INTRODUCTION
The Saurauia vulcani plant was used as traditional medicine in North Sumatra. This plant is called a pirdot, which grows wild on the forest edge. This plant is generally used as an antidiabetic medication. This plant has been planted on critical lands such as Merek, Karo Regency (Pasaribu et al., 2020).
This plant is quite easy to propagate in terms of cultivation, so its development as herbal biomass will not encounter problems. Various studies have investigated this species and its genus and have revealed its bioactivities, such as antidiarrheal, antidiabetic, anticholesterol, antihyperglycemic, and antioxidant effects (br Ginting et al., 2018; Gurning et al., 2020; Hutahaean et al., 2018; Lovena et al., 2018; Musa et al., 2019; Sitorus et al., 2018). Phytochemically, the extract contains saponins, tannins, flavonoids, glycosides, steroids, and triterpenoids (Rosidah et al., 2021). Several types of compounds reported are 3-hydroxyolean-12-en-28-oic acid and 3,19-dihydroxyurs-12-en-28-oic acid (Musa et al., 2019).
Some previous studies reported that S. vulcani leaf extract has anticancer activities against WiDr and HCT 116 cell lines. The WiDr cell line exhibits cytotoxicity when exposed to fractions of n-hexane, ethyl acetate, and methanol; the 50% inhibition concentration (IC50) values for these fractions are 456.19, 97.41, and 191.92 ppm, respectively. Our previous research showed that the leaf extract’s cytotoxicity activity against the HCT cell lines is achieved at an IC50 of 777.35, 568.53, and 529.39 ppm, respectively, with fractions of n-hexane, ethyl acetate, and methanol (Pasaribu et al., 2021).
Anticancer research using natural ingredients for cancer drugs will continuously be investigated worldwide due to the rising number of cancer patients, particularly those with colorectal cancer. Today, colorectal cancer is the third leading cause of death, while ovarian and breast cancers are in the first and second places, respectively (Granados-Romero et al., 2017).
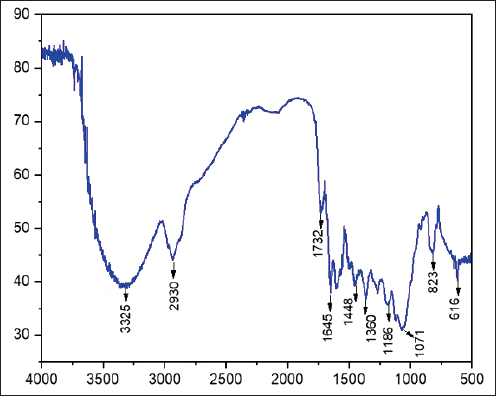 | Figure 1. FTIR spectrum of isolated compound 1. [Click here to view] |
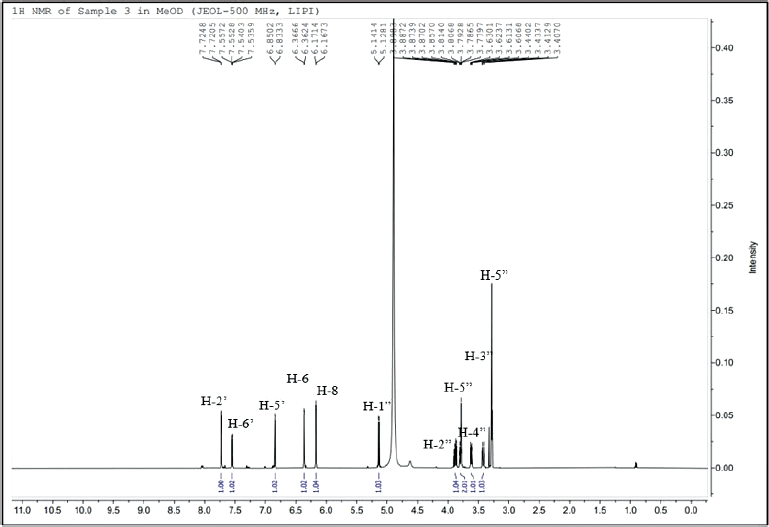 | Figure 2. 1H-NMR spectrum of the isolated compound 1. [Click here to view] |
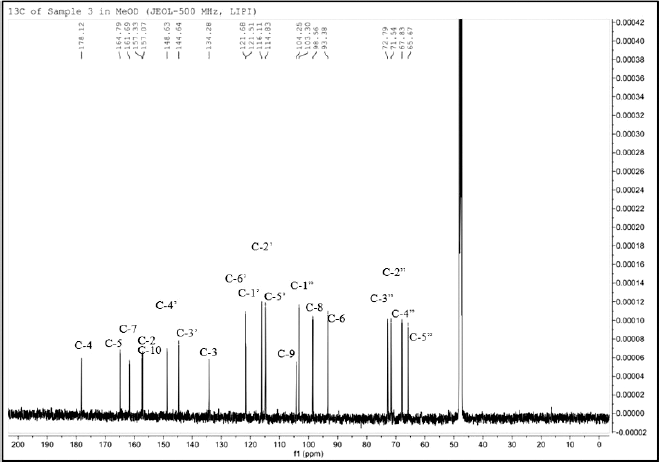 | Figure 3. 13C-NMR spectrum of the isolated compound 1. [Click here to view] |
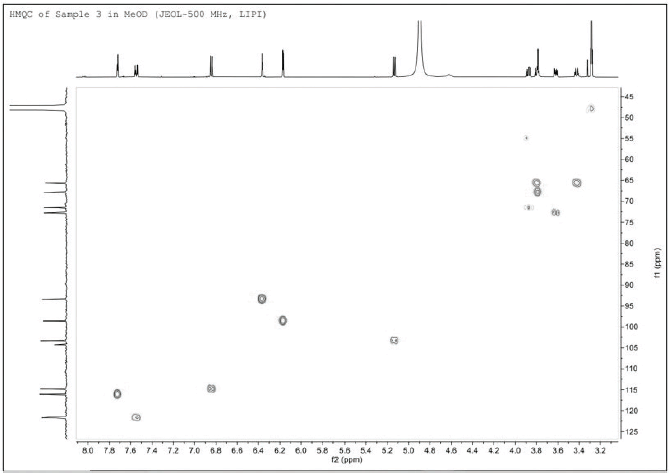 | Figure 4. 1H↔13C HSQC NMR spectrum of isolated compound 1. [Click here to view] |
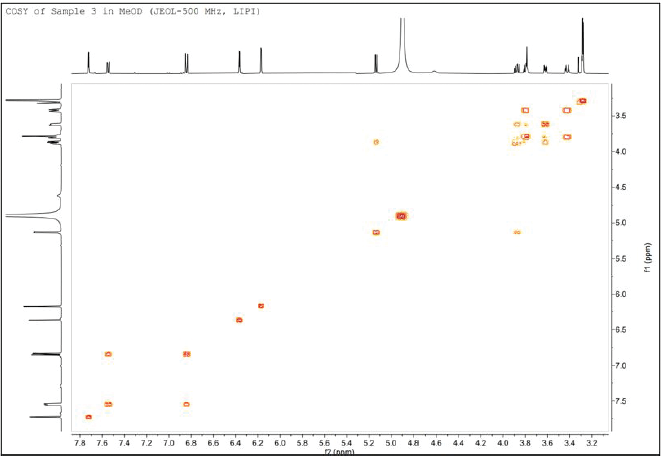 | Figure 5. 1H↔1H COSY NMR spectrum of isolated compound 1. [Click here to view] |
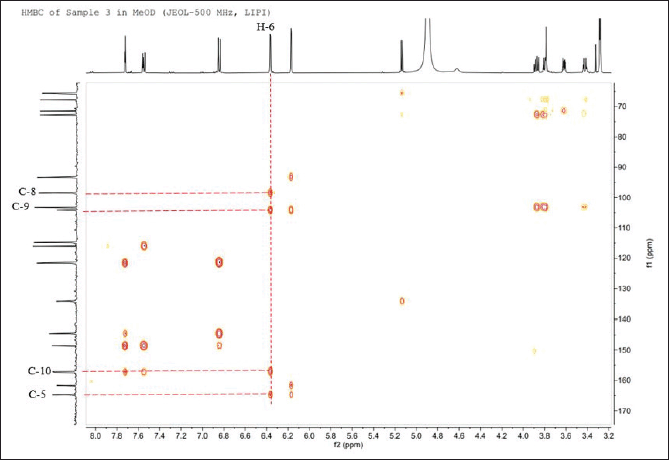 | Figure 6. 1H↔13C HMBC NMR spectrum of the isolated compound 1. [Click here to view] |
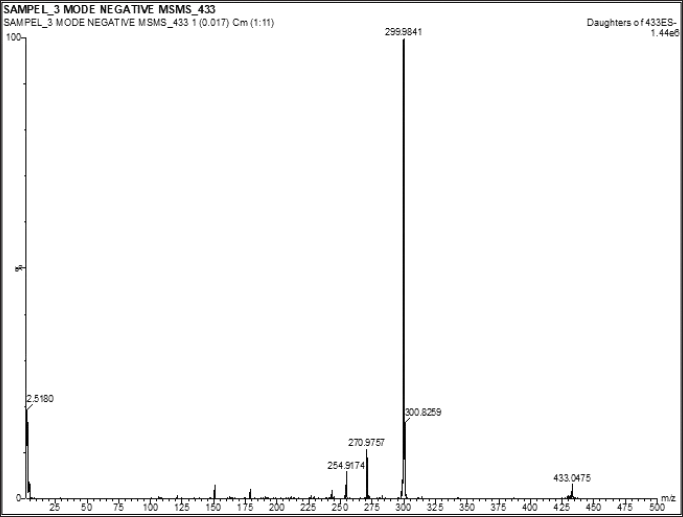 | Figure 7. MS spectra of compound 1. [Click here to view] |
Colorectal cancer is very commonly found in people worldwide. The main causes of colorectal cancer are bad habits, a poor diet, smoking, colitis, inherited risk factors, and aging (Benarba and Pandiella, 2018; Hashim et al., 2016).
Conventional cancer treatment with chemotherapy sometimes gives unexpected side effects that cause drug resistance (Kuipers et al., 2015). The adverse effects of chemotherapy for colorectal cancer have been reduced in several ways, including renewable natural substances like plants and other living things.
Herbal medicines can be developed by finding active compounds from the plant extracts produced. This research aims to isolate an active compound from ethyl acetate fractions in WiDr cancer cells and determine the activities of the isolated compound as a colorectal anticancer.
MATERIAL AND METHODS
Materials
The plant materials were collected from the Indonesian Ministry of Environment and Forestry research forest in the Sipiso-piso area, Karo Regency. For authentication, samples were tested at Herbarium Bogoriensis, Research Center for Biology LIPI, Indonesia. Meanwhile, WiDr cells made up the cancer cell components.
Experimental procedures
Fractionation
The S. vulcani leaf was extracted in the previous studies (Pasaribu et al., 2021). In the next step, the fractionation was carried out based on the level of polarity. The test results show that the ethyl acetate fraction gives the best bioactivity. Thus, in the next stage, the ethyl acetate fraction becomes the material to isolate and test its bioactivity.
Isolation
The ethyl acetate fraction was separated using vacuum liquid chromatography and purified using radial chromatography (RC) with a solvent system used as the eluent ethyl acetate: methanol: Aquadest = 4.6:0.2:0.2 to yield 50 mg of the isolated compound. To improve the separation process, the stationary phase is compacted by lowering the mobile phase and the column walls are tapped slowly. After that, the concentrated extract is added gradually with a dropper pipette through the column wall, then eluted with the eluent determined previously by TLC. The fractions were accommodated in test tubes, and each tube was identified by TLC. Next, the fractions with the same spots are combined into one fraction, and the eluent is evaporated using a rotary evaporator. Afterward, RC and Merck Kieselgel 60 PF254 round glass plates were used to perform the isolation. All of the prior profiles were detected using fragments from aluminum Merck TLC silica gel 60 F254 sheets measuring 20 × 20 cm and having a thickness of 0.25 mm. The profiles were then checked at UV light (254 nm) or using an H2SO4 spray heating after the reaction.
Test of anti-colorectal cancer activities
The stages of in vitro anti-colorectal cancer tests followed the procedures and were conducted in previous studies (Pasaribu et al., 2021). The avicularin compound’s cytotoxicity was measured using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay. The method depended on the breakdown of the yellow tetrazolium salt, MTT, into a soluble blue formazan product by mitochondrial enzymes. The amount of formazan was proportional to the number of live cells present during the MTT exposure. Human colon adenocarcinoma WiDr cells were used in the test. The cells were cultivated in DMEM, 10% fetal bovine serum, and 1% antibiotic (Penicillin Streptomycin) containing media in a suitable environment (37°C, 5% CO2, 90% humidity). In total, 5,000 cells/well of the subcultured cells were placed into a 96-well tissue culture plate to adhere for 18–20 hours in a growth medium. Then triplicate tests on each concentration were conducted. The media was removed from the wells the next day and replaced with an avicularin compound in a growth medium. To make the sparingly soluble avicularin compound homogenous, dimethyl sulfoxide (DMSO) was added; the final concentration of DMSO was 0.5% in each well. The avicularin compound was then added to the plates at varied concentrations for 48 hours: 400, 200, 100, 50, 25, and 12.5 mg/l. Each well in the plates received a dose of MTT (10 l of 5 mg/ml in PBS). The plates underwent an additional 4 hours of incubation. After that, the medium was taken out, and 100 l of 1 N HCl in isopropanol was added. Finally, a microplate reader was used to read the plates’ absorbance at 562 nm. The absorbance results were calibrated using 1 N HCl in isopropanol as a blank, and the absorbance of the cells exposed to the corresponding medium was interpreted as 100% cell viability (taken as negative control). Based on the relationship between percent inhibitions and concentrations, the IC50 of the avicularin compound was calculated for each cell.
RESULTS AND DISCUSSION
Isolation major compound
This study has discovered that the obtained compound is a yellow-pale solid, and the melting point is 215°C–217°C. The ultraviolet (UV) spectrum of compound 1 shows maximum absorption at λmax (MeOH) (log ε) 255–358 nm, which experiences a bathochromic shift with the addition of NaOH. The UV spectral pattern indicates that this compound has a phenol chromophore with an extended conjugation system.
Meanwhile, the infrared (IR) spectrum denotes strong absorption at max 3,252 cm−1 for the hydroxyl group, 2,956 cm−1 (aliphatic CH), and 1,645 (conjugated C=O) and νmax 1,612, 1,516, and 1,442 cm−1for aromatic units (Fig. 1).
NMR spectroscopy was used to determine the chemical structure of the compound. The 1H NMR spectrum data (Fig. 2) indicated 11 signals. Five signals are related to the aromatic system, with H-6, H-8, H2’, H-5’, and H-6’ having chemical shifts at δH 6.36, 6.17, 7.72, 6.84, and 7.55 ppm, respectively. The other six signals were deshielded area and appear at δH 5.13 (H-1”), 3.88 (H-2”), 3.62 (H-3”), 3.78 (H-4”), 3.81 (H-5”a), and 3.42 (H-5”b) ppm.
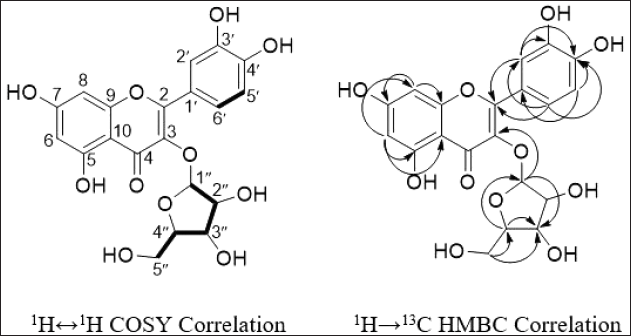 | Figure 8. 1H↔1H COSY and 1H→13C HMBC-NMR correlation of compound 1. [Click here to view] |
Furthermore, it is also necessary to know the amount of carbon from this compound. The 13C NMR spectrum (Fig. 3) shows that 20 carbon signals represent 20 carbon atoms, consisting of one carbonyl area of carbon atoms at δC 178.1 (C-4) ppm and 14 signals for olefinic carbons at δC 157.3 (C-2), 134.3 (C-3), 164.8 (C-5), 98.6 (C-6), 161.7 (C-7), 93.4 (C-8), 104.3 (C-9), 157.1 (C-10), 121.5 (C-1′), 116.1 (C-2′), 144.6 (C-3′), 148.6 (C-4), 114.8 (C-5′), and 121.7 (C-6′) ppm. The other five carbons are specific chemical shifts for sugar group at δC 103.3 (C-1?), 72.8 (C-2?), 71.5 (C-3?), 67.8 (C-4?), and 65.7 (C-5?) ppm (Fig. 4)).
Based on COSY spectrum, the H-6 and H-8 protons can be explained (Table 1). Although both protons have a doublet signal, the COSY spectrum does not indicate that they are correlated or adjacent (Fig. 5). This is because the value of J is 2.1 Hz for each proton suggests that they are interacting as a meta-coupling in the aromatic system. On the other hand, H-5′ and H-6′ are adjacent and show an ortho coupling in the aromatic system, with J = 8.5 Hz each. H-6′ has a dd multiplicity, and the second J = 2.2 Hz is meta coupled with H-2′. Finally, the remaining five protons H-1?–H-5? are part of the arabinofuranoside units, as indicated by this spectrum’s correlation.
The heteronuclear multiple bond correlation (HMBC) spectrum is utilized to establish the relationship between structural units and confirm that the aglycone of the compound is quercetin (C6-C3-C6). The correlation between H-6 and C-5, C-8, C-9, and C-10 (Fig. 6) establishes the presence of 2,4-dihydroxyphenyl (ring A) and carbonyl at C-4 (ring B). Moreover, the correlation between H-2’ and carbons C-2, C-3’, C-4′, and C-6′ confirm the presence of 3,4-dihydroxyphenyl (ring C) (Fig. 8). Finally, the last HMBC correlation confirms that the arabinofuranoside group is bound to C-2 (Ugheighele et al., 2022)
The mass spectrum (MS) data displays an [M-H]- ion with a value of m/z 433.0475 (Fig. 7), suggesting that the molecular formula C20H17O11 (calculated value for C20H18O11 is 434.0475). The double bond equivalent (DBE) value is determined to be 12, obtained from the sum of 8 DBEs of the double bond (sp2) and 4 DBEs of the ring. Based on the analysis of the whole spectroscopy data and comparisons with existing literature, it can be inferred that this compound is similar to avicularin (Mohammed et al., 2022, 2021; Moharram et al., 2018).
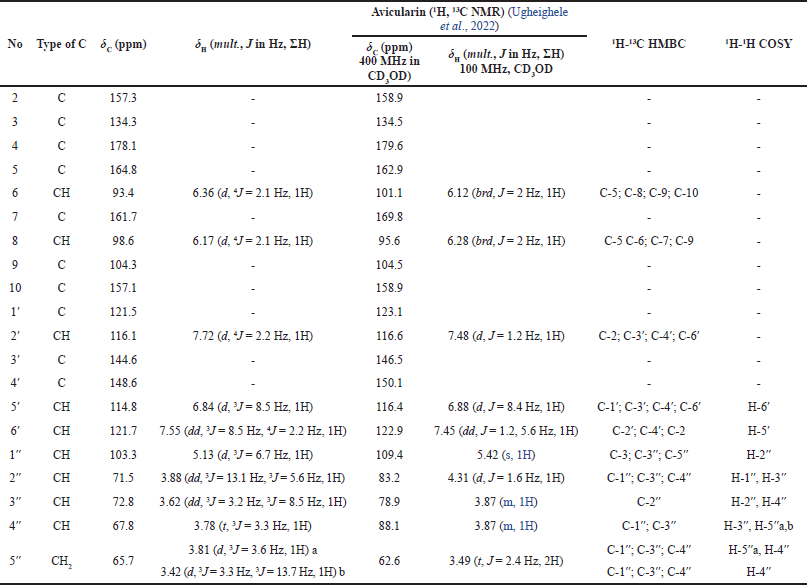 | Table 1. 1D and 2D NMR of isolated compound 1. [Click here to view] |
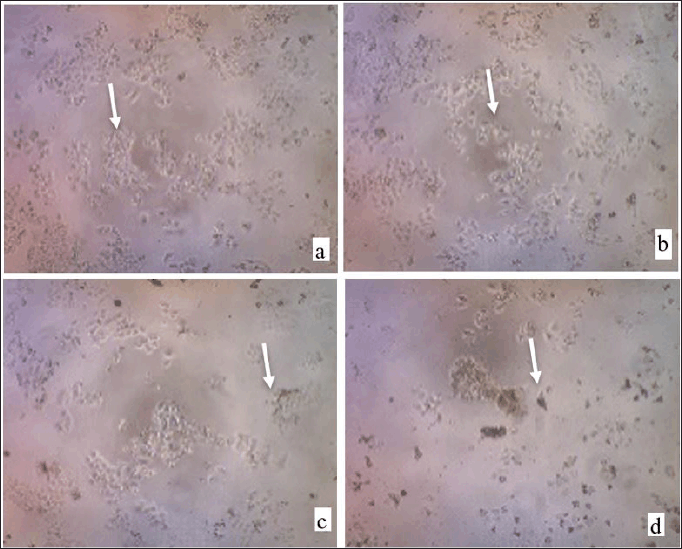 | Figure 9. The appearance of WiDr cancer cells as observed under a microscope following therapy at the isolated compound; control cell (a); concentration 100 ppm (b); 200 ppm (c); 400 ppm (d). [Click here to view] |
Avicularin is a compound widely found in natural products and has bioactivities, such as antioxidant (Lee et al., 2019), anti-inflammation (Hamdy et al., 2020; Vo et al., 2012; Wang et al., 2018), and anticancer activities (Wang et al., 2019), enhancement of insulin sensitivity (Amadi et al., 2021), and insulin resistance (Amadi et al., 2021).
Anticolorectal cancer activities
The cytotoxicity exerted by the avicularin compound in WiDr cells is 521.14 μg/ml. This material is derived from ethyl acetate of leaf fraction that has good cytotoxic activity against cancer cells. An extract or chemical compound is declared to provide very strong cytotoxic activity if the IC50 value is below 10 μg/ml. Meanwhile, the cytotoxic activity is classified as strong if the IC50 value is 10–100 μg/ml. The level of toxicity to cells is considered to be moderate if the concentration at which 50% of cells are killed is between 100 and 500 μg/ml. This study has discovered that the IC50 value has leaf extract at the lowest possible dose to stop the growth of cancer cells; therefore, the growth of cell capacity is decreased or inhibited up to 50% (Weerapreeyakul et al., 2012).
The differences in the morphological changes in the control cells and the variations in concentrations were carried out (Fig. 9). Healthy cells are characterized by round cells and nuclei, protected by clear cell walls, and shine under a microscope (Kusuma et al., 2010). Meanwhile, dead cells appear dark with damaged cell nuclei. The morphological changes were pronounced at high isolate concentrations. In the control cells, the cells appeared in clusters, while at various concentrations, the growth of WiDr cancer cells was inhibited.
CONCLUSION
Avicularin was successfully isolated from the ethyl acetate fraction of S. vulcani extract originating from North Sumatra, Indonesia. Using UV-Vis, IR, 1D, and 2D NMR spectroscopies and an MS spectrometric method, the chemical structure of the isolated product is clarified. The cytotoxicity of the avicularin compound against the WiDr cell line has an IC50 value of 521.14 μg/ml. Meanwhile, the bioactivity of an ethyl acetate fraction is stronger than that of the isolated compound (avicularin).
ACKNOWLEDGEMENT
This study was sponsored by the 2022 project of the doctoral dissertation research (Penelitian Disertasi Doktor) of the Ministry of Education, Research, and Technology, Republic of Indonesia.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Amadi JA, Amadi PU, Njoku UC, Onitchi CL. Lettuce-avicularin treatment reverses insulin resistance through stimulation of glycolytic kinases and insulin signaling molecules. Iran J Basic Med Sci, 2021; 24(2):232–39; doi:10.22038/ijbms.2020.49770.11368
Benarba B, Pandiella A. Colorectal cancer and medicinal plants: principle findings from recent studies. Biomed Pharmacother, 2018; 107:408–23; doi: 10.1016/j.biopha.2018.08.006
br Ginting GA, Rosidah, Sitorus P, Satria D. Wound healing activity of Saurauia vulcani Korth aqueous leaves extract evaluation on excision wound in hyperglycemia rats. J Innov Pharm Biol Sci, 2018; 5(3):52–7. Available via https://jipbs.com/index.php/journal/article/view/331
Granados-Romero JJ, Valderrama-Treviño AI, Contreras-Flores EH, Barrera-Mera B, Herrera Enríquez M, Uriarte-Ruíz K, Ceballos-Villalba JC, Estrada-Mata AG, Alvarado Rodríguez C, Arauz-Peña G. Colorectal cancer: a review. Int J Res Med Sci, 2017; 5(11):4667–76; doi: 10.18203/2320-6012.ijrms20174914
Gurning K, Boangmanalu R, Simanjuntak HA, Singarimbun N, Rahmiati R, Lestari W. Identification of secondary metabolites and antidiarrheal activity of pirdot leaves ethanol extract (Saurauia vulcani Korth.) from West Pakpak, North Sumatra province, Indonesia. Rasayan J Chem, 2020; 13(4):2385–9; doi: 10.31788/RJC.2020.1345984.
Hamdy SA, Menze ET, El Hefnawy HM, Azzam SM, Aboutabl EA. In-vivo anti-inflammatory activity of Hydrocotyle umbellata aerial parts and isolation of the main phytochemicals. Iran J Pharm Res, 2020; 19(3):34–44; doi: 10.22037/ijpr.2020.1101154
Hashim YZHY, Grill CIR, Latimer C, Ternan N, Abbas P. Studies of Malaysian plants in prevention and treatment of colorectal cancer. In: Rodrigo L (Ed.). Colorectal cancer—from pathogenesis to treatment, InTech Open, pp 377–94, 2016; doi: 10.5772/62868
Hutahaean S, Tanjung M, Sari DP, Ningsih VE. Antihyperglycemic and antihyperlipidemic effects of pirdot (Saurauia vulcani Korth.) leaves extract in mice. IOP Conf Ser Earth Environ Sci, 2018; 130; doi: 10.1088/1755-1315/130/1/012042
Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers, 2015; 1:15065; doi: 10.1038/nrdp.2015.65.COLORECTAL
Kusuma AW, Nurulita NA, Hartanti D. Efek sitotoksik dan antiproliferatif kuersetin pada sel kanker kolon WiDr. Pharmacy, 2010; 07(03):107–22; doi: 10.30595/pji.v7i3.584
Lee JS, Lee AY, Quilantang NG, Geraldino PJ, Cho EJ, Lee S. Antioxidant activity of avicularin and isovitexin from Lespedeza cuneata. J Appl Biol Chem, 2019; 62(2):143–7; doi: 10.3839/jabc.2019.020
Lovena TN, Harahap U, Sitorus P, Satria D. Antioxidant activity and α-glucosidase inhibition effect of water extract of Saurauia vulcani Korth leaves. J Innov Pharm Biol Sci, 2018; 5(3):47–51. Available via https://jipbs.com/index.php/journal/article/view/330
Mohammed HA, Almahmoud SA, El-Ghaly ES, Khan FA, Emwas AH, Jaremko M, Almulhim F, Khan RA, Ragab EA. Comparative anticancer potentials of taxifolin and quercetin methylated derivatives against HCT-116 cell lines: effects of O-methylation on taxifolin and quercetin as preliminary natural leads. ACS Omega, 2022; 7:46629–39; doi: 10.1021/acsomega.2c05565
Mohammed SA, Ali HM, Mohammed HA, Al-Omar MS, Almahmoud SA, El-Readi MZ, Ragab EA, Sulaiman GM, Aly MS, Khan RA. Roles of Suaeda vermiculata aqueous-ethanolic extract, its subsequent fractions, and the isolated compounds in hepatoprotection against paracetamol-induced toxicity as compared to silymarin. Oxid Med Cell Longev, 2021:Article ID 6174897; doi: 10.1155/2021/6174897
Moharram FA, Al-Gendy AA, El-Shenawy SM, Ibrahim BM, Zarka MA. Phenolic profile, anti-inflammatory, antinociceptive, anti-ulcerogenic and hepatoprotective activities of Pimenta racemosa leaves. BMC Complement Alternat Med, 2018; 18(1):1–15; doi: 10.1186/s12906-018-2260-3
Musa WJ, Situmeang B, Sianturi J. Anti-cholesterol triterpenoid acids from Saurauia vulcani korth. (Actinidiaceae). Int J Food Prop, 2019; 22(1):1439–44; doi:10.1080/10942912.2019.1650762
Pasaribu G, Budianto E, Cahyana H, Saepudin E. A review on genus Saurauia: chemical compounds and their biological activity. Pharmacogn J, 2020; 12(3):657–66; doi: 10.5530/pj.2020.12.97
Pasaribu G, Hudiyono S, Budianto E, Cahyana AH, Pari G. In-vitro anticancer activity of Saurauia vulcani leaf extracts against human colon WiDr and HCT-116 cancer cells. Rasayan J Chem, 2021; 2021:165–74; doi: 10.31788/RJC.2021.1456449
Rosidah R, Widjaja SS, Pertiwi D, Simanjuntak NJ, Satria D. Phytochemical constituent and subchronic toxicity evaluation of ethanol extract of Saurauia vulcani Korth Lour. leaves in wistar rats. Rasayan J Chem, 2021; 14(3):1441–6; doi: 10.31788/RJC.2021.1436135
Sitorus P, Rosidah, Satria D. Hypoglycemic activity of ethanolic extract of Saurauia vulcani Korth leaves. Asian J Pharm Clin Res, 2018; 11(1):35–6; doi: 10.22159/ajpcr.2018.v11s1.26561
Ugheighele SE, Imafidon KE, Choudhary MI, Shakil A, Okoro EE. Isolation of quercetin and avicularin from Dennettia tripetala (G. baker) seeds, and evaluation of the oxidative stress management capacity and cytotoxic activities of its acetone extract and fractions. Chem Afr, 2022; doi: 10.1007/s42250-022-00413-5
Vo VA, Lee JW, Chang JE, Kim JY, Kim NH, Lee HJ, Kim SS, Chun W, Kwon YS. Avicularin inhibits lipopolysaccharide-induced inflammatory response by suppressing ERK phosphorylation in raw 264.7 macrophages. Biomol Ther, 2012; 20(6):532–7; doi: 10.4062/biomolther.2012.20.6.532
Wang W, Zheng H, Zheng M, Liu X, Yu J. Protective effect of avicularin on rheumatoid arthritis and its associated mechanisms. Exp Ther Med, 2018; 16(6):5343–9; doi: 10.3892/etm.2018.6872
Wang Z, Li F, Quan Y, Shen J. Avicularin ameliorates human hepatocellular carcinoma via the regulation of NF?κB/COX?2/PPAR?γ activities. Mol Med Rep, 2019; 19(6):5417–23; doi: 10.3892/mmr.2019.10198
Weerapreeyakul N, Nonpunya A, Barusrux S, Thitimetharoch T, Sripanidkulchai B. Evaluation of the anticancer potential of six herbs against a hepatoma cell line. Chin Med, 2012; 7(15):1–7; doi: 10.1186/1749-8546-7-15