INTRODUCTION
Herbal/traditional medicine has long been utilized as an official alternative treatment for various illnesses in Southeast Asia regions, especially in developing countries (Nguyen et al., 2021; Tran et al., 2022) during the COVID-19 pandemic was explored in this study. The aims were to examine (1. In fact, more than 40% of drug formulations consist of natural products and standard drugs such as artemisinin and aspirin originated from traditional medicine (WHO Global Centre for Traditional Medicine n.d., 2022). Moreover, the World Health Organization recently fostered the use of scientifically proven herbal/traditional medicines, in adjunction to modern medicine, because of their potential low toxicity, ease of accessibility, and diverse therapeutic effects (Traditional, Complementary and Integrative Medicine n.d., 2022). To this end, Vietnam, a country with a rich history of traditional medicinal uses and a diverse therapeutic plant resource, possesses promising conditions for researching and developing herb-related pharmaceutical products (Huynh et al., 2022; Pham et al., 2022, 2019). As such, an interesting medicinal plant that has gained much attention is the Houttuynia cordata Thunb. (HC, generally known as “Diep ca” in Vietnamese).
Belonged to the family Saururaceae, HC mainly distributes in the tropics and subtropics areas of the Asian continent, from India to China, Japan, Thailand, and Indochina countries (Yang and Jiang, 2009). In Vietnam, HC grows wildly and naturally in clusters at the river banks, streams, or fields of numerous places, from low mountains to midlands and plains. Ethnopharmacologically, in the rural areas of Vietnam, HC extracts, commonly in the form of tea, have long been utilized as a folk medicine for treating acne, inflammation, bacterial infection, and digestive disorders. Scientifically, HC has been proven its therapeutic efficacies since its essential oil components possess anti-inflammatory, antibacterial, and antiviral effects (Hayashi et al., 1995; Lu et al., 2006), its flavonoid contents have anticancer, antioxidative, antimutagenic, and anti-free radical properties (Chen et al., 2003; Fu et al., 2013; Laldinsangi, 2022), and its alkaloid compounds demonstrate remarkably potent antiplatelet and cytotoxic effects (Kim et al., 2001). In addition, the HC extract itself exhibits a wide range of pharmaceutical activities, including antibacterial, antiviral, anti-inflammatory, anti-cancer, and antioxidative effects (Fu et al., 2013; Laldinsangi, 2022; Wu et al., 2021). However, to the best of our knowledge, limited research has reported the therapeutic actions of HC extract and its isolated compounds on leukemia (i.e., blood cancer) treatment and the human immune system.
Therefore, this present work first explored the optimal conditions for HC extraction and its flavonoid isolations, purifications, and structure elucidations. Then, the extract was tested for its long-term stability using accelerated storage conditions. Finally, the HC extract and its isolated pure flavonoids were in vitro tested for their anticancer effect on the leukemia cell lines of K562 and HL60, and in vivo evaluated their immunostimulatory action on the cyclophosphamide (CPA)-induced immunosuppression mice model.
MATERIALS AND METHODS
Materials
HC (whole plant) was collected at four Vietnam cities/provinces of Ha Noi, Dak Lak, Bien Hoa, and Long An, from January 2018 to March 2018. To identify the HC, the collected plant materials were morphologically determined by a botanical expert based on the book “Plants of Vietnam” and the Vietnamese pharmacopeia. The HC voucher specimens were kept at the Faculty of Pharmacy, Can Tho University of Medicine and Pharmacy, Can Tho, Vietnam. The standard compounds hyperoside and quercitrin were purchased from TRC company, Canada. Absolute ethanol (EtOH), ethyl formate, toluene, formic acid, acetonitrile (ACN), methanol (MeOH), chloroform, sulfuric acid (98%), and phosphoric acid (85%) were bought from Merck, Germany and J.T. Baker, USA. 3-(4,5-Dimethylthiazol-2-yl)-2,5- diphenyl tetrazolium bromide) (MTT) was imported from Sigma-Aldrich, USA. Cells culture materials, namely, Dulbecco′s modified eagle′s medium (DMEM) medium, fetal bovine serum phosphate buffer saline (PBS), Penicillin-Streptomycin (PenStrep), and trypsin-Ethylenediaminetetraacetic acid (EDTA), were supplied by Gibco, USA. All other chemicals used were of reagent grade or higher.
Plant extractions
The collected HC was dried at ambient temperature, ground to fine powder, and sieved through a 355-μm mesh. Then, 1 gram of the HC powder was macerated twice, with sonication, with 20 ml of the extraction solvents for 30 minutes. Finally, the extract was rotavapor at 60°C until dry solid mass. In this study, the effects of six different extraction solvents (absolute EtOH, EtOH 96%, EtOH 70%, EtOH 50%, EtOH 30%, and water) on the extract yields were investigated.
Flavonoid isolations and purifications
The optimal extract was then undergone column chromatographic and preparative high-performance liquid chromatographic (HPLC) processes to isolate the bioactive flavonoids. To this end, the dried extract (15.05 g) was first dispersed in ethyl acetate, followed by column chromatographic eluted with a mobile phase of n-hexane–ethyl acetate (9:1 to 3:7 v/v). The fractions were checked with thin layer chromatography (TLC) using the solvent mixture of toluene–ethyl acetate–acetone–MeOH (10:5:5:0.6 v/v/v/v) under the UV 235 nm and UV 365 nm. Fractions with similar TLC patterns were pooled and the appropriate pooled fractions were subjected to preparative HPLC (Shimadzu LC-20AP, detector UV SPD-20A, column Agilent Prep–C18 (21.2 × 250 mm × 10 μm), detected wavelength of 210 nm, injection volume of 900 μl, flow rate of 5.0 ml/minute, and mobile phase of MeOH–water (gradient mode, 0–39 minute: 40:60 v/v; 40–49 minute: 70:30 v/v; 50–75 minute: 80:20 v/v). All HPLC analyses were critically validated, regarding the specificity, accuracy, precision, linearity, range, and limit of detection/limit of quantitation, following the International Council for Harmonisation (ICH) guidelines.
The isolated compounds obtained from the preparative HPLC were tested for their purities prior to structure elucidation. For this, HPLC-Photodiode array analysis [Shimadzu LC-203°C machine, column Phenomenex Gemini NX C18 (250 × 21.2 mm × 5 μm), detected wavelength of 210 nm, injection volume of 10 μl, a flow rate of 1 ml/minute, and a mobile phase of ACN–water].
Flavonoid structure elucidations
The flavonoid chemical structures were elucidated by spectroscopic methods, including Fourier-transform infrared spectroscopy (FTIR), mass spectrometry (MS), and nuclear magnetic resonance spectroscopy nuclear magnetic resonance (13C-NMR and 1H-NMR).
For the FTIR, the KBr pellet technique was utilized on the Bruker Alpha T spectrophotometer (Bruker, USA), connected with a Deuterated triglycine sulfate detector. The FTIR spectra were obtained at a wavenumber range of 4,000–400 cm−1, with a 4.0 cm−1 resolution. For the MS, the flavonoid masses were determined using a Fourier-transform ion cyclotron resonance apparatus (Perkin-Elmer, USA) at room temperature with the electrospray ionization method. For the NMR, the compounds dissolved in CDCl3 (δH 7.27 s; δC 77.0) were analyzed on a Bruker Avance 500 MHz instrument. Signals were shown on a ppm scale with tetramethylsilane as an internal standard.
Extract stability test
Before the therapeutic activity evaluations, the HC extract was subjected to the stability test using the accelerated storage condition at a temperature of 40°C ± 2°C and relative humidity of 75% ± 5% (KBF 720 Binder constant climate chamber, Germany) for 6 months. At each time interval of 0, 1, 3, and 6 months, the extracts were tested their physicochemical properties: (1) appearance, (2) water content, (3) chemical determinations (by HPLC), (4) chemical quantitation (assays, by HPLC, based on two biomarkers hyperoside and quercitrin), and (5) microbial limit (aerobic bacteria, yeast and mold, Gram (−) bacteria, and pathogenic bacteria). The extracts were considered stable when the qualitative factors did not different than the initial extract, and the quantitative values were in the range of ±10% compared to those of the initial extract. All extract parameters must pass the requirements in the Vietnamese pharmacopeia.
Extract standardization
Qualitative (TLC) and quantitative (HPLC) methods were developed and validated to standardize the HC extract and its biomarkers. To this end, the TLC of the HC extract and the reference biomarkers of quercitrin and hyperoside were run with a mobile phase consisting of ethyl acetate–butan-2-one–formic acid–water (24:3.6:1.5:0.9 v/v/v/v). The AlCl3 2.5% ethanolic solution was used to visualize the layer at 105°C under normal or UV light (254 and 366 nm). For the HPLC analysis, the HC extract and its markers were subjected to the HPLC system of Shimadzu LC-203°C machine, column Phenomenex Gemini NX C18 (250 × 21.2 mm × 5 μm), injection volume of 10 μl, a flow rate of 1 ml/minute, and a mobile phase of ACN–water.
In vitro cytotoxicity test
The cytotoxicity activities on the leukemia K562 (ATCC CCL-243™, USA) and HL60 cell line (ATCC CCL-240™, USA) of the HC extract and the pure isolated flavonoids were measured using the MTT assay. To this end, cells were cultured in DMEM medium, supplemented with 10% FBS and 1% PenStrep, followed by incubation at 37°C, 5% CO2 until 70%–80% coverage. Then, the HL60 cells were collected using trypsin-EDTA 0.25 M and redispersed in 96-well culture plates (2.5 × 104 cells/well). The plate was then incubated for another 24 hours at 37°C, 5% CO2 for cell growth and adhesion. After that, the HC extract and the pure compounds, dissolved in DMSO at various concentrations of 5–100 μg/ml, were added to the wells and incubated for 72 hours. The negative control (solvent) and the positive control (doxorubicin) were conducted similarly to the test samples. After 72 hours, the cells were washed with PBS twice and incubated with 150 μl MTT solution in serum-free culture medium (0.5 mg/ml) at 37°C, 5% CO2 for another 3 hours. Finally, the formazan products were dissolved in DMSO–water (5:5 v/v), and the solutions’ absorbance values were determined using UV-Vis spectroscopy at 570 nm by a microplate reader. The cell viability percentage is calculated based on the following equation:
In vivo immunostimulatory activity test
To evaluate the immunostimulatory action of the HC extract and the pure flavonoids, in vivo CPA-induced immunosuppression mice model was utilized. For this, 80 healthy mice (Mus musculus, Pasteur Institute, Vietnam, 6 weeks old, body weight 25–35 g) were acclimatized for 7 days, fed standard rodent meals and water ad libitum, allocated in cages at a temperature of 20°C–25°C and a humidity of 70%–75%, with controlled 12 hours light/dark cycles. Then, the mice were separated into 10 groups, 8 mice/groups, as follows: (1) the CPA control group (normal/physiologic mice), (2) the negative control group (mice treated with CPA to induce immunosuppression), (3) the vehicle control group (CPA-induced mice treated with the extract/compounds solvent, DMSO 1%), (4) the reference control group (CPA-induced mice treated with the reference immunostimulant, filgrastim 50 μg/kg), (5 and 6) the extract-treated group (CPA-induced mice treated with the HC extract, at a dose of 240 and 480 mg/kg), (7, 8, 9, and 10) the flavonoid-treated group (CPA-induced mice treated with the pure flavonoids, at different doses). To induce the immunosuppression mice, CPA, at a dose of 150 mg/kg, was intraperitoneally injected 1 day before the test compound administrations, respectively. The reference/test substances were orally administered five times at 24-hour intervals.
Alterations in the mice’s body weights; the relative weights of the mice’s immune system organs such as liver, spleen, thymus, and adrenal glands; and the mice leukocyte/white blood cell (WBC) formula, during and after the treatments, were recorded, compared, and reported.
All in vivo animal experiments were conducted based on international regulations for the usage and welfare of laboratory animals. The study protocol and other relevant documentation were ethically approved by the University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam (Approval Number 3716/QD-DHYD).
Statistical analysis
All quantitative experiments were conducted in triplicate, and the results were presented as mean ± SD (Mean ± SD). Student’s t-test and the analysis of variance test were employed to compare the sample values, with p < 0.05 for significant comparisons.
RESULTS AND DISCUSSION
HC (Fig. 1) is a popular medicinal plant in Vietnam and Southeast Asia, which has been ethnopharmacologically utilized in treating various diseases (Fu et al., 2013; Laldinsangi, 2022; Wu et al., 2021). Nevertheless, limited research has reported using HC extract and its flavonoid contents as a leukemia chemotherapeutic agent and an immunostimulant. Therefore, this study critically investigated the Vietnamese HC extraction processes and their flavonoid content isolation, purification, and structure elucidation. Then, the optimal extract was further evaluated for its long-termed stability in accelerated storage conditions for 6 months. Finally, the HC extract and its isolated flavonoids were in vitro determined their antileukemia effect on K562 and HL60 cell lines, and in vivo determined their immunostimulatory effect on the CPA-induced immunosuppression mice model.
First, the optimal extraction solvent was investigated based on the extraction yields. For this, the absolute EtOH, EtOH 96%, EtOH 70%, EtOH 50%, EtOH 30%, and water yielded extraction efficiencies of 11.93%, 19.28%, 34.79%, 30.24%, 33.15%, and 26.74%, respectively. Thus, the EtOH 70% was considered the best solvent to extract HC with the highest efficiency. This fact was in accordance with the previous studies, which utilized EtOH 70% (Lee et al., 2015) or EtOH 80% (Chiow et al., 2016; Kim et al., 2007) as an extraction solvent for HC. The multipurpose solvent EtOH could extract various types of compounds such as alkaloids, sterols, terpenoids, and phenolics and their glycoside derivatives (Agarwal et al., 2016; Azmir et al., 2013). In EtOH–water mixtures, water could enhance the extractions of polar compounds such as phenolics (i.e., flavonoids and their glycosides) (Azmir et al., 2013). Therefore, mixtures of EtOH–water are often appropriate for extracting polyphenol compounds such as flavonoids. Conclusively, EtOH 70% was the optimal solvent for HC extraction.
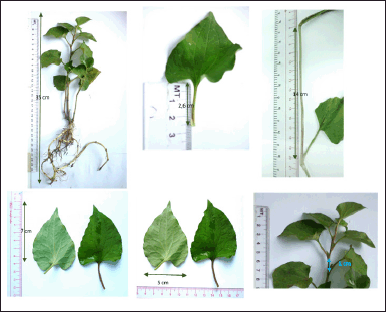 | Figure 1. HC Thunb. plant. [Click here to view] |
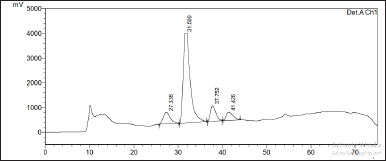 | Figure 2. Preparative HPLC chromatogram of the HC Thunb. extract. [Click here to view] |
Second, the HC extract was further fractionated to isolate its flavonoid content. After utilizing the preparative HPLC, three distinct compounds (Q1, Q2, and Q3) were collected corresponding to the separate peaks of the chromatogram (Fig. 2). The elutes were then added to a rotavapor to solid mass to obtain 55.3 mg of Q1, 171 mg of Q2, and 30 mg of Q3, respectively. These compounds were structure-elucidated using FTIR (Fig. 3), MS, and NMR spectroscopy methods. To this end, the MS ion peaks [M-H]− of Q1 was 463.0817 (theory 463.0800), corresponding to the chemical formula C21H20O12; of Q2 was 447.0910 (theory 447.0933), corresponding to the formula C21H20O11; and of Q3 was 431.0817 (theory 431.0878), corresponding to the formula C21H20O10. The 13C-NMR (125 MHz, DMSO-d6) and 1H-NMR (500 MHz, DMSO-d6) spectra of Q1, Q2, and Q3 demonstrated chemical shifts similar to those in the literature of the hyperoside (Olszewska, 2005), quercitrin (Bose et al., 2013), and afzelin (Trinh et al., 2006) spectra, respectively (Table 1). This information, together with the FTIR and MS data, confirmed that Q1 is hyperoside, Q2 is quercitrin, and Q3 is afzelin (Fig. 4). Our results reconfirmed the presence of these three main compounds in the HC extract, which has been previously reported in the literature (Chen et al., 2012; Chou et al., 2009; Lee et al., 2015), and have already been isolated from HC. Nevertheless, the HC extract and these flavonoid activities on blood cancer (leukemia) and the immune system have not been thoroughly investigated, to the best of our knowledge. Thus, these actions will be discussed in the last section.
Third, natural products, due to their complexity, are commonly deteriorated (i.e., compound degradations), especially during storage at ambient temperature, leading to a decrease in bioactive component contents and, consequently, therapeutic efficiency. Therefore, the HC extract was tested for its stability prior to therapeutic activity tests. For this, the HC extract long-term stability was determined using accelerated storage conditions (temperature of 40°C ± 2°C and humidity of 75% ± 5%). Physicochemically, the extract was stable for at least 6 months, as all properties of (1) appearance, (2) water content, (3) chemical determinations, (4) chemical quantitation (assays), and (5) microbial limit were similar (i.e., in the range of ±10%) compared to the initial extract (Table 2). Furthermore, all extract parameters passed the requirements in the Vietnamese pharmacopeia, indicating their suitability for the therapeutic tests.
Fourth, the HC extract and its biomarkers were standardized using the qualitative (TLC) and quantitative (HPLC) methods. For the TLC method (Fig. 5), the HC extract has six spots, of which two main spots have Rf values of 0.40 and 0.62, corresponding to the hyperoside and quercitrin Rf, respectively, in the reference samples. The chromatograms of the placebo sample were spotless. Therefore, the TLC method for the qualitative determination of HC has specificity and could be used to quantify quercitrin and hyperoside in this extract. Regarding the quantitative HPLC method, all parameters were ICH evaluated and were in the acceptable criteria (Table 3), indicating the suitability of this method on the quantification of the two biomarkers, quercitrin, and hyperoside, in the HC extract.
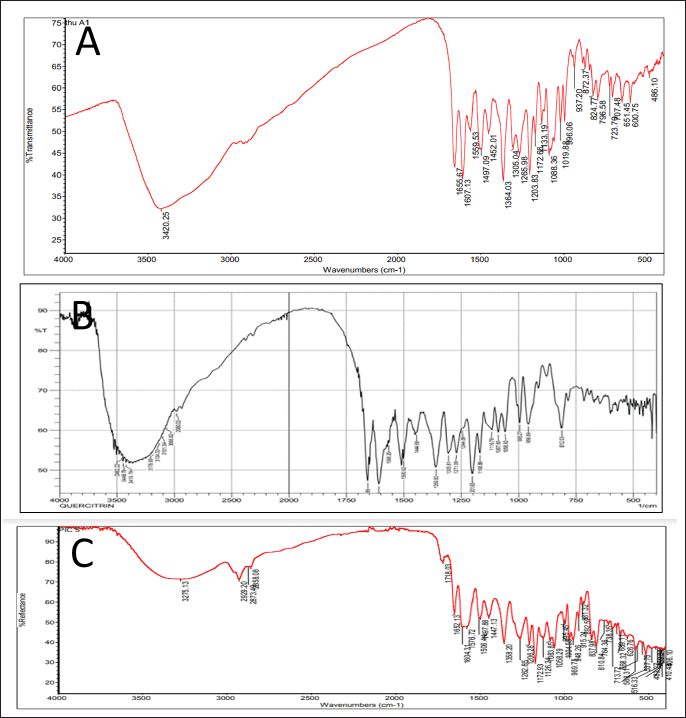 | Figure 3. FTIR spectra of the flavonoid compounds isolated from the HC Thunb. extract. (A) Q1, hyperoside; (B) Q2, quercitrin; (C) Q3, afzelin. [Click here to view] |
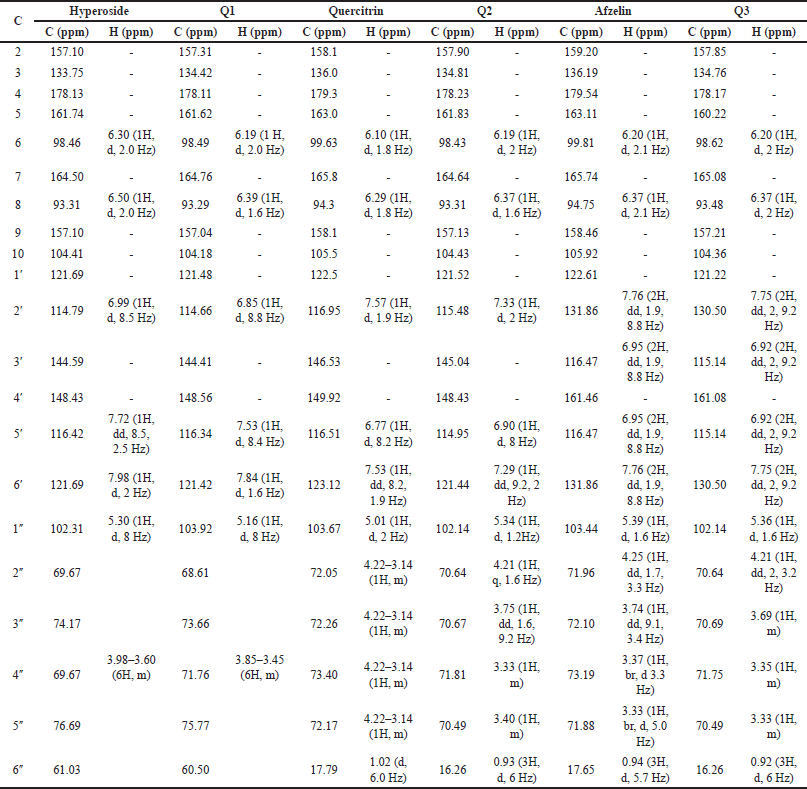 | Table 1. Comparison of 13C-NMR (125 MHz, DMSO-d6) and 1H-NMR (500 MHz, DMSO-d6) of compound Q1 and hyperoside (Olszewska, 2005), Q2 and quercitrin (Bose et al., 2013), and Q3 and afzelin (Trinh et al., 2006). [Click here to view] |
 | Figure 4. Chemical structures of (A) Q1, hyperoside; (B) Q2, quercitrin; (C) Q3, afzelin. [Click here to view] |
Finally, the HC extract and its isolated flavonoids, hyperoside, and quercitrin, were evaluated for their therapeutic efficiencies using two assays, the MTT assay for the in vitro leukemia cytotoxicity test and the in vivo CPA-induced immunosuppression mice model for the immunostimulatory test.
Regarding the MTT assay, the HC extract, hyperoside, and quercitrin, at concentrations of 5–100 μg/ml, were nontoxic to both the HL60 and K562 leukemia cell lines, with <50% of cell deaths after 72 hours of incubations. Thus, these extracts/compounds might not be suitable for treating leukemia.
 | Table 2. Stability test of the HC Thunb. extract, under the accelerated storage condition at a temperature of 40°C ± 2°C and relative humidity of 75% ± 5% for 6 months (n = 3). [Click here to view] |
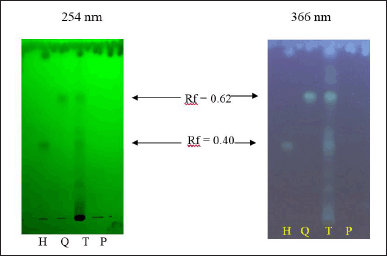 | Figure 5. TLC, under the UV light 254 and 366 nm, of the HC Thunb. extract (T) and its biomarkers of quercitrin (Q) and hyperoside (H), and the placebo sample (P). [Click here to view] |
For the immunostimulatory test, the extract/compounds demonstrated potential actions. Prior to the test, the WBC counts of all mice were measured, and the results revealed that these parameters were within the limits of the normal range (data not shown), indicating that the mice were suitable for the experiments. First, for all the mice characteristics, compared to the physiologic control group (group 1), the CPA-induced group (group 2) showed a significant decrease in the mice weights (p < 0.05) (Table 4), the weights of the immune system tissues/organs (liver, spleen, thymus, and adrenal glands) (p < 0.05) (Table 5), and the WBC counts, for both the neutrophils (NEU), lymphocytes (LYM), and monocytes (MONO) (p < 0.05) (Table 6), indicating the immunosuppression effects of CPA on the mice. Second, the mice characteristics in the vehicle control group (group 3) were not statistically different from those in group 2, suggesting that the solvent DMSO used to dissolve the extracts/compounds did not affect the mice. Third and most importantly, both the HC extract (480 mg/kg), the hyperoside (5 and 10 mg/kg), and the quercitrin (10 and 20 mg/kg) significantly (p < 0.05) increased the CPA-induced mice WBC counts, for both the NEU, the LYM, and the MONO. Interestingly, at high doses of 480 mg/kg for the extract, 10 mg/kg for hyperoside, and 20 mg/kg for quercitrin, these substances possessed comparable effects with the standard drug filgrastim, with no significant differences in the WBC increment (Table 6). These data suggest that the HC extract and its isolated flavonoids, hyperoside, and quercitrin could be potential immunostimulants. Last but not least, it is worth noting that both the reference drug, filgrastim, and the extracts/compounds could not restore the mice’s body weights and their immune organs’ weights to the initial state. This might be attributed to the short investigation time of the study (i.e., only 05 days after the treatments), which could not be adequate for the mice to recover fully.
CONCLUSION
This study investigated the HC optimal extraction condition (EtOH 70%), its flavonoid isolations, purifications, and structure elucidations (to be quercitrin, hyperoside, and afzelin), the extract long-term stability (stable for at least 6 months), its in vitro anticancer effect on the leukemia cell lines of K562 and HL60, and in vivo immunostimulatory action on the CPA-induced immunosuppression mice model. Although the HC extract and its flavonoid compositions, at concentrations of 5–100 μg/ml, possessed no potential cytotoxicity on the leukemia cell lines, the HC extract (480 mg/kg), hyperoside (10 mg/kg), and quercitrin (20 mg/kg) significantly increased the CPA-induced mice WBC counts comparably with the standard drug filgrastim, indicating these extract/compound had potential immunostimulatory action. Conclusively, the HC extract and its flavonoids (quercitrin and hyperoside) could be further researched to become novel immunostimulants.
 | Table 3. HPLC analysis validation of the HC Thunb. extract, in terms of its biomarkers of quercitrin and hyperoside. TR: retention time; RSD: relative standard deviation; N: theoretical plates; As: asymmetric factor. [Click here to view] |
 | Table 4. Mice body weight (g) alterations after 5 days of treatment between the immunostimulatory activity testing groups (n = 8). CPA: cyclophosphamide; HC: Houttuynia cordata Thunb. [Click here to view] |
ACKNOWLEDGMENTS
The authors would like to thank Can Tho University and Can Tho University of Medicine and Pharmacy for supporting this research.
LIST OF ABBREVIATIONS
ACN, Acetonitrile; CPA, Cyclophosphamide; EtOH, Ethanol; HC, Houttuynia cordata Thunb.; HPLC, High-performance liquid chromatography; ICH, International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; LYM, Lymphocyte; MeOH, Methanol; MONO, Monocyte; MS, Mass spectrometry; MTT, 3-(4,5-Dimethylthiazol-2-yl)-2,5- diphenyl tetrazolium bromide); NEU, Neutrophil; NMR, Nuclear magnetic resonance; PBS, Phosphate buffer saline; TLC, Thin layer chromatography; WBC, White blood cell.
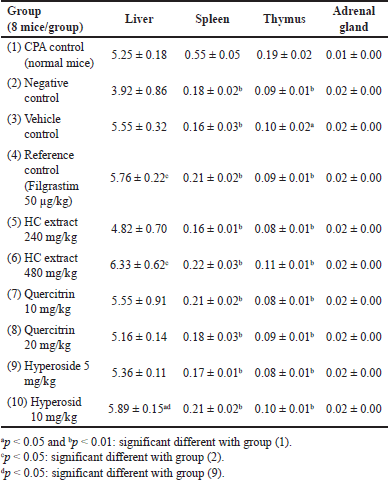 | Table 5. Alterations in the relative weights (%g/g) of the mice liver, spleen, thymus, and adrenal glands after 5 days of treatment between the immunostimulatory activity testing groups (n = 8). CPA: cyclophosphamide; HC: Houttuynia cordata Thunb. [Click here to view] |
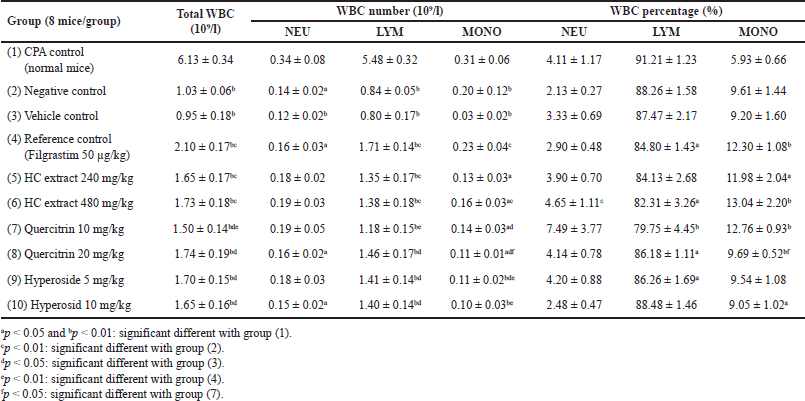 | Table 6. Alterations in the mice WBC formula after 5 days of treatment between the immunostimulatory activity testing groups (n = 8). CPA: cyclophosphamide; HC: Houttuynia cordata Thunb. NEU: neutrophil; LYM: lymphocyte; MONO: monocyte. [Click here to view] |
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The animal experiments were ethically approved by the University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam(Approval Number 3716/QD-DHYD).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Agarwal A, Kumar A, Singh BK, Trivedi N, Jha KK. A review on extraction and phytochemical screening methods. Res Pharm Heal Sci, 2016; 2:130–7; https://doi.org/10.32463/RPHS.2016.V02I02.25
Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NAN, Omar AKM. Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng, 2013; 117:426–36; https://doi.org/10.1016/J.JFOODENG.2013.01.014
Bose S, Maji S, Chakraborty P. Quercitrin from Ixora coccinea leaves and its anti-oxidant activity. J PharmaSciTech, 2013; 2:72–4.
Chen SD, Gao H, Zhu QC, Wang YQ, Li T, Mu ZQ, Wu HL, Peng T, Yao XS. Houttuynoids A-E, anti-herpes simplex virus active flavonoids with novel skeletons from Houttuynia cordata. Org Lett, 2012; 14:1772–5; https://doi.org/10.1021/OL300017M
Chen YY, Liu JF, Chen CM, Chao PY, Chang TJ. A study of the antioxidative and antimutagenic effects of Houttuynia cordata Thunb. using an oxidized frying oil-fed model. J Nutr Sci Vitaminol (Tokyo), 2003; 49:327–33; https://doi.org/10.3177/JNSV.49.327
Chiow KH, Phoon MC, Putti T, Tan BKH, Chow VT. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac J Trop Med, 2016; 9:1–7; https://doi.org/10.1016/J.APJTM.2015.12.002
Chou SC, Su CR, Ku YC, Wu TS. The constituents and their bioactivities of Houttuynia cordata. Chem Pharm Bull (Tokyo), 2009; 57:1227–30; https://doi.org/10.1248/CPB.57.1227.
Fu J, Dai L, Lin Z, Lu H. Houttuynia cordata Thunb: a review of phytochemistry and pharmacology and quality control. Chin Med, 2013; 4:101–23; https://doi.org/10.4236/CM.2013.43015
Hayashi K, Kamiya M, Hayashi T. Virucidal effects of the steam distillate from Houttuynia cordata and its components on HSV-1, influenza virus, and HIV. Planta Med, 1995; 61:237–41; https://doi.org/10.1055/S-2006-958063
Huynh DTM, Le MNT, De Tran V, Pham DT. Antibacterial hydrogel containing Piper betle L. extract for acne treatment, an ex vivo investigation. Pharm Sci Asia, 2022; 49:381–9; https://doi.org/10.29090/PSA.2022.04.22.061
Kim IS, Kim JH, Kim JS, Yun CY, Kim DH, Lee JS. The inhibitory effect of Houttuynia cordata extract on stem cell factor-induced HMC-1 cell migration. J Ethnopharmacol, 2007; 112:90–5; https://doi.org/10.1016/J.JEP.2007.02.010
Kim SK, Ryu SY, No J, Choi SU, Kim YS. Cytotoxic alkaloids from Houttuynia cordata. Arch Pharm Res, 2001; 24:518–21; https://doi.org/10.1007/BF02975156
Laldinsangi C. The therapeutic potential of Houttuynia cordata: a current review. Heliyon, 2022; 8:e10386; https://doi.org/10.1016/J.HELIYON.2022.E10386
Lee JH, Ahn J, Kim JW, Lee SG, Kim HP. Flavonoids from the aerial parts of Houttuynia cordata attenuate lung inflammation in mice. Arch Pharm Res, 2015; 38:1304–11; https://doi.org/10.1007/S12272-015-0585-8
Lu HM, Liang YZ, Yi LZ, Wu XJ. Anti-inflammatory effect of Houttuynia cordata injection. J Ethnopharmacol, 2006; 104:245–9; https://doi.org/10.1016/J.JEP.2005.09.012
Nguyen PH, De Tran V, Pham DT, Dao TNP, Dewey RS. Use of and attitudes towards herbal medicine during the COVID-19 pandemic: a cross-sectional study in Vietnam. Eur J Integr Med, 2021; 44:101328; https://doi.org/10.1016/J.EUJIM.2021.101328
Olszewska M. Flavonoids from Prunus serotina Ehrh. Acta Pol Pharm, 2005; 62:127–33.
Pham DT, Saelim N, Tiyaboonchai W. Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy. Colloids Surf B, 2019; 181:705–13; https://doi.org/10.1016/j.colsurfb.2019.06.011
Pham DT, Thao NTP, Thuy BTP, De Tran V, Nguyen TQC, Nguyen NNT. Silk fibroin hydrogel containing Sesbania sesban L. extract for rheumatoid arthritis treatment. Drug Deliv, 2022; 29:882–8; https://doi.org/10.1080/10717544.2022.2050848
Traditional, Complementary and Integrative Medicine. n.d. Available via https://www.who.int/health-topics/traditional-complementary-and-integrative-medicine#tab=tab_1 (Accessed 18 October 2022).
Tran VD, Pham DT, Cao TTN, Bahlol M, Dewey RS, Le MH, Nguyen VA. Perspectives on COVID-19 prevention and treatment using herbal medicine in Vietnam: a cross-sectional study. Ann Ig, 2022; 34; https://doi.org/10.7416/AI.2021.2484
Trinh TT, Tran VS, Nguyen TH. Study on the chemical constituents of Fissistigma pallens. Vietnam J Chem, 2006; 44:412–7.
WHO Global Centre for Traditional Medicine . n.d. Available via https://www.who.int/initiatives/who-global-centre-for-traditional-medicine (Accessed 18 October 2022).
Wu Z, Deng X, Hu Q, Xiao X, Jiang J, Ma X, Wu M. Houttuynia cordata Thunb: an ethnopharmacological review. Front Pharmacol, 2021; 12:714694; https://doi.org/10.3389/FPHAR.2021.714694
Yang L, Jiang JG. Bioactive components and functional properties of Hottuynia cordata and its applications. Pharm Biol, 2009; 47(12):1154–61; https://doi.org/10.3109/13880200903019200