INTRODUCTION TO SCHIZOPHRENIA (SPR)
SPR is a leading cause of disability [1]. It is characterized by delusions, hallucinations, disordered speech and behavior, and other symptoms that cause social or occupational impairment [2]. As per the World Health Organization (WHO), SPR affects about 24 million individuals globally, with a prevalence of 1 in 300 individuals. The most common age for onset is late adolescence and early adulthood. Patients with SPR often have a poor long-term prognosis, which includes psychotic symptoms, poor social functioning, and low quality of life [3–5]. The global burden of disease is substantial since the ailment impairs several life domains and generally lasts for decades [6].
SEARCH STRATEGY
A systematic literature search of the PubMed database from the beginning to January 1, 2018, i.e., the previous 5 years, was carried out. The following key phrase was entered into the search bar: [probiotics or gut microbiota (GM) or dysbiosis] and SPR. The search covered both original research articles and review articles that dealt with both human and animal investigations. Each article’s references were also examined. Additionally, cross-references and manual searches were evaluated to find other related articles that might have been overlooked during the initial database search.
ETIOLOGICAL FACTORS RELATED TO SPR
The specific cause of SPR is still unknown, although it is considered that a mix of genetic, physical, physiological, and environmental factors are to blame despite advances in science and technology. It is believed that this condition develops in utero. The increased risk of SPR in adulthood has been related to poor pregnancy circumstances, emergency cesarean sections, and low birth weight [7]. Prenatal and postnatal environmental vulnerabilities and immune system responses are the best-studied etiopathogeneses of SPR. For instance, maternal immune activation (MIA), which is the term for the stimulation of the mother’s immune system in reaction to infection or infection-like impetuses, has recently been proposed as a “neurodevelopmental primer” [8]. A cascade of cytokines and immunologic changes are passed down to the fetus, resulting in negative phenotypes, particularly in the central nervous system (CNS) [9]. These studies also suggested that maternal respiratory infections, influenza, Toxoplasma gondii infections, and other infections might be biomarkers of MIA and the inflammatory response. It is crucial to keep in mind that some environmental variables, such as advanced paternal age, prenatal insults, obstetric problems, early stress, or illegal substances, are known to raise the risk of psychosis. These factors may also work by altering the gut flora [10].
GUT MICROBIOTA
The GM is the collective name for the enormous number of microbes found in the human gastrointestinal (GI) tract (GIT), other than bacteria there are also viruses, protozoa, fungi, and archaea in it, whereas the genes carried by these cells make up the human microbiome [7].
Both disease-causing and disease-preventing microbes can be found in the microbiome [11]. GM has about 1,000 to 5,000 distinct species, 99% of which are phyla. Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia are the major phyla of the microbiota. These microorganisms are essential for maintaining homeostasis, and an imbalance can result in several diseases [12]. An estimate indicates that the number of bacteria in the human gut at about 3.8 × 1013, which is somewhat higher than the total number of human cells [13]. These bacteria have a significant role in the physiologies of both health and sickness. These bacteria not only benefit the metabolic process but also help us avoid illnesses, strengthening, and control of our immune system (adaptive and innate) in the process. They also carry out vital tasks including the metabolism of xenobiotics, the synthesis of vitamin B, the promotion of digestion, and the acceleration of neuronal activities [7]. It directly or indirectly affects the majority of our physiological activities through these imperative acts [14].
A newborn is sterile until delivery because the inside of the uterus is a sterile environment, with bacterial invasion beginning shortly after delivery, while the infant transits the birth canal. There is the colonization of the conjunctiva, oral cavity, digestive tract, and skin. The neonatal microbiome differs depending on the mode of birth, with vaginally born children having a microbiome similar to the vaginal microbiome and those delivered through abdominal delivery having a microbiome similar to the maternal skin microbiome. Bifidobacterium, Lactobacillus, and Prevotella are the most common genera found in the GM acquired following vaginal birth. The most common bacteria found in neonates delivered by cesarean section are Staphylococcus, Corynebacterium, and Clostridioides difficile [15] indicating the mode of technique has a significant impact on the early microbial settling. The GM will further differ depending on whether breastfed or not, the use of antibiotics, and diet gut [15,16].
THE MICROBIOTA–GUT–BRAIN AXIS (MGBA)
The gut–brain axis is a bidirectional communication system that connects the central and enteric nervous systems (ENS), linking the brain’s affective and cognitive centers with digestive processes [17]. The GIT, the microorganisms that live there, and the peripheral and CNS all have sophisticated communication systems. This continuous transfer and interpretation of information from the periphery to the brain and back are termed the MGBA.
The MGBA has a significant impact on mood and behavior [18]. This axis consists of two-way communication between the brain and GM through immunological and inflammatory pathways, neurotransmitters, microbial by-products, neuroendocrine and enteroendocrine signaling [vagus nerve and ENS, cortisol, and hypothalamic–pituitary–adrenal (HPA) axis], the stress response, and the vagus nerve; however, the processes are still being understood [19]. There is a healthy resting inflammatory state that exists under normal physiological and homeostatic settings, and the GM triggers the synthesis of cytokines and chemokines that maintain microbial inhabitants in the gut [20]. The luminal–mucosal interface, which is predominantly made by the epithelial layer of the GI system, is where most host-microbiota interactions occur. The innate immune response is necessary for this interaction to occur. Intestinal enterocytes produce chemokines and cytokines, contain innate immune receptors, and can affect regional immune cells [21]. As many GI bacteria have a polysaccharide coating, the host immune system may also keep an eye on the GM through Toll-like receptors (TLRs), recognizing prospective infections if exposed [22].
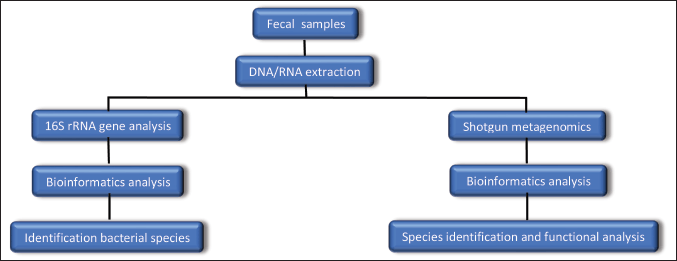 | Figure 1. Laboratory methods for fecal sample analysis. [Click here to view] |
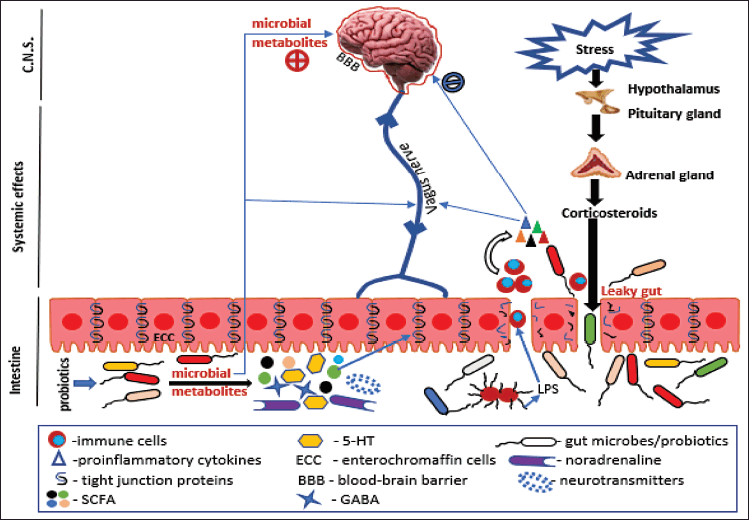 | Figure 2. GM and brain are interconnected by the vagus nerves. Stress and emotions disrupt the HPA axis and ultimately the gut barrier integrity through the release of corticosteroids causing a leaky gut, leading to the migration of bacteria with immune cells releasing proinflammatory cytokines and triggering inflammation. These cytokines further compromise the BBB’s integrity and allow potentially harmful and inflammatory substances to enter, which alters mood and cognition and leads to SPR. Probiotics work by (1) Increasing production of SCFA, neurotransmitters, and CA. (2) Inducing expression of tight junction proteins. (3) Restoring the integrity of the gut barrier and BBB and also modulating the neurotransmission in the ENS. (4) Releasing anti-inflammatory cytokines. [Click here to view] |
DYSBIOSIS
A microbial population imbalance caused by alterations to the gut microbiome is frequently referred to as gut dysbiosis or microbial dysbiosis [23].
GUT AND BRAIN BARRIERS
• The blood–brain barrier (BBB) and the intestinal epithelial barrier are two distinct, specialized vascular barriers that connect the gut and brain [24].
• Studies show that gut bacteria can increase the production of cytokines and chemokines, which can affect the HPA axis and cause a neuroinflammatory response. These cytokines and chemokines can then travel to the brain via the bloodstream, lymphatic system, and vagus nerve and increase BBB permeability [19]. When the BBB is compromised, cytokines can have an impact on the hypothalamus and circumventricular organs, among other areas of the brain. The HPA axis can also be stimulated by IL-1 and IL-6 [25]. In investigations on animals, it was shown that the GM regulates the integrity of the BBB, and a varied GM is crucial for maintaining and maturing microglia [19].
• It is found that both fetal and adult mice can have altered BBB permeability due to alterations in the microbiota. In comparison to pathogen-free (PF) mice, who have normal GM, germ-free (GF) animals have a larger BBB permeability, which is also true for the GF mice’s embryos. The cause of this anomaly is decreased expression of occludin and claudin-5—which control BBB activities in endothelial tissue and are involved in tight junction disorganization. When the GM of PF mice was transferred to adult GF mice, they exhibited increased expression of tight junction proteins and a reduction in BBB permeability. Transferring microorganisms that synthesize short-chain fatty acids (SCFAs) or feces of PF animals to GF animals aids in maintaining the BBB’s integrity [24].
Major factors and pathways altering the MGBA their connection with SPR
Stress
• Stress on the body, both psychological and physical, affects the makeup of the gut microbiome.
• Stress can lead to elevation of inflammation which further can lead to damage to the intestinal barrier (leaky gut), causing bacterial infiltration and a rise in plasma lipopolysaccharide (LPS) [19] (Fig. 2).
• Stress changes the way mucus is secreted, and this can have a serious effect on the growth of intestinal microbes that prebiotics and dietary fibers promote [26].
• The HPA axis is activated by stress, which has a significant impact on GM through the process of microbial dysbiosis. In SPR, there is more neuroinflammation after extended HPA axis activity [19] (Fig. 2).
Leaky gut
• Stress can weaken the epithelium and make it porous thereby increasing the intestinal barrier’s permeability, resulting in a “leaky gut” [24]and loss of bacteria and their by-products [16,27].
• It leads to the translocation of gram-negative microbes LPS, which activates the immune system (TLRs) and regulates the production of proinflammatory cytokines [IL-6, IFN-γ, C-reactive protein (CRP), and TNF-α] [24,27] (Fig. 2).
• In SPR, this bacterial translocation can result in autointoxication, which fuels the chronic inflammatory state. SPR patients have been found to have elevated inflammatory cytokines in addition to LPS, which may help to modify gut permeability and create a “leaky gut” [19].
• Gliadin, b-lactoglobulin, and casein IgA levels were shown to be higher in SPR patients [28]. In large research on SPR patients and nonpsychiatric comparative controls, SPR patients exhibited moderate-to-high levels of IgA antigliadin antibodies (AGA). In another study, patients who had recently developed psychosis and those with numerous-episode of SPR exhibited greater levels of IgG and IgA antibodies to gliadin [28,29].
VAGUS NERVE AND SEROTONIN INTERCONNECTION
Vagal afferent fibers are directly contacted by enteroendocrine cells (EECs) (Fig. 2) by the production of serotonin, which activates 5-hydroxytryptamine-3 (5-HT3) receptors, or indirectly through gut hormone. In addition to having SCFA receptors, EECs also express TLRs, which are involved in the detection of microbes. Thus, by controlling GI motility, secretion, and food intake, these cells can detect bacterial chemicals and indirectly affect vagal afferent fibers. TLR4, which is triggered by LPS, allows bacteria to directly detect the vagus nerve. LPS stimulates cytokines like IL-1, which then cause disease by activating the vagus nerve. The induction of cytokines is stopped after vagotomy [30].
VAGUS NERVE AND GAMMA-AMINOBUTYRIC ACID (GABA) INTERCONNECTION
Antibiotic combinations cause gut dysbiosis by altering GABA-mediated neurotransmission [8], indicating the significance of normal GM in relating N-Methyl D-Aspartate Receptor (NMDAR)-GABA activity to hippocampus (HPC) memory, motor control, and cognitive flexibility; reduction in NMDAR-GABA levels can cause impairment in cognitive function similar to SPR and can increase psychotic symptoms because GM development is essential to fuel brain plasticity via the expressions of the adequate NMDA and brain-derived neurotrophic factor (BDNF) receptors [7,8].
VAGUS NERVE AND CYTOKINES INTERCONNECTION
The vagus nerve transmits environmental signals that the CNS continually reacts to maintain homeostasis. Peripheral cytokine synthesis sets off the vagal anti-inflammatory reflex, which produces acetylcholine and stops excessive cytokine release from causing tissue damage [31] (Fig. 2). Recent studies have shown that people with depression, anxiety disorders, and SPR have altered gut microbiome as well as vagal tone. Some probiotics, including Bifidobacterium, use vagal pathways to communicate with the brain. While certain probiotics lose their beneficial effects on the brain and behavior when the vagus nerve is severed [32].
Microbial by-products
Numerous bioactive compounds, including bacteriocins, bile acids, choline, and SCFAs, can be secreted by bacteria present in the gut microbiome (Fig. 2). SCFAs are the resultant of the fermentation of polysaccharides by inducing the synthesis of neurotransmitters. They contribute to neurological and mental conditions like SPR [19] (Fig. 2).
The cecum and colon contain GM, which metabolizes fiber, protein, and peptides that are not broken down by digestive enzymes in the upper gut. SCFAs, such as acetate, propionate, and butyrate, are their major products [33], although fermented foods can be a supplemental source. The host’s metabolism of long-chain fatty acids, the conversion of pyruvate to acetate, and the degradation of proteins by the microbiota are all endogenous sources of SCFAs. SCFAs are digested by cells through the Krebs cycle of citric acid to provide energy [34]. The main energy source for colonocytes is butyrate, which prevents inflammation by preventing histone deacetylases [35]. Butyrate has been used as an experimental medication for neuropsychiatric diseases because it affects particular receptors and transporters, whereas all SCFAs have inhibitory effects on histone deacetylase [34].
The CNS appears to be affected by SCFAs produced by the GM that act on glial cells, including microglia and astrocytes, although the precise effect varies on the kind of SCFA and the target cell. It has been claimed that butyric acid caused the LPS-induced microglial cells in rats to exhibit anti-inflammatory properties [36]. According to another study, SCFA treatment of GF mice led to the restoration of microglial malformation and immaturity when FFAR2 was activated [37]. Propionic acid had an impact on cytoskeletal integration and elevated glial fibrillary acidic protein in cultured astrocytes in rat studies [38]. Rats were given injections of propionic acid made from bacteria, which resulted in cognitive and motor damage [39].
Inflammation
SPR is linked to chronic systemic and GI inflammation, oxidative stress, and metabolic dysfunction [40]. According to several research, SPR is linked to increased serological indicators of microbial translocation [41–43], implying higher intestinal lumen permeability affecting physiological functioning. Inflammation and neuropsychiatric diseases are inextricably related [44]. Patients with SPR have elevated levels of proinflammatory cytokines, inflammation-inducing molecules like damage-associated molecular patterns, activated sensors like TLR, inflammasomes, acute-phase proteins like CRP, and adhesion molecules in their blood and cerebrospinal fluid [30]. Similarly, two different studies on mice demonstrated attenuation of the main signs of ketamine-induced SPR, and enhanced memory by modifying the oxido-inflammatory and neurotransmitter-related pathways, with the use of Carpolobia lutea extract and diosmin [45,46]. Chronic inflammation brought on by the GM can develop as a result of structural damage to the intestine. A study of SPR patients’ autopsies identified several inflammatory bowel diseases that might cause instability in the intestinal wall structure [47].
Research also indicates that around 30% of individuals with SPR have higher AGA of the IgG type, suggesting that this subset of individuals may also have increased gut permeability. AGA IgG in particular suggests that these antibodies may be crossing the BBB, leading to neuroinflammation since new findings have demonstrated a strong correlation of IgG-mediated antibodies between the peripheral and cerebral spinal fluid in SPR but not healthy controls. Other aberrant translational indicators have been strongly linked to SPR, suggesting greater intestinal permeability in this condition. Metabolic syndrome (MS) is seen in >1 in 5 and persistent low-grade peripheral inflammation in 1/3rd of people with SPR. This inflammation has also been linked to SPR-major depression and is a reliable indicator of central inflammation [48].
Immune system and neuronal inflammation in SPR
The intestinal immune system keeps up immunity to dangerous bacteria and tolerance to commensals, and an imbalance between the host immune system and microbiota can influence inflammation and lead to several disorders. Many bacterial substances, including peptidoglycan, lipoteichoic acid (a component of Gram-positive bacteria’s cell wall), LPS, flagellum (which facilitates bacterial motility), pilus (which mediates bacterial attachment to cells), DNA, and cell wall fragments, are regarded as pathogen-associated molecular patterns. Pattern-recognition receptors and nonpattern-recognition receptors, which are crucial elements of the immune system, can identify the molecular patterns associated with pathogens. Immune receptors’ detection of pathogen-associated molecular patterns starts a chain reaction of signaling pathways that turns on several transcription factors and increases the production of inflammatory mediators, which are necessary for the eradication of invasive pathogens, including cytokines, chemokines, and antimicrobial peptides. This host-immune response raises intestinal permeability, which makes it easier for drugs to enter circulation [30]. It also causes a systemic inflammatory response, which raises BBB permeability and activates microglial cells. Cytokines in the blood compromise the immune system. The TLR-4 receptor, which is abundantly present in brain monocytes, macrophages, and microglia, is responsible for recognizing LPS. It has been documented that the GM in inflammatory bowel syndrome patients with depression activates TLR-4-mediated inflammatory responses [26,49]. Alterations in circulation levels of proinflammatory and anti-inflammatory cytokines can result from gut bacteria and probiotics’ indirect effects on the innate immune system, which in turn have direct effects on brain functioning [26].
Growing evidence points to the immune system’s important role in SPR via changing innate and adaptive defensive systems. Immune cells may invade the brain and mediate neuroimmune interaction to cause neuroinflammation by releasing inflammatory cytokines and reactive oxygen species, leading to neurodegenerative and neuro-progressive alterations in SPR [7]. SPR and other associated psychoses exhibit a variety of immune system disorders that often overlap with one another.
A highly intriguing new result from a human investigation revealed a correlation between alterations in the right middle frontal gyrus volume and certain bacteria linked with SPR, suggesting a possible relationship between GM and brain anatomy in SPR. Data on altered GM in SPR sufferers are contradictory overall, especially when it comes to Proteobacteria and Firmicutes (at the family level) and Clostridia (at the class level). The most consistent observation so far seems to be an increase in Lactobacilli abundance [24].
Neurotransmitters
Gut microbiome bacteria generate a variety of neurotransmitters like GABA (Lactobacillus and Bifidobacterium), norepinephrine (Escherichia coli, Bacillus, and Saccharomyces spp.), dopamine (Bacillus), acetylcholine (Lactobacillus), and serotonin (Escherichia, Enterococcus, Candida, and Streptococcus), tryptophan (Clostridium, Burkholderia, Streptomyces, Pseudomonas, and Bacillus), a precursor to serotonin are the most frequently generated neuroactive substances which interact with various host systems to maintain homeostasis. There are distinct metabolizing bacterial routes found to differ between healthy and those with SPR [19]. Through enterochromaffin cells (ECs) and enteric nerve receptors, these neurotransmitters can communicate with the CNS [26].
GABA
Lactobacillus brevis and Bifidobacterium dentium effectively generate GABA, a key inhibitory neurotransmitter whose malfunction is linked to sadness, anxiety, autism, and SPR. In a preclinical investigation, it was proposed that GABA generated by gut bacteria passes the BBB and reaches the CNS. Mice’s anxiety and depression-related behaviors have also been shown to decrease when given Lactobacillus rhamnosus, and the concentration of GABA in the HPC has been observed to rise. Given that these effects only manifest when the vagus nerve is unharmed, it is conceivable that gut bacteria indirectly control GABA transmission (discovered as an SPR endophenotype]) via the vagus nerve [26].
Histamine
Some gut microbes can make histamine. Histidine decarboxylase is expressed by Lactobacillus reuteri, which also produces histamine [50], and suppresses TNF-α by generating histamine in myeloid progenitor cells. Histamine has been proven to play a similar immunomodulatory effect in intestinal lymphoid organs, where it controls Yersinia enterocolitica infection. Additionally, it has been discovered that an H2-receptor blockade decreases mucus production and increases intestinal barrier dysfunction, which may help to facilitate the transfer of germs from the intestinal lumen to the bloodstream [26].
REGULATION OF SEROTONIN AND DOPAMINE BY THE GM
Lower serotonin levels result in less permeability of the gut wall and reduce occludin expression, which raises gut wall permeability. Another study found that giving Lactobacillus to GF mice boosted their levels of 5-HT as well as dopamine in the striatum, suggesting the potential for employing bacterial transplants to treat Parkinson’s disease. Additionally, the MGBA allows the GM to influence the enzymes that control dopamine production. Data from rat research indicates a small but significant role for the gut lumen in dopamine synthesis [51].
NOREPINEPHRINE
The fact that specific-PF mice had much higher amounts of dopamine and noradrenaline in the cecum than GF mice suggests GM is a source of catecholamine (CA) [52]. A gene for a transcript with a sequence similar to that of tyrosine hydroxylase, the rate-limiting enzyme in the production of noradrenaline and dopamine, can be found in some bacterial species [53]. Dopamine synthesis by Lactobacillus bacteria is known to occur during culture [54]. Dopamine generated in the peripheral nervous system cannot cross the BBB, hence there is currently no proof that CA produced by microbes affect the CNS. However, tyrosine levels are found to be lower in GF mice than in ex-GF animals, suggesting that GM may increase dopamine levels in GF mice’s brains [55]. Supported another study showing the brains of GF mice had higher levels of CA as compared to ex-GF animals, but that restoring the GM reduced those levels through modulating dopamine and noradrenaline turnover in the brain [56].
GLUTAMATE
Numerous neuropathological illnesses, including SPR, have been linked to neurotransmission dysfunction of glutamate and disturbance in iGluRs signaling. Alterations in glutamate metabolism have been linked to changes in gut flora. For instance, Campylobacter jejuni increases the production of glutamate, and its decreased abundance in the GIT influences the synthesis of glutamate, which indirectly affects the metabolism of glutamate [57].
LABORATORY METHODS FOR MICROBIOME ANALYSIS
Numerous instruments are used to examine the processes of communication between the gut microbiome and the brain, as well as the fundamental laboratory procedures needed to detect bacteria in a fecal sample (Fig. 1) [16]. In the past, the only way to study bacteria was through culture procedures that entailed plating samples on the right media and recognizing the bacterial growth that appeared [58]. Numerous microbes were incompatible with culture, which made it difficult to identify them using this approach. All the microorganisms present may now be identified thanks to the development of “metagenomics,” a culture-independent method that allows for direct examination of the genetic material in a sample [59].
Microbe, DNA, and mRNA level analyses are the three main forms of analysis. Culturome, amplicon, metagenome, metavirome, and metatranscriptome analyses are some of the relevant study methods. A high-throughput technique for cultivating and detecting bacteria at the microbe level is called culturome. It is the best way to produce bacterial stocks, but it is expensive and laborious [60]. The microbiome of human beings [61,62], mice [63], marine sediment [64], Arabidopsis thaliana [65], and rice [66] have all been studied using this technique.
Almost all sample types can be used for amplified sequencing. For prokaryotes, this approach mostly uses 16S ribosomal DNA (rDNA), while for eukaryotes, it primarily uses 18S rDNA and internal transcribed spacers [60].
While more expensive than amplicon sequencing, metagenomic sequencing offers more information. For “pure” samples like human excrement, 6 to 9 terabytes (GB) of sequencing data per sample is considered acceptable [67].
Technically, metagenome and metatranscriptome analysis are included in metavirome research since viruses have either DNA or RNA as their genetic material. To obtain enough viral DNA or RNA for analysis because of the low biomass of viruses in a sample, virus enrichment [68], or the removal of host DNA [69], is a necessary step.
DIFFERENT OMICS APPROACHES FOR THE IDENTIFICATION OF BACTERIA IN THE GM
Metagenomics is shotgun sequencing of DNA from samples which yields a collection of genomes and genes, analyzed using a metagenomics program. Following the assembly or mapping of sequences to a reference database typically includes an annotation step. The microbiome’s ability to function and the identification of bacteria are revealed by this extensive and expensive procedure. Additionally, it helps in gathering genomic information for gut bacteria that cannot be cultured [70].
Metataxonomics is a high-throughput method for characterizing the entire microbiota majority of which relies on the amplification and sequencing of marker genes, such as the bacterial 16S rRNA gene, which contain both conserved and variable sections. Community-wide taxonomic classifications are made possible by metataxonomic tree-based hierarchical clustering studies, which also show the evolutionary relationships among all collected sequences. The organization and make-up of the bacterial communities in the guts of humans, mice, and insects have therefore been clarified via metataxonomics. It can be used for gut archaeal community studies in addition to bacterial microbiota identification [70].
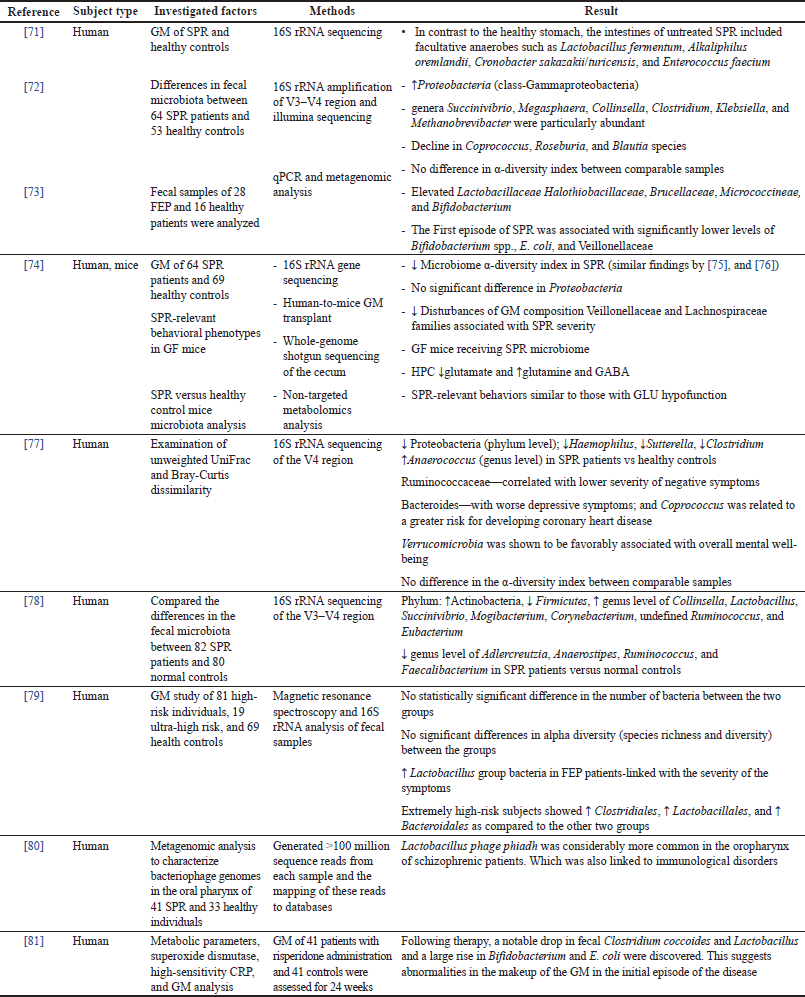 | Table 1. Alteration of the GM and their link to SPR—evidence from clinical research. [Click here to view] |
Metatranscriptomics—its goal is to study how RNA is expressed and regulated in complex organisms in their natural habitats. In general, samples’ RNA is extracted after synthetic clonal DNA is created, and then high-throughput sequencing is applied.
Other omics approaches: Gut microbial activity is tracked by transcriptional and proteomics principles to produce gut metabolites. Studies on GM usually employ the omics technique known as metabolomics, which identifies the metabolite profiles in any particular strain or solo tissue. The measurement of all metabolite amounts (or concentrations) and locations in cells or tissues is known as metabolomics [70].
Various software along with their links used in amplicon and metagenomic sequencing: QIIME (http://qiime.org/), QIIME 2 (https://qiime2.org/), USEARCH (http://www.drive5.com/usearch), VSEARCH (https://github.com/torognes/vsearch), Trimmomatic (http://www.usadellab.org/cms/index.php?page=trimmomatic), Bowtie 2 (http://bowtie-bio.sourceforge.net/bowtie2), MetaPhlAn2 (https://bitbucket.org/biobakery/metaphlan2), Kraken 2 (https://ccb.jhu.edu/software/kraken2), HUMAnN2 (https://bitbucket.org/biobakery/humann2), MEGAN(https://github.com/husonlab/megan-ce), MEGAHIT (https://github.com/voutcn/megahit), metaSPAdes (http://cab.spbu.ru/software/spades), MetaQUAST (http://quast.sourceforge.net/metaquast), MetaGeneMark (http://exon.gatech.edu/GeneMark/), Prokka (http://www.vicbioinformatics.com/software.prokka.shtml), CD-HIT (http://weizhongli-lab.org/cd-hit), Salmon (https://combine-lab.github.io/salmon), metaWRAP (https://github.com/bxlab/metaWRAP), and DAS Tool (https://github.com/cmks/DAS_Tool) [60].
ALTERATION OF THE GM AND THEIR LINK TO SPR—EVIDENCE FROM CLINICAL RESEARCH
One of the initial precise documentation of GI inflammation related to SPR was where an autopsy examination showed that 41 of the 82 SPR patients had gastritis, and 73 and 76 had enteritis, and colitis, respectively (Table 1). Interestingly, reports of mental comorbidities among persons with intestinal diseases with an inflammatory component show the opposite trend [7]. The first study that discovered disturbed GM in SPR patients had 25 chronic SPR patients who differed from 25 healthy controls [28].
The frequency of SPR and autism has been observed to be greater in C. difficile infected. A phenylalanine derivative generated and released by the same bacteria in the stomach that is known to control CA levels in the brain helped to explain this link. Twin and adoption genetic studies further support the SPR and GM relationship. In monozygotic twins, the microbial commonality is higher than in dizygotic twins, which is consistent with the frequency of SPR in twin studies. Premature infants have also been found to have a higher chance of having SPR later in life [82]. Several investigations found an increase in species belonging to the gram-negative bacteria Fusobacterium, Megasphaera, and Prevotella genera [4] in SPR.
ALTERATION OF THE GM AND THEIR LINK TO SPR—EVIDENCE FROM PRECLINICAL RESEARCH
When GF animals were given the SPR microbiome showed altered glutamate/glutamine and GABA levels in the HPC, demonstrating SPR-like behaviors comparable to previous mouse models of SPR with glutamatergic hypofunction [76,83] (Table 2).
An in vitro study that looked at how LPS affected intestinal epithelial cells discovered that acute LPS injection changed and decreased the distribution of tight junctions. LPS has been demonstrated to be a successful mouse neurodevelopmental model of SPR [4].
The majority of preclinical research on the MGBA has focused on unfolding how GM may impact brain function through immunological, neural, and endocrine mechanisms [84].
ANTIPSYCHOTICS IN SPR
A wide array of typical and atypical antipsychotics are used for the treatment of SPR. The introduction of second-generation antipsychotics, often known as “atypical” antipsychotics, was spurred by the fact that typical antipsychotics like haloperidol and fluphenazine did not have an impact on the negative and cognitive symptoms and the severity of their side effects. Atypical antipsychotics heralded a significant shift and the development of fresh strategies in psychiatric pharmacology. When compared to “typical” or “conventional” antipsychotics, they exhibit distinct prominent features, which is what the name “atypical” refers to. Contrarily, there are significant variations in the risk of specific adverse effects among antipsychotic medications. Atypical antipsychotics like olanzapine, risperidone, and clozapine appear to have much fewer adverse effects like prolactinemia, pseudoparkinsonism, and dystonia after being taken on a long-term basis, but they are still effective in reducing positive symptoms [85]. The variations in antipsychotic medication efficacy across different drugs, at least at group levels, are rather minimal, except for clozapine, which is more successful in SPR [87,88]. The risk of metabolic problems, such as hypoglycemia and weight gain, is increased with certain atypical antipsychotics [87].
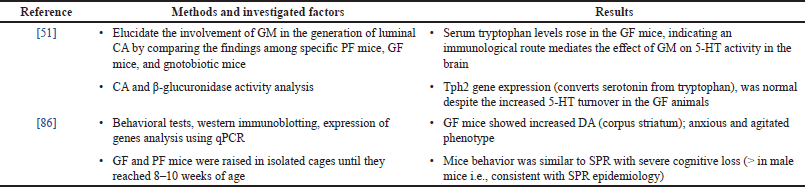 | Table 2. Alteration of the GM and their link to SPR—evidence from preclinical research. [Click here to view] |
The clinical antipsychotic trials of intervention effectiveness [89] and cost utility of the latest antipsychotic drugs in SPR study [90] revealed no differences in the treatment of psychotic symptoms and quality of life by either type of antipsychotic drugs, in contrast to initial enthusiasm and optimism for the therapeutic advantage of atypical over typical antipsychotics. A meta-analysis separating antipsychotic drugs into typical and atypical antipsychotic groups revealed that amisulpride, clozapine, olanzapine, and risperidone, four atypical antipsychotics, were more effective in treating SPR than the typical antipsychotics studied. In terms of how they attach to dopamine receptors, they are different from traditional medicines. These antipsychotics are more potent at D2 receptors due to their affinity for 5-HT2A receptors, which raises their overall effectiveness [7]. Additionally, there is mounting proof that antipsychotic usage affects gut microbial species via their antimicrobial action [19].
EFFECT OF ANTIPSYCHOTICS ON THE GUT MICROBIOME
The antipsychotic drug olanzapine has been demonstrated to increase Firmicutes and reduce Bacteroidetes in rats. Although not always [81], this effect was replicated in human antipsychotic trials. Antipsychotics do have strong anticommensal action [91]. This has the consequence that if antipsychotic therapy damages the GM, then supplemental therapies meant to restore microbial function would be helpful [92].
A systematic review article published that both olanzapine and risperidone were found to enhance the number of Firmicutes in comparison to Bacteroidetes. These changes in the GM were connected to weight growth, lipogenesis, and increased levels of free fatty acids and acetate all at the same time. Since this research is primarily based on animal models, clinical trial data are less in line. For instance, following 6-week treatment with olanzapine in individuals with SPR, not many substantial alterations were noted in gut flora. Another research found that when compared to healthy controls, drug-naive individuals with first-episode psychosis (FEP) had higher concentrations of Proteobacteria and lower concentrations of bacteria that produce SCFAs (Faecalibacterium and Lachnospiraceae) [93]. Importantly, several changes in GM have been linked to regional brain volume. For example, drug-naive FEP individuals have decreased Actinobacillus and Veillonellaceae abundance [83].
Atypical antipsychotics may be able to affect serotonin, which is disrupted in SPR. A meta-analysis of autopsy studies on SPR found that there were more prefrontal 5-HT1A receptors and fewer prefrontal 5-HT2A receptors. Moreover, the regulation of brain serotonin levels and the gut-brain axis is interlinked. Dopamine and serotonin levels were shown to be higher in research using GF mouse models, indicating that the CNS is influenced by gut microorganisms. In a similar vein, higher serotonin levels have been seen in the hippocampi of GF mice. Another research claimed that by stimulating the synthesis of serotonin in ECs in the GIT, gut microorganisms have contributed to elevated blood levels of the neurotransmitter [7,94].
Patients with SPR who were taking risperidone had significantly reduced fecal Bifidobacterium spp., E. coli, and Lactobacillus spp., when compared to untreated controls. Suggestive of metabolic changes due to risperidone therapy and it alters specific fecal microbes. Okubo et al. [95] looked into how the probiotic Bifidobacterium breve A-1 affected SPR patients’ symptoms of anxiety and depression as well as how it affected immune substances including cytokines and chemokines. Four weeks of probiotic therapy helped SPR patients perform better on anxiety/depression tests, signifying probiotics’ role in SPR. Individuals on either risperidone or olanzapine had different levels of Akkermansia, Sutterella, and Lachnospiraceae than healthy controls [96].
The first rats to receive olanzapine had an altered microbial composition [30,97]. A rise in the relative abundance of Firmicutes, and a drop in Bacteroidetes, Actinobacteria, and Proteobacteria were all detected. Body weight gain, a rise in the amount of adipose tissue, and changes in inflammatory and metabolic markers all occurred in conjunction with the changes in microbial composition. Some of the impacts were gender-specific, i.e., stronger in females than in males [97]. Administration of olanzapine reduced total microbial diversity decreased Bacteroidia abundance and raised Erysipelotrichia, Actinobacteria, and Gammaproteobacteria abundances. It is noteworthy to note that olanzapine-induced weight growth was reliant on the presence of GM, it was supported by the lack of a substantial rise in body weight in GF mice treated with the drug and a quick weight gain after microbial colonization [98]. Due to decreased energy expenditure, risperidone therapy led to excessive weight gain in rats, and this effect was linked to changes in the gut flora. More specifically, it was shown that Firmicutes were more prevalent than Bacteroidetes [99].
PROBIOTICS
In the early twentieth century, Elie Metchnikoff, popularly known as the “father of modern probiotics,” discovered that frequent intake of lactic acid bacteria found in fermented dairy products was linked to improved health and longevity in humans. This prompted scientists to investigate the microbiology of human processes further. In 1953, Werner Kollath coined the term “probiotics”, combining the Greek terms “pro,” which means “for,” and “biotos,” which means “life”. Fuller recommended probiotics as “live microbial supplements that benefit the host by enhancing its microbial balance.” Later in 2001, the United Nations Food and Agriculture Organization and the WHO agreed on a consensus definition of probiotics. The 2001 definition is still valid today, with slight changes. Probiotics are currently described as “live organisms that, when administered in adequate amounts, confer a health benefit to the host” [100]. Bacteria and yeasts are among the biologically active organisms found in probiotics. Lactobacillus and Bifidobacterium are the two types used Gram-positive lactic acid bacteria found in probiotics [101]. These two species are often utilized in animal and human investigations. Lactococcus, Bacillus, Pediococcus, and Streptococcus are among the other microbes commonly used in probiotic research. Other genera include Kluyveromyces, Pichia, and Candida, as well as common yeasts like Saccharomyces boulardii [102]. Today, probiotics may be found in a variety of foods, including yogurt, cheese, wafers, fermented milk or other beverages, pills, capsules, sachets, and even chocolates. You may get them from pharmacies, drugstores, supermarkets, health food stores, or online sites [28].
Even though commensal microbes in the gut are frequently the source of probiotic strains, these strains cannot be deemed probiotics unless they have been secluded, and recognized, and a convincing argument for their health aids has been made [103]. A probiotic supplement should list the genus, species, and strain of the microorganisms it contains. This is significant because, one species or strain of Bifidobacterium or Lactobacillus may be useful for reducing anxiety or elevating mood, while another strain may not. The product should also list the number of colony-forming units (CFU), or live bacteria, that are present. The majority of human psychobiotic trials employ products containing at least one billion CFU/day, even though optimum probiotic doses have not yet been measured [16]. They are used alone or in combination as cocktails of different bacterial species and strains.
Overall, the available information on microbiome changes in SPR is extremely erratic and insufficient to draw any firm conclusions about whether these changes are linked to an elevated risk of the condition or are merely the product of environmental variables or medical interventions. Pro/prebiotic supplementation has shown some hopeful outcomes, although there is conflicting data about its effectiveness in treating SPR.
PSYCHOBIOTICS
The term “psychobiotics” was coined by Dinan et al. [104] and is commonly described as any “living organism that, when consumed in sufficient proportions, improves the health of individuals suffering from psychiatric disorder. As a result, psychobiotics are defined as a subclass of probiotics with a focus on mental health. GABA, CA, and 5-HT regulate the brain–gut axis and mental health, and most psychobiotics may produce or promote their endogenous synthesis [50]. Hence, all microbiota-targeted therapies, such as probiotics and prebiotics, that affect the connections between bacteria and the brain and have an impact on mood, anxiety, and cognitive performance are referred to as “psychobiotics” [105]. Psychobiotics also influence metabolism and hormone production, strengthen the immune system, and more [106,107]. Today, these are being thoroughly studied as a complementary therapy for mental diseases.
Effect of psychobiotics on MGBA
Since GM is a target that can be altered and can alter epigenetic processes [108], it may be utilized to treat and lessen the symptoms of mental illnesses. Prebiotics, probiotics, live bacteria, antibiotics, synbiotics (combinations of pre and probiotics), postbiotics (bacterial fermentation products such as SCFAs, and fecal microbiota transplantation (FMT) are some of the methods that might change the MGBA [109].
CLINICAL EVIDENCE ON OUTCOMES OF PROBIOTICS SUPPLEMENTATION IN SPR
The first probiotic investigation was carried out by Benton et al. [110] utilizing milk with Lactobacillus casei strain Shirota (LcS) in depressed and normal participants (Table 3). Primary conclusions were that it enhanced cognition in all individuals and mood in 1/3rd of the patients with depression. In a 3-fortnight human experiment, probiotic yogurt (which comprise B. lactis and Lactobacillus acidophilus) and probiotic capsules (which comprise L. casei, L. acidophilus, L. rhamnosus, L. delbrueckii bulgaricus, Bifidobacterium breve, Bifidobacterium longum, and Streptococcus thermophiles) significantly reduced anxiety and depression compared to placebo groups [111]. In another two investigations, a probiotic mixture of B. longum and Lactobacillus helveticus alleviated anxiety and depression [112]. Probiotics can significantly reduce depression in subjects with mental illnesses, according to a meta-analysis [113], although it is yet unknown if the reduction continues once probiotic use is stopped [48].
It has been discovered that Lactobacillus supplementation in asymptomatic obese people reduces the fat content of the subcutaneous and visceral belly. Probiotics’ ability to cure obesity and dyslipidemia may have the same promise for schizophrenic patients, as they also have the likelihood of developing MS [82]. Probiotics and prebiotics have been found to be effective in treating psychotic diseases in recent years. Probiotics are routinely given for gut inflammation as they have anti-inflammatory qualities. A study revealed that probiotic supplements might stimulate the vagus nerve and cause cytokines to have immunomodulatory effects [7].
According to a systematic review of 32 studies on the eating habits of SPR patients analyzed these individuals tend to consume diets that are of poor quality, with excess calories and processed foods, foods high in saturated fats, refined sugar, and salt, and little fruit and fiber consumption [114]. These variables have been associated with the emergence of MS in the general population. Patients with SPR may find it difficult to maintain a balanced diet due to several variables, including the weight gain brought on by antipsychotic medications, poor socioeconomic position, unpleasant symptoms, and drug addiction, particularly cigarettes. According to a new meta-analysis, dietary therapies can improve the physical health of those with severe mental illnesses [115].
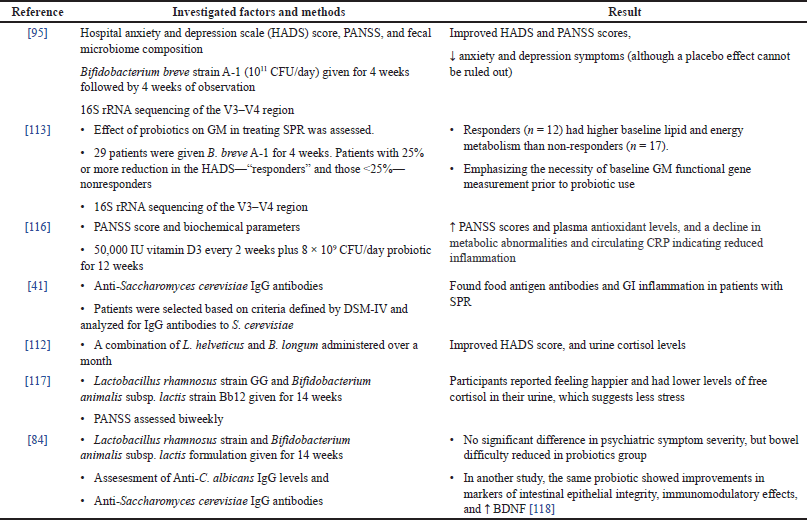 | Table 3. Clinical evidence on outcomes of probiotics supplementation in SPR. [Click here to view] |
A pilot study’s findings suggest a link between Candida albicans infection and worsening positive mental symptoms, and this connection was later verified in a bigger group of 384 men with SPR. Then concluded that probiotics can benefit many men with C. albicans-related GI pain and normalize their C. albicans antibody levels. GI epithelial and immunological diseases were improved by probiotics in SPR patients, they are also capable of restoring the GM and reducing the growth of Candida spp. [84].
It would be advisable to conduct a thorough metabolic and immunological study of people who have taken probiotics because various pieces of research imply that the role of probiotics in stress and mood regulation may be strain-specific.
Several pieces of research suggest that probiotics in stress and mood regulation might be strain-specific, detailed metabolic and immunological examination of probiotics-treated individuals would be advised. For instance, male and female participants given LcS for 3-week reported increased levels of happiness than depression. Similarly, to this, 40 male and female healthy patients administered probiotics showed less rumination and aggressive cognition than those who took placebos [119]. It is unknown precisely which probiotic strain is in charge of these behavioral modifications. Consuming LcS and Lactobacillus gasseri, respectively, led to reduced academic stress in students and improved mood in student-athletes. Students who were given probiotics in the classroom had lower plasma cortisol levels than the placebo group, indicating less stress. A probiotic-fed athletic group of kids performed better, demonstrating the influence of probiotics on fundamental life functions. Bifidobacterium longum was shown to be able to reduce the excitability of enteric neurons in a different investigation [120].
There are very few clinical studies for SPR, and those that have been done have always included antipsychotics along with dietary changes and probiotics [104,121]. Dietary modifications can alter the gut microflora’s makeup and activity. However, the inability to produce a meaningful shift in SPR patients indicates the use of probiotics and/or prebiotics [104]. To obtain better outcomes in terms of health, functionality, and quality of life, psychiatry must adopt a multimodal strategy in the future [122]. Dietary change is the best strategy; however, it is quite difficult when dealing with SPR. More effective interventions than those based on a single nutrient can be achieved with formulations that mix multiple nutrients [115].
As a treatment for mental illness, modulating the MGBA with probiotics appears to promise, but there are still several obstacles to overcome. First, randomized control trials reported so far exhibit methodological variation and comparatively small sample sizes. Numerous studies also rely solely on selfreported symptomatology characteristics without adequately evaluating patients, confirming a clinical diagnosis, or screening for comorbidities. Second, not every patient may see the same results from probiotics. For instance, a clinical trial has demonstrated that, depending on the environment in the gut, ingested probiotics may change and adapt in a good or harmful way under specific circumstances. Probiotics are live things that go through natural selection. For instance, E. coli Nissle probiotic increases the use of mucin in low diversity conditions, which may harm the gut lining [123]. The wide range of investigated strains and strain combinations is a significant factor in the high degree of results variability in probiotic investigations. Different strains of the same species, for instance, have shown conflicting results regarding mental symptoms: L. rhamnosus did not affect mood or anxiety levels in healthy males [124], LcS improved mood in those with low baseline mood scores [110].
PRECLINICAL RESEARCH
Preclinical research has shown that probiotics can restore corticosterone, norepinephrine, and BDNF levels, as well as immune regulation when they are given chronically (Table 4). In an animal model of separation from the mother, the probiotic Bifidobacterium infantis was given, and when compared to a placebo, it regulated the immunological response, corrected behavioral impairments, and returned norepinephrine levels in the brain stem to normal [30]. Studies examining the impact of probiotics on the HPC BDNF, which was linked to lower levels of hippocampal BDNF in a model of low-grade colitis (AKR mice), revealed that the aberrant behavior was restored. They show that in rats probiotics enhance the expression of the neurotrophin under circumstances of chronic stress, inflammation, and aging, most likely through lowering microglia activation [28].
Unresolved is the question of whether probiotic colonization of the gut is a permanent process or only a temporary one. Prebiotics and probiotics are frequently advised to be used continuously to maintain a healthy GM and increase natural immunity; however, more thorough research is needed to determine their effectiveness, identify the most potent microbial strains, and determine the right number of fiber/prebiotics to promote their growth. Furthermore, probiotics and prebiotics must be evaluated using cutting-edge technologies such as genotype identification, epigenetic marker focusing, neuropsychology, biochemical marker evaluation, and other techniques to determine their efficacy as an adjunctive therapy to recognize the associated pathways, GI activity, metabolism disparities, and cognition dysfunction in SPR subjects. Environmental aspects of this therapy (food, hydration, age, gender, and comorbidities) should also be taken into consideration.
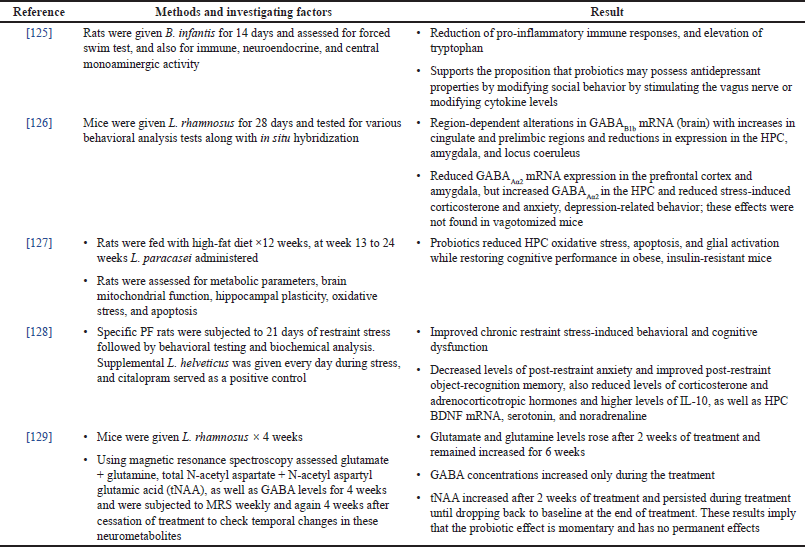 | Table 4. Preclinical evidence of probiotics in SPR. [Click here to view] |
INTERCONNECTION BETWEEN CONSTIPATION-SPR AND PROBIOTICS
Constipation is a frequent sign of SPR. Probiotics have been demonstrated to relieve constipation in a variety of groups, but SPR has not yet been the subject of research on them [28]. Probiotics may thus be used as an additional therapy for SPR, particularly in those with high intestinal permeability.
Constipation is lessened by butyrate generated by Clostridium butyricum, which stimulates the release of intestinal hormones [130]. An improvement in insulin resistance with probiotics may have been indicated by the sequential shift in the triglycerides (TGs)/HDL-C ratio from 3.44 at baseline to 2.00 at 1 month and 2.05 at 2 months. Even more powerful than the total cholesterol/high-density lipoprotein cholesterol (HDL-C) ratio and low-density lipoprotein cholesterol/HDL-C ratio, the TG/HDL-C ratio is an atherogenic index that is a highly significant independent predictor of myocardial infarction. This is because it has been demonstrated that in people with insulin resistance, TG levels rose while HDL-C levels fell. This outcome is consistent with the finding that probiotics may improve dysbiosis and stimulate insulin signaling. Promising outcomes have been observed as more research on probiotics’ impact on metabolism and constipation has been conducted in recent years. However, animal research accounts for the vast bulk of the available data. A combination probiotic may help SPR patients with constipation and insulin resistance, according to this early clinical trial.
Regulating bowel movements and treating metabolic problems are two advantages of probiotics. SPR subjects were given Streptococcus faecalis 2 × 108 CFU/day, Bacillus mesentericus 1 × 107 CFU/day, and C. butyricum 5 × 107 CFU/day mixture for constipation for 60 days and found improvement in excretion score and there was substantial relief from constipation at the end of the therapy [131].
According to some research using animal models, probiotics may enhance brain activity and signaling and hence aid in the treatment of psychiatric illnesses [132]. Probiotics’ effectiveness in treating SPR and their capacity to lessen illness symptoms must yet be shown via extensive research. Before prescribing probiotics as a treatment for SPR, it may also be wise to take strain-specific and temporary effects into account. Because bacteria do not receive enough nourishment to proliferate quickly and sustain their population in the gut, probiotics may only have a temporary effect. Additionally, it is never easy for a new strain to establish itself in an equilibrium microbial community since it would have to remove autochthonous strains with likely stronger adaptation advantages in their particular niche. Daily fluctuations in GM can be managed by providing them with dietary fiber/prebiotics [7].
In SPR, bacteria from the Lactobacillaceae family and the genus Lactobacillus had beneficial health outcomes. One explanation might be that various species in this genus have varied effects. One study found that the rise in psychosis and SPR was due to a subspecies not normally found in a healthy gut [133]. Increased Lactobacillus, on the other hand, has been linked to antipsychotic usage in the past. This was partially confirmed here since four psychosis and SPR studies that found increased Lactobacillus were done in medicated groups, whereas the one that indicated decreased Lactobacillus was undertaken in a treatment-naive group [134].
FECAL MICROBIOTA TRANSPLANTATION
FMT: This procedure entails the transfer of fecal microorganisms from a healthy subject to a receiver [135].
A study showed FMT from SPR individuals and healthy controls in GF mice resulted in decreased glutamate levels, whereas glutamine and GABA levels were increased in the HPC. Furthermore, recipient animals exhibited behaviors reminiscent of mice with glutamatergic hypofunction in SPR [76]. Another study using a similar experimental technique found that FMT from drug-naive SPR subjects to antibacterial-treated mice causes behavioral complications in recipient animals, including hyperactivity, poor learning, and memory functions. These behavioral impairments were accompanied by higher values of baseline extracellular dopamine in the prefrontal cortex and 5-HT in the HPC, as well as activation of the kynurenine–kynurenic acid pathway in peripheral tissues and the brain. According to the same study transplantation of Streptococcus vestibularis (a bacterium prevalent in people with SPR) causes hyperkinetic behavior and affects social interactions in mice [83].
In a human investigation, a decline in the number of Faecalibacterium can cause an increase in gut TH17 cells in SPR patients. It has been suggested that these cells may pass the BBB and stimulate the microglia in the HPC, causing aberrant behavior [93].
Experimental research unequivocally demonstrates that the GM of untreated SPR patients may be transplanted into GF mice and cause a variety of behavioral as well as altered neurotransmission. Clinical study results, however, do not substantiate probiotics as supplemental therapy for SPR [83]. To get a conclusion on the causal relationships between GM and SPR/psychosis, longitudinal research is still required.
CONCLUSION
Numerous studies in the last decade have emphasized the importance of GM in brain function and dysfunction. The to-and-fro interaction between microbes and the brain (interconnected by the vagus nerve) modulates immune, enteroendocrine, and neuronal inflammation system pathways impacting stress response, behavior, mood, and cognition. It can be hypothesized that probiotics can be targeted at neurobehavioral disorders. As several preclinical and clinical trials showed the efficacy of probiotics in alleviating the symptoms of SPR and other mental diseases by modulating the MGBA. Even though the preclinical studies have promising data on the interconnection between gut dysbiosis and SPR, further comprehensive human studies are needed to conclude on the efficacy and usage of probiotics both in preventing and treating SPR and other psychiatric disorders. Effects of probiotics in modulating endocrinal, inflammatory, immunochemical, and neuronal changes in both physiological and pathological states should be evaluated to identify their thorough mechanism of action in preventing/treating SPR. This will assist in determining the probiotics supplement’s dose, duration, and side effects. It is critical to discover novel gut microbial species that can maintain the diversity of the gut microbial population. As the present literature is insufficient to conclude specific microbial species or probiotics that can benefit SPR.
FMT, an emerging microbiome-focused strategy of one-time transplantation of completely new human GM is prophesied to be the future of GM-targeted strategies in SPR.
We also noticed a grey area in gene-specific microbial therapy. Since the role of genetics and epigenetics in SPR is not clearly understood. Hence, we stress the identification of functional genes which might lead to the recognition of individualized probiotic treatment of psychiatric disorders through GM analysis in the future.
AUTHOR CONTRIBUTIONS
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Syed Mushraf and Veena Nayak. The first draft of the manuscript was written by Syed Mushraf and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
CONSENT FOR PUBLICATION
All the authors have consent for publication.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects. Hence ethical clearance is not required.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35:549–62. CrossRef
2. American Psychiatric Association. Substance-related and addictive disorders. In: Diagnostic and statistical manual of mental disorders [Internet]. Washington, DC: American Psychiatric Association; 2013. Available from: https://psychiatryonline.org/doi/10.1176/appi.books.9780890425596.dsm16 CrossRef
3. Lavretsky H. History of schizophrenia as a psychiatric disorder history of clinical diagnosis of schizophrenia. New York, NY: Guilford Publications; 2008.
4. Liu JCW, Gorbovskaya I, Hahn MK, Müller DJ. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021 Apr 1;13(4):1152. CrossRef
5. Ijaz S, Bolea B, Davies S, Savovi? J, Richards A, Sullivan S, et al. Antipsychotic polypharmacy and metabolic syndrome in schizophrenia: a review of systematic reviews. BMC Psychiatry [Internet]. 2018 Sep 3;18(1):275. Available from: https://pubmed.ncbi.nlm.nih.gov/30176844 CrossRef
6. Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. The global burden of mental, neurological and substance use disorders: an analysis from the global burden of disease study 2010. PLoS One. 2015 Feb 6;10(2):e0116820. CrossRef
7. Munawar N, Ahsan K, Muhammad K, Ahmad A, Anwar MA, Shah I, et al. Hidden role of gut microbiome dysbiosis in schizophrenia: antipsychotics or psychobiotics as therapeutics? Int J Mol Sci. 2021;22(14):7671. doi: https://doi.org/10.3390/ijms22147671 CrossRef
8. Patrono E, Svoboda J, Stuchlík A. Schizophrenia, the gut microbiota, and new opportunities from optogenetic manipulations of the gut-brain axis. Behav Brain Funct. 2021;17(1):7. CrossRef
9. Reisinger S, Khan D, Kong E, Berger A, Pollak A, Pollak DD. The Poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol Ther. 2015;149:213–26. CrossRef
10. Kelly JR, Minuto C, Cryan JF, Clarke G, Dinan TG. The role of the gut microbiome in the development of schizophrenia. Schizophr Res. 2021 Aug 1;234:4–23. CrossRef
11. Obrenovich M, Jaworski H, Tadimalla T, Mistry A, Sykes L, Perry G, et al. The role of the microbiota–gut–brain axis and antibiotics in ALS and neurodegenerative diseases. Microorganisms. 2020;8(5):784. CrossRef
12. Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev [Internet]. 2019;99:1877–2013. Available from: www.prv.org
13. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016 Aug 19;14(8):e1002533. CrossRef
14. Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol. 2015;31:69–75. CrossRef
15. Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017;5(1):1–19. CrossRef
16. Butler MI, Mörkl S, Sandhu KV, Cryan JF, Dinan TG. The gut microbiome and mental health: what should we tell our patients?: Le microbiote Intestinal et la Santé Mentale?: que Devrions-Nous dire à nos Patients? Can J Psychiatry. 2019;64:747–60. CrossRef
17. Carabotti M, Scirocco A, Antonietta Maselli M, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems [Internet]. Ann Gastroenterol. 2015;28(2):203–9. Available from: www.annalsgastro.gr
18. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci [Internet]. 2012;13(10):701–12. doi: https://doi.org/10.1038/nrn3346 CrossRef
19. Halverson T, Alagiakrishnan K. Gut microbes in neurocognitive and mental health disorders. Ann Med. 2020;52:423–43. CrossRef
20. Rea K, Dinan TG, Cryan JF. Gut microbiota: a perspective for psychiatrists. Neuropsychobiology. 2020;79:50–62. CrossRef
21. Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol [Internet]. 2005;2(9):416–22. doi: https://doi.org/10.1038/ncpgasthep0259 CrossRef
22. Royet J, Gupta D, Dziarski R. Peptidoglycan recognition proteins: modulators of the microbiome and inflammation. Nat Rev Immunol [Internet]. 2011;11(12):837–51. doi: https://doi.org/10.1038/nri3089 CrossRef
23. Grochowska M, Wojnar M, Radkowski M. The gut microbiota in neuropsychiatric disorders. Acta Neurobiol Exp (Wars). 2018;78(2):69–81. CrossRef
24. Soca?a K, Doboszewska U, Szopa A, Serefko A, W?odarczyk M, Zieli?ska A, et al. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol Res. 2021;172:105840. CrossRef
25. El Aidy S, Dinan TG, Cryan JF. Immune modulation of the brain-gut-microbe axis. Front Microbiol. 2014;5(APR):146. CrossRef
26. Kim YK, Shin C. The microbiota-gut-brain axis in neuropsychiatric disorders: pathophysiological mechanisms and novel treatments. Curr Neuropharmacol. 2018 Mar 7;16(5):559–73. CrossRef
27. Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am [Internet]. 2017;46(1):77–89. Available from: https://www.sciencedirect.com/science/article/pii/S0889855316300826 CrossRef
28. Genedi M, Janmaat IE, Haarman BCM, Sommer IEC. Dysregulation of the gut-brain axis in schizophrenia and bipolar disorder: probiotic supplementation as a supportive treatment in psychiatric disorders. Curr Opin Psychiatry. 2019;32:185–95. CrossRef
29. Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Leister F, et al. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry [Internet]. 2010;68(1):100–4. Available from: https://www.sciencedirect.com/science/article/pii/S0006322310002507 CrossRef
30. Generoso JS, Giridharan VV, Lee J, Macedo D, Barichello T. The role of the microbiota-gut-brain axis in neuropsychiatric disorders. Revista brasileira de psiquiatria (Sao Paulo, Brazil: 1999). 2021;43:293–305. CrossRef
31. Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science (1979). 2011 Oct 7;334(6052):98–101. CrossRef
32. Mörkl S, Butler MI, Holl A, Cryan JF, Dinan TG. Probiotics and the microbiota-gut-brain axis: focus on psychiatry. Curr Nutr Rep. 2020;9:171–82. CrossRef
33. Koh A, de Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–45. CrossRef
34. Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int [Internet]. 2016;99:110–32. Available from: https://www.sciencedirect.com/science/article/pii/S0197018616301747 CrossRef
35. Allen AP, Hutch W, Borre YE, Kennedy PJ, Temko A, Boylan G, et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry. 2016 Nov 1;6(11):e939. CrossRef
36. Huuskonen J, Suuronen T, Nuutinen T, Kyrylenko S, Salminen A. Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br J Pharmacol. 2004 Mar;141(5):874–80. CrossRef
37. Erny D, de Angelis ALH, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015 Jun 25;18(7):965–77. CrossRef
38. de Almeida LMV, Funchal C, Gottfried C, Wajner M, Pessoa-Pureur R. Propionic acid induces cytoskeletal alterations in cultured astrocytes from rat cerebral cortex. Metab Brain Dis. 2006 Mar;21(1):51–62. CrossRef
39. Shultz SR, MacFabe DF, Martin S, Jackson J, Taylor R, Boon F, et al. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the long–evans rat: further development of a rodent model of autism. Behav Brain Res [Internet]. 2009;200(1):33–41. Available from: https://www.sciencedirect.com/science/article/pii/S0166432808007274 CrossRef
40. Nguyen TT, Kosciolek T, Eyler LT, Knight R, Jeste DV. Overview and systematic review of studies of microbiome in schizophrenia and bipolar disorder. J Psychiatr Res. 2018;99:50–61. CrossRef
41. Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CL, et al. Candida albicans exposures, sex specificity and cognitive deficits in schizophrenia and bipolar disorder. NPJ Schizophr. 2016 May 4;2(1):1–7. CrossRef
42. Severance EG, Alaedini A, Yang S, Halling M, Gressitt KL, Stallings CR, et al. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res. 2012 Jun;138(1):48–53. CrossRef
43. Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM, et al. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res. 2013 Aug;148(1–3):130–7. CrossRef
44. Sethi R, Gómez-Coronado N, Walker AJ, Robertson OD, Agustini B, Berk M, et al. Neurobiology and therapeutic potential of cyclooxygenase-2 (COX-2) inhibitors for inflammation in neuropsychiatric disorders. Front Psychiatry. 2019;10:605. CrossRef
45. Omeiza NA, Bakre A, Ben-Azu B, Sowunmi AA, Abdulrahim HA, Chimezie J, et al. Mechanisms underpinning Carpolobia lutea G. Don ethanol extract’s neurorestorative and antipsychotic-like activities in an NMDA receptor antagonist model of schizophrenia. J Ethnopharmacol [Internet]. 2023;301:115767. Available from: https://www.sciencedirect.com/science/article/pii/S0378874122008066 CrossRef
46. Eneni AEO, Ben-Azu B, Ajayi AM, Aderibibge AO. Lipopolysaccharide exacerbates ketamine-induced psychotic-like behavior, oxidative stress, and neuroinflammation in mice: ameliorative effect of diosmin. J Mol Neurosci [Internet]. 2023;73(2):129–42. doi: https://doi.org/10.1007/s12031-022-02077-9 CrossRef
47. Hemmings G. Schizophrenia. Lancet [Internet]. 2004 Oct;364(9442):1312–3. Available from: https://linkinghub.elsevier.com/retrieve/pii/S014067360417181X CrossRef
48. Fond GB, Lagier JC, Honore S, Lancon C, Korchia T, De Verville PLS, et al. Microbiota-orientated treatments for major depression and schizophrenia. Nutrients. 2020;12(4):1024. CrossRef
49. Daulatzai MA. Chronic functional bowel syndrome enhances gut-brain axis dysfunction, neuroinflammation, cognitive impairment, and vulnerability to dementia. Neurochem Res. 2014;39:624–44. CrossRef
50. Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, et al. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One. 2012 Feb 22;7(2):e31951. CrossRef
51. Lombardi VC, De Meirleir KL, Subramanian K, Nourani SM, Dagda RK, Delaney SL, et al. Nutritional modulation of the intestinal microbiota: future opportunities for the prevention and treatment of neuroimmune and neuroinflammatory disease. J Nutr Biochem. 2018;61:1–16. CrossRef
52. Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol [Internet]. 2012;303:1288–95. Available from: http://www.ajpgi.org CrossRef
53. Hernández-Romero D, Sanchez-Amat A, Solano F. A tyrosinase with an abnormally high tyrosine hydroxylase/dopa oxidase ratio: role of the seventh histidine and accessibility to the active site. FEBS J. 2006 Jan;273(2):257–70. CrossRef
54. Kuley E, Bal?kc? E, Özo?ul ?, Gökdogan S, Özo?ul F. Stimulation of cadaverine production by foodborne pathogens in the presence of Lactobacillus, Lactococcus, and Streptococcus spp. J Food Sci [Internet]. 2012 Dec 1;77(12):M650–8. doi: https://doi.org/10.1111/j.1750-3841.2012.02825.x CrossRef
55. Matsumoto M, Kibe R, Ooga T, Aiba Y, Sawaki E, Koga Y, et al. Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study. Front Syst Neurosci. 2013 Apr 1;7(Apr 2013):9. CrossRef
56. Nishino R, Mikami K, Takahashi H, Tomonaga S, Furuse M, Hiramoto T, et al. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol Motil. 2013;25(6):521–e371. CrossRef
57. van der Stel AX, van Mourik A, ?aniewski P, van Putten JPM, Jagusztyn-Krynicka EK, Wösten MMSM. The Campylobacter jejuni RacRS two-component system activates the glutamate synthesis by directly upregulating γ-glutamyltranspeptidase (GGT). Front Microbiol. 2015;6(June):567. CrossRef
58. Lagier JC, Edouard S, Pagnier I, Mediannikov O, Drancourt M, Raoult D. Current and past strategies for bacterial culture in clinical microbiology. Clin Microbiol Rev. 2015 Jan 1;28(1):208–36. CrossRef
59. Hugenholtz P, Goebel BM, Pace NR. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity [Internet]. J Bacteriol. 1998;180(18):4765–74. Available from: http://crab2.berkeley.edu/pacelab/176.htm. CrossRef
60. Liu YX, Qin Y, Chen T, Lu M, Qian X, Guo X, et al. A practical guide to amplicon and metagenomic analysis of microbiome data. Protein Cell. 2021;12:315–30. CrossRef
61. Goodman AL, Kallstrom G, Faith JJ, Reyes A, Moore A, Dantas G, et al. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Nat Acad Sci [Internet]. 2011 Apr 12;108(15):6252–7. doi: https://doi.org/10.1073/pnas.1102938108 CrossRef
62. Zou Y, Xue W, Luo G, Deng Z, Qin P, Guo R, et al. 1,520 reference genomes from cultivated human gut bacteria enable functional microbiome analyses. Nat Biotechnol [Internet]. 2019;37(2):179–85. doi: https://doi.org/10.1038/s41587-018-0008-8 CrossRef
63. Liu C, Zhou N, Du MX, Sun YT, Wang K, Wang YJ, et al. The mouse gut microbial biobank expands the coverage of cultured bacteria. Nat Commun [Internet]. 2020;11(1):79. doi: https://doi.org/10.1038/s41467-019-13836-5 CrossRef
64. Mu DS, Liang QY, Wang XM, Lu DC, Shi MJ, Chen GJ, et al. Metatranscriptomic and comparative genomic insights into resuscitation mechanisms during enrichment culturing. Microbiome [Internet]. 2018;6(1):230. doi: https://doi.org/10.1186/s40168-018-0613-2 CrossRef
65. Bai Y, Müller DB, Srinivas G, Garrido-Oter R, Potthoff E, Rott M, et al. Functional overlap of the Arabidopsis leaf and root microbiota. Nature [Internet]. 2015;528(7582):364–9. doi: https://doi.org/10.1038/nature16192 CrossRef
66. Zhang J, Liu YX, Zhang N, Hu B, Jin T, Xu H, et al. NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat Biotechnol [Internet]. 2019;37(6):676–84. doi: https://doi.org/10.1038/s41587-019-0104-4 CrossRef
67. Xu Y, Zhao F. Single-cell metagenomics: challenges and applications. Protein Cell [Internet]. 2018 May 1;9(5):501–10. doi: https://doi.org/10.1007/s13238-018-0544-5 CrossRef
68. Metsky HC, Siddle KJ, Gladden-Young A, Qu J, Yang DK, Brehio P, et al. Capturing sequence diversity in metagenomes with comprehensive and scalable probe design. Nat Biotechnol [Internet]. 2019;37(2):160–8. doi: https://doi.org/10.1038/s41587-018-0006-x CrossRef
69. Charalampous T, Kay GL, Richardson H, Aydin A, Baldan R, Jeanes C, et al. Nanopore metagenomics enables rapid clinical diagnosis of bacterial lower respiratory infection. Nat Biotechnol [Internet]. 2019;37(7):783–92. doi: https://doi.org/10.1038/s41587-019-0156-5 CrossRef
70. Whon TW, Shin NR, Kim JY, Roh SW. Omics in gut microbiome analysis. J Microbiol. 2021;59:292–7. CrossRef
71. Zhu F, Guo R, Wang W, Ju Y, Wang Q, Ma Q, et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol Psychiatry. 2020 Nov 1;25(11):2905–18. CrossRef
72. Shen Y, Xu J, Li Z, Huang Y, Yuan Y, Wang J, et al. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: a cross-sectional study. Schizophr Res [Internet]. 2018;197:470–7. Available from: https://www.sciencedirect.com/science/article/pii/S0920996418300021 CrossRef
73. Schwarz E, Maukonen J, Hyytiäinen T, Kieseppä T, Oreši? M, Sabunciyan S, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res [Internet]. 2018;192:398–403. Available from: https://www.sciencedirect.com/science/article/pii/S0920996417302049 CrossRef
74. Ma X, Asif H, Dai L, He Y, Zheng W, Wang D, et al. Alteration of the gut microbiome in first-episode drug-naïve and chronic medicated schizophrenia correlate with regional brain volumes. J Psychiatr Res [Internet]. 2020;123:136–44. Available from: https://www.sciencedirect.com/science/article/pii/S0022395619311628 CrossRef
75. Xu R, Wu B, Liang J, He F, Gu W, Li K, et al. Altered gut microbiota and mucosal immunity in patients with schizophrenia. Brain Behav Immun. 2020 Mar 1;85:120–7. CrossRef
76. Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. CrossRef
77. Nguyen TT, Kosciolek T, Maldonado Y, Daly RE, Martin AS, McDonald D, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019 Feb 1;204:23–9. CrossRef
78. Li S, Zhuo M, Huang X, Huang Y, Zhou J, Xiong D, et al. Altered gut microbiota associated with symptom severity in schizophrenia. Peer J. 2020;8:e9574. CrossRef
79. He Y, Kosciolek T, Tang J, Zhou Y, Li Z, Ma X, et al. Gut microbiome and magnetic resonance spectroscopy study of subjects at ultra-high risk for psychosis may support the membrane hypothesis. Eur Psychiatry [Internet]. 2018;53:37–45. Available from: https://www.sciencedirect.com/science/article/pii/S0924933818301068 CrossRef
80. Yolken RH, Severance EG, Sabunciyan S, Gressitt KL, Chen O, Stallings C, et al. Metagenomic sequencing indicates that the oropharyngeal phageome of individuals with schizophrenia differs from that of controls. Schizophr Bull. 2015 Sep 1;41(5):1153–61. CrossRef
81. Yuan X, Zhang P, Wang Y, Liu Y, Li X, Kumar BU, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res [Internet]. 2018;201:299–306. Available from: https://www.sciencedirect.com/science/article/pii/S0920996418302743 CrossRef
82. Grover S, Patil A, Kaur A, Garg G. Probiotics: a potential immunotherapeutic approach for the treatment of schizophrenia. J Pharm Bioallied Sci. 2019;11:321–7. CrossRef
83. Samochowiec J, Misiak B. Gut microbiota and microbiome in schizophrenia. Curr Opin Psychiatry. 2021;34:503–7. CrossRef
84. Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CLG, et al. Probiotic normalization of Candida albicans in schizophrenia: a randomized, placebo-controlled, longitudinal pilot study. Brain Behav Immun. 2017 May 1;62:41–5. CrossRef
85. Tripathi KD. Essentials of medical pharmacology. 8th ed. New Delhi, India: Jaypee Brothers Medical; 2019.
86. Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):3047–52. CrossRef
87. Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009 Jan 3;373(9657):31–41. CrossRef
88. Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet [Internet]. 2013;382(9896):951–62. Available from: https://www.sciencedirect.com/science/article/pii/S0140673613607333 CrossRef
89. Lieberman JA, Stroup TS, Mcevoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia [Internet]. N Engl J Med. 2005;353(12):1209–23. Available from: www.nejm.org CrossRef
90. Jones PB, Barnes TRE, Davies L, Dunn G, Lloyd H, Hayhurst KP, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: cost utility of the latest antipsychotic drugs in schizophrenia study (CUtLASS 1). Arch Gen Psychiatry [Internet]. 2006 Oct 1;63(10):1079–87. doi: https://doi.org/10.1001/archpsyc.63.10.1079 CrossRef
91. Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson E, et al. Extensive impact of non-antibiotic drugs on human gut bacteria Europe PMC Funders Group. Nature [Internet]. 2018;555(7698):623–8. Available from: http://www.nature.com/authors/editorial_policies/license.html#terms http://dx.doi.org/10.6084/m9.figshare.4813882 https://git.embl.de/mkuhn/drug_impact_gut_bacteria.A CrossRef
92. Szeligowski T, Yun AL, Lennox BR, Burnet PWJ. The gut microbiome and schizophrenia: the current state of the field and clinical applications. Front Psychiatry. 2020;11:156. CrossRef
93. Zhang X, Pan LY, Zhang Z, Zhou YY, Jiang HY, Ruan B. Analysis of gut mycobiota in first-episode, drug-naïve Chinese patients with schizophrenia: a pilot study. Behav Brain Res [Internet]. 2020;379:112374. Available from: https://www.sciencedirect.com/science/article/pii/S016643281931530X CrossRef
94. Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015 Apr 9;161(2):264–76. CrossRef
95. Okubo R, Koga M, Katsumata N, Odamaki T, Matsuyama S, Oka M, et al. Effect of Bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: a proof-of-concept study. J Affect Disord [Internet]. 2019;245:377–85. Available from: https://www.sciencedirect.com/science/article/pii/S0165032718313600 CrossRef
96. Flowers SA, Evans SJ, Ward KM, McInnis MG, Ellingrod VL. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017 Mar 1;37(3):261–7. CrossRef
97. Davey KJ, O’Mahony SM, Schellekens H, O’Sullivan O, Bienenstock J, Cotter PD, et al. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology (Berl). 2012 May;221(1):155–69. CrossRef
98. Murakami E, Shionoya T, Komenoi S, Suzuki Y, Sakane F. Cloning and characterization of novel testis-specific diacylglycerol kinase η splice variants 3 and 4. PLoS One. 2016 Sep 1;11(9):e0162997. CrossRef
99. Bahr SM, Weidemann BJ, Castro AN, Walsh JW, deLeon O, Burnett CML, et al. Risperidone-induced weight gain is mediated through shifts in the gut microbiome and suppression of energy expenditure. EBioMedicine [Internet]. 2015;2(11):1725–34. Available from: https://www.sciencedirect.com/science/article/pii/S235239641530181X CrossRef
100. Leem C, Martirosyan DM. The bioactive compounds of probiotic foods/supplements and their application in managing mental disorders. Bioact Compounds Health Dis. 2019;2:206–20. CrossRef
101. Nath A, Haktanirlar G, Varga Á, Molnár MA, Albert K, Galambos I, et al. Biological activities of lactose-derived prebiotics and symbiotic with probiotics on gastrointestinal system. Medicina (Lithuania). 2018;54(2):18. CrossRef
102. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017 May 1;62:46–52. CrossRef
103. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. CrossRef
104. Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry [Internet]. 2013;74(10):720–6. Available from: https://www.sciencedirect.com/science/article/pii/S0006322313004083 CrossRef
105. Fuller R. Probiotics: the scientific basis [Internet]. Dordrecht, The Netherlands: Springer; 2012. Available from: https://books.google.co.in/books?id=i8brCAAAQBAJ
106. El Aidy S, Dinan TG, Cryan JF. Gut microbiota: the conductor in the orchestra of immune-neuroendocrine communication. Clin Ther. 2015;37:954–67. CrossRef
107. Patterson EE, Ryan PM, Cryan JF, Dinan TG, Paul Ross R, Fitzgerald GF, et al. Gut microbiota, obesity and diabetes. Postgrad Med J. 2016;92:286–300. CrossRef
108. Miro-Blanch J, Yanes O. Epigenetic regulation at the interplay between gut microbiota and host metabolism. Front Genet. 2019;10:638. CrossRef
109. Niv Z, Eliran S, Eran E. Transforming medicine with the microbiome. Sci Transl Med [Internet]. 2019 Jan 30;11(477):eaaw1815. doi: https://doi.org/10.1126/scitranslmed.aaw1815 CrossRef
110. Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007 Mar;61(3):355–61. CrossRef
111. Mohammadi AA, Jazayeri S, Khosravi-Darani K, Solati Z, Mohammadpour N, Asemi Z, et al. The effects of probiotics on mental health and hypothalamic–pituitary–adrenal axis: a randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr Neurosci. 2016 Nov 8;19(9):387–95. CrossRef
112. Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2(4):256–61. CrossRef
113. Yamamura R, Okubo R, Katsumata N, Odamaki T, Hashimoto N, Kusumi I, et al. Lipid and energy metabolism of the gut microbiota is associated with the response to probiotic Bifidobacterium breve strain for anxiety and depressive symptoms in schizophrenia. J Pers Med. 2021 Oct 1;11(10):987. CrossRef
114. Dipasquale S, Pariante CM, Dazzan P, Aguglia E, McGuire P, Mondelli V. The dietary pattern of patients with schizophrenia: a systematic review. J Psychiatr Res. 2013;47:197–207. CrossRef
115. Balanzá-Martínez V. Nutritional supplements in psychotic disorders. Actas Esp Psiquiatr. 2017;45:16–25.
116. Ghaderi A, Banafshe HR, Mirhosseini N, Moradi M, Karimi MA, Mehrzad F, et al. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry. 2019 Feb 21;19(1):1–10. CrossRef
117. Dickerson FB, Stallings C, Origoni A, Katsafanas E, Savage CLG, Schweinfurth LAB, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion J Clin Psychiatry. 2014;16(1):26294. CrossRef
118. Tomasik J, Yolken RH, Bahn S, Dickerson FB. Immunomodulatory effects of probiotic supplementation in schizophrenia patients: a randomized, placebo-controlled trial. Biomark Insights. 2015 Jan 1;10:47–54. CrossRef
119. Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258–64. CrossRef
120. Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011 Dec;23(12):1132–9. CrossRef
121. Arroll MA, Wilder L, Neil J. Nutritional interventions for the adjunctive treatment of Schizophrenia: a brief review. Nutr J. 2014;13(1):1–9. CrossRef
122. Brown HE, Roffman JL. Emerging treatments in schizophrenia: highlights from recent supplementation and prevention trials. Harv Rev Psychiatry [Internet]. 2016;24(2):e1–7. Available from: https://journals.lww.com/hrpjournal/Fulltext/2016/03000/Emerging_Treatments_in_Schizophrenia__Highlights.9.aspx CrossRef
123. Crook N, Ferreiro A, Gasparrini AJ, Pesesky MW, Gibson MK, Wang B, et al. Adaptive strategies of the candidate probiotic E. coli Nissle in the mammalian gut. Cell Host Microbe. 2019 Apr 10;25(4):499–512.e8. CrossRef
124. Kelly JR, Allen AP, Temko A, Hutch W, Kennedy PJ, Farid N, et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun. 2017 Mar 1;61:50–9. CrossRef
125. Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res [Internet]. 2008;43(2):164–74. Available from: https://www.sciencedirect.com/science/article/pii/S0022395608000745 CrossRef
126. Bravo JA, Forsythe P, Chew MV., Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011 Oct 20;108(38):16050–5. CrossRef
127. Chunchai T, Thunapong W, Yasom S, Wanchai K, Eaimworawuthikul S, Metzler G, et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J Neuroinflammation. 2018 Jan 9;15(1):1–15. CrossRef
128. Liang S, Wang T, Hu X, Luo J, Li W, Wu X, et al. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience [Internet]. 2015;310:561–77. Available from: https://www.sciencedirect.com/science/article/pii/S0306452215008520 CrossRef
129. Janik R, Thomason LAM, Stanisz AM, Forsythe P, Bienenstock J, Stanisz GJ. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage [Internet]. 2016;125:988–95. Available from: https://www.sciencedirect.com/science/article/pii/S105381191501040X CrossRef
130. Zhuang M, Shang W, Ma Q, Strappe P, Zhou Z. Abundance of probiotics and butyrate-production microbiome manages constipation via short-chain fatty acids production and hormones secretion. Mol Nutr Food Res [Internet]. 2019 Dec 1;63(23):1801187. doi: https://doi.org/10.1002/mnfr.201801187 CrossRef
131. Nagamine T, Nakamura M. The effect of probiotics on bowel movement and metabolic parameters in schizophrenia patients: a retrospective chart review. Biosci Microbiota Food Health. 2020;39(4):197–8. CrossRef
132. Cuomo A, Maina G, Rosso G, Crescenzi BB, Bolognesi S, Muro AD, et al. The microbiome: a new target for research and treatment of schizophrenia and its resistant presentations? A systematic literature search and review. Front Pharmacol. 2018;9:1040. CrossRef
133. Bahr SM, Tyler BC, Wooldridge N, Butcher BD, Burns TL, Teesch LM, et al. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl Psychiatry. 2015 Oct 6;5(10):e652. CrossRef
134. Nikolova VL, Hall MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry. 2021;78:1343–54. CrossRef
135. Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology [Internet]. 2011;141(2):599–609.e3. Available from: https://www.sciencedirect.com/science/article/pii/S001650851100607X CrossRef