaxanthin is a keto-carotenoid that naturally occurs. Raman spectroscopy data incorporated into the data fusion approach of mid-level fusion prior to PLSR regression was successfully used for quantification EPA + DHA in krill oil samples with R2 calibration = 0.92, R2 validation = 0.90, RMSEC = 5.7%, and RMSEP = 4.5%. The reference for EPA and DHA concentration was determined using the GC-MS method [38]. Several data fusion techniques can be applied to multivariate data, such as low-level, mid-level, and high-level fusion [39]. The application of data fusion in analytical chemistry has recently increased, such as its application for the analysis of olive oil and honey [40,41]. It also has been used to predict meat spoilage [42].
NMR spectroscopy for analysis of ω-3 Fas
The application of NMR spectroscopy for food analysis has increased because of its versatility. Even though NMR spectroscopy is quite expensive, it allows for fingerprinting analysis to analyze both primary and secondary metabolites simultaneously without being time-consuming [43]. It has been applied to the analysis of fatty acids in food samples, including omega fatty acids [44]. A proton NMR (1H-NMR) spectroscopy has been used to determine the content of omega-3 PUFA fatty acids in four types of oils, namely capelin oil, tuna oil, omega-3 oil, and cod liver oil, which contains high content of omega-3 PUFA. Different conditions were applied to oxidize the oils, then the changes in fatty acid composition were monitored using a 1H-NMR spectrometer. The result showed that 1H-NMR spectroscopy was successfully used to determine both certain and total ω-3 Fas in the oxidized oils rapidly and accurately. The results were comparable with the results obtained using the GC-MS method [45]. Another study reported using 1H-NMR spectroscopy combined with chemometrics to classify omega-3 fish oil in fish oil supplements. Unsupervised pattern recognition of PCA and supervised pattern recognitions of both PLS-DA and OPLS-DA were successfully applied to classify omega-3 fish oils in triacylglycerol (TAG) and ethyl ester (EE) forms. The chemical shift used as variables for chemometrics analysis ranged from 4.0 to 4.5 ppm. PCA analysis demonstrated clear differentiation between TAG and EE in the PC1. Meanwhile, the supervised analysis could be used for the correct classification of ω-3 Fas in TAG and EE forms with 100% accuracy. The model had good model fitting, indicated by its R2Y value (≥0.997) and good predictivity, indicated by its Q2 value (≥0.953). Moreover, high precision was obtained, shown by its low error for RMSEP (≤0.009) [46].
1H-NMR spectroscopy method has been reported to be successfully used for the quantification of DHA and EPA in fish oils. Fish oil soft gels from four different types were used for EPA and DHA analysis using the 1H-NMR spectroscopy method. The chemical shift of 2.391 ppm was used for DHA quantification, whereas the chemical shift of 1.697 was applied to quantify EPA. The result showed that the 1H-NMR spectroscopy technique could be used as a non-destructive analytical technique for quantifying DHA and EPA in fish oil samples rapidly and accurately. Quantification was performed using dimethyl terephthalate as the internal standard. The parameters of specificity, stability, and precision were measured to validate the developed method. The results of the method were in accordance with the China Food and Drug Administration; therefore, it is potential as a rapid method for quality control of fish oils [47]. NMR spectroscopy combined with PLS regression has been successfully used to rapidly measure DHA and EPA in algal oils. Among other techniques used in this study, such as visible and short-wave near-infrared, long-wave near-infrared, mid-infrared, and NMR spectroscopy combined with PLS regression showed the best model to determine DHA and EPA with a high R2 value of 0.963 and 0.967 for DHA and EPA, respectively. The PLS model for DHA was carried out using 15 variables with successive projections algorithm . In contrast, PLS for EPA was performed using 13 variables using an uninformative variable elimination-successive projections algorithm. The PLS regression for DHA and EPA demonstrated low error associated with good precision indicated by lower RMSEC (0.864 and 0.051, for DHA and EPA, respectively) and RMSEP (1.271 and 0.069, for DHA and EPA, respectively) values. It demonstrated that NMR spectroscopy combined with multivariate calibrations has a great potential for determining DHA and EPA in algal oil, further applied to other types of oils [48].
Determination of polyunsaturated ω-3 Fas of DHA, DPA, and EPA using NMR spectroscopy in combination with multivariate calibration has also been successfully performed. The selection of important variables for creating PLS regression used a combination of uninformative variable elimination and successive projections algorithm. Before chemometrics analysis, the data preprocessing was carried out. SNV and MSC were chosen. Results showed that NMR and PLS could be applied for rapid determination of DHA, DPA, and EPA with high R2 of cross-validation, that is, 0.982, 0.983, and 0.970, respectively. The obtained RMSECV values were 4.73, 0.77, and 11.48 mg/g for DHA, DPA, and EPA, respectively. It showed that NMR spectroscopy, in combination with chemometrics, could be used as a potential alternative method to the GC-MS-based method for the analysis of omega fatty acids [49]. Another study reported that the 1H-NMR spectroscopy was successfully used to analyze the relative concentration of DHA and EPA in commercial samples of omega-3 supplements. The samples were dissolved in deuterated chloroform containing tetramethylsilane as the internal standard. The quantification of DHA was performed using a chemical shift of 2.37 ppm, whereas EPA was quantified using a chemical shift of 1.75 ppm. The obtained results of DHA and EPA quantification using the 1H-NMR spectroscopy method were comparable with the DHA and EPA concentration in the product labels. Therefore, the 1H-NMR spectroscopy technique provides great potential and promising to be used to determine EPA and DHA in omega-3 supplements [29].
Future prospect
Different molecular spectroscopic methods, including FTIR, NIR, Raman, and NMR spectroscopy, have been discussed in this review. These spectroscopic techniques could be used to analyze ω-3 fatty acids, such as quantification of EPA and DHA with minimum sample preparation steps and short analysis time compared to other methods, such as gas chromatography and liquid chromatography. Table 1 shows different chromatographic-based methods that have been previously used for the analysis of ω-3 fatty acids, including DHA and EPA, in fish and fish oil samples. It demonstrated that liquid chromatography/tandem mass spectrometry (LC–MS/MS) and GC-MS-based methods provided high sensitivity for ω-3 fatty acids analysis with high accuracy and high precision results. However, compared to the molecular spectroscopic techniques, these methods are quite laborious due to complex sample preparation steps and require many chemical reagents compared to FTIR, NIR, Raman, and NMR methods. Molecular spectroscopic methods, especially FTIR, NIR, and Raman, are also more effective and efficient in terms of cost reduction. In addition, results prove that the quantification results from molecular spectroscopic methods combined with chemometrics have comparable results with the reference results obtained from GC-MS measurement. These methods also provided high accuracy and high precision for analyzing ω-3 fatty acids. Therefore, molecular spectroscopic methods are very promising and can be used as analytical techniques for quantifying ω-3 fatty acids, including EPA and DHA, in fish and fish oil-based products for quality control and authentication purposes.
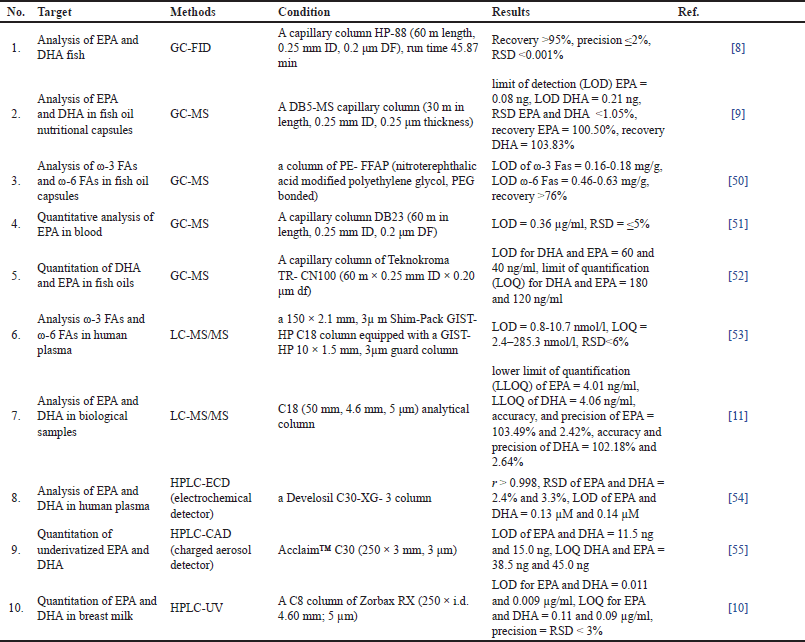 | Table 1. Quantitative analysis of omega-3 and omega-6 fatty acids using chromatographic-based methods. [Click here to view] |
CONCLUSION
Omega fatty acids are important sources of human nutrition. Therefore, the determination of omega fatty acids such as ω-3 fatty acids, EPA, and DHA is important to know the omega fatty acids content in samples. In addition, it is also necessary for quality control purposes. Molecular spectroscopic techniques such as NIR, FTIR, Raman, and NMR have been widely used for food analysis and determining omega fatty acids in food products because of their advantages. They allow for simple sample preparation, short-time analysis, and a small volume of solvents, which support the green chemistry principle. Advanced tools such as chemometrics are crucial to handle and process the huge amounts of data obtained from spectroscopic analysis. The application of NIR, FTIR, Raman, and NMR spectroscopy in combination with chemometrics has been widely used to determine omega fatty acids such as DHA, EPA, and ω-3 Fas. Chemometrics of PLS showed high accuracy and high precision results in the determination of omega fatty acids; therefore, it has become the most common methods chosen for the determination of omega fatty acids obtained from NIR, FTIR, Raman, and NMR spectroscopy. It can be concluded that spectroscopic techniques in combination with MDA are promising for the determination of omega fatty acids and provides great potential to be used as a rapid, effective, and efficient analytical technique for the determination of omega fatty acids for quality control purposes. Further action on the standardization of spectroscopic techniques (NIR, FTIR, Raman, and NMR) combined with chemometrics for the determination of omega fatty acids is required to warrant reliability and reproducibility.
ACKNOWLEDGEMENT
The authors thank the Directorate of Research Universitas Gadjah Mada for the financial support during this study through Program Post-Doctoral 2022 with a commitment letter of 5836/UN1/DITLIT/Dit-Lit/PT.01.05/2022.
LIST OF ABBREVIATIONS
DHA: Docosahexaenoic acid (C22:6, ω-3); EPA: Eicosapentaenoic acid (C20:5, ω-3); ω-3 FAs: Omega-3 fatty acids; NIRS: Near-infrared spectroscopy; PCA: Principal component analysis; PLSR: Partial least squares regression; RMSEC: Root mean square error of calibration; RMSECV: Root mean square error of cross-calibration; RMSEP: Root mean square error of prediction; SV-R: Support vector regression.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Park SE, Yu HY, Ahn S. Development and validation of a simple method to quantify contents of phospholipids in krill oil by Fourier-transform infrared spectroscopy. Foods. 2022;11:41. CrossRef
2. Pasini F, Gómez-Caravaca AM, Blasco T, Cveji? J, Caboni MF, Verardo V. Assessment of lipid quality in commercial omega-3 supplements sold in the French market. Biomolecules. 2022;12:1361. CrossRef
3. Vongsvivut J, Heraud P, Gupta A, Puri M, McNaughton D, Barrow CJ. FTIR microspectroscopy for rapid screening and monitoring of polyunsaturated fatty acid production in commercially valuable marine yeasts and protists. Analyst. 2013;138:6016–31. CrossRef
4. Innes JK, Calder PC. Marine omega-3 (N-3) fatty acids for cardiovascular health: an update for 2020. Int J Mol Sci. 2020;21:1–21. CrossRef
5. Mozaffari H, Daneshzad E, Larijani B, Bellissimo N, Azadbakht L. Dietary intake of fish, n-3 polyunsaturated fatty acids, and risk of inflammatory bowel disease: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2020;59:1–17. CrossRef
6. Giacobbe J, Benoiton B, Zunszain P, Pariante CM, Borsini A. The anti-inflammatory role of omega-3 polyunsaturated fatty acids metabolites in pre-clinical models of psychiatric, neurodegenerative, and neurological disorders. Front Psychiatry. 2020;11:122. CrossRef
7. Gao F, Han LJ, Liu X. Vibration spectroscopic technique for species identification based on lipid characteristics. Int J Agric Biol Eng. 2017;10:255–68.
8. Alinafiah SM, Azlan A, Ismail A, Rashid NKMA. Method development and validation for omega-3 fatty acids (DHA and EPA) in fish using gas chromatography with flame ionization detection (GC-FID). Molecules. 2021;26:6592. CrossRef
9. Yi T, Li SM, Fan JY, Fan LL, Zhang ZF, Luo P, et al. Comparative analysis of EPA and DHA in fish oil nutritional capsules by GC-MS. Lipids Health Dis. 2014;13:1–6. CrossRef
10. Kie?basa A, Buszewski B, Gadza?a-Kopciuch R. A novel non-derivatization HPLC/UV method for the determination of some n-3 free fatty acids in breast milk matrix. Microchem J. 2022;181:107789. CrossRef
11. Viswanathan S, Verma PRP, Ganesan M, Manivannan J. A novel liquid chromatography/tandem mass spectrometry (LC–MS/MS) based bioanalytical method for quantification of ethyl esters of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and its application in pharmacokinetic study. J Pharm Biomed Anal. 2017;141:250–61. CrossRef
12. Rohman A, Rawar EA, Sudevi S, Nurrulhidayah AF, Windarsih A. The use of chemometrics in combination with molecular spectroscopic and chromatographic methods for authentication of curcuma species: a review. Food Res. 2020;4:1850–8. CrossRef
13. Breuer D. Molecular spectroscopy in the ultraviolet and visible range. Hoboken (NJ): John Wiley & Sons. pp 1–5.
14. Rohman A, Windarsih A. The application of molecular spectroscopy in combination with chemometrics for halal authentication analysis: a review. Int J Mol Sci. 2020;21:1–18. CrossRef
15. Pollo BJ, Teixeira CA, Belinato JR, Furlan MF, Cunha IC de M, Vaz CR, et al. Chemometrics, comprehensive two-dimensional gas chromatography and “omics” sciences: basic tools and recent applications. Trends Anal Chem. 2021;134:116111. CrossRef
16. Moros J, Garrigues S, De Guardia M. Vibrational spectroscopy provides a green tool for multi-component analysis. Trends Anal Chem. 2010;29:578–91. CrossRef
17. Paul A, De P, Harrington B. Chemometric applications in metabolomic studies using chromatography-mass spectrometry. Trends Anal Chem. 2021;135:116165. CrossRef
18. Lee LC, Liong CY, Jemain AA. A contemporary review on data preprocessing (DP) practice strategy in ATR-FTIR spectrum. Chemom Intell Lab Syst. 2017;163:64–75. CrossRef
19. Rinnan Å, Berg F van den, Engelsen SB. Review of the most common pre-processing techniques for near-infrared spectra. Trends Anal Chem. 2009;28:1201–22. CrossRef
20. Rohman A, Che Man YB, Nurrulhidayah AF. Fourier-transform infrared spectra combined with chemometrics and fatty acid composition for analysis of pumpkin seed oil blended into olive oil. Int J Food Prop. 2015;18:1086–96. CrossRef
21. Peris-Díaz MD, Kr??el A. A guide to good practice in chemometric methods for vibrational spectroscopy, electrochemistry, and hyphenated mass spectrometry. Trends Anal Chem. 2021;135:116157. CrossRef
22. Rohman A, Windarsih A, Hossain MAM, Johan MR, Ali ME, Fadzilah NA. Application of near- and mid-infrared spectroscopy combined with chemometrics for discrimination and authentication of herbal products: a review. J Appl Pharm Sci. 2019;9:137–47. CrossRef
23. Brereton RG. Consequences of sample size, variable selection, and model validation and optimisation, for predicting classification ability from analytical data. Trends Anal Chem. 2006;25:1103–11. CrossRef
24. Saad AS, Elzanfaly ES, Halim MK, Kelani KM. Comparing the predictability of different chemometric models over UV-spectral data of isoxsuprine and its toxic photothermal degradation products. Spectrochim Acta Part A Mol Biomol Spectrosc. 2019;219:444–9. CrossRef
25. Bekhit MY, Grung B, Mjøs SA. Determination of omega-3 fatty acids in fish oil supplements using vibrational spectroscopy and chemometric methods. Appl Spectrosc. 2014;68:1190–200. CrossRef
26. Cascant MM, Breil C, Fabiano-Tixier AS, Chemat F, Garrigues S, de la Guardia M. Determination of fatty acids and lipid classes in salmon oil by near infrared spectroscopy. Food Chem. 2018;239:865–71. CrossRef
27. Karunathilaka SR, Choi SH, Mossoba MM, Yakes BJ, Brückner L, Ellsworth Z, et al. Rapid classification and quantification of marine oil omega-3 supplements using ATR- FTIR, FT-NIR and chemometrics. J Food Compos Anal. 2019;77:9–19. CrossRef
28. Van der Merwe S, Manley M, Wicht M. Enhancing near infrared spectroscopy models to identify omega-3 fish oils used in the nutraceutical industry by means of calibration range extension. J Near Infrared Spectrosc. 2018;26:245–61. CrossRef
29. Lopes TIB, Pereira ES, dos Freitas DS, Oliveira SL, Alcantara GB. Spectral profiles of commercial omega-3 supplements: an exploratory analysis by ATR-FTIR and 1H NMR. J Food Sci Technol. 2020;57:1251–7. CrossRef
30. Toyoda K, Yamanoue M, Ihara I, Hu X. ATR-FTIR evaluation of important fatty acid profile in Japanese black cattle beef. 58th International Congress of Meat Science and Technology; 2012 August 12–17; Montreal, Canada.
31. Hernández-Martínez M, Gallardo-Velázquez T, Osorio-Revilla G, Almaraz-Abarca N, Ponce- Mendoza A, Vásquez-Murrieta MS. Prediction of total fat, fatty acid composition and nutritional parameters in fish fillets using MID-FTIR spectroscopy and chemometrics. LWT Food Sci Technol. 2013;52:12–20. CrossRef
32. Karunathilaka SR, Yakes BJ, Choi SH, Brückner L, Mossoba MM. Comparison of the performance of partial least squares and support vector regressions for predicting fatty acids and fatty acid classes in marine oil dietary supplements by using vibrational spectroscopic data. J Food Prot. 2020;83:881–9. CrossRef
33. Vongsvivut J, Miller MR, McNaughton D, Heraud P, Barrow CJ. Rapid discrimination and determination of polyunsaturated fatty acid composition in marine oils by FTIR spectroscopy and multivariate data analysis. Food Bioprocess Technol. 2014;7:2410–22. CrossRef
34. Daoud S, Bou-maroun E, Dujourdy L, Waschatko G, Billecke N, Cayot P. Fast and direct analysis of oxidation levels of oil-in-water emulsions using ATR-FTIR. Food Chem. 2019;293:307–14. CrossRef
35. Daoud S, Bou-Maroun E, Waschatko G, Horemans B, Mestdagh R, Billecke N, et al. Detection of lipid oxidation in infant formulas: application of infrared spectroscopy to complex food systems. Foods. 2020;9:1432. CrossRef
36. Killeen DP, Marshall SN, Burgess EJ, Gordon KC, Perry NB. Raman spectroscopy of fish oil capsules: polyunsaturated fatty acid quantitation plus detection of ethyl esters and oxidation. J Agric Food Chem. 2017;65:3551–8. CrossRef
37. Killeen DP, Card A, Gordon KC, Perry NB. First use of handheld Raman spectroscopy to analyze omega-3 fatty acids in intact fish oil capsules. Appl Spectrosc. 2020;74:365–71. CrossRef
38. Ahmmed F, Fuller ID, Killeen DP, Fraser-Miller SJ, Gordon KC. Raman and infrared spectroscopic data fusion strategies for rapid, multicomponent quantitation of krill oil compositions. ACS Food Sci Technol. 2021;1:570–8. CrossRef
39. Márquez C, López MI, Ruisánchez I, Callao MP. FT-Raman and NIR spectroscopy data fusion strategy for multivariate qualitative analysis of food fraud. Talanta. 2016;161:80–6. CrossRef
40. Borràs E, Ferré J, Boqué R, Mestres M, Aceña L, Calvo A, et al. Olive oil sensory defects classification with data fusion of instrumental techniques and multivariate analysis (PLS-DA). Food Chem. 2016;203:314–22. CrossRef
41. Tahir HE, Xiaobo Z, Zhihua L, Jiyong S, Zhai X, Wang S, et al. Rapid prediction of phenolic compounds and antioxidant activity of Sudanese honey using Raman and Fourier transform infrared (FT-IR) spectroscopy. Food Chem. 2017;226:202–11. CrossRef
42. Fengou LC, Spyrelli E, Lianou A, Tsakanikas P, Panagou EZ, Nychas GJE. Estimation of minced pork microbiological spoilage through Fourier transform infrared and visible spectroscopy and multispectral vision technology. Foods. 2019;8:238. CrossRef
43. Pacholczyk-Sienicka B, Ciepielowski G, Albrecht ?. The application of NMR spectroscopy and chemometrics in authentication of spices. Molecules. 2021;26:382. CrossRef
44. Li J, Vosegaard T, Guo Z. Applications of nuclear magnetic resonance in lipid analyses: an emerging powerful tool for lipidomics studies. Prog Lipid Res. 2017;68:37–56. CrossRef
45. Tyl CE, Brecker L, Wagner KH. 1H NMR spectroscopy as tool to follow changes in the fatty acids of fish oils. Eur J Lipid Sci Technol. 2008;110:141–8. CrossRef
46. Amorim TL, Granato ÁS, de Oliveira Mendes T, de Oliveira MAL, Amarante GW, de la Fuente MA, et al. Lipid classification of fish oil omega-3 supplements by 1H NMR and multivariate analysis. J Food Compos Anal. 2021;102:104060. CrossRef
47. Lv J, Wang C, Zhang X, Lv Z, Yu M. 1H NMR quantification of DHA and EPA in fish oil. J Ocean Univ China. 2020;19:1193–7. CrossRef
48. Wu D, He Y. Potential of spectroscopic techniques and chemometric analysis for rapid measurement of docosahexaenoic acid and eicosapentaenoic acid in algal oil. Food Chem. 2014;158:93–100. CrossRef
49. Wu D, Chen X, Cao F, Sun DW, He Y, Jiang Y. Comparison of infrared spectroscopy and nuclear magnetic resonance techniques in tandem with multivariable selection for rapid determination of ω-3 polyunsaturated fatty acids in fish oil. Food Bioprocess Technol. 2014;7:1555–69. CrossRef
50. Brotas MSC, Carvalho GA, Pereira PAP. Determination, through derivatization and GC-MS analysis, of omega-3 and omega-6 fatty acids in fish oil capsules sold in salvador, Bahia. J Braz Chem Soc. 2020;31:447–55. CrossRef
51. Abu EO, Oluwatowoju I. Omega-3 index determined by gas chromatography with electron impact mass spectrometry. Prostaglandins Leukot Essent Fat Acids. 2009 Apr;80(4):189–94. CrossRef
52. Khedr A, Alahdal AM. Optimized gas chromatography-mass spectrometric method to profile esterified fatty acids in fish roe and fish oil. Indian J Pharm Sci. 2018;80:628–36. CrossRef
53. Serafim V, Tiugan DA, Andreescu N, Mihailescu A, Paul C, Velea I, et al. Development and validation of a LC–MS/MS-based assay for quantification of free and total omega 3 and 6 fatty acids from human plasma. Molecules. 2019;24:360. CrossRef
54. Kotani A, Watanabe M, Yamamoto K, Kusu F, Hakamata H. Determination of eicosapentaenoic, docosahexaenoic, and arachidonic acids in human plasma by high-performance liquid chromatography with electrochemical detection. Anal Sci. 2016;32:1011–4. CrossRef
55. Acworth I, Plante M, Crafts C, Bailey B. Quantitation of underivatized omega-3 and omega-6 fatty acids in foods by HPLC and charged aerosol detection. Planta Med. 2011;77(12):PJ1. CrossRef