INTRODUCTION
Color pigments of natural extracts (organic colors) have been of interest for cosmetic applications such as lipsticks and blushes for health and beauty. Weak points in chemical pigments that are composed of heavy metals or have metal contamination have been reported and should be avoided (Azwanida et al., 2015; Borowska and Brzoska, 2015; da Fanca et al., 2015; Mohana et al., 2020). The accumulation of heavy metals can cause melasma (Granstein and Sober, 1981) and several acute and chronic toxic effects, such as gastrointestinal and kidney dysfunction, nervous system disorders, skin lesions, vascular damage, immune system dysfunction, birth defects, and cancer (Balali-Mood et al., 2021).
Many researchers have introduced the application of natural extracts as ingredients in lipstick products to counteract the side effects of chemical pigments, such as cinnamon bark powder, turmeric powder, beetroot powder, pomegranate juice, carrot juice, tomato juice, Clitoria ternatea, and Pandanus amaryllifolius (Azwanida et al., 2015; Rasheed et al., 2020).
Oryza sativa Linn. (red jasmine rice) and Carissa carandas Linn. are widely found in Thailand, and are eaten as local foods (Fig. 1). The extract of C. carandas Linn. had the ability of antioxidant activities at 80.75% ± 0.02% in the in 2,2-diphenyl-1-picrylhydradzyl (DPPH) assay. In addition, the 100% ethanol extract had anti-tyrosinase activity at 71.84% ± 0.00% (Khunchalee and Charoenboon, 2019).
A previous study found that jasmine rice had antioxidant activities (the half maximal inhibitory concentration: IC50) at 314.51 μg/ml in ABTS assay, 145.67 μg/ml in DPPH assay, and 21.26 ± 0.21 μM Fe (II)/μg in FRAP assay (Widowati et al., 2016). Moreover, the extract of rice berries, which was germinated, could inhibit melanin synthesis (Sangsila et al., 2018), indicating the potential of rice extracts in cosmetic applications for skin brightening and rejuvenation. However, the report of the anti-tyrosinase activity of red jasmine rice has not been found.
 | Figure 1. Images of (A) C. carandas Linn. and (B) O. sativa Linn. [Click here to view] |
The antioxidant and anti-tyrosinase activities above are interesting for use as natural pigments in lipstick, not only for their activities but also for their beautiful colors. Lipsticks containing active compounds that have anti-tyrosinase and antioxidant activities induce rejuvenation and reduce darkness on lips. The ethanol extract of O. sativa Linn. has a yellowish-brown color, while the ethanol extract of C. carandas Linn. has a dark red color. However, their application as natural pigments in gel lipsticks has not been reported. Also, the stabilities of their antioxidant, anti-tyrosinase activities, and color stability after adding them to gel lipsticks are here determined by in vitro study and sensory test.
METHODS AND MATERIALS
Materials
Mushroom tyrosinase, DPPH, 3,4-dihydroxyphenylalanine (L-Dopa), quercetin, Trolox (TE), aluminum chloride, ABTS, tripyridyltriazine, and ferric chloride were purchased from Sigma-Aldrich, Darmstadt, Germany. Isododecane, silicone film, dimethicone, nylon 12, silica dimethyl silylate, matte silica powder, polyethylene wax, titanium dioxide, and phenoxyethanol were purchased from Chanjao Longevity Co., Ltd., Bangkok, Thailand. Whatman No.1 was purchased from GE Healthcare UK Limited, Shanghai Amersham, China. Ethanol was purchased from ACL Labscan Limited, Bangkok, Thailand. Sodium acetate, potassium acetate, and potassium persulfate were obtained from Carlo Erba, Cornaredo, Italy. Gallic acid was purchased from Fluka, Buchs, Switzerland. Folin–Ciocalteau reagent was purchased from Loba Chemie, Mumbi, India. Sodium dihydrogen phosphate (NaH2PO4) and disodium hydrogen phosphate (Na2HPO4) were purchased from Himedia, Mumbai, India. Sodium carbonate (Na2CO3) was purchased from Kemaus, New South Wales, Australia. Acetic acid was purchased from ACL Labscan Limited, Bangkok, Thailand.
Plant extraction
Ripe fruits (black color) of C. carandas Linn. were harvested, as can be seen in Figure 1. Both plant materials (O. sativa Linn. and C. carandas Linn.) were cleaned using tap water. The materials were chopped into small pieces and dried in a hot air oven at 50°C for 2 days. After that, they were ground to a powder and macerated in 95% ethanol in the plant-ethanol ratio of 1 (g):25 (ml) by stirring every day. After maceration for 1 week at room temperature (28°C–35°C), the materials were filtered using a Buchner funnel with Whatman No. 1 filter paper. The filtrates were dried by pouring them on a tray, put in a hot air oven at 50°C, and kept at −20°C for approximately 1 month before use.
Gel lipstick preparation
The lipstick base was prepared by mixing isododecane, silicone film, and dimethicone in a beaker A and was stirred by a glass rod and heated at approximately 85°C until clearer for approximately 5 minutes. A beaker B contained nylon 12, silica dimethyl silylate, and matte silica powder was heated at approximately 85°C and was stirred by a glass rod until clearer for approximately 5 minutes. After that, the mixture in beaker B was added to beaker A and stirred by the glass rod until homogeneous. After that, the polyethylene wax was melted in a beaker E at approximately 85°C, and then the mixture of the beaker A and B was quickly added to the beaker E because the wax was quickly changed to be solid at approximately 80°C–83°C and stirred until homogeneous. As shown in Table 1, the lipstick base was mixed by the glass rod with the plant extracts in the ratio of 1:0.3 (lipstick base: plant extract), resulting in five gel lipstick formulas, as detailed in Table 2.
Phenolic content assay
A colorimetric method was used for phenolic content, which was adapted from a previous study (Pekal and Pyrzynska, 2004). Shortly, the extract, gallic acid (a standard phenolic substance in 0.01–0.09 mg/ml), and ethanol (a negative control) in various concentrations were introduced at 150 μl in each microtube. After that, 10% Folin-Ciocalteu reagent, 375 μl, was added to each microtube, following 7.5% Na2CO3, 75 μl, which was mixed in each microtube. After incubation in the dark at room temperature for 1 hour, each sample had its absorbance measured at 715 nm using a spectrophotometer (Thermo Fisher Scientific, Genesys 10-s, USA). The phenolic content of all extracts was calculated from a gallic acid standard graph.
 | Table 1. Composition of lipstick base used for five gel lipstick formulas. [Click here to view] |
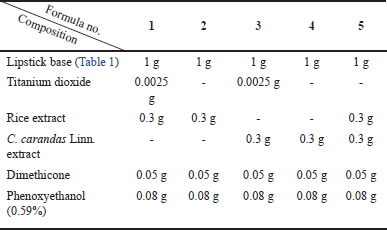 | Table 2. Composition of five gel lipstick formulas. [Click here to view] |
Flavonoid content
A colorimetric method was adapted from a previous study (Pourmorad et al., 2005) for determining the aluminum chloride content, indicating a flavonoid content. Briefly, 0.3 ml of the extracts, quercetin (a standard substance in 20–120 μg/ml), and ethanol (a negative control) were added to each microtube. Next, 0.10 ml of 10.00% aluminum chloride was mixed in each tube, followed by 1 M potassium acetate 0.10 and 2.8 ml of distilled water additions. After incubation at room temperature for 30 minutes, each sample had its absorbance measured at 415 nm using a spectrophotometer (Thermo Fisher Scientific, Genesys 10-s, USA). The flavonoid contents of the extracts were calculated from a standard graph of quercetin.
DPPH scavenging assay
A colorimetric method was adapted slightly from a previous study (Manosroi et al., 2010). Then, 2 mM DPPH, dissolved in ethanol, 250 μl, was added to each microtube. Moreover, 250 μl of the extracts, the standard substance (TE) in 2–16 μg/ml, and ethanol (a negative control) were added to microtubes and mixed with 250 μl of DPPH solution in each tube. After incubation for 30 minutes, all samples had their absorbance measured at 517 nm using a spectrophotometer (Thermo Fisher Scientific, Genesys 10-s, USA). The antioxidant activities of each extract were calculated from a TE standard graph and expressed in terms of TE equivalent antioxidant capacity (TEAC), mg TE/g dry weight (DW).
ABTS scavenging assay
The antioxidant activity of ABTS free radicals was determined using a previous method (Re et al., 1999) with some modifications. A mix of 500 μl, 7.4 mM ABTS dissolved in distilled water and 500 μl of 2.6 mM potassium persulfate dissolved in distilled water was left for approximately 14 hours at room temperature in the dark. Then, the ABTS solution was diluted with 95% ethanol to give an absorbance at 732 nm of approximately 7.2, and it was added to each microtube at 250 μl. Next, the extracts, a positive control (TE) in 1–6 μM, and ethanol (a negative control) were added in microtubes at 250 μl and then were incubated at room temperature and in the dark for 30 minutes. After that, all treatments were measured at the absorbance of 732 nm using a spectrophotometer (Thermo Fisher Scientific, Genesys 10-s, USA). The antioxidant activities of each extract were calculated from a standard graph of TE and expressed in terms of TEAC (mg TE/g DW).
FRAP assay
A colorimetric method for ferric-reducing power by Zhang et al. (2014) was slightly modified. The 120 μl of the extracts, TE (a positive control in 20–100 μM), and ethanol (a negative control) were added in microtubes. The 450 μl of FRAP solution [300 mM acetate buffer (pH 3.6): 10 mM tripyridyltriazine: 20 mM ferric chloride in the ratio of 10:1:1] was added and mixed in each tube. After incubation for 30 minutes in the dark, all samples had their absorbance at 593 nm measured using a spectrophotometer (Thermo Fisher Scientific, Genesys 10-s, USA) and subtracted from the absorbance of a sample color (a mixture of solvent and plant extracts). After that, the antioxidant activities of each extract were calculated from a standard graph of TE and expressed in terms of TEAC (mg TE/g DW).
Anti-tyrosinase activity
The method of anti-tyrosinase activity was adapted slightly from a previous method (Di Petrillo et al., 2016). To begin with, the 50 μl extracts and 171.25 μl phosphate buffer (pH 6.8, 0.5 mM) were mixed in each microtube and incubated with 3.75 μl tyrosinase enzyme (2,500 units/ml) for 5 minutes. After that, 25 μl L-Dopa (2.5 mM) was treated, mixed, and incubated for 10 minutes. The anti-tyrosinase activity of each sample was determined by measuring absorbance at 495 nm using a spectrophotometer (Thermo Fisher Scientific, Genesys 10-s, USA) and then calculated as a percentage of anti-tyrosinase activity as in the following formula.
where A represents the plant extract incubating with the mixture described above, B represents a color blank (The mixture described above, but adding a phosphate buffer instead of the enzyme.), C represents a positive control (The mixture described above, but adding ethanol instead of the plant extract.), and D represents a negative control (The mixture described above, but adding ethanol and buffer instead of the plant extract and the enzyme.)
Color stability
In total, 6 g of each lipstick formula was put into a transparent plastic bag (7.00 × 8.70 cm), and then the color stability was measured at 1-, 2-, 3-, and 4-month periods of storage and compared with that the start time. The color meter (Konica Minolta, CR-400, Japan) was calibrated with a standard white tile, as specified by the manufacturer. It was then used to measure L*(lightness), a*(redness), and b*(yellowness) values at the same position three times.
Antioxidant activity and anti-tyrosinase activity of the gel lipsticks
Each lipstick formula was prepared in 95% ethanol to obtain 10 mg/ml and was then centrifuged at 5,000 rpm for 5 minutes. The supernatant was kept at −20°C until required for measuring the antioxidant activities in the DPPH scavenging assay, ABTS scavenging assay, FRAP assay, and anti-tyrosinase activity.
Sensory test
The sensory test has been approved by the human research ethics committee of Rajamankala University of Technology of Isan (HEC-01-65-028), the Helsinki Declaration, the Belmont Report, CIOMS guidelines, and the International Conference on Harmonization in Good Clinical Practice (ICH-GCP). Twenty female participants (19–41 years old) were informed how to test the gel lipsticks and their benefits before research participation (Esposito and Kirilov, 2021). All participants had all the gel lipsticks smeared on their forearms in an area of approximately 1.00 × 2.00 cm at room temperature (25°C ± 2°C) and answered the questions with a nine-point hedonic scale.
Statistical analysis
All experiments were performed in triplicate, and their data were expressed as means ± standard deviation (SD). The statistical analysis was performed by Statistical software (SPSS for Windows, version 23, SPSS Inc., Chicago, IL, USA) was used to analyze the data. The differences in phenolic contents, flavonoid contents, antioxidant activities (ABTS, DPPH, FRAP), and anti-tyrosinase activities between the plant extracts were analyzed by an independent sample t-test, p < 0.05. The differences in antioxidant activities, colors, and anti-tyrosinase activities among the lipstick extracts were analyzed by one-way analysis of variance (ANOVA), Duncan, p < 0.05. The sensory test was statistically analyzed using the one-way ANOVA in randomized complete block design (RCBD) with Duncan, p < 0.05.
RESULTS AND DISCUSSION
Phenolic and flavonoid content assay
Phenolic compounds consist of aromatic rings and hydroxy groups. They were found in plants to defend against ultraviolet, pathogens, and other predators (Balasundram et al., 2006; Shah et al., 2018). They have diverse bioactivities such as antioxidants, anti-collagenase, anti-tyrosinase, and anti-elastase. They can scavenge free radicals by hydrogen atom transfer, transfer of a single electron, sequential proton loss electron transfer, and chelation of transition metals (Zeb, 2020). Also, they can inhibit melanin synthesis such as vitamin E analogs, including TE and coumarin derivatives (Kamei et al., 2009; Lu et al., 2023). Therefore, many pharmaceutical products are composed of phenolic compounds as active compounds for their beneficial effects on health.
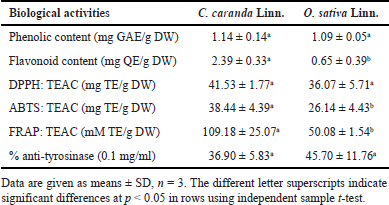
The phenolic contents of C. carandas Linn. and O. sativa Linn. extracts were almost equal (not significant), which were 1.14 ± 0.14 and 1.09 ± 0.05 mg gallic equivalent (GAE)/g DW, respectively (Table 3). However, the phenolic content of C. carandas Linn., which is commonly found in Thailand and was harvested in Nakhon Ratchasima province, had lower phenolic content than those in previous studies (Khunchalee and Charoenboon, 2019; Pewlong et al., 2014; Thitilertdecha and Pintathong, 2022), which reported the phenolic content of C. carandas Linn. were 20.20 ± 0.00 mg GAE/g DW (100% ethanol extraction), 4.67 ± 0.59 mg GAE/g (40% ethanol extraction), 16.18 ± 2.21 mg GAE/g by absolute ethanol extraction. Also, the phenolic content of O. sativa Linn. extract of this study was lower than those of previous reports, which were approximately 81.91 mg GAE/g by 95% ethanol extraction (Suantai et al., 2022), 3.4119 mg GAE/g DW by 80% ethanol extraction (Kammapana, 2023), 1,018 ± 0.095 mg GAE/g by 70% ethanol extraction (Thitipramote et al., 2016). The different planting areas and solvent extractions may be the reason for the lower phenolic content.
 | Table 3. Antioxidant activities and anti-tyrosinase activities of O. sativa Linn. and C. carandas Linn. extracts. [Click here to view] |
Flavonoid compounds, a polyphenolic secondary metabolite, are composed of 15 carbon atoms arranged in three rings such as C6–C3–C6. The differences in the structure of each subgroup are partly attributed to the pattern and degree of hydroxylation, prenylation, glycosylation, or methoxylation, such as quercetin, catechin, naringenin (Dai and Mumper, 2010; D’Archivio et al., 2007). Many flavonoids have antioxidant activity, such as quercetin and catechin (Bernatoniene and Kopustinskiene, 2018; Xu et al., 2019). Some flavonoids can inhibit melanin synthesis, such as quercetin and catechin. Quercetin is bound to tyrosinase by hydrophobic interaction resulting in a conformational change and malfunction (Fan et al., 2017).
Both extracts used in this study consisted of flavonoid compounds. The flavonoid content of C. carandas Linn. was significantly higher than that of the O. sativa Linn. extract, which was 2.39 ± 0.33 and 0.65 ± 0.39 mg quercetin equivalent (QE)/g DW, respectively (Table 3). However, many previous studies reported the flavonoid content of C. carandas Linn., which was approximately 43.75 mg catechin equivalent (CE)/g extract (Nanasombat et al., 2019). Moreover, the flavonoid content of other Thai rice (Sang-Yod, Mun-Poo, Hom-Nin, Rice Berry, and Luem-Pua) was in the range of 86–341 mg CE/g extract (Teeranachaideekul et al., 2018). However, the active compound contents in the previous reports cannot be compared with the results of this study because of the different units and solvent extraction.
DPPH scavenging assay
Both extracts and the gel lipsticks containing the extracts were evaluated for antioxidant activities by DPPH assay. DPPH radicals are purple and stable in ethanol. The decrease in purple color indicates antioxidant activity. The antioxidant activities of the C. carandas Linn. and O. sativa Linn. extracts were 41.53 ± 1.77 and 36.07 ± 5.71 mg TE/g DW, respectively (Table 3). Previous studies have reported the antioxidant activity of C. carandas Linn. extract at 80.75% ± 0.02% by 100% ethanol extraction (Khunchalee and Charoenboon, 2019), 0.56 ± 0.05 mg/ml (IC50) by 95% ethanol extraction (Sudjaroen et al., 2021), and 16.86 ± 2.31 mg TE/g by absolute ethanol extraction (Thitilertdecha and Pintathong, 2022). In addition, O. sativa Linn. extract had DPPH antioxidant activity (IC50) at 314.51 μg/ml (Widowati et al., 2016), 0.3477 mM TE/g DW by 80% ethanol extraction (Kammapana, 2023), and 7,528 mg TE/g by 70% ethanol extraction (Thitipramote et al., 2016). However, the antioxidant activities in the previous reports cannot be compared with the results of this study because of the different units and solvent extraction.
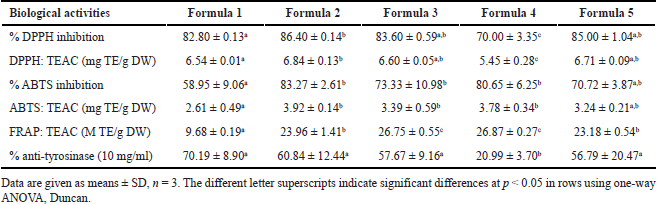
Moreover, the DPPH antioxidant activities of all gel lipstick formulas (Table 4) at 10 mg/ml had antioxidant activities in the range of 5.45 ± 0.28 to 6.84 ± 0.13 mg TE/g DW and antioxidant percentages in the range of 70.00% ± 3.35% to 86.40% ± 0.14%. The highest antioxidant activity was the gel lipstick 2, which used the O. sativa Linn. extract and titanium dioxide as a brown and white pigment, respectively, while the lowest antioxidant activity was the gel lipstick 4, which used C. carandas Linn. extract as a red pigment. The results suggest beneficial effects such as rejuvenation and antiaging on the lips.
ABTS scavenging assay
The extracts and the gel lipsticks containing the extracts were evaluated for antioxidant activities by ABTS assay. ABTS are free radicals and are blue in water or ethanol. The antioxidant ability was obtained by measuring the reduction of blue color. The antioxidant activities of the C. carandas Linn. and O. sativa Linn. extracts were 38.44 ± 4.39 and 26.14 ± 4.43 mg TE/g DW, respectively (Table 3). In addition, previous studies have reported the antioxidant activities in ABTS of C. carandas Linn. (100% ethanol extraction) at approximately 61.72% ± 0.00% (Khunchalee and Charoenboon, 2019), 131.8 ± 3.9 μM/g by 95% ethanol extraction (Neimkhum et al., 2021), and 19.67 ± 0.07 mg TE/g DW by water extraction (Jiangseubchatveera et al., 2021). Also, previous studies have reported the antioxidant activity in ABTS of O. sativa Linn. at approximately 189.45 ± 11.58 mg TE/g by 95% ethanol extraction (Suantai et al., 2022) and 84,476 mg TE/g by 70% ethanol extraction (Thitipramote et al., 2016). The reason why the activity in this study was lower than that in the previous report of Suantai et al. (2022) is possibly due to the different environmental conditions. However, the antioxidant activities in other previous reports cannot be compared with the results of this study because of the different units and extraction processes.
Moreover, the ABTS antioxidant activities of all gel lipstick formulas (Table 4) at 10 mg/ml had antioxidant activities in the range of 2.61 ± 0.49 to 3.92 ± 0.14 mg TE/g DW and antioxidant percentages in the range of 58.95% ± 9.06% to 83.27% ± 2.61%. The highest antioxidant activity was the gel lipstick 2, which used only the O. sativa Linn. extract as a brown pigment. In contrast, the lowest antioxidant activity was the gel lipstick 1, which used O. sativa Linn. extract and titanium dioxide as a brown and white pigment. The results indicate that the other lipstick components had no major effects on the antioxidant activity of the lipsticks and confirmed the potential of the extracts for anti-aging activity.
FRAP assay
The FRAP of the extracts also determined the hydrogen electron transfer by FRAP; the results are shown in Table 3. The antioxidant activity of the C. carandas Linn. (109.18 ± 25.07 mM TE/g DW) was significantly higher than that of the O. sativa Linn. extract (50.08 ± 1.54 mM TE/g DW). However, previous studies have reported that the O. sativa Linn. extract had IC50 at 21.26 μM Fe(II)/μg by 70% ethanol extraction (Widowati et al., 2016) and 3,292.46 g FeSO4/g by 95% ethanol extraction (Suantai et al., 2022) and 10,792 mg TE/g by 70% ethanol extraction (Thitipramote et al., 2016). For C. carandas Linn., previous studies have shown the extract had 2,085.23 g FeSO4/g by 100% ethanol extraction (Khunchalee and Charoenboon, 2019) and 198.50 ± 5.6 μM FeSO4/g by 95% ethanol extraction (Neimkhum et al., 2021). However, the antioxidant activities in the previous reports cannot be compared with the results of this study because of the different units and solvent extractions.
Also, the FRAP antioxidant activities of all gel lipstick formulas (Table 4) at 10 mg/ml, had antioxidant activities in the range of 9.68 ± 0.19 to 26.87 ± 0.27 M TE/g DW. The highest antioxidant activity was the gel lipstick 4, which used the C. carandas Linn. extract as a red pigment, while the lowest antioxidant activity was the gel lipstick 1, which used the O. sativa Linn. extract and titanium dioxide as brown and white pigments.
 | Table 4. Antioxidant activities and anti-tyrosinase activities of all lipstick formulas added with O. sativa Linn. and C. carandas Linn. extracts added. [Click here to view] |
Anti-tyrosinase activity
The activity in tyrosinase inhibition is one of the favorite bioactivities for cosmeceuticals in Asia. The anti-tyrosinase activities of the C. carandas Linn. and O. sativa Linn. extracts were 36.90% ± 5.83% and 45.70% ± 11.76%, respectively (Table 3). The anti-tyrosinase activity of the C. carandas Linn. was consistent with that of previous studies, in which the anti-tyrosinase activity was 71.84% ± 0.00% by 100% ethanol extraction (Khunchalee and Charoenboon, 2019). The C. carandas Linn. extract at 1 mg/ml inhibited tyrosinase activity at 6.55% ± 0.16% by water extraction (Jiangseubchatveera et al., 2021). For O. sativa Linn., the anti-tyrosinase activity corresponds to a previous study (Kanlayavattanakul et al., 2016), which reported that white jasmine rice extracted by a different extract method could inhibit melanogenesis via tyrosinase inhibition at 61.66% ± 0.12%. In addition, the activity remained after adding extracts into gel lipsticks (20.99% ± 3.70% to 70.19% ± 8.90%), as can be seen in Table 4, indicating the potential as cosmeceutical lipsticks.
Sensory test
The colors of the gel lipsticks in each formula (Table 2) were observed by eye and the color meter (Fig. 2). In eye observation (Fig. 3), the color of gel lipstick formula 1, which had O. sativa Linn. extract and titanium dioxide added as pigments, was yellow. The color of gel lipstick formula 2, which had O. sativa Linn. extract added as a pigment, was brown. The color of gel lipstick formula 3, which had C. carandas Linn. extract and titanium dioxide added as pigments, was pink. The color of gel lipstick formula 4, which had C. carandas Linn. extract added as a pigment, was red. The color of gel lipstick formula 5, which had C. carandas Linn. extract and O. sativa Linn. Extract, added as pigments was brown-red. The parameters, which are absorption, color, odor, spreadability, and overall acceptability, were determined by 20 women participants, and the results are shown in Figure 4. In color parameter, lipstick formulas 3, 4, and 5, which were not significantly different, had the higher scores. Formulas 1 and 2 were not significantly different and had lower scores than others. In the odor parameter, the formulas that had the highest scores were formulas 4 and 5 (not significantly different), followed by formulas 2 and 3 (not significantly different). The lowest score is formula 1. In the overall acceptability parameter, the highest scores which were not significantly different were formulas 3, 4, and 5, followed by formulas 1, 2, and 3 (not significantly different). The lipstick which had C. carandas Linn. extract added had a higher score than those lipsticks which had O. sativa Linn. extract added in color, odor, and overall acceptability scores, indicating the greater flavor of C. carandas Linn in dark color lipsticks. Also, the O. sativa Linn. extract is suggested as a pigment for tint lipsticks (light color). The spreadability and absorption scores of all lipstick formulas were not significantly different. However, the spreadability and flakiness of all formulas should be further developed.
Color stability
All lipstick formulas were tested for color stability by measuring the color at the start time compared with that of the storage times (4 months), as can be seen in Figure 2. Comparing their storage times, the yellowness of all formulas was slightly increased, but the redness and lightness of all formulas decreased. The redness of all formulas significantly decreased after one month of storage, corresponding to the results measured by the color meter. Similarly, the yellowness trend of all formulas increased slightly but significantly since the 1-month storage. Also, the lightness trend of formulas 1, 2, and 5 decreased slightly but significantly since the 1-month of storage. However, the lightness trend of formulas 3 and 4 decreased slightly but significantly since the 2-month storage. Normally, natural pigments are less stable than synthetic pigments because of microbial contaminations, light, and heat (Azwanida et al., 2015; da Fanca et al., 2015; Mohana et al., 2020).
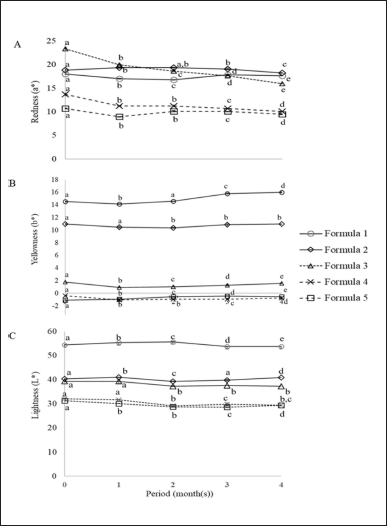 | Figure 2. Color stabilities (redness, yellowness, and lightness) of five lipstick formulas. The different letters (a, b, c, d, and e) in the set for each period represent significant differences by one-way ANOVA (Duncan). [Click here to view] |
 | Figure 3. Lipstick colors examined by eye for five formulas. The lipstick formula 1 had O. sativa Linn. extract and titanium dioxide added as pigments. The lipstick formula 2 had O. sativa Linn. extract added as a pigment. The lipstick formula 3 had C. carandas Linn. extract and titanium dioxide added as pigments. The color lipstick formula 4 had C. carandas Linn. extract added as a pigment. The lipstick formula 5 had C. carandas Linn. extract and O. sativa Linn. extract added as pigments. [Click here to view] |
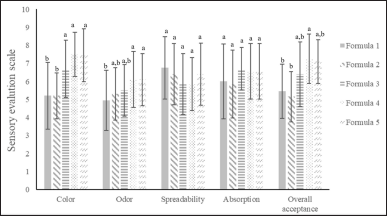 | Figure 4. Sensory scores for odor, color, spreadability, absorption, and overall acceptability of each lipstick. The different letters (a, b, c, d, and e) in the columns for each characteristic represent significant differences by using one-way ANOVA in RCBD with Duncan, p < 0.05. [Click here to view] |
The color instability of these lipsticks suggests that perhaps the percentage of a preservative should be higher, but no more than 1.00%. Therefore, the color stability of the lipsticks using the C. carandas Linn. extract as a red pigment should be further studied for more stability.
CONCLUSION
The antioxidant and anti-tyrosinase activities of O. sativa Linn. and C. carandas Linn. extracts remain active after adding into gel lipstick as pigments, indicating that they may reduce wrinkles and melanin on lips. Also, in the sensory test, the lipstick with C. carandas Linn. extract added had a higher score than those lipsticks with O. sativa Linn. extract added in terms of color, odor, and overall acceptability scores. Oryza sativa Linn. extract was suitable for tint lipsticks because of its light color. Carissa carandas Linn. extract was appropriate for use to darken lipsticks as a pigment because of its darker red color. However, the spreadability, flakiness, and color stability of all lipsticks should be developed for better quality.
ACKNOWLEDGEMENT
The authors would like to thank Plant Genetic Conservation Project Under the Royal Initiative of Her Royal Highness Princess Maha Chakri Sirindhorn (RSPG) for financial support. Also, we would like to thank the Rajamangala University of Technology Isan, Nakhon Ratchasima, Thailand, for their facilities.
AUTHOR CONTRIBUTIONS
The authors confirm their contribution to the article as follows: study conception and design: Thitiporn Anunthawan was responsible for data collection, analysis, and interpretation of results. Thitiporn Anunthawan and Thipwarin Rimlumduan contributed to the draft manuscript preparation. All authors reviewed the results and approved the final version of the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
The sensory test has been approved for ethics by the human research ethics committee of Rajamankala University of Technology of Isan (HEC-01-65-028), the Declaration of Helsinki, the Belmont Report, CIOMS guideline, and the ICH-GCP.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Azwanida NN, Hui MS, Afandi A, Mohamed S, Zulhisyam AK, Ayob A, Rusli N, Rasat MSM, Mohamed M. Color stability evaluation of pigment extracted from Hylocereus polyrhizus, Clitorea ternatae, and Pandanus amaryllfolius as cosmetic colorants and premarket survey on customer acceptance on natural cosmetic product. J Trop Resour Sustain Sci, 2015; 3(1):61–7.
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol, 2021; 13(12):1–19.
Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem, 2006; 99(2006):191–203.
Bernatoniene J, Kopustinskiene DM. The role of catechins in cellular responses to oxidative stress. Molecules, 2018; 23(4):1–11.
Borowska S, Brzoska M. Metals in cosmetics: implications for human health. J Appl Toxicol, 2015; 35(6):551–72.
da Fanca SA, Dario MF, Esteves VB, Baby AR, Velasco MVR. Types of hair dyes and their mechanism of action. Cosmetics, 2015; 2(2):110–26.
Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules, 2010; 15(10):7313–52.
D’Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R. Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita, 2007; 43(4):348–61.
Di Petrillo A, González-Paramás AM, Era B, Medda R, Pintus F, Santos-Buelga C, Fais A. Tyrosinase inhibition and antioxidant properties of Asphodelus microcarpus extracts. BMC Complement Altern Med, 2016; 16:453–62.
Esposito CL, Kirilov P. Preparation, characterization and evaluation of organogel-based lipstick formulations: application in cosmetics. Gels, 2021; 7(97):1–15.
Fan M, Zhang G, Hu X, Xu X, Gong D. Quercetin as a tyrosinase inhibitor: inhibitory activity, conformational change and mechanism. Food Res Int, 2017; 100:226–233.
Granstein RD, Sober AJ. Drug and heavy metal induced hyperpigmentation. J Am Acad Dermatol, 1981; 5(1):1–18.
Jiangseubchatveera N, Saechan C, Leelakanok N, Petchsomrit A. The evaluation of antioxidant and antityrosinase efficacy of Carissa carandas fruit extracts and the development of a preliminary skincare product. J Appl Pharm Sci, 2021; 11(07):153–7.
Kamei Y, Otsuka Y, Abe K. Comparison of the inhibitory effects of vitamin E analogues on melanogenesis in mouse B16 melanoma cells. Cytotechnology, 2009; 59(3):183–90.
Kammapana L. Physical characteristics, phytochemical contents and antioxidant activity of ten organic-pigmented rice varieties from Surin province. Trends Sci, 2023; 20(4):1–14.
Kanlayavattanakul M, Lourith N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol, 2016; 193:607–16.
Khunchalee J, Charoenboon P, Study of free radical scavenging, total phenolic contents and tyrosinase inhibition activity of crude extract from Carissa carandas Linn. SNRUJST, 2019; 11(1):26–34.
Lu L, Zhang X, Kang Y, Xiong Z, Zhang K, Xu X, Bai L, Li H. Novel coumarin derivatives as potential tyrosinase inhibitors: synthesis, binding analysis and biological evaluation. Arab J Chem, 2023; 16(104724):1–12.
Manosroi A, Jantrawut P, Akihisa T, Manosroi W, Manosroi J. In vitro anti-aging activities of Terminalia chebula gall extract. Pharm Biol, 2010; 48(4):469–81.
Mohana PM, Chidambara RP, Lavanya M. Use of natural pigments as colorants in cosmetics. J Emerg Technol Innov Res, 2020; 7(3):907–17.
Nanasombat S, Yansodthee K, Jongjaited I. Evaluation of antidiabetic, antioxidant and other phytochemical properties of Thai fruits, vegetables and some local food plants. Walailak J Sci Tech, 2019; 16(11):851–66.
Neimkhum W, Anuchapreeda S, Lin WC, Lue SC, Lee KH, Chaiyana W. Effects of Carissa carandas Linn. fruit, pulp, leaf, and seed on oxidation, inflammation, tyrosinase, matrix metalloproteinase, elastase, and hyaluronidase inhibition. Antioxidants (Basel), 2021; 10(9):1345.
Pekal A, Pyrzynska K. Evaluation of aluminum complexation reaction for flavonoid content assay. Food Anal, 2004; 7:1776–82.
Pewlong W, Sajjabut S, Eamsiri J, Chookaew S. Evaluation of antioxidant activities, anthocyanins, total phenolic content, vitamin C content and cytotoxicity of Carissa carandas Linn. CMUJ NS, 2014; 13(1):509–17.
Pourmorad FS, Hosseinimehr SJ, Shahabimajd N. Antioxidant activity of phenol and flavonoid contents of some Iranian medicinal plants. Afr J Biotechnol, 2005; 5(11):1142–5.
Rasheed N, Rahman SA, Hafsa S. Formulation and evaluation of herbal lipsticks. Res J Pharm Tech, 2020; 13(4):1693–700.
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med, 1999; 26(9):1231–7.
Sangsila A, Promden W, Pimda W. Antioxidant and antityrosinase activities in germinated brown rice of indigenous Thai cultivars. Int J Agric Technol, 2018; 14(7):1883–92.
Shah SR, Ukaegbu CI, Hamid HA, Alara OR. Evaluation of antioxidant and antibacterial activities of the stems of Flammulina velutipes and Hypsizygus tessellatus (white and brown var.) extracted with different solvents. J Food Meas Charact, 2018; 12:1947–61.
Suantai B, Jantakee K, Kaewkod T, Sangboonruang S, Chitov T, Tragoolpua Y. Anthocyanins in red jasmine rice (Oryza sativa L.) extracts and efficacy on inhibition of herpes simplex virus, free radicals and cancer cell. Nutrients, 2022; 14(9):1905–23.
Sudjaroen Y, Petcharaporn K, Moolwong J, Jodnok K, Kakatum N. Biological activities of ethanol extract from Karanda (Carissa carandas L.) fruits. J Pharm Res Int, 2021; 33(35):20–5.
Teeranachaideekul V, Wongrakpanich A, Leanpolchareanchai J, Thirapanmethee K, Sirichaovanichkarn C. Characterization, biological activities and safety evaluation of different varieties of Thai pigmented rice extracts for cosmetic applications. Pharm Sci Asia, 2018; 45(3):140–53.
Thitilertdecha N, Pintathong P. The antioxidant and anti-tyrosinase activities of Carissa carandas Linn. fruit extracts and their applications in cosmetics. Indian J Pharm Sci, 2022; 84(5):1218–26.
Thitipramote N, Pradmeeteekul P, Nimkamnerd J. Chaiwut P, Pintathong P, Thitilerdecha N. Bioactive compounds and antioxidant activities of red (Brown Red Jasmine) and black (Kam Leum Pua) native pigmented rice. Int Food Res J, 2016; 23(1):410–4.
Widowati W, Fauziah N, Herdiman H, Afni M, Afifah E, Kusuma HSW, Nufus H, Arumwardana S, Rihibiha DD. Antioxidant and anti-aging assays of Oryza Sativa extracts, vanillin and coumaric acid. J Nat Remedies, 2016; 16(3):88–99.
Xu D, Hu MJ, Wang YQ, Cui YL. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules, 2019; 24(1123):1–15.
Zeb A. Concept, mechanism, and applications of phenolic antioxidants in foods. J Food Biochem, 2020; 44(9):e13394.
Zhang XL, Zhang YD, Wang T, Guo HY, Liu QM, Su HX. Evaluation on antioxidant effect of Xanthohumol by different antioxidant capacity analytical methods. J Chem, 2014; 2014:1–6.