INTRODUCTION
Marine floras and faunas, including seaweeds, are increasingly recognized as a major source of healthy ingredients for various applications [1]. Seaweeds comprising thousands of species represent a considerable part of the littoral biomass, and they can be classified, depending on their chemical composition and pigment profiles, into three phyla, namely, red algae (Rhodophyta), brown algae (Phaeophyta), and green algae (Chlorophyta) [2,3]. Recent information shows an increase in global seaweed production due to high demands for foods, nutraceuticals, pharmacies, and cosmetic applications[4,5].
Seaweed has been used as food, spices, and folk medicine in many East Asian countries for many years [6,7]. In Japan, seaweeds have been consumed since the 4th century and in China afterward. More recently, the popularity and consumption of seaweeds in Western countries have risen [8–10]. Seaweeds are excellent sources of polysaccharides, amino acids, proteins, essential fatty acids (FAs), and other minor components, such as minerals, vitamins, trace elements, polyphenols (PPs), and photosynthetic pigments (PGs) [10,11]. Seaweeds are increasingly being viewed as potential sources of bioactive compounds which are not found in terrestrial plants with immense pharmaceutical, biomedical, and nutraceutical importance [12].
Among seaweed phyla, brown seaweeds (BS) are the most investigated phyla owing to their bioactive compounds. In a marine environment, several BS species form underwater forests, and the forests have become the habitat for marine invertebrates [13]. The BS are the most seaweed-producing phyla, and these seaweeds are considered to be economic natural resources. They are widely distributed from temperate to tropical areas with Japan and China as the primary producers. More than 1,000 BS species have been identified with Laminariales, Fucales, and Ectocapales as the most studied orders [14]. These orders are considered rich in primary metabolites and secondary metabolites, such as polysaccharides (sulfated and non-sulfated), sterols, FAs, chlorophylls (Chls), carotenoids (Cars), and PPs [14–16]. Because of their health-promoting features, biologically active compounds have sparked substantial interest in a variety of disciplines, particularly food sciences. The BS PGs, including Chls and Cars, have gained more interest in food sectors as food colorants [2,17]. In addition, not only due to their potency as food dyes, BS natural pigments also showed potential bioactivities and health benefit value.
This contribution discusses BS bioactive compounds, focusing on PPs and PGs. The recent characteristics, bioavailability and functionality, will be presented in the following sections. In addition, technologies to improve their stability and bioavailability are also discussed. Pursuing our interest in the biological potential of PPs phlorotannins (Phls) and PGs (Chls and Cars), this review is aimed at exploring their recent updates on characteristics, potential development, and prospects, particularly in food industries.
BS AS A POTENTIAL SOURCE OF PHLS AND PGS
The marine environment is a home for organisms to produce a multitude of bioactive compounds. A wide variety of bioactive compounds have been isolated from seaweeds, including PPs class, e.g., Phls and PGs, such as beta carotene (β-Car) and fucoxanthin (FUCOX) [18,19] (Table 1). Those bioactive compounds are diverse in their molecular structures and chemical properties, which will further affect their biological or physiological activities. Due to their health-promoting properties, those bioactive compounds have attracted considerable interest in several fields (e.g., pharmaceuticals, cosmetics, nutraceuticals, and foods).
Phenolic compounds
BS is known to be the best source of PPs among other seaweed phyla [11,20,21]. Structurally, Phls have high molecular weights of up to 650 kDa, constructed with phloroglucinol monomers and hydroxyl groups (OH) in the structural backbone [24–24]. Based on their structure, they can be classified into six different types, including phlorethols (aryl-ether bonds), fuhalols (ortho- and para-arranged ether bonds with an additional OH group), fucols (aryl-aryl bonds), fucophlorethols (ether and phenyl linkages), eckols (dibenzodioxin elements substituted by a phenoxyl group at C-4), and carmalols (derivatives of phlorethols with a dibenzodioxin moiety) (Fig. 1) [24,25]. The Phls are known to accumulate mostly in physodes (i.e., specialized membrane-bound vesicles of the cell cytoplasm) and are majorly found in Fucales, with 25% of seaweed’s dry weight (DW) [25–27]. The content of Phls in BS is related to the chelating activity of Phls to arsenic in BS due to the higher level of arsenic content [23]. These secondary metabolites play pivotal roles in BS cell wall components as biosynthetic precursors and defensive mediators against natural enemies, acting as herbivore deterrents, inhibitors of digestion, and agents against bacteria and protecting seaweeds from ultraviolet exposure in the BS [25]. Besides these functions, secondary metabolites have displayed various biological activities which are related to human health, including antioxidants, anti-cancer, anti-bacterial, anti-diabetics, and neuroprotective properties [22,25,27,34–36]. The concentrations of Phls in brown algae are easily recognized because they are highly accumulated in the fucaceae family, including Eisenia bicyclis, Ecklonia cava, Ecklonia kurome, Fucus vesiculosus, and Ascophyllum nodosum [25,27].
 | Table 1. PPs and PGs in BS. [Click here to view] |
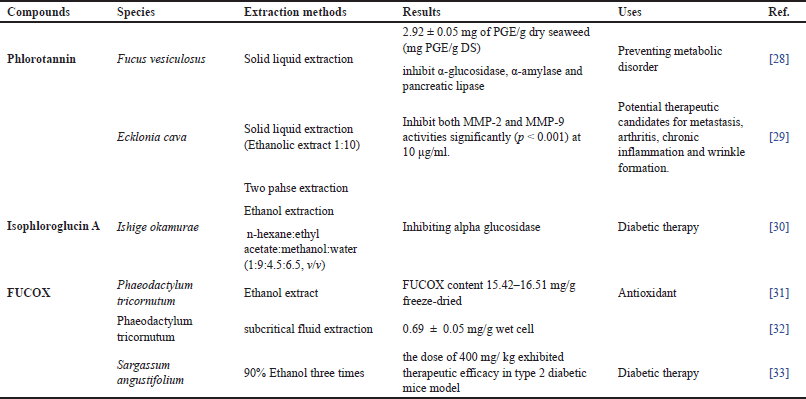
 | Figure 1. Possibility of absorption mechanism of Phls, Chls, β-carotene, and FUCOX in the enterocyte cell. The BS pigments, cars, are possibly captured from mixed micelles and carrier protein by apical membrane lipid transporters, CD36, NPC1L1, and SR-B1, except ABC transporters (MDR1). SR-B1 have shown to be involved in the absorption of derivative Chls, Pheo s, in the enterocyte cells. Breast cancer resistance protein (BCRP) (ABCB2) transporter is responsible for pumping back Pheo in the apical site enterocytes cells. The absorption of these compounds is low in humans. The ABCA1 is responsible for the efflux of cars in the basolateral site of enterocyte cells. In addition, the BS PPs, Phls, possess different mechanisms on their absorption in the enterocytes cells. Instead of the passive diffusions, the uptake of phlorotannin from the micelles possibly is related to the involvement of MCT-1 and SGLT-1 intestinal transporter. ABC type B 1 ABCB1 (MDR1) transporter may be responsible for the efflux back of the Phls from the apical site of enterocytes cells. [Click here to view] |
Photosynthetic pigments
The PGs are biologically active compounds responsible for capturing solar energy required for photosynthesis [37,38]. Nowadays, BS PGs, including Chls and Cars, have gained a lot of interest due to their multiple roles, not only for photosynthesis but also for other purposes, including renewable energy, food colorant, cosmetics, nutraceuticals, functional foods, as well as pharmaceutical industry [2,17]. In the following sections, we will describe PGs in BS, their characteristics, as well as biological properties.
Chlorophylls
PGs in BS are composed of lipophilic pigments, Chls, and Cars, which play an important role in the photosynthesis of the BS [17]. The major pigment, Chlorophyll (Chl), is constructed of tetrapyrrole porphyrin ring and phytol tail except for Chl c and Pheo (pheophorbides). The pigment creates a protein complex in photosystem I and photosystem II [39]. During photosynthesis, Chls plays an important role in the natural light-harvesting complex using solar energy to convert CO2 into carbohydrates through transferring the excitation energy to the reaction centers, to generate membrane potential, as well as to stimulate reluctant generation and adenosine triphosphate to produce energy [40]. Two predominant Chls families exist in BS, Chls a and Chls c series, which are different in their structure. Structurally, Chls a is characterized by tetrapyrrole porphyrin rings with chelated magnesium (Mg and esterified phytol) in the center [41,42]. Chls a is one of the abundant PGs which serve to harvest and transfer light energy to the photochemical reaction center. Chl a molecules are associated with intrinsic membrane proteins, and are able, upon excitation, to donate electrons to an acceptor molecule and accomplish charge separation, hence the primary photochemistry of photosynthesis [43]. Chl a has a methyl attached to R1 position [44]. Chl a molcules are soluble in organic solvents such as ethanol and methanol and insoluble in water.
Chls c are characterized by tetrapyrrole chlorin rings containing Mg ions in the center without esterified phytol [39]. The structurally different rings can modify their properties, their wavelength, absorption, and bioactivity. Chls a can be detected to be dominant at 430 nm and small absorption at 660 nm, whereas Chls c are predominated by Soret band absorption around 450 nm and Q band at 630 nm [39]. In BS, only Chl c1 and c2 are detected, while Chl c3 is found in Haptophyta organisms [18,19,39,41]. Instead of parental forms, they also contain derivative forms in the forms of fresh BS, including pheophytin a [45], Pheo a (Pheo) a, and Pheo c1/c2 [19]. Chl c is characterized by the lack of a phytol ester. The residue attached to the porphyrin ring system results in the group of Chl c1, c2, or c3 that are generally available in nature. Compared to Chl a, the spectra of Chl c are dominated by a huge Soret band absorption (around 450 nm in acetone) and a small QY absorption close to 630 nm. Chl c2 and c3 contain an additional double bond which is part of the conjugated system. Hence, they absorb at slightly longer wavelengths compared to Chl c1 [39].
Furthermore, Chl is prone to degrading and forming its derivatives as an effect of several factors, such as light, heat, and pH [9,42]. In general, Chls are predominantly contained more in BS than in other seaweed phyla [19]. The Chls contain porphyrin rings bound to magnesium as a central cavity. They are green colored and serve as non-polar pigments [46]. The major Chl a and Chl c are the most important pigments for photosynthesis.
Carotenoids
The term “Cars” is used to describe the common pigments found in animals and plants, which are responsible to express the red, orange, and yellow colors. These groups of pigments are fat-soluble [47]. The pigments are synthesized by plants, seaweeds, and other photosynthetic organisms as well as some non-photosynthetic bacteria [48]. Structurally, cars are classified according to the number of carbon atoms that constitute their structure. It may have specific linear C30, C40, C45, and C50 molecular backbone, but only the cars with C40 are available in abundance in nature. The tetraterpenoid carotene (C40) consists of 8 units of isoprenoids linked to each other and form a linear and symmetric molecule [47].
More than 600 variant structures of Cars have been reported [49]. They can be classified as carotenes and xanthophylls. The most common abundant carotenes found in BS are β-carotene, while FUCOX, zeaxanthin, violaxanthin, beta-cryptoxanthin, antheraxanthin, violaxanthin, and neoxanthin are grouped as xanthophylls [19,49,50]. The β-carotene has a tetraterpenoid structure, which consists of 40 carbon atoms in its core structure of double bonds, which substitute with 2-β-ionone rings. The extension of 9 fully conjugated double bonds in beta-carotene structures is given a major peak absorbance with a maximum at ~450 nm in the visible spectrum, and it is designated for the orange to red color of the compounds [51]. The conjugated carbon-carbon bonds in the core system of beta-carotene make these compounds become an efficient singlet oxygen quencher, and the compounds prevent the formation of singlet oxygen by quenching excited triplet sensitizer and, they designate well as an antioxidant. In the light-harvesting system of photosynthetic organisms, β-Car and other cars play protection in preventing photooxidative damage by inhibiting singlet oxygen generation [59]. Xanthophyll is another subclass of cars that cover the polar compounds in their group members. FUCOX (3’-acetoxy-5,6-epoxy-3,5’-dihydroxy-6’7’-didehydro-5,6,7,8,5’,6’-hexahydro-ββ-caroten-8-on) is one of the most prominent members of xanthophylls which have a unique carotenoid structure, including an allenoic band and a 5,6-monoepoxide. FUCOX is a major non provitamin A Cars isolated from BS [53]. FUCOX has been found abundantly (approximately 10% of the estimated total production of Cars in nature). This pigment is responsible for giving brown colors [49,54,55].
Bioavailability and metabolisms of Phls and PGs
The bio-functionality of secondary metabolites is related to their bioavailability in human organs. Bioavailability is the amount of compound which is absorbed, metabolized, and stored in organ membranes for physiological utilization, while metabolism is a chemical process in the human body [56]. Generally, after digestion, the nutritional compounds will be released in the gastrointestinal tract followed by absorption in the small and large intestines, then followed by metabolites in the liver before distributing to all organs. Phls, Chls, and Cars (β-car and FUCOX) are ingested in humans together when consuming BS. Information on their bioavailability and metabolism will be discussed in this review.
Generally, the increase in BS consumption results in the occurrence of bioactive/nutritional compounds and their metabolites. The annual consumption of Phls has been reported to reach 66–652 g/person. This means in 1 day about 183 mg are consumed by every person. However, the investigation of Phls and their metabolites is very limited; this may be due to the fast metabolism of PPs, which makes it difficult to identify. The investigation of Phls absorption, bioavailability, and metabolism is initiated by Corona et al. [57]. They found that Phls metabolites were detected in urine and plasma of healthy volunteers, in the form of conjugated (glucoronids and or sulfates) and unconjugated, such as Phls oligomer (hymetrifuhalol, 7-hydroxyeckol, co-c dimer of phloroglucinol), after 6–24 hours of A. nodosum capsule consumption [57]. This result indicates that Phls can absorb in the human with variation in every respondent. The variation of PPs bioavailability is related to some factors, such as intestinal microbiota and digestive enzymes, food matrix, which may affect its bioacessibilities, uptake, and metabolism of PPs.
The PGs are ingested by humans when they consumed BS. Historically, humans have consumed Chls-containing seaweeds for thousands of years. However, there is a lack of information about Chl, derivative absorption, and mechanism, possibly due to the fact that these pigments are prone to their derivatives. Research on the bioavailability of Chls started 8 decades ago by Brugsch and Keys [58], who found the bioavailability of Porphyrin in human urine. Later on, Chls were found in the visceral organs of rabbits. Then, Ferruzzi et al. [59] reported the absorption of spinach Chl in Caco-2 cells, which gives clear information about the pheophytinize and phehorbidation process during in vitro study by using Caco-2 cells [59]. Gandul and Rojas [60] found that Pheo a, polar Chl derivatives, demonstrated higher absorption in Caco-2 cells, with different absorption mechanisms, at lower concentrations facilitated uptake, while at higher concentrations, it was dominated by passive diffusions [59,61]. Furthermore, animal and human studies have proven the bioavailability of spinach Chls, which were found in the gallbladder, spleen, kidney, liver, and plasma of rabbits [62]. Furthermore, the recent study by Bradbury [26] has shown the occurrence of Chls and derivatives in human plasma in the form of Pheo a; however, its content was lower than lutein. The study of in vitro digestion and bioavailability of BS Chls has been initiated by Chen and Roca [63]. BS is rich in Chl a and c series, with different characteristics during in vitro digestion and absorption. This study found the absorption and bioavailability of Chls a and c containing BS. During digestion, pheophitinization and pheophorbidation of BS Chls occurred. Chl a series was more prone to pheophorbidation and oxidation process compared to Chl c series. Pheophorbidation process of BS Chls results in some derivative compounds, such as pheo a and c, while the oxidation process during digestion produced 132-hydroxy pheo a, 132-hydroxy phy a, 151-OH-lact. phy a, 151-OH-lact. pheo a, and oxidized pheo c. Chl c series is more resistant during in vitro digestions, and it is directly transformed into Pheo by removing ion Mg2+ in pyrrole rings due to the lack of phytol tails in their structure [63].
In addition, in the absorption of Chls over Caco-2 cells study, BS Chls exhibited different absorption rates with c series as the most favored absorption rate in Caco-2 cells. During the absorption process in Caco-2 cells, dephytillated and oxidized Chl derivatives become the most absorbable BS Chls. Some factors possibly affect the different rates of BS Chls absorption in Caco-2 cells, such as chemical structures, hydrophobicity, molecular weight, polarity of molecules, and ionization process [63].
FUCOX is known to have multiple biological effects in reducing non-communicable diseases. Those functionalities are possibly related to FUCOX and its metabolites, which are present in the human target organs after ingestion. Information about FUCOX and its metabolites available in humans is required to be known. Caco-2 is the best cell culture model to study upon the absorption and metabolism of drugs and dietary compounds, including FUCOX by human intestinal cells. The micellar FUCOX, which is composed of FUCOX (1 μmol/l), sodium taurocholate (2 mmol/l), monoacylglycerol (100 μmol/l), FAs (33.3 μmol/l) and phospholipid (0–200 μmol/l) is used in the absorption study in dCaco-2 cells. The study demonstrates that FUCOX is able to be uptaken by Caco-2 cells [64]. Another study conducted by the same researcher using micellar FUCOX, which composes FUCOX: sodium taurocholate: monoacylglycerol: FAs: lipophosphatidylcholine in ratio of 1:2:100:33.3:50 w/v). The composition was incubated for 24 hours in a DMEM medium, and dCaco-2 cells also show the absorption of FUCOX and their metabolites. Fucoxanthinol (FUCOL), cis-isomer of FUCOL, and FUCOX were detected in the dCaco-2 cell in all treatments. FUCOL significantly increased (p < 0.05 after 6 hours) up to 24 hours of incubation, while FUCOX significantly decreased (p < 0.05) until the end of the study. Furthermore, in vivo study, male Institute of Cancer Research (ICR) mice (7 week-old), were orally administered of Fx (40 nmol). Then, after 1 hour of being administered, the plasma was collected, and FUCOL and unknown metabolites were detected in the mice plasma. These results demonstrated that FUCOX is hydrolyzed into FUCOL, and unknown metabolites (alter known as Amaroxiaxanthin (AMX) [65]. Further in vivo study by the same researcher showed that all FUCOX was converted into its metabolites (FUCOX and FUCOL). After ingestion of FUCOX in male ICR mice, FUCOX was hydrolyzed in gastro intestinal tract (GIT) into FUCOL; then it was converted into AMX in the liver through dehydrogenation/isomerization with the participation of dehydrogenase [53]. A report by Sangeetha et al. [66] found other metabolites after administrations of FUCOX extracted from Padina tetrastromaica, such as FUCOL(m/z 600.6; FUCOX–H2O), AMX m/z 597 (AMX–H2O), other metabolites (deacetylated m/z 579; AMX–2H2O + 1), hydrolyzed (m/z 551; AMX–2H2O–2CH3+2) and demethylated metabolites (m/z 523; AMX–2H2O–4CH3 + 4)) in plasma and liver by liquid chromatography-mass spectrometry (atmospheric pressure chemical ionization) [66,64].
A study on humans with the intervention of 6 g wakame (6.1 mg/fx/d) for 1 week exhibited lower absorption of FUCOX. The lower amount of FUCOX 0.8 and 0.4 nmol/l was detected in human plasma, but AMX and other metabolites were not detected in the human plasma. This result is different from the previous studies on the FUCOX metabolites in mice and rats [67]. This research was supported by other groups which found lower absorption of FUCOX in rats. This phenomenon was possibly related to the fast metabolism of FUCOX, and possibly to the formation of other metabolites. Furthermore, as lipid-soluble Cars, the solubility is lower; this is possibly another reason for the accumulation of FUCOX and its metabolites in targeted organs [68].
The incorporation of FUCOX into modified nanoencapsulation and nanoemulsion will increase the bioavailability and stability of FUCOX in human and animal intestinal cells. Encapsulation with chitosan + FUCOX + GL is able to improve their bioavailability in vitro and in vivo [69,70]. In addition, the fortifications of FUCOX with milk powder exhibited better absorption and bioavailability both in vitro (dcaco-2 cells) and in vivo (male C57BL/6 mice). The higher bioavailability present is possibly due to high bioaccessibility and cellular uptake of FUCOX-SM and higher protein and Ca2+ ion, and it was dependent on fat efficient, so they all resulted in the increase of the bioavailability [71].
The β-Cars, lipophilic cars, are the most frequently studied on bioavailability and metabolism among other Cars. The investigation of β-Car bioavailability and metabolism has been conducted in the last 3 decades ago. The study on the bioavailability and metabolism of β-Car in humans has progressively increased, due to the highest activity of among other pro vitamin A Cars. The highest activity of β-carotene is related to the conversion of these Cars into two molecules of vitamin A [72–74]. The bioavailability of β-car plant sources is reported to be absorbed in humans by about 5% to 65%, the differences in β-car absorption, which is related to some factors, including the concentration of β-car, food matrix, food preparations, the amount of other compounds, such as lipids, fibers, and other cars in food, nutritional human status, and health and genetic characteristics of humans [75]. However, to date, the bioavailability and metabolism of β-car extracted from BS are not known; most studies use β-car extracted from carrots or synthesized β-car. The authors hypothesize that due to the different cell wall structures between terrestrial vegetables and BS, the bioavailability of this carotenoid will possibly be different. However, there is an opportunity to compare the bioavailability of β-car plant sources and that of BS sources.
Small and large intestines are the first gate before the distribution into all human organs. In these organs, nutritional absorption mechanisms, passive diffusions, and facilitated or active transport occur, depending possibly on chemical structures and molecular weights. The lower molecular weight of PPs is absorbed through passive diffusions based on in vitro studies using Caco-2 cells. While glycoside PPs are taken up to the enterocyte by sodium-glucose transport protein transporter 1. These results need to be reconfirmed, due to the fact that not all studies show the involvement of these intestinal transporters. In addition, monocarboxylic and transport have been reported as substrate PPs which are composed of monocarboxylic groups and non-polar sodium chain aromatic hydrophobic moieties [24]. However, the Phls absorption mechanisms in the human body is still unclear. Phls have a wide range of metabolites from low molecular weights to high molecular weights. Passive diffusions and facilitated transporters are possibly involved in the Phls absorption in human intestines. Further investigations are needed to confirm the absorption mechanism of Phls.
The different chemical structures and metabolism products turn out different absorption rates, which may suggest various routes of intestinal absorption and efflux mechanisms of Phls and PGs (Fig. 1). The hydrophilic (polar) Chls are possibly absorbed by both simple diffusions and facilitated transport as well as by the involvement of SR-B1 and BCRP2 [60,76,77]. Meanwhile, the parental Chl and phytillated Chls need miscelarization for their absorptions. More effort should be addressed to disclosing their absorption, metabolism, and mechanism. Instead of Chls, BS cars’ bioavailability and metabolism also require further discussion.
Initially, cars uptake in human intestines has been hypothesized by passive diffusions. However, some research groups have demonstrated the involvement of the facilitated transporter in the uptake and transport of cars in Caco-2 cells. The lipid intestinal transporters, abundantly found in the apical side of enterocyte cells, have been proved to be involved in carotenoid uptakes and transports. The involvement of this transporter class is reliable because cars are lipophilic pigments. SR-B1, CD-36, and Niemann-Pick C1-Like 1 (NPC1L1) are responsible for the apical uptake of cars. Reboul [78] has shown the involvement cluster of differentiation 36 (CD36) (FAs transporter) and NPC1L1 (sterol transporter) in the uptake of β-car in enterocyte cells. The different mechanism of β-car absorption and uptake occur possibly depend on its concentrations. The lower concentrations are dominated by facilitated diffusion, and the higher concentrations are possibly uptaken by passive diffusions [78].
The absorption of β-car in the wild type and SR-B1 knock-out mice fed with a high-fat diet demonstrates the facilitation of SR-B1 on the absorption of β-car. Furthermore, the β-car absorption on in vitro and human studies shows the involvement of SR-B1 [79]. After the uptake from the apical side of enterocyte cells, β-car is packed into cyclomicron, and then it is secreted into the lymph to the bloodstream through the basolateral side. The ABCA1 may be involved in the efflux of β-car from enterocyte to the lymph [88].
Some proteins, including lipid transporter (i.e. Cd36, SR-B1, and NPC1L1) cleavage enzyme BCO1, and transcription factor intestine-specific homeobox, have been demonstrated to take part in the β-car uptake and metabolism [78,81]. However, in the case of β-car absorption and metabolism, the involvement of intestinal transporters and other genes needs to be confirmed and identified.
Strategies to improve stability, protect the functionalities of BS PPs and PGs
PPs and PGs have been reported as low bioacessibility and bioavailability in the GIT. Emerging technology approaches have been developed to increase their stability, bioaccessibility, bioavailability, and biofunctionality, such as nano encapsulation and nano emulsion.
Nanoencapsulation
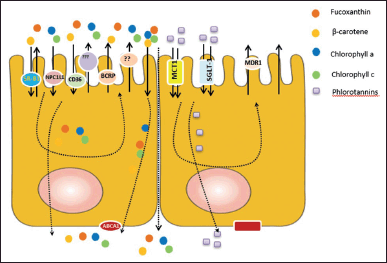
Encapsulation is a process of forming a capsule consisting of an outer layer covering the nucleus, where this nucleus is a certain functional compound. The protected compound is trapped in an inner matrix called the core [82]. Based on their size, it is divided into two groups micro and nano encapsulation. Microencapsulation is the potential to control bioactive compounds during their release in the gastrointestinal tract (Fig. 2), while nano encapsulation is able to protect bioactive compounds from the external environment and increase their bioavailability in targeted organs [83].
Phls and PGs possess multi-functionalities such as antioxidants, anti-inflammation, anticancer, and anti-diabetics [84]. These functional properties can be optimized if the bioactive compounds can be maximized, regarding their absorption and bioavailability, in targeted organs. Phls are easily oxidized by environmental factors; therefore, it is unstable, and it has low bioavailability and solubility [85]. Meanwhile, β-car and FUCOX are low molecular weights of Cars, which are easily absorbed in the body. Cars are transported by lipoproteins, and they are released into the bloodstream and stored in organs and tissues, such as skin, adipose tissues, kidneys, testes, and adrenal glands. After releasing from the food matrix, these cars are unstable due to the abundance of unsaturated bonds, which are easy to undergo oxidation [86]. Therefore, encapsulation can be used to increase their stability and bioavailability.
 | Figure 2. Encapsulation of bioactive compounds and release in GIT. The encapsulated bioactive compounds with coating materials are able to increase their stabilities during storage, ingestion, and absorption. The encapsulated bioactive compounds are stable during the ingestion and absorption process. The encapsulated bioactive compounds are released and absorbed in the small intestine; therefore, it can increase their bioavailability in the organ target. [Click here to view] |
The encapsulation process is used as a delivery system for PPs and PGs. PPs possess low solubility and limited application in nutraceutical and functional foods because they have a bitter taste when they are directly consumed [86]. Meanwhile, PGs are commonly used as an alternative to natural food dyes and functional ingredients. However, it is susceptible to damage due to less stability. Several kinds of coating materials can be used to protect PPs and PGs, which may increase their stability and control the release of the coated materials (core) at the target organ. Encapsulation efficiency is related to the coating materials formulation and the ratio of the core and coating materials. The formulation of the coating material and the ratio of the core and coating material are very influential in generating encapsulation efficiency [87,88]. Carbohydrates, such as starch, cellulose, pectin, maltodextrin, gum arabic, and alginate, are commonly used as encapsulation materials. In addition, gelatin, whey protein, casein, and phospholipids are also used as coating materials. The choice of coating material depends on the hydrophilic or lipophilic properties of the bioactive compounds being coated and the properties of the final products [73].
Limited information has been identified as it is related to Phls encapsulation. Encapsulated Phls with sodium alginate and nano-fibers produce microcapsules that are able to reduce the growth of Salmonella enteritidis in chicken meat stored at 4°C and 25°C [89]. Furthermore, another work has shown that nanoencapsulation of Phls with polyvinylpyrrolidone can produce a uniform size of Phls, increasing solubility in water. The nanoencapsulation did not form a precipitate during storage at room temperature for 15 days [84]. Based on the simulation results of gastrointestinal conditions, the nanocapsules showed increased solubility and bioavailability according to their release time. The encapsulation process is able to protect Phls from H2O2 in cells, which induces the formation of reactive oxygen species causing oxidative stress. These nanocapsules are suitable for application on the skins. Further development is needed regarding the process of releasing PPs nanocapsules, both in vitro and in vivo to disclose its stability and bioavailability in the digestion system.
It has been verified that alginate and chitosan are suitable materials for β-car encapsulation to boost β-car bioavailability. Encapsulation of β-car with alginate and chitosan can be released in small intestines, and it still remains in the stomach during digestion [73]. Chitosan has good stability and compatibility, which makes it a good biopolymer for drug delivery, which has hydroxyl and amino groups in its structures so that it forms a balance of hydrophilic and hydrophobic properties [90]. Regarding information about the bioavailability of FUCOX, Sun et al. [91] proved the use of maltodextrin, gum arabic, and whey protein isolation to increase FUCOX stability, protect FUCOX from an acid environment, and increase the release time in the gastrointestinal tract. In addition, a similar study has demonstrated that the combination of gum arabic, gelatin and alginate is able to increase the bioavailability and bioactivity of FUCOX in small intestines [92]. Encapsulated FUCOX treatment in mice was able to decrease blood lipid concentrations in the mice, which may affect lipolysis and lipogenesis.
Nanoemulsion
Emulsions are made up of two immiscible liquid phases, including oil (o) and water (w), in which the droplets of one liquid are distributed into the droplets of the other to produce an emulsion. An oil-in-water (o/w) emulsion is one in which oil droplets are dispersed in the aqueous phase, whereas a water-in-oil emulsion is one in which water droplets are dispersed in the oil phase [56,93]. Emulsions are categorized into three types based on droplet size: macroemulsions, nanoemulsions, and microemulsions.
The term “nanoemulsion” refers to an emulsion system with comparatively small droplets (200 nm) [94]. The nanoemulsions are also thermodynamically unstable (like macroemulsions), but they are kinetically more stable [95]. Many bioactive substances are lipophilic, with low solubility in dietary triglyceride oils and fats, making them difficult to integrate into diets [96]. They are also highly susceptible to degradation when subjected to harsh conditions (for example oxygen, high temperature, light, pH, and other reactive substances) [96,97].
To increase water dispersion, chemical stability, and bioavailability, bioactive substances must be encapsulated using delivery techniques. In recent years, there has been a lot of research published on nanoemulsions as bioactive substance delivery vehicles. Some of the advantages are as follows: (a) small particle size that is physically stable; (b) large surface area that improves water dispersion and bioavailability; (c) protection of bioactive compounds towards degradation; (d) optical clarity that allows bioactive compounds to be incorporated into food systems (e.g., water, drinks) without changing their original appearance; and (e) masking undesirable flavors and odors of some bioactive compounds.
Some research groups have prepared nanoemulsions encapsulating several Cars within their oil phase using different carrier oils, such a long-chain triglycerides, medium-chain triglycerides and indigestible oils (e.g., orange and mineral oils), emulsifiers, and various natural ingredients (such as tea PPs, whey protein, carrot) [98–102]. Microfluidization is used to stabilize betacarotene nanoemulsions utilizing betalactoglobulin or Tween20 as the carrier. The mean particle size formed was 156 nm in diameter, and the rate of encapsulated β-car degradation in nanoemulsions was found to be slower when betalactoglobulin was used as the emulsifier than when Tween-20 was used, indicating that the type of emulsifier can affect the chemical stability of bioactive compounds in nanoemulsions used [98,103].
Emerging technology to extract and improve bioavailability of brown seaweed PPs and pigments
Extraction
Seaweed phenolics and pigments have gained a lot of attention due to their outstanding antioxidant properties with valuable applications for humankind. In order to get the health benefits of seaweed phenolics and pigments and utilize them in the various carrier systems, these bioactives need to be extracted, analyzed, and purified. Many scientific studies on the extraction method of seaweed phenolics and pigments have been reported over the last years [104–106], these include ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), supercritical fluid extraction (SFE), pressurized liquid extraction (PLE), and enzyme-assisted extraction (EAE). The mentioned extraction methods are known as novel extraction techniques for seaweed bioactive, which provide several advantages, such as rapid extraction, high extraction yield, less solvent used, and environmentally friendly.
Ultrasound-assisted extraction
The UAE is defined by mechanical sound waves with a high frequency exceeding human hearing capacity, ranging from less than 20 kHz to 10 MHz [107,108]. In this method, the sound waves cause the creation of cavitation bubbles with a certain size, while at high pressure these cavitation bubbles implode. Lower frequency ultrasound produces huge cavitation bubbles and as the frequency increases the bubble decreases [108,109]. The implosion of cavitation bubbles on a material’s surface causes cell disruption, particle breakdown, and enhances mass transfer, which means that bioactive compounds are released from biological materials. UAE is safe and inexpensive, making it easy to handle. Besides, UAE makes a time-efficient and clean process because it requires low amounts of solvent [110]. The schematic diagram of UAE is shown in Figure 3. Generally, UAE can be performed using an ultrasonic probe (Fig. 3A) or ultrasonic bath (Fig. 3B), which fits with a transducer as a source of ultrasound power. The ultrasonic bath works at a frequency of 40 to 50 kHz, with power of 50 to 500 W, and can be equipped with temperature control. The ultrasonic probe is operated at a frequency of 20 kHz. In the ultrasonic bath system, the samples are immersed, and the ultrasound power delivered into the samples is low. The ultrasonic probe is inserted into the samples, and it delivers high-power ultrasound [117,119]. For extraction applications, the use of a high-power ultrasonic probe is preferable to an ultrasonic bath since it is more powerful with minimal ultrasonic energy loss [111]. In UAE process, several parameters may affect the recovery yield of PPs and PGs, such as BS species, ultrasound power, the solvent used and its ratio, temperature, time, and the particle size of BS samples.
 | Figure 3. The schematic representation of UAE: ultrasonic probe (A); ultrasonic bath (B). [Click here to view] |
UAE has been successfully employed to isolate bioactive materials from BS, including PPs and PGs, at laboratory and industrial scales. Dang et al. [112] successfully applied and optimized the UAE to obtain phenolic compounds from BS Hormosira banksii. The ultrasonic conditions to obtain the highest phenolic content were optimized using response surface methodology. They found that the optimum conditions were at the temperature of 30°C, time of 60 minutes, and power of 150 W. The total phenolic compound from three BS (A. nodosum, F. vesiculosus, and Bifurcaria bifurcata were also successfully recovered using UAE [113]. The experiment was conducted in an ultrasonic bath using a mixture consisting of water/ethanol (50:50, v/v), at room temperature for 30 minutes. The highest total phenolic content (TPC) was presented in B. bifurcata (5.74 g phloroglucinol equivalents (PGE) /100 g DW); then it is followed by that in A. nodosum and F. vesiculosus with a TPC concentration of 4.66 and 1.92 PGE/100 g DW, respectively. Based on their observation, the TPC of three BS was higher than two species of green microalgae, which results in a higher antioxidant effect as well. This result indicates that the higher TPC of BS could be due to the presence of phlorotannin compounds in BS. Another study of the isolation of BS PPs and FUCOX using UAE was conducted by Kumar et al. [114]. The UAE was operated at a frequency of 40 kHz and temperature of 37°C for 120 minutes to extract 69.86 mg/kg of phloroglucinol and 1.45 mg/kg of FUCOX from BS Sargassum wightii. Vázquez-Rodríguez et al. [115] reported the optimal conditions of the UAE process to obtain highly concentrated phlorotannin extracts from BS Silvetia compressa. The maximum yield of Phls (7.3 mg PGE/g dry seaweed) was reached at the extraction temperature of 50°C, ultrasound power density of 3.8 W/cl, solvent/seaweed ratio of 30 ml/g, and ethanol concentration in water of 32.3%. Based on their experimental data, they concluded that the ultrasound power density was the most dominant parameter to enhance the extraction yield of Phls. More recently, the optimum UAE conditions for extracting higher total phenolic compounds from BS Padina australis (807.20 mg gae/g) were also determined by Hassan et al. [116]. The UAE optimal conditions were determined to be an ultrasonic temperature of 60°C, ultrasonic time of 60 minutes, solvent concentration of 60% (v/v) aqueous ethanol, and sample-to-solvent ratio of 1 g/100 ml. Moreover, they found that the temperature and sample-to-solvent ratio significantly enhanced the TPC of BS.
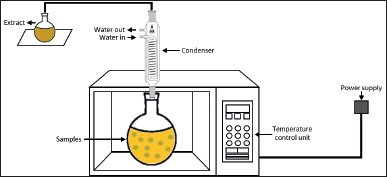 | Figure 4. Scheme of MAE. [Click here to view] |
Microwave-assisted extraction
MAE is one of the suitable alternatives to conventional technologies in developing an environmentally friendly extraction process of valuable compounds from marine seaweeds. It is known as a promising innovative technology for it is simple and inexpensive. MAE allows a faster extraction process and uses less solvent to obtain high yields of bioactive compounds [117]. This technology, using a nonionizing electromagnetic wave spectrum with a frequency ranging from 300 MHz to 300 GHz, transfers the heat to the system by two mechanisms occurring simultaneously: dipole rotation and ionic conduction [108] (Fig. 4). The heat transfer leads to cell disruption and results in higher penetration of the solvent into the cell wall, which releases the target compound into the solvent. In other words, microwave heating helps the solvent to penetrate deeper into the sample matrix to obtain the highest yield of the target compounds. In addition, both the heat and mass gradients work in the same direction toward the outside of the cells, allowing the process to increase extraction yields while reducing the time of the process [126].
Recently, MAE has been explored to extract phenolic compounds and pigments from several BS species. Magnusson et al. [119] reported that the extraction of PPs from BS Carpophyllum flexuosum using MAE with the optimum condition could increase the yield of PPs content up to 70% compared to conventional solid-liquid extraction. The optimum condition of MAE was performed using microwave synthesis reactor monowave 300 MHz at the temperature of 160°C, for 3 minutes, and H2O was applied as solvent extraction, with a solvent-to-sample ratio of 1:30. Another study of PPs recovery using MAE was reported by Amarante et al. [120]. The optimal MAE conditions were operated at the temperature of 75°C, time of 5 minutes, and used a hydroethanolic solution as a solvent with an ethanol concentration of 57% (v/v). These MAE conditions resulted in the optimum recovery of total phlorotannin from BS F. vesiculosus (9.8 mg PGE/g DW). According to the studies described above, several parameters of MAE play important roles to extract polyphenols, including the microwave power, the types of solvent used, the solvent ratio, the extraction time, and the temperature [107,121].
Microwave-assisted extraction has been reported to be effective for the extraction of FUCOX. Xiao et al. [122] used MAE to isolate FUCOX from three economically important pigments of BS, namely, Undaria pinnatifida, Saccharina japonica, and Sargassum fusiforme. The optimum MAE conditions were that ethanol was used as extraction solvent, the solvent/sample ratio was 15:1 ml/g, and the extraction temperature and time were 60°C and 10 minutes, respectively, where the microwave power had insignificant influence. The optimal yield of FUCOX obtained from dry U. pinnatifida, fresh S. japonica, and dry S. fusiforme were 109.3, 5.13, and 2.12 mg/100 g, respectively. These results indicated MAE is an attractive sample preparation method and has good potential for the extraction of FUCOX from BS [123].
Supercritical fluid extraction
SFE is defined as a separation technique of valuable compounds from natural resources using a supercritical fluid as a solvent. A supercritical fluid refers to any substance at a temperature and pressure above its critical point (Tc and Pc) and has several properties which are intermediate between those of gases and liquids. Under the critical state, the viscosity is lower and the diffusion rate is relatively high, which results in a more rapid solid matrices penetration and solute mass transfer from the matrices to the solvent. Additionally, the physical properties of supercritical fluids, such as viscosity, density, diffusivity, and dielectric constant, can be modified by simply changing the pressure and temperature. The tunable solvation power of supercritical fluid makes it a good extraction agent [124,125].
Carbon dioxide is the most commonly used solvent in SFE (referred to as supercritical CO2 extraction) because it is nontoxic, nonflammable, and inexpensive. Carbon dioxide is easily available, and it has low critical points; in addition, it can be easily separated from the extract, without chemical residue [126]. Supercritical carbon dioxide (Sc-CO2) provides a nonpolar environment, and its polarity can be occasionally modified by using co-solvents (Fig. 5). Several studies of Sc-CO2 extracting BS PPs and FUCOX have been reported. Phenolic and flavonoid contents of BS Sargassum horneri have been successfully recovered using Sc-CO2 with ethanol as a co-solvent [127]. The Sc-CO2 operated at a temperature of 45°C, a pressure of 250 bar could obtain 0.64 mg/g of total phenolic and 5.57 mg/g of total flavonoid. Similar work has been done by Sivagnanam et al. [128], in which FUCOX was extracted from BS S. japonica and S. horneri using Sc-CO2. The amount of FUCOX recovered from the SC-CO2 extracts of S. japonica and S. horneri were 0.41 and 0.77 mg/g, respectively. According to their work, the Sc-CO2 extracts of those BS showed higher phenolic content and stronger antioxidant capacity than those obtained by solid-liquid extraction. Another previous study reported that sun flower oil as Sc-CO2 co-solvent increased the recovery of Cars and FUCOX, while water as a co-solvent with Sc-CO2 increased the yield of phlorotannin of BS S. japonica [129]. The sunflower oil as a co-solvent could improve the extraction yield because it has low viscosity, and the FUCOX could easily dissolve in it. Then, the higher yield of phlorotannin might attribute to the density of the fluid mixture. Water as a co-solvent increased the density and diffusion rate facilitating the phlorotannin solubilization. Moreover, tannins were known as a chemically heterogeneous group which could dissolve very well in water. The latest work of FUCOX extraction using Sc-CO2 was reported by Getachew et al. [130]. The FUCOX of BS S. japonica and the oil of roasted coffee Coffea arabica were concurrently extracted using Sc-CO2. According to their investigation, the synergetic effect between the roasted coffee oil and Sc-CO2 resulted in the increasing amount of FUCOX up to fourfold. Additionally, several research have been shown that vegetable oil as Sc-CO2 co-solvent could enhance efficiency of Cars and pigments from marine and terrestrial resources [129,131,132].
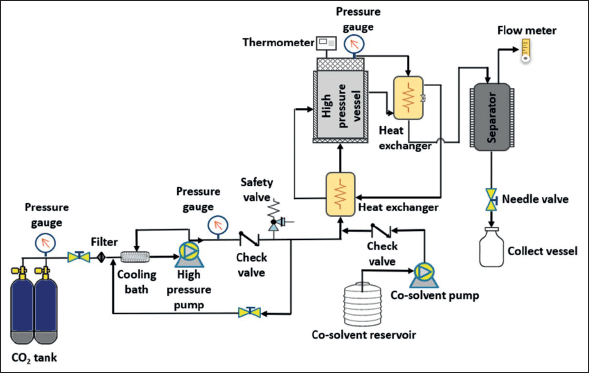 | Figure 5. Schematic diagram of Sc-CO2 extraction with an option of adding a co-solvent. Liquefied carbon dioxide was pumped to the extraction vessel by a high-pressure pump (PU-2-88, Jasco, Japan) up to the desired pressure which was regulated by a back-pressure regulator. The extraction temperature was monitored by the thermocouples at the inlet and outlet of the extractor. [Click here to view] |
According to the investigations described above, in the SFE process, the interaction between the temperature and the pressure is the fundamental key to improving the extraction efficiency of BS phenolic compounds and pigments, for both significant effects, the physical properties, including viscosity, diffusivity, and density of the solvent.
Pressurized liquid extraction
PLE, also called accelerated solvent extraction, or pressurized hot solvent extraction, has been acknowledged as a feasible green process for extracting bioactive compounds from natural resources. The principle of this extraction is to keep the solvent used at high pressures and temperatures above the solvents’ boiling point but under their critical temperature. The high temperature reduces the viscosity and surface tension of the solvent and increases the diffusivity and solubility of the desired analytes as well as mass transfer. This condition promotes several advantages in using PLE, including less sample and reagent required, rapid extraction, and higher extraction yields. Various organic solvents have been applied in PLE, such as ethanol, toluene, hexane, and water [133]. When PLE used water as an extractant, the extraction process is called subcritical water extraction (SWE).
In SWE, the water is maintained in the subcritical state (temperatures ranging from 100°C to 373°C), and its physical properties can be modified by tuning the pressure and the temperature. In the form of subcritical liquid, the dielectric constant of water is reduced from 80 (at ambient temperature) to 33 (at 200°C), which is close to the dielectric constant of organic solvents like ethanol and methanol. This fact makes SWE suitable to be used in extracting bioactive compounds which are difficult to be extracted by water at normal conditions [107,110,134]. Meillisa et al. [135] reported the recovery of total phenolic and flavonoid from BS S. japonica obtained by using SWE. The highest yield of total phenolic and flavonoid was 9.63 mg/l and 1.92 mg/l, while the SWE operated at the temperature of 180°C, with a ratio sample to water of 1:25 (w/v). More recently, Bordoloi and Goosen [136] found that SWE could improve the yield of the phenolic content from BS Ecklonia maxima up to 46%. According to these findings, it can be concluded that SWE has shown to be an efficient technology for the extraction of BS PPs.
Enzyme-assisted extraction
EAE is considered a potential alternative to conventional solvent extraction methods since it offers several advantages including low-temperature process, short time extraction, and nontoxic. In this process, the enzymes are used to break down the complex cell walls and to increase cell permeability; thus, the desired compound could be released more completely, leading to improved yield [108,137]. The important parameters in operating EAE are reaction time, pH, temperature, type and concentration of enzyme, types of solvent, and particle size of the material [125].
Phenolics are produced by plants, microorganisms, and algal materials in various forms and structures, such as in free soluble forms, or as in soluble complex forms [110,138]. BS presents the highest amount of phenolics compared to red and green seaweeds, ranging from simple phenolics (phenolics acids) to complex forms, such as tannins. BS Phls are often covalently bound to other macromolecules like proteins or other cell wall polysaccharides, which cannot be easily extracted using conventional methods. Besides, in most extraction studies, BS phenolics are also found to be more hydrophobic, in which the polyphenol content in ethanol extracts is higher than in water extracts [139]. These physical barriers result in the limitation of releasing the phenolic compounds from BS cell walls. In recent years, enzymatic degradation of cell wall polymers has become an attractive alternative to isolate bioactive compounds from seaweeds. The use of the appropriate enzyme could disrupt the cell wall, release the phenolic compounds into the extractant, and thus increase the extraction yields. In addition, EAE could convert water-insoluble compounds into water-soluble compounds, which makes EAE more suitable for polyphennol extraction of BS.
Several studies have reported the application of EAE for BS polyphenol isolation. Several enzymes including Alcalase 2.4 l food grade (FG), Carezyme 4,500l, protease from Streptomyces griseus, pectinase from Aspergillus niger, Celluclast 1.5 l, protease from Bacillus licheniformis have been used to isolated PPs and Phls from BS Lobophora variegata [140]. The highest PPs and Phls values were obtained by protease from Bacillus licheniformis and Celluclast 1.5l, respectively. Reaction temperature at 50°C and pH 5 were found to be the most optimum to isolate Phls and PPs. It has been reported that EAE was optimum at a relatively low temperature and moderate pH. The extraction time and mild conditions minimize the isomerization or destruction of active compounds.
EAE applied on BS Lessonia nigrescens, Macrocystis pyrifera, and Durvillaea antartica presented the higher yield of total phenol and the highest ACE inhibition (95.6%) [45]. The enzymatic extraction of BS Sargassum boveanum, Sargassum angustifolium, and Feldmannia irregularis has been done by using five carbohydrases (Viscozyme l, AMG300 l, Celluclast 1.5 l FG, termamyl 120 l, and Ultraflo l) and three proteases (flavourzyme 500M G, alcalase 2.4 l FG, and Neutrase 0.8 l), and showed high total phnolic content in almost all the enzymatic extracts. The extracts of S. bovanum using Viscozyme and Alcalase were found to be effective in inhibiting of lipid oxidation due to high amount of polyphenol [141]. These results suggested that the EAE caused not only an improvement in the extraction yield but also an improvement in the bioactivity. Some authors have combined the EAE with other green extraction processes like UAE, MAE, PLE, and SFE, in order to improve the extraction. When EAE is combined with PLE and SFE, the pretreatment of materials, together with the enzyme, breaks the cell walls, and it releases the phenolic compounds easily from the matrix [110,142]. Aside from the advantages of EAE, it is unfortunate that EAE has limitations for industrial-scale application due to the high cost and the availability of necessary enzymes.
Seaweeds-derived PPs and PGs potential uses in the food industry
The popular phrase of Hippocrates, “Let food be the medicine, and medicine be the food”, seems to be more relevant to current lifestyle. People tend to look for natural and healthy food products in order to get health benefits from their dietary intake. The growing demand for healthy food has forced the food industry to develop novel products with functional ingredients. It has been acknowledged that seaweeds are a high and sustainable source of macro-micronutrients in human diets. They have become a major ingredient in food and nutraceutical products in East Asia for centuries due to their valuable bioactive compounds. The fact is contrary to the one where seaweed is still under-exploited corps in food terms in other parts of the world. Now, seaweeds are being inserted into Western diets and are increasingly being explored due to the health-food industry [143].
Global health is increasingly conscious; therefore, the demand for bioactive compounds, such as functional foods and nutraceutical ingredients, is growing. Bioactive-containing BS, i.e., Phls and PPs, are valuable bioactive compounds required for the functional food industry. Over recent years, the interest in brown seaweed PPs, such as Phls, has raised market demand, and PPs are considered a precious source of functional foods (Fig. 6).The main target of dietary supplements containing brown seaweed PPs is cardiovascular disease management through atherosclerosis prevention and enhancement of High-Density Lipoprotein cholesterol protection [144]. Some polyphenol products have been produced as food supplements and distributed in the market (Table 2). Among Cars in BS, β-carotene and FUCOX are considered to be pivotal Cars in the application of nutraceuticals and biomedicals.
The BS cars, FUCOX are known as potential antioxidant compounds [18,19]. The potential therapeutics of FUCOX on reducing metabolites syndrome-associated diseases is well documented to have increased its charm for the nutraceutical and pharmaceutical industry. Its antioxidant activity is related to its chemical structures, which are able to modulate specific genes and protein expression in the biological system. These properties are related to FUCOX main activities in reducing metabolite syndromes [16,54,145].
Commercially, the production of FUCOX-rich fractions from U. pinnatifida and S. horneri, Japanese BS, has been extensively launched in the Japanese and US market. The commercial application of FUCOX-rich fraction or powder is not only for Japanese and Korean markets, but it is also for other parts of the world. The global market of FUCOX from BS is not only dependent on the two mentioned species, but it can also be extracted from other BS, which are grown in temperate and tropical areas [18,19]. FUCOX is the most popular commercial dietary ingredient derived from BS. Unlike other cars, such as β-carotene, which are commonly used as food colorants, pure FUCOX is not sold as a bulk ingredient [145]. Currently, FUCOX is available to retail consumers in the form of dietary products. Dietary products containing FUCOX mostly support healthy weight management (Table 3). In several studies, FUCOX is also used as natural food coloration, such as shrimp paste and catfish sausage [147]. According to those studies, the most preferable concentration of FUCOX application for shrimp paste and catfish sausage coloring was 12% and 1%, respectively.
Among bioactive photosynthetic pigmented BS, Chls are less investigated than Cars, particularly FUCOX. The lower stability and less bioavailability of Chls are probably the main reasons for its applications. Chls are being used for photodynamic therapy, anti-cancer as well as natural dyes. The utilization of brown seaweed Chls is very rare, so most studies use spinach Chls as their standard. Much research has investigated the bioavailability and bio-accessibility of seaweed Chls with Chls c and Pheo c because they are more absorbable than other Chls. However, their biological function is not investigated yet; they have been studied only as anti-allergics. Even the seaweed Chls are more complex than terrestrial plant Chls; so, more effort will be needed to prove their functionality. BS, fast-growing organisms, are potential sources of PGs, particularly Chls.
The limitation and prospective applications
The utilization and application of BS bioactive compounds are globally growing in various fields. However, there are many limitations and challenges which must be resolved. The easy farming and growing of seaweeds are the opportunity for BS to be the functional ingredients for various applications [24]. However, several challenges of bioactive compounds are present in the polyphenol class, e.g., Phls and PGs. Meanwhile, β-car and FUCOX from BS, used as functional ingredients in foods, are limited due to their poor solubility, chemical instability, and undesirable sensory attributes (e.g., astringent, bitter, off-odors), leading to poor oral bioavailability and low absorption rate as well as reduced consumer acceptance [93].
As evident from the increasing scientific literature and the number of patents filed each year, Phls represents a multifunctional group of natural products. Owing to their potential antioxidant properties, Phls are being exploited as curative and/or preventive agents in several disease areas. The present invention related to seaweed phlorotannin with anti-viral properties has been published by Evans et al. [167]. Phlorotannin extracted from brown seaweed, in particular those with a molecular mass of about 1,000 g/mol to about 3,000 g/mol could inhibit enveloped and non-enveloped viruses’ replication. Another work of seaweed phlorotannin against skin aging has been performed by Martins Da Silva et al. [168]. This invention showed that phlorotannin-enriched extract obtained from Fucus spiralis owned anti-enzymatic action that slow down skin aging.
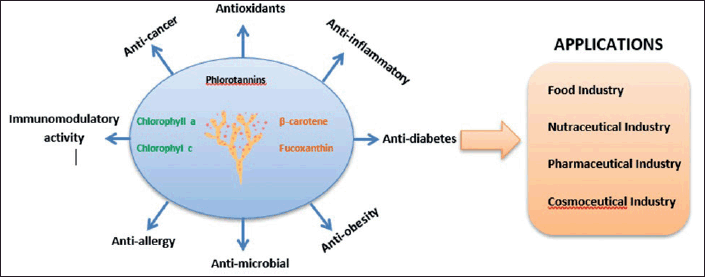 | Figure 6. Brown seaweed bioactive compounds, and their functionality and application in industry. The brown seaweed pigments and PPs possess multiple biological activities, which are beneficial for human health. Those bioactive compounds can be applied in the food, nutraceuticals, pharmaceuticals, and cosmeceutical industries. [Click here to view] |
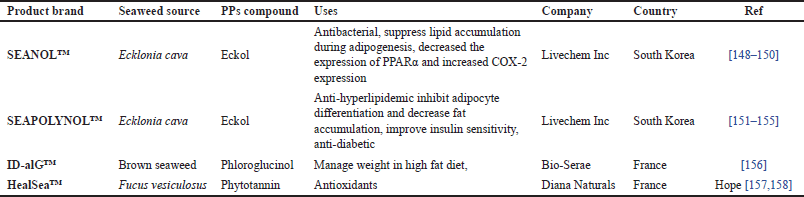 | Table 2. Food supplement product of BS PPs in the market. [Click here to view] |
 | Table 3. Commercial dietary products containing BS FUCOX. [Click here to view] |
From an industrial perspective, phlorotannins have proved their candidature as nutraceuticals, pharmaceuticals, and cosmeceuticals in global markets. Their drug-like functions have prompted many food and drug industries to promote the Phls-rich formulations as over-the-counter (OTC) products. These OTC products are being “safely” used as functional foods in many parts of the world. Unlike plant-derived natural products, the vast arena of marine resources is still open for screening, isolation, identification, and pharmacological characterization of these compounds. Despite the excitement generated by the spectrum of the biological potential of Phls, the structural complexity of these compounds has probably led to reduced attention to their synthesis. The synthesis of pharmacologically active Phls is an important area requiring the attention of chemists.
Several studies have demonstrated the potential of multiple emulsions as delivery systems to encapsulate food components and to control their release, such as water-o/w, lecithin-chitosan, etc. [169]. Although these systems are more suitable for the encapsulation of hydrophilic ingredients (e.g., water-soluble pigments, amino acids, and phenolic compounds), they may be used as a delivery system to encapsulate both lipophilic and hydrophilic components in the same system. However, there are still several important issues that need to be considered before using this emulsification technique in the food industry. Most prepared multiple emulsions are not suitable for use in food systems due to difficulty in scale-up and cost; they are not suitable because of the use of non-FG ingredients, which make them unsuitable for human consumption. Thus, during the fabrication of multiple emulsions, consideration must be given to nature, and concentration must be paid to the ingredients used. The main challenge with commercial multiple emulsions has been to make products that have a sufficiently long shelf-life for utilization within the food industry, and those which are capable of withstanding the fairly harsh processing operations involved. Also, once incorporated in a food matrix, multiple emulsions must have compatible structures which help to give the products the desired characteristics, without presenting unwanted features in terms of technological and sensory viability.
β-carotene, a natural colorant and an antioxidant, is a compound beneficial for human health through its ability to decrease the risk of cancer, cardiovascular diseases, and cataracts. Encapsulating beta-carotene in emulsion-based delivery systems can help in overcoming drawbacks, such as poor water solubility and chemical instability.
CONCLUSIONS AND FUTURE CHALLENGES
BS have been studied and used for various applications. The application of BS is now shifted from the food industry application to nutraceuticals, cosmeceuticals, and pharmaceuticals. Several BS are known to be economically seaweeds as staple foods and industrial raw materials; whereas many BS species are categorized as under-exploited, and they open huge potential to be explored. In addition, Phls and PGS from BS exhibit multifunctional properties to protect from various diseases. They have advantages such as a safe ingredient, and they are easy to utilize for various purposes. The development and commercialization of bioactive compounds from BS for industrial applications have several limitations and challenges regarding their bioavailability and stability in the gastrointestinal tract and erythrocyte, which are important to promote commercialization. The utilization of technology, such as nano-encapsulation and nano-emulsion, is prospective to boost their bioavailability and bioactivity. Further investigation in human study is needed to make a clear biofunction for human health. In addition, until now there has been less information about the metabolism of Phls and PGs metabolism in the biological system. Therefore, utilization and development of advanced methods, such as Maldi-TOF, LCHRMS, and LC-MS/MS are needed. There is still a huge opportunity to improve their utilization in various sectors through innovative research and development in industries, universities, and research institutions.
LIST OF ABBREVIATIONS
AMX = Amaroxiaxanthin; BS = Brown seaweeds; β-car = Beta carotene; Chl = Chlorophyll; Chls = Chlorophylls; Cars = Carotenoids; CD36= Cluster of differentiation 36; DW = Dry weight; EAE = Enzyme-assisted extraction; FAs = Fatty acids; FUCOL = Fucoxanthinol; FUCOX = Fucoxanthin; GIT = gastrointestinal tract; MAE = Microwave-assisted extraction; NPC1L1= Niemann-Pick C1-Like 1; OH = Hydroxyl group; (o) = oil; (o/w) = oil-in-water; PGE = Phloroglucinol equivalents; PGs = Photosynthetic pigments; Pheo = Pheophorbide; Phls = Phlorotannins; PLE = Pressurized liquid extraction; PPs = Polyphenols; SC-CO2 = Supercritical carbon dioxide; SGLT-1 = Sodium Glucose Transport Protein Transporter 1; SFE = Supercritical fluid extraction; UAE = Ultrasound-assisted extraction; (w) = water.
ACKNOWLEDGMENTS
The authors gratefully thank Professor Se-Kwon Kim (Hanyang University, Korea), for his support in preparing the manuscript.
AUTHOR CONTRIBUTIONS
Conceptualization: Ratih Pangestuti, Eko Susanto, Evi Amelia Siahaan; Writing original draft: Ratih Pangestuti, Eko Susanto, Evi Amelia Siahaan, Heli Siti Halimatul Munawaroh, Andriyanti Ningrum, Lukita Purnamayati; Review and editing: Ratih Pangestuti, Evi Amelia Siahaan; Supervision and final approval: Ratih Pangestuti. All authors have read and agreed to the published version of the manuscript.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data generated and analyzed are included within this report.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Pangestuti R, Shin KH, Kim SK. Anti-photoaging and potential skin health benefits of seaweeds. Mar Drugs. 2021;19(3):172. CrossRef
2. Pangestuti R, Kim SK. Biological activities and health benefit effects of natural pigments derived from marine algae. J Funct Foods. 2011;3(4):255–66. CrossRef
3. García-Poza S, Leandro A, Cotas C, Cotas J, Marques JC, Pereira L, et al. The evolution road of seaweed aquaculture: cultivation technologies and the industry 4.0. Int J Environ Res Public Health. 2020;17(18):6528. CrossRef
4. Wijesekara I, Pangestuti R, Kim SK. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr Polym. 2011;84(1):14–21. CrossRef
5. Pangestuti R, Siahaan EA, Kim SK. Photoprotective substances derived from marine algae. Mar Drugs. 2018;16(11):399. CrossRef
6. Murakami K, Yamaguchi Y, Noda K, Fujii T, Shinohara N, Ushirokawa T, et al. Seasonal variation in the chemical composition of a marine brown alga, Sargassum horneri (Turner) C. Agardh. J Food Compos Anal. 2011;24(2):231–6. CrossRef
7. Ferdouse F, Holdt SL, Smith R, Murúa P, Yang Z. The global status of seaweed production, trade and utilization. Globefish Res Programme. 2018;124:I.
8. Bouga M, Combet E. Emergence of seaweed and seaweed-containing foods in the UK: focus on labeling, iodine content, toxicity and nutrition. Foods. 2015;4(2):240–53. CrossRef
9. Chen K, Roca M. Cooking effects on bioaccessibility of chlorophyll pigments of the main edible seaweeds. Food Chem. 2019;295:101–9. CrossRef
10. Nova P, Martins AP, Teixeira C, Abreu H, Silva JG, Silva AM, et al. Foods with microalgae and seaweeds fostering consumers health: a review on scientific and market innovations. J Appl Phycol. 2020;32(3):1789–802. CrossRef
11. Pangestuti R, Kim SK. Neuroprotective effects of marine algae. Mar Drugs. 2011;9(5):803–18. CrossRef
12. Veena CK, Josephine A, Preetha SP, Varalakshmi P. Beneficial role of sulfated polysaccharides from edible seaweed Fucus vesiculosus in experimental hyperoxaluria. Food Chem. 2007;100(4):1552–9. CrossRef
13. Yesson C, Bush LE, Davies AJ, Maggs CA, Brodie J. The distribution and environmental requirements of large brown seaweeds in the British Isles. J Mar Biol Assoc. 2015;95(4):669–80. CrossRef
14. Gupta S, Abu-Ghannam N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci Technol. 2011;22(6):315–26. CrossRef
15. Pádua D, Rocha E, Gargiulo D, Ramos A. Bioactive compounds from brown seaweeds: phloroglucinol, fucoxanthin and fucoidan as promising therapeutic agents against breast cancer. Phytochem Lett. 2015;14:91–8. CrossRef
16. Miyashita K, Beppu F, Hosokawa M, Liu X, Wang S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch Biochem Biophys. 2020;686:108364. CrossRef
17. Aryee AN, Agyei D, Akanbi TO. Recovery and utilization of seaweed pigments in food processing. Curr Opin Food Sci. 2018;19:113–9. CrossRef
18. Susanto E, Fahmi AS, Abe M, Hosokawa M, Miyashita K. Lipids, fatty acids, and fucoxanthin content from temperate and tropical brown seaweeds. Aquat Procedia. 2016;7:66–75. CrossRef
19. Susanto E, Fahmi AS, Hosokawa M, Miyashita K. Variation in lipid components from 15 species of tropical and temperate seaweeds. Mar Drugs. 2019;17(11):630. CrossRef
20. Sanz-Pintos N, Pérez-Jiménez J, Buschmann AH, Vergara-Salinas JR, Pérez-Correa JR, Saura-Calixto F. Macromolecular antioxidants and dietary fiber in edible seaweeds. J Food Sci. 2017;82(2):289–95. CrossRef
21. Peñalver R, Lorenzo JM, Ros G, Amarowicz R, Pateiro M, Nieto G. Seaweeds as a functional ingredient for a healthy diet. Mar Drugs. 2020;18(6):301. CrossRef
22. Li Y-X, Wijesekara I, Li Y, Kim SK. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011;46(12):2219–4. CrossRef
23. Isaza Martínez JH, Torres Castañeda HG. Preparation and chromatographic analysis of phlorotannins. J Chromatogr Sci. 2013;51(8):825–38. CrossRef
24. Barbosa M, Valentão P, Ferreres F, Gil-Izquierdo Á, Andrade PB. In vitro multifunctionality of phlorotannin extracts from edible Fucus species on targets underpinning neurodegeneration. Food Chem. 2020;333:127456. CrossRef
25. Catarino MD, Silva AM, Cardoso SM. Fucaceae: a source of bioactive phlorotannins. Int J Mol Sci. 2017;18(6):1327. CrossRef
26. Bradbury LM, Shumskaya M, Tzfadia O, Wu SB, Kennelly EJ, Wurtzel ET. Lycopene cyclase paralog CruP protects against reactive oxygen species in oxygenic photosynthetic organisms. Proc Natl Acad Sci USA. 2012;109(27):E1888–97. CrossRef
27. Shibata T, Kawaguchi S, Hama Y, Inagaki M, Yamaguchi K, Nakamura T. Local and chemical distribution of phlorotannins in brown algae. J Appl Phycol. 2004;16(4):291–6. CrossRef
28. Catarino MD, Silva AM, Mateus N, Cardoso SM. Optimization of phlorotannins extraction from Fucus vesiculosus and evaluation of their potential to prevent metabolic disorders. Mar Drugs. 2019;17(3):162. CrossRef
29. Kim MM, Van Ta Q, Mendis E, Rajapakse N, Jung WK, Byun HG, et al. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006;79(15):1436–43. CrossRef
30. Ryu B, Jiang Y, Kim HS, Hyun JM, Lim SB, Li Y, et al. Ishophloroglucin A, a novel phlorotannin for standardizing the anti-α-glucosidase activity of Ishige okamurae. Mar Drugs. 2018;16(11):436. CrossRef
31. Kim SM, Jung YJ, Kwon ON, Cha KH, Um BH, Chung D, et al. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl Biochem Biotechnol. 2012;166:1843–55. CrossRef
32. Guler BA, Deniz I, Demirel Z, Yesil-Celiktas O, Imamoglu E. A novel subcritical fucoxanthin extraction with a biorefinery approach. Biochem Eng J. 2020;153:107403. CrossRef
33. Oliyaei N, Moosavi-Nasab M, Tamaddon AM, Tanideh N. Antidiabetic effect of fucoxanthin extracted from Sargassum angustifolium on streptozotocin-nicotinamide-induced type 2 diabetic mice. Food Sci Nutr. 2021;9(7):3521–9. CrossRef
34. Imbs T, Zvyagintseva T. Phlorotannins are polyphenolic metabolites of brown algae. Russ J Mar Biol. 2018;44(4):263–73. CrossRef
35. Wang J, Zhang M, Zhao S, Liu J, Hu X, editors. In vivo anti-tumor effect of Eckol, a phlorotannin component isolated from brown algae, associated with regulating dendritic cells in sarcoma 180 (S180) xenografts-bearing mice. In Proceedings for Annual Meeting of The Japanese Pharmacol Soc WCP2018 (The 18th World Congress of Basic and Clinical Pharmacology); Japanese Pharmacological Society, 2018. CrossRef
36. Gheda S, Naby MA, Mohamed T, Pereira L, Khamis A. Antidiabetic and antioxidant activity of phlorotannins extracted from the brown seaweed Cystoseira compressa in streptozotocin-induced diabetic rats. Environ Sci Pollut Res. 2021;28(18):22886–901. CrossRef
37. Hamed I, Özogul F, Özogul Y, Regenstein JM. Marine bioactive compounds and their health benefits: a review. Compr Rev Food Sci Food Saf. 2015;14(4):446–65. CrossRef
38. Kuczynska P, Jemiola-Rzeminska M, Strzalka K. Photosynthetic pigments in diatoms. Mar Drugs. 2015;13(9):5847–81. CrossRef
39. Büchel C. Light-harvesting complexes of diatoms: fucoxanthin-chlorophyll proteins. Photosynthesis in algae. Biochemical and physiological mechanisms. Amsterdam, The Netherlands: Springer; 2020. 441–57 pp. CrossRef
40. Cai JQ, Liu XM, Gao ZJ, Li LL, Wang H. Chlorophylls derivatives: photophysical properties, assemblies, nanostructures and biomedical applications. Mater Today. 2021;45:77–92. CrossRef
41. Chen K, Ríos JJ, Pérez-Gálvez A, Roca M. Comprehensive chlorophyll composition in the main edible seaweeds. Food Chem. 2017;228:625–33. CrossRef
42. Hayes M, Ferruzzi MG. Update on the bioavailability and chemopreventative mechanisms of dietary chlorophyll derivatives. Nutr Res. 2020;81:19–37. CrossRef
43. Alberte RS, Friedman AL, Gustafson DL, Rudnick MS, Lyman H. Light-harvesting systems of brown algae and diatoms. Isolation and characterization of chlorophyll ac and chlorophyll afucoxanthin pigment-protein complexes. J Biochim Biophys Acta Bioenerg. 1981;635(2):304–16. CrossRef
44. Roca M, Chen K, Pérez-Gálvez A. Chlorophylls. Handbook on natural pigments in food and beverages. Amsterdam, The Netherlands: Elsevier; 2016. 125–58 p. CrossRef
45. Olivares-Molina A, Fernández K. Comparison of different extraction techniques for obtaining extracts from brown seaweeds and their potential effects as angiotensin I-converting enzyme (ACE) inhibitors. J Appl Phycol. 2016;28:1295–302. CrossRef
46. Takaichi S. Carotenoids in algae: distributions, biosyntheses and functions. Mar Drugs. 2011;9(6):1101–8. CrossRef
47. Mezzomo N, Ferreira SR. Carotenoids functionality, sources, and processing by supercritical technology: a review. J Chem. 2016;2016. CrossRef
48. Balboa EM, Conde E, Moure A, Falqué E, Domínguez H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013;138(2-3):1764–85. CrossRef
49. Mikami K, Hosokawa M. Biosynthetic pathway and health benefits of fucoxanthin, an algae-specific xanthophyll in brown seaweeds. Int J Mol Sci. 2013;14(7):13763–81. CrossRef
50. Zorofchian Moghadamtousi S, Karimian H, Khanabdali R, Razavi M, Firoozinia M, Zandi K, et al. Anticancer and antitumor potential of fucoidan and fucoxanthin, two main metabolites isolated from brown algae. Sci World J. 2014;2014. CrossRef
51. Stahl W, Sies H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J Nutr. 1992;122(11):2161–6. CrossRef
52. Demmig-Adams B, Adams WW. Antioxidants in photosynthesis and human nutrition. Science. 2002;298(5601):2149–53. CrossRef
53. Asai A, Sugawara T, Ono H, Nagao A. Biotransformation of fucoxanthinol into amarouciaxanthin A in mice and HepG2 cells: formation and cytotoxicity of fucoxanthin metabolites. Drug Metab Dispos. 2004;32(2):205–11. CrossRef
54. Hosokawa M, Kudo M, Maeda H, Kohno H, Tanaka T, Miyashita K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells. Biochim Biophys Acta. 2004;1675(1-3):113–9. CrossRef
55. Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem Biophys Res Commun. 2005;332(2):392–7. CrossRef
56. Choi SJ, McClements DJ. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci Biotechnol. 2020;29:149–68. CrossRef
57. Corona G, Ji Y, Anegboonlap P, Hotchkiss S, Gill C, Yaqoob P, et al. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br J Nutr. 2016;115(7):1240–53. CrossRef
58. Keys A, Brugsch J. The distribution coefficients of porphyrins between ether and hydrochloric acid and applications to problems of quantitative separation. J Am Chem Soc. 1938;60(9):2135–9. CrossRef
59. Ferruzzi MG, Failla ML, Schwartz SJ. Assessment of degradation and intestinal cell uptake of carotenoids and chlorophyll derivatives from spinach puree using an in vitro digestion and Caco-2 human cell model. J Agric Food Chem. 2001;49(4):2082–9. CrossRef
60. Gandul-Rojas B, Gallardo-Guerrero L, Mi?nguez-Mosquera MI. Influence of the chlorophyll pigment structure on its transfer from an oily food matrix to intestinal epithelium cells. J Agric Food Chem. 2009;57(12):5306–14. CrossRef
61. Aparicio-Ruiz Rn, Gandul-Rojas B, Roca M. Pigment profile in non-Spanish olive varieties (Olea europaea L. Var. Coratina, Frantoio, and Koroneiki). J Agric Food Chem. 2009;57(22):10831–6. CrossRef
62. Hsu CY, Yeh TH, Huang MY, Hu SP, Chao PY, Yang CM. Organ-specific distribution of chlorophyll-related compounds from dietary spinach in rabbits. Biochem Biophys. 2014;51(5):388–95.
63. Chen K, Roca M. In vitro digestion of chlorophyll pigments from edible seaweeds. J Funct Foods. 2018;40:400–7. CrossRef
64. Sugawara T, Kushiro M, Zhang H, Nara E, Ono H, Nagao A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J Nutr. 2001;131(11):2921–7. CrossRef
65. Sangeetha RK, Bhaskar N, Divakar S, Baskaran VJ. Bioavailability and metabolism of fucoxanthin in rats: structural characterization of metabolites by LC-MS (APCI). Mol Cell Biochem. 2010;333:299–310. CrossRef
66. Sangeetha R, Bhaskar N, Baskaran V. Comparative effects of β-carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Mol Cell Biochem. 2009;331:59–67. CrossRef
67. Asai A, Yonekura L, Nagao A. Low bioavailability of dietary epoxyxanthophylls in humans. Br J Nutr. 2008;100(2):273–7. CrossRef
68. Zhang Y, Wu H, Wen H, Fang H, Hong Z, Yi R, et al. Simultaneous determination of fucoxanthin and its deacetylated metabolite fucoxanthinol in rat plasma by liquid chromatography-tandem mass spectrometry. Mar Drugs. 2015;13(10):6521–36. CrossRef
69. Ravi H, Baskaran V. Biodegradable chitosan-glycolipid hybrid nanogels: a novel approach to encapsulate fucoxanthin for improved stability and bioavailability. Food Hydrocoll. 2015;43:717–25. CrossRef
70. Ravi H, Kurrey N, Manabe Y, Sugawara T, Baskaran V. Polymeric chitosan-glycolipid nanocarriers for an effective delivery of marine carotenoid fucoxanthin for induction of apoptosis in human colon cancer cells (Caco-2 cells). Mater Sci Eng C. 2018;91:785–95. CrossRef
71. Mok IK, Lee JK, Kim JH, Pan CH, Kim SM. Fucoxanthin bioavailability from fucoxanthin-fortified milk: In vivo and in vitro study. Food Chem. 2018;258:79–86. CrossRef
72. Grune T, Lietz G, Palou A, Ross AC, Stahl W, Tang G, et al. β-Carotene is an important vitamin A source for humans. J Nutr. 2010;140(12):2268S–85S. CrossRef
73. Donhowe EG, Kong F. Beta-carotene: digestion, microencapsulation, and in vitro bioavailability. Food Bioproc Tech. 2014;7(2):338–54. CrossRef
74. Donhowe EG, Flores FP, Kerr WL, Wicker L, Kong F. Characterization and in vitro bioavailability of β-carotene: effects of microencapsulation method and food matrix. LWT-Food Sci Technol. 2014;57(1):42–8. CrossRef
75. Haskell MJ. The challenge to reach nutritional adequacy for vitamin A: β-carotene bioavailability and conversion—evidence in humans. Am J Clin Nutr. 2012;96(5):1193S–203S. CrossRef
76. Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci USA. 2002;99(24):15649–54. CrossRef
77. Viera I, Pérez-Gálvez A, Roca M. Bioaccessibility of marine carotenoids. Mar Drugs. 2018;16(10):397. CrossRef
78. Reboul E. Mechanisms of carotenoid intestinal absorption: where do we stand? Nutrients. 2019;11(4):838. CrossRef
79. Van Vliet T. Absorption of beta-carotene and other carotenoids in humans and animal models. Eur J Clin Nutr. 1996;50:S32–7.
80. During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr. 2005;135(10):2305–12. CrossRef
81. Widjaja-Adhi MAK, Lobo GP, Golczak M, Von Lintig J. A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Hum Mol Genet. 2015;24(11):3206–19. CrossRef
82. Vidhyalakshmi R, Bhakyaraj R, Subhasree R. Encapsulation “the future of probiotics”-a review. Adv Biol Res. 2009;3(3-4):96–103.
83. Bratovcic A, Suljagic J. Micro-and nano-encapsulation in food industry. Croat J Food Sci Technol. 2019;11(1):113–21. CrossRef
84. Bai Y, Sun Y, Gu Y, Zheng J, Yu C, Qi H. Preparation, Characterization and antioxidant activities of kelp phlorotannin nanoparticles. Molecules. 2020;25(19):4550. CrossRef
85. Ozkan G, Kostka T, Esatbeyoglu T, Capanoglu E. Effects of lipid-based encapsulation on the bioaccessibility and bioavailability of phenolic compounds. Molecules. 2020;25(23):5545. CrossRef
86. Elvira-Torales LI, García-Alonso J, Periago-Castón MJ. Nutritional importance of carotenoids and their effect on liver health: a review. Antioxidants. 2019;8(7):229. CrossRef
87. Cilek B, Luca A, Hasirci V, Sahin S, Sumnu G. Microencapsulation of phenolic compounds extracted from sour cherry pomace: effect of formulation, ultrasonication time and core to coating ratio. Eur Food Res Technol. 2012;235:587–96. CrossRef
88. Yang M, Liang Z, Wang L, Qi M, Luo Z, Li L. Microencapsulation delivery system in food industry—Challenge and the way forward. Adv Polym Technol. 2020;2020. CrossRef
89. Surendhiran D, Cui H, Lin L. Encapsulation of phlorotannin in alginate/PEO blended nanofibers to preserve chicken meat from Salmonella contaminations. Food Packag Shelf Life. 2019;21:100346. CrossRef
90. Pisoschi AM, Pop A, Cimpeanu C, Turcu? V, Predoi G, Iordache F. Nanoencapsulation techniques for compounds and products with antioxidant and antimicrobial activity-a critical view. Eur J Med Chem. 2018;157:1326–45. CrossRef
91. Sun X, Xu Y, Zhao L, Yan H, Wang S, Wang D. The stability and bioaccessibility of fucoxanthin in spray-dried microcapsules based on various biopolymers. RSC Adv. 2018;8(61):35139–49. CrossRef
92. Li Y, Dou X, Pang J, Liang M, Feng C, Kong M, et al. Improvement of fucoxanthin oral efficacy via vehicles based on gum Arabic, gelatin and alginate hydrogel: delivery system for oral efficacy enhancement of functional food ingredients. J Funct Foods. 2019;63:103573. CrossRef
93. Greiner R, Roohinejad S, Oey I, Wen J. Emulsion-based systems for delivery of food active compounds: formation, application, health and safety. 2018. CrossRef
94. Kumar M, Bishnoi RS, Shukla AK, Jain CP. Techniques for formulation of nanoemulsion drug delivery system: a review. Prev Nutr Food Sci. 2019;24(3):225. CrossRef
95. Donsì F, Sessa M, Mediouni H, Mgaidi A, Ferrari G. Encapsulation of bioactive compounds in nanoemulsion-based delivery systems. Procedia Food Sci. 2011;1:1666–71. CrossRef
96. Odriozola-Serrano I, Oms-Oliu G, Martín-Belloso O. Nanoemulsion-based delivery systems to improve functionality of lipophilic components. Front Nutr. 2014;1:24. CrossRef
97. Liu Q, Huang H, Chen H, Lin J, Wang Q. Food-grade nanoemulsions: Preparation, stability and application in encapsulation of bioactive compounds. Molecules. 2019;24(23):4242. CrossRef
98. Qian C, Decker EA, Xiao H, McClements DJ. Nanoemulsion delivery systems: influence of carrier oil on β-carotene bioaccessibility. Food Chem. 2012;135(3):1440–7. CrossRef
99. Castro-López C, Sánchez-Alejo EJ, Saucedo-Pompa S, Rojas R, Aranda-Ruiz J, Martínez-Avila GCG. Fluctuations in phenolic content, ascorbic acid and total carotenoids and antioxidant activity of fruit beverages during storage. Heliyon. 2016;2(9):e00152. CrossRef
100. Meng Q, Long P, Zhou J, Ho CT, Zou X, Chen B, et al. Improved absorption of β-carotene by encapsulation in an oil-in-water nanoemulsion containing tea polyphenols in the aqueous phase. Food Res Int. 2019;116:731–6. CrossRef
101. López-Monterrubio D, Lobato-Calleros C, Vernon-Carter E, Alvarez-Ramirez. Influence of β-carotene concentration on the physicochemical properties, degradation and antioxidant activity of nanoemulsions stabilized by whey protein hydrolyzate-pectin soluble complexes. Lwt. 2021;143:111148. CrossRef
102. Jalali-Jivan M, Fathi-Achachlouei B, Ahmadi-Gavlighi H, Jafari SM. Improving the extraction efficiency and stability of β-carotene from carrot by enzyme-assisted green nanoemulsification. Innov Food Sci Emerg Technol. 2021;74:102836. CrossRef
103. Zhou X, Wang H, Wang C, Zhao C, Peng Q, Zhang T, et al. Stability and in vitro digestibility of beta-carotene in nanoemulsions fabricated with different carrier oils. Food Sci Nutr. 2018;6(8):2537–44. CrossRef
104. Cotas J, Leandro A, Monteiro P, Pacheco D, Figueirinha A, Gonçalves AM, et al. Seaweed phenolics: from extraction to applications. Mar Drugs. 2020;18(8):384. CrossRef
105. Carreira-Casais A, Otero P, Garcia-Perez P, Garcia-Oliveira P, Pereira AG, Carpena M, et al. Benefits and drawbacks of ultrasound-assisted extraction for the recovery of bioactive compounds from marine algae. Int J Environ Res Public Health. 2021;18(17):9153. CrossRef
106. Gan A, Baroutian S. Current status and trends in extraction of bioactives from brown macroalgae using supercritical CO2 and subcritical water. J Chem Technol Biot. 2022;97(8):1929–40. CrossRef
107. Cikoš AM, Joki? S, Šubari? D, Jerkovi? I. Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae. Mar Drugs. 2018;16(10):348. CrossRef
108. Siahaan EA, Chun BS. Innovative alternative technology for fucoxanthin recovery. In: Kim SK, editor. Encyclopedia of Marine Biotechnology. Hoboken, NJ: John Wiley & Sons Ltd; 2020. 3213–27 pp. CrossRef
109. Leong T, Johansson L, Juliano P, McArthur SL, Manasseh R. Ultrasonic separation of particulate fluids in small and large scale systems: a review. Ind Eng Chem Res. 2013;52(47):16555–76. CrossRef
110. Getachew AT, Jacobsen C, Holdt SL. Emerging technologies for the extraction of marine phenolics: opportunities and challenges. Mar Drugs. 2020;18(8):389. CrossRef
111. Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason Sonochem. 2017;34:540–60. CrossRef
112. Dang TT, Van Vuong Q, Schreider MJ, Bowyer MC, Van Altena IA, Scarlett CJ. Optimisation of ultrasound-assisted extraction conditions for phenolic content and antioxidant activities of the alga Hormosira banksii using response surface methodology. J Appl Phycol. 2017;29:3161–73. CrossRef
113. Agregán R, Munekata PE, Franco D, Carballo J, Barba FJ, Lorenzo JM. Antioxidant potential of extracts obtained from macro-(Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and micro-algae (Chlorella vulgaris and Spirulina platensis) assisted by ultrasound. Medicines. 2018;5(2):33. CrossRef
114. Kumar Y, Singhal S, Tarafdar A, Pharande A, Ganesan M, Badgujar PC. Ultrasound assisted extraction of selected edible macroalgae: Effect on antioxidant activity and quantitative assessment of polyphenols by liquid chromatography with tandem mass spectrometry (LC-MS/MS). Algal Res. 2020;52:102114. CrossRef
115. Vázquez-Rodríguez B, Gutiérrez-Uribe JA, Antunes-Ricardo M, Santos-Zea L, Cruz-Suárez LE. Ultrasound-assisted extraction of phlorotannins and polysaccharides from Silvetia compressa (Phaeophyceae). J Appl Phycol. 2020;32:1441–53. CrossRef
116. Hassan IH, Pham HNT, Nguyen TH. Optimization of ultrasound-assisted extraction conditions for phenolics, antioxidant, and tyrosinase inhibitory activities of Vietnamese brown seaweed (Padina australis). J Food Process Preserv. 2021;45(5):e15386. CrossRef
117. Gomez L, Tiwari B, Garcia-Vaquero M. Emerging extraction techniques: microwave-assisted extraction. Sustainable Seaweed Technologies. Amsterdam, The Netherlands: Elsevier; 2020. 207–4 pp. CrossRef
118. Veggi P, Martinez J, Angela M, Meireles AJ. Chapter 2: fundamentals of microwave extraction. In: Chemat F, Cravotto G, editor. Microwave-assisted extraction for bioactive compounds: theory and practice. Food Engineering Series; 2013. CrossRef
119. Magnusson M, Yuen AK, Zhang R, Wright JT, Taylor RB, Maschmeyer T, et al. A comparative assessment of microwave assisted (MAE) and conventional solid-liquid (SLE) techniques for the extraction of phloroglucinol from brown seaweed. Algal Res. 2017;23:28–36. CrossRef
120. Amarante SJ, Catarino MD, Marçal C, Silva AM, Ferreira R, Cardoso SM. Microwave-assisted extraction of phlorotannins from Fucus vesiculosus. Mar Drugs. 2020;18(11):559. CrossRef
121. Daglia M. Polyphenols as antimicrobial agents. Curr Opin Biotechnol. 2012;23(2):174–81. CrossRef
122. Xiao X, Si X, Yuan Z, Xu X, Li G. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J Sep Sci. 2012;35(17):2313–7. CrossRef
123. Pangestuti R, Siahaan EA. Seaweed-derived carotenoids. Bioactive Seaweeds for Food Applications. Cambridge, MA: Academic Press; 2018. 95–107 pp. CrossRef
124. Topuz B, Günal G, Guler S, Aydin HM. Use of supercritical CO2 in soft tissue decellularization. Methods Cell Biol. 2020;157:49–79. CrossRef
125. Matos GS, Pereira SG, Genisheva ZA, Gomes AM, Teixeira JA, Rocha CM. Advances in extraction methods to recover added-value compounds from seaweeds: sustainability and functionality. Foods. 2021;10(3):516. CrossRef
126. Mussatto SI. Generating biomedical polyphenolic compounds from spent coffee or silverskin. Coffee in health and disease prevention. Amsterdam, The Netherlands: Elsevier; 2015. 93–106 pp. CrossRef
127. Yin S, Woo HC, Choi JH, Park YB, Chun BS. Measurement of antioxidant activities and phenolic and flavonoid contents of the brown seaweed Sargassum horneri: comparison of supercritical CO 2 and various solvent extractions. Fish Aquatic Sci. 2015;18(2):123–30. CrossRef
128. Periaswamy Sivagnanam S, Yin S, Choi JH, Park YB, Woo HC, Chun BS. Biological properties of fucoxanthin in oil recovered from two brown seaweeds using supercritical CO2 extraction. Mar Drugs. 2015;13(6):3422–42. CrossRef
129. Saravana PS, Getachew AT, Cho YJ, Choi JH, Park YB, Woo HC, et al. Influence of co-solvents on fucoxanthin and phlorotannin recovery from brown seaweed using supercritical CO2. J Supercritic Fluids. 2017;120:295–303. CrossRef
130. Getachew AT, Saravana PS, Cho YJ, Woo HC, Chun BS. Concurrent extraction of oil from roasted coffee (Coffea arabica) and fucoxanthin from brown seaweed (Saccharina japonica) using supercritical carbon dioxide. J CO2 Util. 2018;25:137–46. CrossRef
131. Sun M, Temelli F. Supercritical carbon dioxide extraction of carotenoids from carrot using canola oil as a continuous co-solvent. J Supercritic Fluids. 2006;37(3):397–408. CrossRef
132. Krichnavaruk S, Shotipruk A, Goto M, Pavasant P. Supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis with vegetable oils as co-solvent. Bioresour Technol. 2008;99(13):5556–60. CrossRef
133. Meng Y, Anderson J. Use of unconventional solvents for sample preparation in Environmental Analysis. 2012; 3. CrossRef
134. Turner C, Ibañez E. Pressurized hot water extraction and processing. In: Lebovka N, Vorobiev E, Chemat F, Editors. Enhancing Extraction Processes in the Food Industry. Boca Raton, FL: CRC Press; 2011. 223–54 pp. CrossRef
135. Meillisa A, Siahaan EA, Park JN, Woo HC, Chun BS. Effect of subcritical water hydrolysate in the brown seaweed Saccharina japonica as a potential antibacterial agent on food-borne pathogens. J Appl Phycol. 2013;25:763–9. CrossRef
136. Bordoloi A, Goosen NJ. A greener alternative using subcritical water extraction to valorize the brown macroalgae Ecklonia maxima for bioactive compounds. J Appl Phycol. 2020;32:2307–19. CrossRef
137. Mari? M, Grassino AN, Zhu Z, Barba FJ, Brn?i? M, Brn?i? SR. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci Technol. 2018;76:28–37. CrossRef
138. Gligor O, Mocan A, Moldovan C, Locatelli M, Cri?an G, Ferreira IC. Enzyme-assisted extractions of polyphenols–A comprehensive review. Trends Food Sci Technol. 2019;88:302–15. CrossRef
139. Aminina NM, Karaulova EP, Vishnevskaya TI, Yakush EV, Kim YK, Nam KH, et al. Characteristics of polyphenolic content in brown algae of the pacific coast of Russia. Molecules. 2020;25(17):3909. CrossRef
140. Gümü? Y?lmaz G, Gómez Pinchetti JL, Cifuentes A, Herrero M, Ibáñez E. Comparison of extraction techniques and surfactants for the isolation of total polyphenols and phlorotannins from the brown algae lobophora variegata. Anal Lett. 2019;52(17):2724–40. CrossRef
141. K. Habeebullah SF, Alagarsamy S, Sattari Z, Al-Haddad S, Fakhraldeen S, Al-Ghunaim A, et al. Enzyme-assisted extraction of bioactive compounds from brown seaweeds and characterization. J Appl Phycol. 2020;32:615–29. CrossRef
142. Del Pilar Sánchez-Camargo A, Montero L, Stiger-Pouvreau V, Tanniou A, Cifuentes A, Herrero M, et al. Considerations on the use of enzyme-assisted extraction in combination with pressurized liquids to recover bioactive compounds from algae. Food Chem. 2016;192:67–74. CrossRef
143. Leandro A, Pacheco D, Cotas J, Marques JC, Pereira L, Gonçalves AM. Seaweed’s bioactive candidate compounds to food industry and global food security. Life 2020;10(8):140. CrossRef
144. Apostolidis E, Lee CM. Brown seaweed-derived phenolic phytochemicals and their biological activities for functional food ingredients with focus on Ascophyllum nodosum. In: Kim SK, editor. Handbook of marine macroalgae: Biotechnologyapplied phycology; 2011. 356–70 pp. CrossRef
145. Miyashita K, Hosokawa M. Therapeutic effect of fucoxanthin on metabolic syndrome and type 2 diabetes. Nutritional and therapeutic interventions for diabetes and metabolic syndrome. Amsterdam, The Netherlands: Elsevier; 2018. 343–55 pp. CrossRef
146. Abu-Ghannam N, Shannon E. Seaweed carotenoid, fucoxanthin, as functional food. Hoboken, NY: John Wiley & Sons Ltd; 2017. 39–64 pp. CrossRef
147. Zahrah Z, Amin M, Alamsjah M, editors. The effect of fucoxanthin as coloring agent on the quality of Shrimp Paste. In IOP Conference Series: Earth and Environmental Science; 2020. IOP Publishing. CrossRef
148. Choi HS, Jeon HJ, Lee OH, Lee BY. Seanol® and its major compound, dieckol suppress lipid accumulation during adipogenesis through inhibition of mitotic clonal expansion (MCE) and cell cycle arrest. FASEB J. 2013. CrossRef
149. Douglas TE, Dokupil A, Reczy?ska K, Brackman G, Krok-Borkowicz M, Keppler JK, et al. Enrichment of enzymatically mineralized gellan gum hydrogels with phlorotannin-rich Ecklonia cava extract seanol® to endow antibacterial properties and promote mineralization. Biomed Mater. 2016;11(4):045015. CrossRef
150. Han JY, Choi JL, Goh RY, Lim HH, Park JI. Activation mediated by peroxisome proliferator-activated receptor (PPAR) ligands and brown algae-based compounds: different mechanisms between megakaryoblasts and mature platelets. Blood. 2017;130:4832.
151. Jeon HJ, Choi HS, Lee YJ, Hwang JH, Lee OH, Seo MJ, et al. Seapolynol extracted from Ecklonia cava inhibits adipocyte differentiation in vitro and decreases fat accumulation in vivo. Molecules. 2015;20(12):21715–31. CrossRef
152. Jeon HJ, Yoon KY, Koh EJ, Choi J, Kim KJ, Choi HS, et al. Seapolynol and dieckol improve insulin sensitivity through the regulation of the PI3K pathway in C57BL/KsJ-db/db mice. J Food Nutr Res. 2015;3(10):648–52. CrossRef
153. Kim KJ, Song JH, Koh EJ, Choi J, Lee YJ, Hwang JH, et al. Seapolynol attenuates lipid accumulation during adipocyte differentiation in 3T3-L1 and decreases body fat accumulation in vivo. FASEB J. 2016;30:lb252. CrossRef
154. Lee YJ, Choi J, Koh EJ, Hwang JH, Seo YJ, Song JH, et al. Anti-diabetic effect of Seapolynol™ in 3T3-L1 adipocytes and C57BL/KsJ-db/db mice. FASEB J. 2016;30:lb255. CrossRef
155. Yeo AR, Lee J, Tae IH, Park SR, Cho YH, Lee BH, et al. Anti-hyperlipidemic effect of polyphenol extract (Seapolynol™) and dieckol isolated from Ecklonia cava in in vivo and in vitro models. Prev Nutr Food Sci. 2012;17(1):1. CrossRef
156. Terpend K, Bisson JF, Gall CL, Linares E. Effects of ID-alG™ on weight management and body fat mass in high-fat-fed rats. Phytother Res. 2012;26(5):727–33. CrossRef
157. Hope N. Diana naturals introduces new phytonutriance® healSea (TM) cardiovascular ingredient from fucus [cited 2007 Nov 26]. Available from: https://www.newhope.com/managing-your-business/diana-naturals-introduces-new-phytonutriance-healseatm-cardiovascular-ingredi.
158. Zaragozá M, López D, P. Sa?iz M, Poquet M, Pérez J, Puig-Parellada P, et al. Toxicity and antioxidant activity in vitro and in vivo of two Fucus vesiculosus extracts. J Agric Food Chem. 2008;56(17):7773–80. CrossRef
159. Amazon. Brown seaweed extract 500mg - 200 veggie capsules- 200 day supply (100% vegetarian, non-GMO & gluten free) - fucoidan - supports healthy immune system [cited 2021 Jun 1]. Available from: https://www.amazon.com/Seaweed-Extract-Capsules-NON-GMO-Gluten/dp/B079VWF5KM.
160. Amazon. Brown seaweed extract capsules 2000 mg fucoxanthin supplement Non-GMO gluten free [cited 2021 Jun 12]. Available from: https://www.amazon.com/Seaweed-Capsules-Fucoxanthin-Supplement-Horbaach/dp/B07VQ39PXR/ref=sr_1_1_sspa?dchild=1&keywords=fucoxanthin&qid=1623415479&sr=8-1-spons&psc=1&spLa=ZW5jcnlwdGVkUXVhbGlmaWVyPUE3WjVEWDQ4OVZaQTQmZW5jcnlwdGVkSWQ9QTA0MTA2ODMxSzBOM1JXVVRHMlo1JmVuY3J5cHRlZEFkSWQ9QTA0MjE3NzAzUk42V1FSU01QWlc1JndpZGdldE5hbWU9c3BfYXRmJmFjdGlvbj1jbGlja1JlZGlyZWN0JmRvTm90TG9nQ2xpY2s9dHJ1ZQ==.
161. eVitamins. Only natural brown seaweed plus [cited 2021 June 12]. Available from: https://www.evitamins.com/brown-seaweed-plus-only-natural-34930.
162. iHerb. Quality of life labs, fucoxanthin MD [cited 2014 Dec 18]. Available from: https://id.iherb.com/pr/quality-of-life-labs-fucoxanthin-md-30-liquid-veggie-caps/58425.
163. eVitamins. Solaray fucoxanthin special formula [cited 2021 Jun 12]. Available from: https://www.evitamins.com/in/fucoxanthin-special-formula-solaray-36205.
164. Amazon. Garden of life fucoxanthin supplements - fucothin green diet pill for weight loss with green coffee bean extract [cited 2021 Jun 12]. Available from: https://www.amazon.com/Garden-Life-Fucoxanthin-Supplements-FucoThin/dp/B00AJNON3S/ref=sr_1_9?dchild=1&keywords=fucoxanthin&qid=1623415479&sr=8-9.
165. Amazon. Garden of life fucoxanthin supplements - fucothin diet pill for weight loss [cited 2021 Jun 12]. Available from: https://www.amazon.com/Garden-Life-Fucoxanthin-Supplements-FucoThin/dp/B00280M102/ref=sr_1_6?dchild=1&keywords=fucoxanthin&qid=1623415479&sr=8-6.
166. Amazon. Best naturals #1 fucoxanthin with fucoplast blend [cited 2021 Jun 12]. Available from: https://www.amazon.com/Best-Naturals-Fucoxanthin-Fucoplast-Capsules/dp/B006QSZJ3I/ref=sr_1_5?dchild=1&keywords=fucoxanthin&qid=1623415479&sr=8-5.
167. Evans H, Plummer C, Luck M, Mcinerney REP, Burns LM, inventorsAnti-viral composition2020.
168. Martins Da Silva JR, Da Maia Alves CM, Goncalves Pinteus SF, Mendes Martins AI, Freire Freitas RP, Pinto Pedrosa RF, InventorsProcess of obtaining a phlorotannin-enriched extract having anti-enzymatic action for use in dermatology2021.
169. McClements DJ, Decker EA, Weiss DJ. Emulsion-based delivery systems for lipophilic bioactive components. J Food Sci. 2007;72(8):R109–R24. CrossRef