INTRODUCTION
Central nervous system (CNS) disorder refers to any neurological disease which affects the functional and structural integrity of the brain including the spinal cord that makes up the CNS (Cacabelos et al., 2016). The World Health Organization (WHO, 2006) reported that over 1 billion people in the world are suffering from neurological disorders of various kinds which pose a threat to public health. Epilepsy is a condition that is characterized by paroxysmal discharge of cerebral neurons resulting in repeated seizures with complete or partial loss of consciousness (Mucklow, 2000). The etiology of epilepsy could be idiopathic, head injury, neoplasm, or hereditary (Gajjar et al., 2016). Newborns and the elderly are at high risk with males being more susceptible than females (Samba Reddy, 2017). An average of 61 per 100,000 persons suffer from epilepsy worldwide with the highest prevalence in low-income countries (Beghi and Hesdorffer, 2014; Levinson et al., 2020). Despite the availability of various treatment regimens, a sizable number of epileptic patients are still experiencing uncontrolled seizures (White, 2003). The old generational drugs currently used in the management of convulsion, anxiety, and other neurological conditions only relieve the symptoms associated with the condition, and prolonged administration of such drugs is associated with significant adverse effects (Leppik, 2002). The newer generational drugs with better pharmacological action and fewer side effects are expensive. This necessitates the need for extensive exploration of new molecular entities that present better pharmacologic action, fewer side effects, and being user friendly. The challenges associated with gene therapy have necessitated the use of pharmacological agents that modify neurotransmitter receptor signaling in the management of neurological disorders (Bowie, 2008).
Securidaca longipedunculata (SL) Fresen is an all-round and universal medicinal plant widely and traditionally used in Africa for the management of all sexually transmitted diseases and any other health problems (Mongalo et al., 2015). It is a member of the Polygalaceae family often called violet tree in English, “Gazawuro” in Kanuri, “‘sanya or uwar magunguna” in Hausa, and “Alali” in Fulani (Akinniyi and Sultanbawa, 1983). It is traditionally utilized in the management of all imaginable human diseases which include but are not restricted to sexually transmitted diseases, fever, migraine, rheumatoid arthritis, tuberculosis, tumors, and metabolic diseases (Avhurengwi and Walter, 2006). The diverse use of SL is probably why the plant is being referred to as “uwar magunguna,” which is “the mother of all medicines.” Scientifically, the root bark extract of the plant has been found to possess sedative and anticonvulsant activities (Okomolo et al., 2011) and anticonvulsant and anxiolytic activities (Adeyemi et al., 2012). SL is among the top five medicinal plants employed in the management of epileptic seizures by the Hausas, Fulanis, and other ethnic groups in the Northern part of Nigeria (Muazu and Kaita, 2008).
Experimental studies showed that extracellular calcium influx triggers epileptic seizures due to paroxysmal depolarization shift (PDS). Epileptic therapies are therefore designed to inhibit calcium influx which in turn alters the PDS thereby reverting the spread of epileptic seizures (Owoalade et al., 2019). Scientists over the years have devoted their time to identifying and validating plant-derived substances for the management of various ailments affecting humanity. Therefore, this study aims to evaluate the anticonvulsant and anxiolytic activities of SL in mice in order to substantiate the traditional claim of its medicinal use in the management of epilepsy and other neurological conditions.
MATERIALS AND METHODS
Plant collection and identification
The root bark of SL along with its other parts such as flowers, leaves, stems, and seeds were collected from a village called Ngulde in Askira-uba local government area of Borno state which is in the Northeastern part of Nigeria, located at latitude 10° 27’ 18.32” N and longitude 13° 13’ 20.39” E10°. The plant was identified and authenticated by a Botanist in Biological Science Department at the University of Maiduguri, Nigeria. The herbarium of the Department of Toxicology and Pharmacology was used to conserve the sample tagged Vet212A2.
Plant preparation and extraction
The fresh roots of SL were washed, decorticated, sliced, and room dried. The root bark was weighed and crushed into fine particles for ease of extraction (Junaidu et al., 2014). Methanol was used as a solvent during the extraction process at the ratio of 1:5 (w/v), the powdered root bark of SL to the solvent. At this extraction ratio, 1 kg of the powdered root bark was extracted using 5,000 ml of methanol using a soxhlet extractor to get its product (methanol extract) (Tiwari et al., 2011). A hot air oven at a temperature of 40°C – 45°C was used to completely dry up the methanol extract of SL for storage and experimental use. The percentage yield of the extract was 15% w/w, stored at 4°C.
Determination of chromatographic chemical profile using gas chromatography-mass spectrometry (GC-MS)
GC-MS screening was performed in a stepwise direction as described by Mgbeje et al. (2020). GC 7890B, MSD 5977A, Agilent Tech comprising a gas chromatograph hyphenated to a mass spectrophotometer (MS) and an autosampler was used for the analysis. A 25 m × 0.25 mm fused silica capillary column coated with CP-Sil5 and film thickness at 0.15 μm was fitted to the gas chromatograph. The carrier gas used is helium at 1.2 ml/minute. Operating conditions of the MS comprise ion source temperature of 230°C and an ionization voltage of 70 ev. The extract of SL was dissolved in methanol and 1.0 µl was injected into the gas chromatography. The MS data obtained was processed online using MassHunter software. A comparison of the retention indices, fragmentation pattern, and mass spectra with a spectrum of already known components stored in the National Institute of Standard and Technology databank was made (http://chemdata.nist.gov).
Experimental animals
Male and female adult mice weighing about 21.7–40.5g were used for the experiment. The mice were housed in newly constructed aluminum/wooden cages and kept in a very clean/noise-free environment. All mice were given tap water and crumbled commercial poultry grower feed manufactured by Amo Byng Nig. Ltd. The animals were given feed twice daily and water was given ad libitum. Animal housing, feeding, handling, and all experimental procedures were in line with the provisions as enshrined in the guidelines for the care and use of laboratory animals by the National Institute of Health (1985). Ethical approval and clearance were obtained from the Committee of Animal Research Ethics, the University of Maiduguri with reference number REF/FP/092019/PGVP/01, and all experimental procedures were expressly under their supervision.
Chemicals and diluents
Pentylenetetrazole (PTZ), strychnine methanol, ethanol, (Sigma-Aldrich Inc., St. Louis, MO), normal saline (Fidson Healthcare Plc, Sango-Ota, Ogun State, Nigeria), and diazepam (Rotexmedica 22946 Trittau, Germany) were used for the experiments. Methanol was used for extraction and ethanol for cleaning used apparatus for reuse, PTZ, and strychnine was used to induce convulsion and seizures, and diazepam was used as positive control while normal saline was used for extract reconstitution and negative control.
PTZ-induced seizures
The anticonvulsant activity of SL root bark was determined using the technique described by Vellucci and Webster (1984). The capacity of the methanol extract of SL in alleviating seizures was caused by the intraperitoneal (i.p.) administration of PTZ. A dose of 60 mg/kg of PTZ was administered intraperitoneally 30 minutes after normal saline administration at 0.1 ml/10 g i.p., SL extracts with LD50 of 6.92 mg/kg were at 0.5, 1.0, and 1.5 mg/kg to groups A, B, C, and D, respectively (Fomnya et al., 2021) and diazepam at 1 mg/kg i.p. to group E with each group containing five mice randomly grouped. Groups A and E served as the negative and positive controls respectively while B, C, and D served as the treatment groups. Immediately after PTZ administration, each mice group was positioned in a clean transparent plastic container and then carefully monitored for any observable sign of seizures with the aid of a camcorder for a period of 30 minutes. The time spent prior to the observation of active seizures, a time span of active seizures, and the percentage mortality were all documented. The capacity of the extract to stop seizure occurrence, retard or accelerate the latency or onset of convulsive seizures was taken as a sign of anticonvulsant activity.
Strychnine-induced convulsion
The activity of SL against seizures was assessed on strychnine-induced convulsions as described by McAllister (1992). Five groups of mice A, B, C, D, and E were used for the experiment with each group containing five mice randomly grouped. Treatment for each group was the same as for the PTZ-induced seizures above. Strychnine at 2 mg/kg was then given i.p. 30 minutes after treatment to all five groups. After strychnine was given, each group was positioned in a clean transparent device and monitored for 30 minutes for an observable sign of seizure with the aid of a camcorder. The time spent before the occurrence of active clonic convulsions, duration of active observable convulsions, and percentage mortality were all documented. The capacity of the extract to stop seizure occurrence, retard, or accelerate the latency or onset of convulsive seizures was taken as a sign of anticonvulsant activity.
Open field test (OFT)
An OFT was employed to determine the anxiolytic or anxiogenic properties of the extract of SL (Carlini et al., 1986). This was achieved through the evaluation of ambulatory behaviors in mice. The ability of the mice to remain on the peripheral part of the field without going into the central part of the field (known as thigmotaxis) and frequent urination/defecation is usually considered as fearful/apprehensive behavior (Hall, 1934). The apparatus used consists of four similar arenas and each arena measures about 45 (width) × 45 (length) × 35 cm (height). Grouping and treatment are the same as in the section PTZ-induced seizures. Thirty minutes after treatment, each mouse was kept at the central part of an open field zone and was observed to see if the mouse would stay at the center of the open field or would move to the periphery of the field. The number of entries and duration of stay in each part of the field (periphery, center, and corner) was assessed and recorded with the aid of a camcorder in a 30 minutes session (Archer, 1973). 5% ethanol was used to clean the apparatus for the presence of feces, urine, and any other body fluid after each session. Anxiety state was indicated by a diminished ratio of entry and the duration of stay at the center of the field to entry and duration of stay in the periphery of the field.
Elevated plus maze (EPM)
An EPM was employed to determine the anxiolytic activity of the root bark extract of SL. The EPM is an apparatus that is made up of two closed arms measuring about 65 × 10 cm, positioned in opposite directions, and surrounded by walls measuring 40 cm in height. In addition, the apparatus has two open arms each measuring about 65 × 10 cm. All four arms are joined together by a square of 10 × 10 cm at the center which forms a sign of a cross that is elevated up to 75 cm from the ground. Four lightning bulbs of 9 W each were fixed on a stand to lighten the apparatus and the experimental area. Behavioral observations were captured with the aid of a video camera strategically positioned at the center of the apparatus. Grouping and treatment are the same as the ones described in the section PTZ-induced seizures. Each mouse was kept in the center of the apparatus with the head pointed toward one of the closed arms 30 minutes after treatment and carefully observed for a period of 5 minutes (Pellow et al., 1985; Yemitan and Adeyemi, 2003). 5% ethanol was used to clean the apparatus for the presence of feces, urine, and any other body fluid after each session. The duration of time spent in both open and closed arms, the proportion of time spent in open arm, and the frequency of entry into both open and closed arms were documented. Complete entry with all limbs was considered an arm entry. Increased frequency of entries/stay in the center/open arm was considered antianxiety behavior while the increased frequency of entries/stay in the closed arm was considered an anxiety behavior.
Data analysis
All the data obtained during the study were presented as mean ± standard deviation. Analysis of variance was used to analyze the extent of variation between groups and p-values ≤ 0.05 were considered significant. SPSS IBM® version 17 (2016) was used for the analysis.
RESULTS
Fingerprint of methanol extract of SL root bark using GC-MS
Analysis of the methanol extract of SL root bark using GC-MS showed two major peaks at 15.527 (46.789%) and 13.404 minutes (17.914%) as presented in Table 1. The chromatogram of the GC-MS is presented in Figure 1.
Anticonvulsant activities of SL extract in mice using PTZ-induced seizures
The outcomes of the experiment in testing the anticonvulsant activities of the extract of SL root bark in mice using PTZ-induced seizures are presented in Table 2. The i.p. administration of extract of SL root bark in mice showed a dose-dependent anticonvulsant activity in both time-lag (T-lag) and duration when juxtaposed with the negative control showing a significant increase (p < 0.05) in T-lag at 1 and 1.5 mg/kg and a significant reduction (p < 0.05) in duration at 0.5, 1, and 1.5 mg/kg of the extract administered. The T-lag rose from 29.60 ± 3.20 seconds in the negative control to 761.20 ± 132.70 seconds in the group that received extract at 1.5 mg/kg. The duration of convulsion decreased from 292.00 ± 59.11 seconds in the negative control to 91.80 ± 28.56 seconds in the group that received extract at 1.5 mg/kg. The i.p. administration of diazepam at 1 mg/kg as the positive control produced the highest and most significant anticonvulsant activity (p < 0.05) in both T-lag (1,360.80 ± 293.32 seconds) and duration (26.20 ± 13.08 seconds) as compared to the negative control.
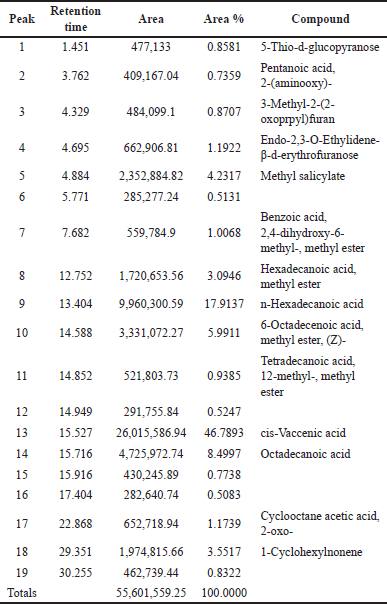 | Table 1. GC-MS fingerprint of methanol extract of SL root barks. [Click here to view] |
Anticonvulsant activities of SL extract in mice using strychnine-induced convulsion
The result of the anticonvulsant activities of the extract of SL root bark employing strychnine-induced convulsion is presented in Table 3. The administration of strychnine- induced episodes of convulsive seizures and the extract conferred protection against the convulsive seizures induced by strychnine. The extract-treated group showed a dose-dependent anticonvulsant activity in T-lag as juxtaposed with the negative control showing a significant increase (p < 0.05) at 1 and 1.5 mg/kg of the extract. The T-lag rose from 240.60 ± 20.48 seconds in the negative control to 503.00 ± 86.86 seconds in a group that received extract at 1.5 mg/kg. The i.p. administration of diazepam at 1 mg/kg as the positive control produced the highest and most significant anticonvulsant activity (p < 0.05) in both T-lag (698.80 ± 84.16 seconds) and duration (130.60 ± 21.37 seconds) as compared to the negative control.
Anxiolytic activities of SL extract in mice using OFT
The result of anxiolytic activities of the extract of SL root bark employing the OFT is presented in Table 4. The extract-treated mice exhibited a dose-dependent anxiolytic-like activity in both times spent in the central and corner zones as juxtaposed with the negative control with a significant increase (p < 0.05) in the duration of time taken in the central zone in all the doses of the extract administered and a significant decrease (p < 0.05) in the duration of time taken in the corner zone at 1 and 1.5 mg/kg extract administration. The time taken in the central zone increased from 99.20 ± 38.31 seconds in the negative control to 251.80 ± 22.11 seconds in the group that received extract at 1.5 mg/kg while the time taken at the corner zone reduced from 901.60 ± 81.31 seconds in the negative control to 527.80 ± 54.29 seconds in the group that received extract at 1.5 mg/kg. The frequency of entries into both the central and corner zones presented a significant increase (p < 0.05) in the frequency of entry into the central zone at 0.5 and 1 mg/kg and a significant decrease (p < 0.05) in the frequency of entry into the corner zone at 1.5 mg/kg extract administrations. The number of entries into the central zone increased from 7.80 ± 2.68 in the negative control to 15.20 ± 2.58 in the group that received extract at 1 mg/kg while the frequency of entry into the corner zone decreased from 29.80 ± 3.83 in the negative control to 24.00 ± 2.73 in the group that received extract at 1.5 mg/kg. The time spent in the peripheral zone was found to increase significantly (p < 0.05) from 799.20 ± 48.23 seconds (negative control) to 996.80 ± 34.49 (1 mg/kg) and 1,020.40 ± 65.60 seconds (1.5 mg/kg). The administration of diazepam i.p. to mice at 1 mg/kg which served as the positive control presented the highest and most significant anxiolytic-like activity (p < 0.05) in the time taken in both the central (293.00 ± 66.02 seconds) and corner (571.60 ± 50.54 seconds) zones while the extract of SL at 1.5 mg/kg produced the highest and significant anxiolytic-like property (p < 0.05) in respect of the duration of time taken in the peripheral zone (1,020.40 ± 65.60 seconds) as compared to the negative control.
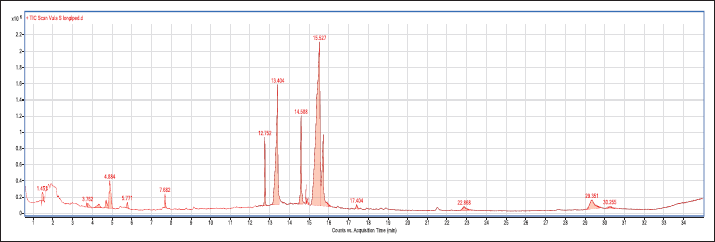 | Figure 1. Chromatogram for the GC-MS analysis of methanol extract of SL root bark. [Click here to view] |
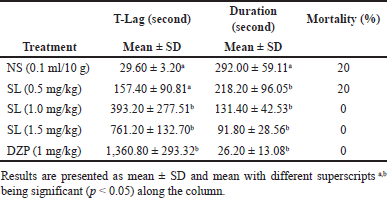 | Table 2. Effects of intraperitoneal administration of methanol extract of SL root bark on PTZ-induced seizures in mice. [Click here to view] |
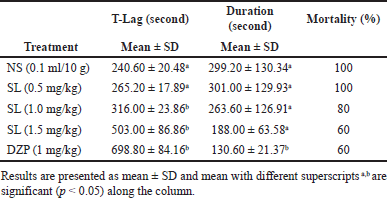 | Table 3. Effects of intraperitoneal administration of methanol extract of SL root bark on strychnine-induced convulsion in mice. [Click here to view] |
Anxiolytic activities of SL extract in mice using EPM
The result of anxiolytic activities of SL root bark extract in mice on various parameters measured in the EPM paradigm is presented in Figures 2 and 3. The SL root bark extract conferred a significant anxiolytic-like property (p < 0.05) in the frequency of open-arm entry and the duration of time taken in the open arm when juxtaposed to the negative control. The anxiolytic-like activity is said to be dose-dependent, and the duration of time taken in the opened arm rose from 8.40 ± 6.34 seconds in the negative control to 61.60 ± 22.83 seconds in the group that received extract at 1.5 mg/kg while the frequency of open arm entry rose from 1.20 ± 0.44 in the negative control to 2.60 ± 0.54 in the group that received extract at 1.5 mg/kg. The SL extract also conferred a dose-dependent anxiolytic-like activity in both the duration of time taken in the closed arm and the frequency of closed arm entry when juxtaposed with the negative control with a significant decrease (p < 0.05) in the duration of time taken in closed arm at all doses of the extract administered and significant increase (p < 0.05) in the frequency of closed arm entry at 1 and 1.5 mg/kg extract administration. The time taken in the closed arm reduced from 291.60 ± 6.34 seconds in the negative control to 238.40 ± 22.83 seconds in the group that received extract at 1.5 mg/kg while the number of closed arm entries increased from 2.00 ± 0.70 in the negative control to 3.20 ± 0.83 in the group that received extract at 1.5 mg/kg. The administration of diazepam i.p. to mice at 1 mg/kg as the positive control produced the highest and most significant anxiolytic-like property (p < 0.05) in the duration of time taken in both the open (64.40 ± 25.71 seconds) and closed (235.60 ± 25.71 seconds) arms and the frequency of opened arm entry (2.60 ± 1.14) while the extract of SL at 1.5 mg/kg produced the highest and significant anxiolytic-like property (p < 0.05) in the frequency of closed arm entry (3.20 ± 0.83) as compared to the negative control.
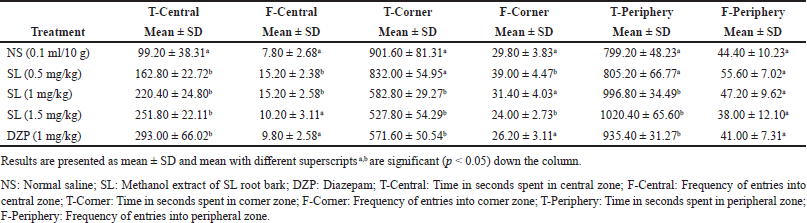 | Table 4. Effects of intraperitoneal administration of methanol extract of SL root bark on OFT parameters in mice. [Click here to view] |
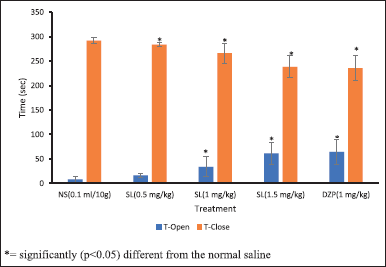 | Figure 2. Mean of total time spent in the open and closed arm (T-open and T-close) due to the i.p. administration of methanol extract of SL root bark on EPM test in mice. * = significantly (p < 0.05) different from the normal saline. NS: Normal saline; SL: Methanol extract of SL root bark; DZP: Diazepam; T-Open: Time spent in open arm; T-Close: Time spent in closed arm. [Click here to view] |
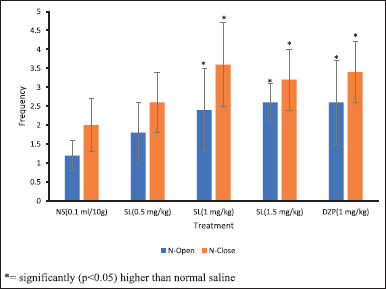 | Figure 3. Mean of the number of entries into the open and closed arm (N-open and N-close) due to the i.p. administration of methanol extract of SL root bark on EPM test in mice. *= significantly (p < 0.05) higher than normal saline. NS: Normal saline; SL: Methanol extract of SL root bark; DZP: Diazepam; N-Open: Number of open arm entries; N-Close: Number of closed arm entries. [Click here to view] |
DISCUSSION
SL is a popular and one of the most utilized plants in African folklore medicine. The root bark extract of SL was used to determine its anticonvulsant as well as its anxiolytic properties in mice. It was revealed that SL and diazepam inhibited PTZ-induced seizures. The PTZ test is known to be a well-grounded prototype used for the determination of seizures in a living system (Loscher and Schmidt, 1988). The processes involved in achieving the epileptogenic property of PTZ are not well understood; however, inhibition of gamma-aminobutyric acid (GABA) neurotransmission by PTZ has been reported to cause seizures (De Sarro et al., 2003). Seizures can be inhibited by the amplification of GABA neurotransmission whereas the propagation of seizure occurrence can be achieved by the impediment of GABA neurotransmission. Diazepam, a known muscle relaxant, inhibits seizures induced by PTZ by amplifying the activities of GABA receptors by increasing the opening frequency of chloride channels (Olsen, 1981). In PTZ-induced seizures, the extract of SL at the dosage of 1.0 and 1.5 mg/kg showed a significant anticonvulsant-like activity by slowing the onset of convulsion and shortening the time span of convulsion with 0% mortality. Diazepam conferred anticonvulsant activity by shortening the onset of convulsion and shortening the time span of convulsion with 0% mortality. Even though the mechanism of action is not known, the anticonvulsant-like property of SL in the PTZ model indicates that the extract of SL possibly achieves the anticonvulsant-like effect through active enhancement of GABA neurotransmission or through the inhibition of glutamate-mediated excitation.
Strychnine is a known antagonist of glycine receptors causing convulsion through the competitive antagonism of glycine at glycinergic receptors in the spinal cord (Yoon et al., 1993). Strychnine particularly obstructs the activities of glycine receptors especially in the spinal cord during the production of excitatory responses within the CNS (Nicoll, 2001; Sayin et al., 1993). The administration of SL extract at the dosage of 1.0 and 1.5 mg/kg in the strychnine-induced-convulsion model showed a significant anticonvulsant-like property by shortening the onset of convulsion with 80% and 60% mortalities, respectively; the extract could not produce any significant effect on the duration of convulsion. Diazepam conferred anticonvulsant activity by shortening the onset of convulsion and shortening the time span of convulsion with 60% mortality. The delay in the onset of the seizure by SL at 1.0 and 1.5 mg/kg may be due to glycine inhibitory mechanisms or effect on the glycinergic receptors, or the mechanism of the delay may be synonymous with that of diazepam which displaces the strychnine molecules from the receptor sites (Nogardy, 1998).
Mice subjected to an open brightly lit environment as it is obtained in the OFT are known to show increased anxiety-like behaviors which include a prolonged stay in the peripheral and corner zones with a correlating reduction in the time taken in the center zone. The frequency of movements and the time taken in the central, peripheral, and corner zones are sensitive to anxiolytics and anxiogenic treatments (Prut and Belzung, 2003; Walsh and Cummins, 1976). The vehicle-treated mice exposed to the OFT showed a significant increase in the time taken at the corner zone with a significant correlating decrease in the time taken in the center zone while the extract-treated mice at 1.0 and 1.5 mg/kg exhibited anxiolytic-like activity in a dose-dependent ratio just like diazepam by significantly increasing the duration of time taken in the center zone as well as the number of entries into the center zone.
The voluntary or involuntary movements of mice to the closed arms of the EPM apparatus have been linked to hormonal changes and it signifies anxious behavior (Adebesin et al., 2015). Eluding the open arms of the mice signifies the expression of fear. In EPM, the time taken is the main determinant of fear or anxiousness. Therefore, according to these assertions, the EPM test can be said to be a dependable way of determining the anxiolytic-like effects of any molecular entity (Adebesin et al., 2015). All the mice administered with normal saline recorded the lowest frequency of entry and lesser time taken in the open arms while the extract of SL at 1.0 and 1.5 mg/kg showed a significant anxiolytic-like activity just like diazepam by increasing the frequency of entry and the time taken in the open arms. The outcome of the study provides evidence that SL possesses significant dose-dependent anticonvulsant and anxiolytic activities in the experimental animal models used. These findings support the result reported by Adeyemi et al. (2012) and Okomolo et al. (2011).
CONCLUSION
Methanol extract of SL has been recognized as a novel extract with potent neuropharmacological effects. Data obtained from this study has provided scientific-based evidence that supports the traditional use of SL in managing CNS disorders through its ability to provide protection against experimentally induced seizures and anxiety in mice. Pentanoic acid, 3-methyl-2-(2-oxopropyl) furan, and methyl salicylate were the major components responsible for the neuropharmacological effects. The findings of this study scientifically revealed the dose-dependent anticonvulsant and anxiolytic activities of the methanol extract of SL root bark in mice. Further study is necessary to confirm these findings with the aim of developing a potent anticonvulsant agent with a superior pharmacological profile better than diazepam used as the positive control.
LIST OF ABBREVIATIONS
CNS, central nervous system; SL, Securidaca longipedunculata; WHO, World Health Organization; w/v, weight of solution in total volume of solution; °C, degrees celsius; g, grams; PTZ, pentylenetetrazole; LD50, median lethal dose; OFT, open field test; EPM, elevated plus maze; i.p., intraperitoneal; GABA, gamma-aminobutyric acid; GC-MS, gas chromatography-mass spectrometry.
ACKNOWLEDGMENTS
The academic and moral support rendered by Prof. Samuel Timothy Yerima and Prof. Garba Uthman Sadiq of the Faculty of Pharmacy, University of Maiduguri, is highly acknowledged and appreciated.
AUTHORS’ CONTRIBUTIONS
S.I-N. provided the plant material. M.S-A. and B.U. prepared and extracted the plant. H.J-F. and S.I-N. performed the biological experiments. S.S. and S.I-N. conceived and supervised the study. H.J-F., S.G-M., and B.U. drafted the manuscript. S.I-N. reviewed the manuscript. All authors read and approved the final manuscript.
FINANCIAL SUPPORT
The authors declare no funding support.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
Ethical approval and clearance were obtained from the Committee of Animal Research Ethics, University of Maiduguri with reference number REF/FP/092019/PGVP/01. No experiment that involved the use of human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Adebesin IF, Akindele AJ, Adeyemi OO. Evaluation of neuropharmacological effects of aqueous leaf extract of Albizia glaberrima (Leguminosae) in mice. J Ethnopharmacol, 2015; 160:101–8; https://doi.org/10.1016/j.jep.2014.11.040
Adeyemi OO, Akindele AJ, Yemitan OK, Aigbe FR, Fagbo FI. Anticonvulsant, anxiolytic and sedative activities of aqueous root extract of Securidaca longipedunculata Fresen. J Ethnopharmacol, 2012; 130(2):191–5; https://doi.org/10.1016/j.jep.2010.04.028
Akinniyi JA, Sultanbawa MUS. A glossary of Kanuri names of plants, with botanical names, distribution and uses. Ann Borno, 1983; 1:85–93.
Archer J. Tests for emotionality in rats and mice: a review. Anim Behav, 1973; 21(2):205–35; https://doi.org/10.1016/s0003-3472(73)80065-x
Avhurengwi PN, Walter S. Securidaca longipedunculata (Fresen). National Botanical Garden, Pietermaritzburg, South Africa, pp 1–3, 2006.
Beghi E, Hesdorffer D. Prevalence of epilepsy-an unknown quantity. Epilepsia, 2014; 55(7):963–7; https://doi.org/10.1111/epi.12579
Bowie D. Central nervous system neuronal disorders. Drug Targets, 2008; 7(2):129–43.
Cacabelos R, Torrellas C, Fernández-Novoa L, López-Muñoz F. Histamine and immune biomarkers in CNS disorders. Mediators Inflamm, 2016; 2016:1924603; https://doi.org/10.1155/2016/1924603
Carlini EA, Contar J, de DP, Silva-Filho AR, da Silveira-Filho NG, Frochtengarten ML, Bueno OF. Pharmacology of lemon grass (Cymbopogon citratus Stapf). I. Effects of teas prepared from leaves on laboratory-animals. J Ethnopharmacol, 1986; 17(1):37–64; https://doi.org/10.1016/0378-8741(86)90072-3
De Sarro G, Ferreri G, Gareri P, Russo E, De Sarro A, Gitto R, Chimirri A. Comparative anticonvulsant activity of some 2,3-benzodiazepine derivatives in rodents. Pharmacol Biochem Behav, 2003; 74(3):595–602; https://doi.org/10.1016/s0091-3057(02)01040-7
Fomnya HJ, Ngulde SI, Sanni S, Malgwi SG, Umaru B, Auwal MS. Phytochemical analysis and acute toxicity studies on the methanol extract of Securidaca longipedunculata (Fresen) root bark in mice. J Vet Biomed Sci, 2021; 3(1):41–8; https://doi.org/10.36108/jvbs/1202.30.0160
Gajjar BM, Shah AM, Patel PM. The pattern of adverse drug events to antiepileptic drugs: a cross-sectional study at a tertiary care teaching hospital. Natl J Physiol Pharm Pharmacol, 2016; 6(6):616–21; https://doi.org/10.5455/njppp.2016.6.0823013082016
Hall CS. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J Comp Psychol, 1934; 18:385–403.
Junaidu S, Shehu K, Aliero AA, Bawa JA, Suleiman I. Evaluation of antifungal and phytochemical properties of Violet Tree (Securidaca longipedunculata Fres). Glob J Sci Front Res, 2014; 14(5):1–6.
Leppik IE. Three new drugs for epilepsy: levetiracetam, oxcarbazepine and zonisamide. J Child Neurol, 2002; 17(suppl 1):s53–7; https://doi.org/10.1177/08830738020170010701
Levinson S, Tran CH, Barry J, Viker B, Levine MS, Vinters HV, Mathern GW, Cepeda C. Paroxysmal discharges in tissue slices from pediatric epilepsy surgery patients: critical role of GABAB receptors in the generation of ictal activity. Front Cell Neurosci, 2020; 14:54; https://doi.org/10.3389/fncel.2020.00054
Loscher W, Schmidt D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res, 1988; 2(3):145–81; https://doi.org/10.1016/0920-1211(88)90054-x
McAllister KH. N-Methyl-D-aspartate receptor antagonists and channel blockers have different effects upon a spinal seizure model in mice. Eur J Pharmacol, 1992; 211(1):105–8; https://doi.org/10.1016/0014-2999(92)90269-a
Mgbeje BIA, Ofem-Out J, Ugoanyanwu FO. Phytochemical components of butanol fraction of Vernonia amygdalina leaf extract using GC-MS analysis. Merit Res J Med Med Sci, 2020; 8(4):74–9; https://doi.org/10.5281/zenodo.3764119
Mongalo NI, McGaw LJ, Finnie JF, Staden JV. Securidaca longipedunculata Fresen (Polygalaceae): a review of its ethnomedicinal uses, phytochemistry, pharmacological properties and toxicology. J Ethnopharmacol, 2015; 165:215–26; https://doi.org/10.1016/j.jep.2015.02.041
Muazu J, Kaita AH. A review of traditional plants used in the treatment of epilepsy amongst the Hausa/Fulani tribes of Northern Nigeria. Afr J Tradit Complement Altern Med, 2008; 5(4):387–90; https://doi.org/10.4314/ajtcam.v5i4.31294
Mucklow JC. Martindale: the complete drug reference. Br J Clin Pharmacol, 2000; 49(6):613; https://doi.org/10.1046/j.1365-2125.2000.00206.x
National Institute of Health. Guidelines for the care and use of laboratory animals. Department of Health Services, Bethesda, MD, pp 83–123, 1985.
Nicoll RA. Introduction to pharmacology of CNS Drugs. In: Katzung BG (ed.). Basic and clinical pharmacology. 8th edition, Lange Medical Books/McGraw-Hill, New York, NY, pp 351–63, 2001.
Nogardy T. Medicinal chemistry-a biochemical approach. 2nd edition, University Press, Oxford, UK, pp 239–41, 1998.
Okomolo FC, Mbafor JT, Bum EN, Kouemou N, Kandeda AK, Talla E, Dimo T, Rakotonirira A, Rakotonirira SV. Evaluation of the sedative and anticonvulsant properties of three Cameroonian plants. Afr J Tradit Complement Altern Med, 2011; 8(Suppl 5):181–90; https://doi.org/10.4314/ajtcam.v8i5S.24
Olsen RW. The GABA postsynaptic membrane receptor-ionophore complex. Site of action of convulsant and anticonvulsant drugs. Mol Cell Biochem, 1981; 39:261–79; https://doi.org/10.1007/BF00232579
Owoalade SA, Martin Lou DM, Yadav K, Poudel S. A review on anti-epileptic activity of seaweed Ecklonia cava. J Drug Deliv Ther, 2019; 9(1-s):425–32; https://doi.org/10.22270/jddt.v9i1-s.2335
Pellow S, Chopin P, File SE, Briely M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods, 1985; 14(3):149–67; https://doi.org/10.1016/0165-0270(85)90031-7
Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol, 2003; 463(1–3):3–33; https://doi.org/10.1016/s0014-2999(03)01272-x
Samba Reddy D. Sex differences in the anticonvulsant activity of neurosteroids. J Neurosci Res, 2017; 95(1–2):661–70; https://doi.org/10.1002/jnr.23853
Sayin U, Cengiz S, Altug T. Vigabatrin as an anticonvulsant against pentylenetetrazole seizures. Pharmacol Res, 1993; 28(4):325–32; https://doi.org/10.1006/phrs.1993.1134
Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. IPS, 2011; 1(1):98–106.
Vellucci SV, Webster RA. Antagonism of caffeine-induced seizures in mice by Ro15-1788. Eur J Pharmacol, 1984; 97(3–4):289–93; https://doi.org/10.1016/0014-2999(84)90462-x
Walsh RN, Cummins RA. The open-field test: a critical review. Psychol Bull, 1976; 83(3):482–504.
White HS. Preclinical development of antiepileptic drugs: past, present and future directions. Epilepsia, 2003; 44(7):2–8; https://doi.org/10.1046/j.1528-1157.44.s7.10.x
World Health Organization. Neurological disorders: public health challenges. World Health Organization, Geneva, Switzerland, vol. 2006, p 9789241563369, 2006.
Yemitan OK, Adeyemi OO. Anxiolytic and muscle-relaxant activities of Dalbergia saxatilis. West Afr J Pharmacol Drug Res, 2003; 19(1–2):42–6; https://doi.org/10.4314/wajpdr.v19i1.14732
Yoon KW, Covey DF, Rothman SM. Multiple mechanisms of picrotoxin block of GABA-induced currents in rat hippocampal neurons. J Physiol, 1993; 464:423–39; https://doi.org/1113/jphysiol.1993.sp019643