INTRODUCTION
Crinum asiaticum Linn. (C. asiaticum) (Amaryllidaceae family) is found in tropical and subtropical regions worldwide. It has long been utilized to treat illnesses related to pain, inflammation, wounds treatment, swellings, pain injuries, and inflamed joints, and as an antidote for poisons or toxins in Southeast Asian and Thai ethnomedicine (Kongkwamcharoen et al., 2021; Ongthanasup et al., 2020; Sharma et al., 2020a, 2020b). In traditional Thai medicine, C. asiaticum leaf is used to treat inflammatory joints, ankle discomfort, and postpartum care (Pholhiamhan et al., 2018). Massage and C. asiaticum hot leaf compresses are used in Thai traditional medicine to treat musculoskeletal discomfort (Dhippayom et al., 2015; Pichiansunthorn et al., 2001). The leaf extract of C. asiaticum had various phytochemicals, including alkaloids, phenolics, terpenoids, and aldehydes (Mahomoodally et al., 2021). Previous research on the bioactivity of C. asiaticum leaves has been published, including anti-inflammatory and pain-relieving effects (Gasca-Silva et al., 2022; Ge et al., 2020; Rahman et al., 2013; Sharma et al., 2020a, 2020b). The major active compound in C. asiaticum leaf is lycorine, which exerts potent anti-inflammatory activities (Ji et al., 2013; Sharma et al., 2020a, 2020b).
The transdermal patch is a drug delivery system that delivers drugs locally or into the bloodstream. It has advantages over other types of dermal drug delivery systems in that it can be administered at high doses and can adhere to the skin for a long time. The transdermal patch consists of three components (the patch matrix, backing layer, and adhesive) and four major types of transdermal patch.
1) Drug in a matrix type: The active pharmaceutical ingredients are directly loaded into the film patch material and the matrix is covered with adhesive and a backing layer.
2) Drug in adhesive type: The drug is loaded in a self-adhesive polymer and covered with a backing layer.
3) Drug in reservoir type: This type combines the drug in a matrix dispersion with a porous polymeric membrane for release rate control.
4) Multilamellar type: There are several layers of a drug-loaded matrix with a membrane in between the layers. This type could provide an initial burst drug release followed by sustained release (Sharadha et al., 2020). Therefore, it is suitable to relieve pain or swelling of muscles (Pastore et al., 2015).
There are three main components of the polymeric film of transdermal patch: polymer, plasticizer, and penetration enhancer (Monika et al., 2012). Several polymers can be incorporated into the film, such as polyvinyl alcohol, hydroxypropyl methacrylate, pectin, polyvinyl pyrrolidone, or the polymer blend (Valenta and Auner, 2004). A plasticizer is added to improve plasticity and flexibility; some common plasticizers in pharmaceuticals such as polyethylene glycol, propylene glycol, and dibutyl phthalate (Goswami and Audett, 2015). Dibutyl phthalate is commonly used as a plasticizer. However, it has been recognized as a possible endocrine disruptor (Heng et al., 2012). Penetration enhancers such as dimethyl sulfoxide, polyethylene glycols, ethanol, and propylene glycol were used to improve skin release of the drug from transdermal patches (Ita, 2020; Suksaeree et al., 2021). Polyethylene glycol 400 (PEG-400) is chosen as a plasticizer for this research as it has low toxicity and acts as a penetration enhancer.
The objectives of this study were to develop a formulation of a transdermal patch containing C. asiaticum leaf extract, including the analysis of physicochemical properties, stability, and the release of essential substances.
MATERIALS AND METHOD
Preparation of C. asiaticum leaf extract
The extraction method was modified from Kongkwamcharoen et al. (2021). Crinum asiaticum leaves were rinsed thoroughly with water and drained off. The leaves were cut into 1-inch pieces and dried in a hot air oven at 40°C for 12 hours. Dry leaves were ground into powder. An amount of 500 mg of powder was macerated in 600 ml of ethanol for 3 days and filtered through filter paper No.1 with a cut-off of 11 mm (Whatman®, GE Healthcare UK Limited, Buckinghamshire, UK). The extraction processes were repeated three times. The extract was pooled and evaporated with a rotary evaporator (Rotavapor® R210, Büchi, Essen, Germany).
Characterization of C. asiaticum leaf extract
Lycorine content assay
Preparation of standard and sample solutions
The standard and sample preparations were adapted from the method reported by Kongkwamcharoen et al. (2021). A precisely weighted lycorine powder was dissolved in methanol to produce a stock standard solution with a 0.5 mg/ml concentration. The stock solution was serially diluted to concentrations of 50, 100, 150, 200, 300, and 500 g/ml for calibration. Each C. asiaticum leaf extract was diluted in methanol to yield a sample solution with about 10 mg/ml of lycorine.
Chromatographic conditions
Determining lycorine in the crude extract was conducted with a minor adaptation of the method reported by Kongkwamcharoen et al. (2021). An high performance liquid chromatography (HPLC) system with a diode array detector (Ultimate 3000, Thermo Scientific, Karlsruhe, Germany) was used to analyze lycorine. The column was C18 reverse-phase 4.6 mm i.d. × 250 mm length (HypersilTM ODS, Thermo Fisher Scientific Inc., MA, USA). The binary gradient mobile phase consisted of (A) 0.1%v/v triethylamine in water and (B) acetonitrile. pH of mobile phase A was adjusted to 3.0 with phosphoric acid. The ratio of mobile phase A:B was programmed as follows: 0–5 minutes, 95%:5%; 30 minutes, 70%:30%; 30.1–35 minutes, 20%:80%; 35.1–40 minutes, 95%:5%. The flow rate was set at 1 ml/minute. The injection volume was 10 μl. The diode array detector wavelength was 290 nm.
Preparation of C. asiaticum leaf extract transdermal patch
Transdermal patches were prepared using the solvent-casting method. The transdermal patches formulation consisted of hydroxypropyl methylcellulose (HPMC) K4M, polyvinyl alcohol (PVA), PEG-400, ethanol, and C. asiaticum leaf extract. The HPMC to PVA ratios were varied at 1:1, 1:0.8, 1:0.5, and 1:0 for formulation F1–F12. Formulations F13 to F15 were PVA only (Table 1). All ingredients were dissolved in water and stirred with a magnetic stirrer (C-MAG HS 7, IKA Works GmbH & Co., Staufen, Germany) for 30 minutes. Then, 15 g viscous solution was poured into a 10 cm Petri dish. The Petri dish was dried in a hot air oven at 50°C for 8 hours. The dried film was removed from the Petri dish, wrapped in aluminum foil, placed in a zip bag, and kept in a desiccator. The optimal formulation will be loaded with C. asiaticum leaf extract equivalent to 10 mg of lycorine.
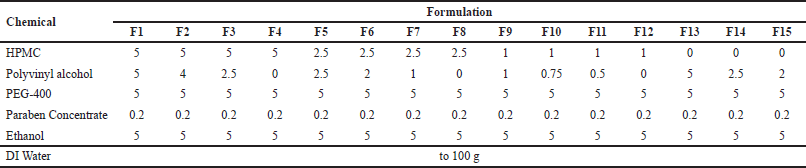 | Table 1. Composition of formulation F1–F15. [Click here to view] |
Evaluation of C. asiaticum leaf extract transdermal patch
Physical appearance
The physical appearance of transdermal patches was evaluated by observation of the roughness and consistency of the film. The thickness of the transdermal patch was measured using a vernier caliper at three different locations. The transdermal patches were cut into 1 cm2 in size and measured the weight using an analytical balance.
Moisture absorption
The transdermal patches of 1 cm2 were weighed and put in a controlled humidity chamber at 75% ± 5% RH at 25°C using saturated sodium chloride solution for 24 hours. The transdermal patches were weighed and calculated for percentage moisture absorption.
Tensile strength
The transdermal patches were cut into pieces as described in ASTM E8 standard tensile strength test specimen. The specimens were measured for tensile strength using a texture analyzer (LR5K, Lloyd instrument, West Sussex, UK) with a pulling speed of 50 mm/minute.
Folding endurance
The folding endurance test was conducted on the final optimization of the formulation. The test procedure of Singh and Bali (2016) was slightly modified. The film was cut into a 2 × 2 cm strip. The folding endurance was performed manually by repeatedly folding the film strip at the same location up to 500 times or until it broke.
In vitro drug release testing and mathematical modeling of release kinetics
In vitro drug release testing was evaluated using a modified Franz diffusion cell (Model FDC-6, Logan Instrument Corp., Somerset, NJ) with an effective diffusion area of 1.77 cm2. Transdermal patches containing C. asiaticum leaf extract were placed on top of the polyimide membrane in the donor compartment. The receiver medium was 30% ethanol, and the temperature was maintained at 32°C. 1 ml samples were drawn from the receiver medium and replaced with a 1 ml fresh receiver medium at the predefined periods of 0, 1, 2, 4, 6, and 8 hours. All collected samples were then filtered through a 0.45 mm membrane and analyzed using HPLC to determine the amount of lycorine release. All HPLC analyses were performed in triplicate. The regression analysis was performed to evaluate the drug release kinetics by fitting the amount of drug release to the following mathematical models: zero-order, first-order, Korsmeyer–Peppas, and Higuchi model.
Statistical analysis
All experimental data were presented as the mean ± standard deviation (SD). Analysis of variance (ANOVA) and Tukey’s honestly significant difference for post hoc analysis were used to evaluate the difference between groups. The correlation between parameters was performed using linear regression. Differences were considered statistically significant at p < 0.05.
RESULTS AND DISCUSSION
Lycorine content assay
Lycorine standard peak and lycorine in C. asiaticum peak were found at about 9.2 minutes (Fig. 1). Lycorine content in the dry crude extract was 9.53 mg/g of dry crude extract. The result was much lower than around 17–34 mg/g of dry crude extract reported by Kongkwamcharoen et al. (2021). Several factors might affect the lycorine content in C. asiaticum leaves extracts, such as cultivation conditions and pre-extraction treatment (Kongkwamcharoen et al., 2021).
Physical properties of transdermal patches
The formulation F1–F4 with 5% HPMC resulted in a hard thick, rough inflexible film. The formulation F5–F8 with 2.5% HPMC resulted in a moderately rough and flexible film. The formulation F9-F12 was not able to form consistent films due to the total polymer content being too low. The flexible clear films were formed with formulations F13–F15 that consisted of 5%, 2.5%, and 2% of PVA, respectively.
The thickness of transdermal patches ranged from 0.3 to 1.2 mm. The weight of transdermal patches per square centimeter ranged from 0.05 to 0.12 mg (Table 2). The thickness of the transdermal patches directly correlated with the total polymer content in the formulation (R2 = 0.743). In addition, the effect of HPMC and PVA was significant in the correlation model (Thickness ~ HPMC + PVA). However, the weight of the transdermal patches had a weak relation to the total polymer content in the formulation (R2 = 0.605).
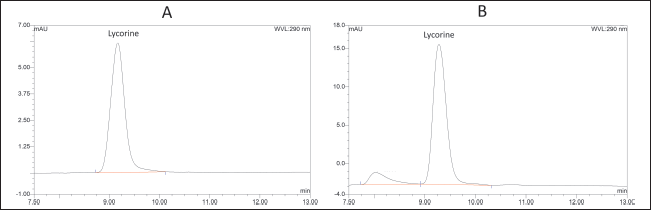 | Figure 1. HPLC chromatogram of (A) lycorine standard and (B) lycorine in C. asiaticum leaf extract. [Click here to view] |
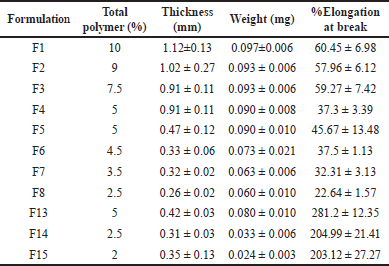 | Table 2. Thickness, weight, and elongation at break (%) of the formulation F1–F15. [Click here to view] |
All formulations were measured for their tensile strength except formulations F9–F12 as they were not able to form consistent films. The tensile of formulations F1–F4 was 28–38 kg/cm2 and not statically different. The tensile of formulations F5–F8 ranged from 13 to 25 kg/cm2 and had no statistical difference. The PVA-only group formulation F13–F15 showed different tensile characteristics. The tensile of formulation F13 was far higher than the other formulation, while the tensile of formulations F14 and F15 was 25–40 kg/cm2 (Fig. 2).
Linear regression was used to evaluate the relationship between PVA, HPMC, and tensile strength. Three regression models were applied; tensile ~ PVA + HPMC, tensile ~ PVA, and tensile ~ HPMC had R2 of 0.61, 0.51, and 0.09, respectively. The independent variable PVA was strongly significant in all models with p-value <0.01.
The formulations with 5% HPMC (F1–F4) showed that the percentage elongation at break for the formulation with PVA was around 60%, while formulation F4 resulted in a significantly lower percentage elongation at break at 37%. Likewise, formulations F5–F8 with 2.5% HPMC and the formulation with PVA percentage elongation at break for formulation with PVA were around 32%–46%. The formulation F8 that PVA was excluded from the formulation exhibited a significantly lower percentage elongation at a break of about 22%. Interestingly, formulations F13–F15 with the absence of HPMC substantially increased percentage elongation at break to around 200%–280%. Thus, F13–F15 were candidates for further formulation optimization.
Regression analysis was performed with the model Elongation ~ HPMC + PVA. The model fitted with R² = 0.77. The regression coefficients of HPMC and PVA were −32.41 and 22.84, respectively (p < 0.05). These results implied that HPMC had negative effects on transdermal patch physical properties when blended with PVA. The phase separation between PVA and HPMC could occur (Saringat et al., 2005).
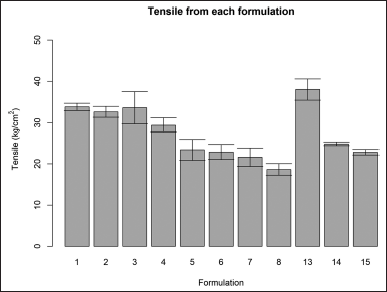 | Figure 2. Tensile of formulation F1–F15 (mean ± SD). [Click here to view] |
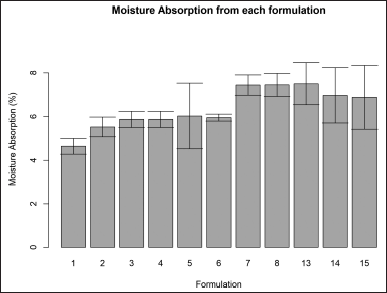 | Figure 3. Moisture absorption for formulation F1–F15 (mean ± SD). [Click here to view] |
Moisture absorption
Moisture absorption ranged from 4.3% to 8.5% (Fig. 3). The ANOVA revealed a statistical difference between formulations with a p-value of 0.006. The post hoc analysis showed that formulation F1 had the lowest moisture absorption among other formulations significantly.
The moisture absorption test shows how much water the patch absorbs from body tissues and the surroundings throughout the application period. High moisture absorption of the patch would result in a negative impact on the mechanical integrity of the patch. All formulations had acceptable moisture absorption properties.
Effect of PEG-400 on physical properties of transdermal patches
Formulation F13 results in the best physical properties. Optimization of plasticizer was conducted by various amounts of PEG-400 in the formulation from 1% to 10% (Table 3). The PVA weight was fixed at 2%. The weights of each formulation range from 9.6 to 33.1 mg/cm2. The moisture absorption ranged from about 1.7% to 9% (Table 3). The weight and moisture absorption had a linear relationship to %PEG-400 with R2 = 0.861 and 0.925, respectively. The moisture absorption of formulations FO1 and FO2 was not statistically different, while the moisture absorption of formulations FO3 and FO4 was significantly higher than that of FO1 and FO2 because PEGs had hygroscopic properties. A higher concentration of PEGs in the formulation leads to increased moisture absorption, particularly at high relative humidity (Baird et al., 2010).
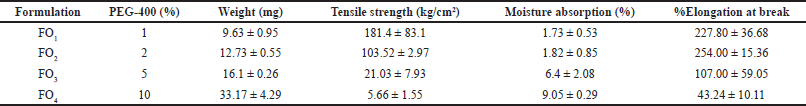 | Table 3. Amount of PEG-400, weight, tensile strength, and moisture absorption of the formulation FO1–FO4. [Click here to view] |
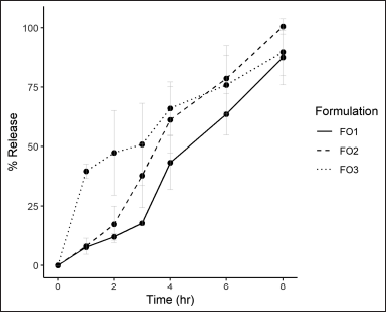 | Figure 4. Lycorine release profile at 1, 2, 3, 4, 6, and 8 hours for formulation FO1, FO2, and FO3 (mean ± SD). [Click here to view] |
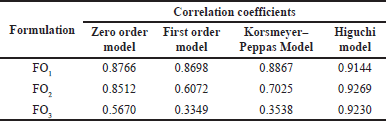 | Table 4. Correlation coefficients of formulation FO1, FO2, and FO3 for the first-order model, Korsmeyer–Peppas model, and Higuchi model. [Click here to view] |
The tensile strength of the transdermal patch film ranged from 5.7 to 181.4 kg/cm2. The tensile strengths of formulation FO1 and FO2 were also not statistically different. However, the tensile strengths of the formulation FO3 and FO4 were significantly different and lower than those of FO1 and FO2.
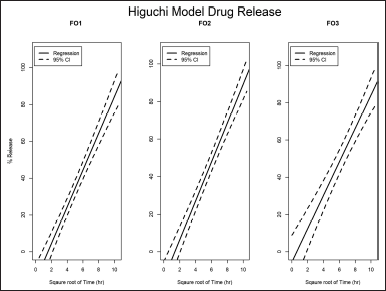 | Figure 5. Higuchi correlation plot of formulation FO1, FO2, and FO3. [Click here to view] |
The percentage elongation at the break of the formulations was statistically different (p = 0.00027). They could be divided into three groups. Firstly, the highest %elongation at break was formulation FO1 and FO2. The medium % elongation at the break group was FO3, and the low % elongation at the break group was FO4 (Table 3). The regression analysis revealed that PEG-400 of more than 2% (100% of polymer weight) had a negative effect on transdermal patch elongation with R² = 0.80, coefficient of −23.29, and p < 0.05. The folding endurance of formulation FO1, FO2, and FO3 was >500, >500, and 176 ± 10.58, respectively. It indicated that the PEG-400 plasticizer was suitable and provided appropriate film flexibility. Since plasticizers functioned by lowering the cohesive intermolecular forces along polymer chains, resulting in a flexible polymer film. However, if the plasticizers were increased beyond a certain threshold, the polymer chains would migrate too widely apart, reducing mechanical strength in terms of tensile strength, elongation at break, and folding endurance (El-Gendy, 2012; Pichayakorn et al., 2020).
The formulations FO1–FO3 were chosen for further evaluation due to the FO4 tensile strengths and the %elongation at break were not satisfied.
Lycorine content and in vitro drug release testing
Lycorine content of the formulation FO1, FO2, and FO3 were 1.88 ± 0.08, 1.51 ± 0.09, and 1.69 ± 0.08 mg/cm2, respectively. In vitro drug release testing showed that all formulations released lycorine for up to 8 hours. Formulations FO1 and FO2 gradually released lycorine over 8 hours, whereas formulation FO3 quickly released 40% at 1 hour. Formulation FO1 lycorine release was the lowest among the three formulations. While formulation FO2 lycorine release was initially lower than that with formulation FO3 up to 6 hours (Fig. 4). When comparing the composition of three formulations, PEG 400 resulted in fast initial drug release in a higher percentage of plasticizers. This result confirmed that PEG 400 increased in vitro drug release (Singh and Bali, 2016).
The release profile was tested among four release models that were the zero-order model, the first-order model, the Korsmeyer–Peppas model, and the Higuchi model (Table 4). Formulations FO1 and FO2 were the best fit with the Higuchi model, with correlation coefficients of more than 0.9. Formulation FO3 was considered correlated to the Higuchi model with correlation coefficients of about 0.8. The homogeneous polymer matrix system of the formulations, lipophilic nature of lycorine, and planar film form followed Higuchi’s conditions (Trucillo, 2022). The active agent release is related to the square root of time with a linear function (Fig. 5). The rate constants of FO1, FO2, and FO3 were 9.94 ± 0.70, 10.36 ± 0.67, and 8.95 ± 0.93 %released/hour1/2, respectively. It indicated that the concentration of the PEG 400 does not have a significant effect on the release rate constants. The optimal formulation for a transdermal patch containing C. asiaticum leaves extract was FO2 because it had good mechanical properties and good drug release.
CONCLUSION
The drug in matrix-type transdermal patches of C. asiaticum leaves extract was prepared, and the effects of the various ratio of HPMC, PVA, and PEG-400 on the physical properties of the transdermal patches were studied. Formulation FO2 has achieved the targets of this study. The tensile and physical appearance of the patches were satisfied. Also, the prepared patches showed good uniformity regarding lycorine content and in vitro release profile. In conclusion, the present data confirm the feasibility of developing C. asiaticum leaves extract transdermal patches based on PVA-PEG-400 film. Further studies are needed to confirm the efficacy of anti-inflammatory and analgesic effects in an animal model.
ACKNOWLEDGMENTS
This research was financially supported by the Research Institute for Health Sciences, Walailak University, Thailand, grant number WU-IRG-64-055. The authors would like to thank the Drug and Cosmetics Excellence Center, Walailak University, Thasala, Nakhon Si Thammarat, Thailand, for providing all research facilities.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Baird JA, Olayo-Valles R, Rinaldi C, Taylor LS. Effect of molecular weight, temperature, and additives on the moisture sorption properties of polyethylene glycol. J Pharm Sci, 2010; 99(1):154–68.
Dhippayom T, Kongkaew C, Chaiyakunapruk N, Dilokthornsakul P, Sruamsiri R, Saokaew S, Chuthaputti A. Clinical effects of thai herbal compress: a systematic review and meta-analysis. Evid Based Complement Alternat Med, 2015; 2015:942378.
El-Gendy NA. Pharmaceutical plasticizers for drug delivery systems. Curr Drug Deliv, 2012; 9(2):148–63.
Gasca-Silva CA, Gomes JVD, Gomes-Copeland KKP, Fonseca-Bazzo YM, Fagg CW, Silveira D. Recent updates on Crinum latifolium L. (Amaryllidaceae): a review of ethnobotanical, phytochemical, and biological properties. S Afr J Bot, 2022; 146:162–73.
Ge X, Meng X, Fei D, Kang K, Wang Q, Zhao M. Lycorine attenuates lipopolysaccharide-induced acute lung injury through the HMGB1/TLRs/NF-κB pathway. 3 Biotech, 2020; 10(8):369.
Goswami T, Audett J. Chemistry, manufacturing and controls in passive transdermal drug delivery systems. Ther Deliv, 2015; 6(9):1071–9.
Heng K, Anand-Ivell R, Teerds K, Ivell R. The endocrine disruptors dibutyl phthalate (DBP) and diethylstilbestrol (DES) influence Leydig cell regeneration following ethane dimethane sulphonate treatment of adult male rats. Int J Androl, 2012; 35(3):353–63.
Ita K. Chapter 5—Chemical permeation enhancers. In: Ita K (ed.). Transdermal drug delivery, Academic Press, Cambridge, MA, pp 63–96, 2020.
Ji YB, Tian P, Dai QC, Wang ST, Chen N. The present research situation of Crinum asiaticum alkaloids active ingredient. App Mech Matter, 2013; 411–4:3181–6.
Kongkwamcharoen C, Itharat A, Pipatrattanaseree W, Ooraikul B. Effects of various preextraction treatments of Crinum asiaticum leaf on its anti-inflammatory activity and chemical properties. Evid Based Complement Alternat Med, 2021; 2021:8850744.
Mahomoodally MF, Sadeer NB, Suroowan S, Jugreet S, Lobine D, Rengasamy KRR. Ethnomedicinal, phytochemistry, toxicity and pharmacological benefits of poison bulb—Crinum asiaticum L. S Afr J Bot, 2021; 136:16–29.
Monika B, Amit R, Sanjib B, Alisha B, Mihir P, Dhanushram T. Transdermal drug delivery system with formulation and evaluation aspects: overview. Res J Pharm Technol, 2012; 5(9):1168–76.
Ongthanasup T, Kanokkangsadal P, Panthong S, Itharat A. Anti-inflammatory activity of extracts from thai herbal compress ball and its plant ingredients. Sci Technol Asia, 2020; 25(3):68–77.
Pastore MN, Kalia YN, Horstmann M, Roberts MS. Transdermal patches: history, development and pharmacology. Br J Pharmacol, 2015; 172(9):2179–209.
Pichayakorn W, Maneewattanapinyo P, Panrat K, Monton C, Suksaeree J. Formulation design of oral strip-films based on PVA/PVP polymer blends for nicotine delivery. J Polym Environ, 2022; 30:4479–91
Pholhiamhan R, Saensouk S, Saensouk P. Ethnobotany of Phu Thai Ethnic Group in Nakhon Phanom Province, Thailand. Khon Kaen Univ J (Grad Stud), 2018; 15:679–99.
Pichiansunthorn C, Chawalit M, Jeerawong V. The describe of Osod Phra Narai textbook. Amarin Publishing Bangkok, Thailand, Bangkok, 2001.
Rahman MA, Ak A, Ahmed N, Islam M. Analgesic and anti-inflammatory effects of Crinum asiaticum leaf alcoholic extract in animal models. Afr J Biotechnol, 2013; 12:212–8.
Saringat HB, Alfadol KI, Khan GM. The influence of different plasticizers on some physical and mechanical properties of hydroxypropyl methylcellulose free films. Pak J Pharm Sci, 2005; 18(3):25–38.
Sharadha M, Gowda DV, Vishal Gupta N, Akhila AR. An overview on topical drug delivery system—updated review. Int J Res Pharm Sci, 2020; 11(1):368–85.
Sharma B, Vasudeva N, Sharma S. Anti-scabies and mosquito repellent activity of Crinum asiaticum linn. leaves extracts. Res J Pharm Technol, 2020a; 13(2):895–900.
Sharma B, Vasudeva N, Sharma S. Phytopharmacological review on Crinum asiaticum: a potential medicinal herb. Nat Prod J, 2020b; 10(4):342–54.
Singh A, Bali A. Formulation and characterization of transdermal patches for controlled delivery of duloxetine hydrochloride. J Anal Sci Technol, 2016; 7(1):1–3.
Suksaeree J, Simchareon W, Pichayakorn W. Effect of glycols permeation enhancer on the release and permeation of meloxicam-natural rubber film through pig skin. J Drug Deliv Sci Technol, 2021; 66:102874.
Trucillo P. Drug carriers: a review on the most used mathematical models for drug release. Processes, 2022; 10(6):1094.
Valenta C, Auner BG. The use of polymers for dermal and transdermal delivery. Eur J Pharm Biopharm, 2004; 58(2):279–89.