INTRODUCTION
Streptomyces belongs to Gram-positive bacteria, having a high guanine and cytosine (GC) content of 72.5% in its genome and being recognized by colony morphology and filamentous growth during its life cycle (Chater et al., 2010). Streptomyces is known as the largest genus of the phylum actinobacteria and a promising source for discovering novel bioactive secondary metabolites contributing to the pharmaceutical industry (Lee et al., 2019). These secondary metabolites possess varied properties containing antibiotic (Bekiesch et al., 2020; Toumatia et al., 2015), antitumor (Shen et al., 2021), antimalarial (Nugraha et al., 2020), and immunosuppressive activity (Yashiro et al., 2019).
In particular, Streptomyces is responsible for producing approximately two-thirds of antibiotics used in current medicine, with major classes such as aminoglycosides, anthracyclines, β-lactams, glycopeptides, and macrolides (Barka et al., 2016; Fernández-Martínez et al., 2014; Petkovi? et al., 2017; Quinn et al., 2020; Takano et al., 2016). Notably, there are various antitumor antibiotics derived from Streptomyces that have been utilized in clinical treatment, including bleomycin (Shen et al., 2001), mitomycin (Tiran et al., 2020), doxorubicin (Benjanuwattra et al., 2020), and dactinomycin (Schink et al., 2020). Several studies reported that Streptomyces possesses different gene clusters encoding multiple polyketide and nonribosomal peptide synthases responsible for secondary metabolite biosynthesis, including antitumor constituents (Risdian et al., 2019; Tang et al., 2004). In fact, the research of cancer treatment attracts more and more interest due to challenges relating to tumor relapse, metastasis, drug resistance, and serious side effects on the whole body system that lower the life quality and expectancy of patients. Therefore, current Food and Drug Administration-approved chemotherapeutic drugs indicate the importance of research and discovery of various novel anticancer compounds from natural sources, including from different strains of Streptomyces.
The increase in exploration from different habitats on earth helps to isolate new taxa of Streptomyces. Some areas in Vietnam have been explored as sources of microbial biodiversity with newly discovered strains of Streptomyces that produce substances owning anticancer potential (Nguyen et al., 2020; Quach et al., 2021; Vu et al., 2018). In that tendency, Streptomyces sp. SS162, recently collected from marine corals in distant islands of central Vietnam, was cultured in a liquid medium and then evaluated for biological activities. Following the initial screening, Streptomyces sp. SS162 exhibited antibacterial activity against the shrimp pathogen Vibrio parahaemolyticus (Chuong Hoang Nguyen, unpublished data), causing acute hepatopancreatic necrosis disease, which would seriously affect shrimp farming. To broaden exploring other activities of this species, the anticancer capacity of Streptomyces sp. SS162 would be investigated on varied cancer cell lines; then, the cytotoxic mechanism was preliminarily evaluated.
MATERIALS AND METHODS
Materials
Streptomyces sp. SS162 was isolated from the coral sample collected at Central Reef, Spratly Islands (GPS: 8° 55′51?N, 112° 21′11?E) by the Centre for Research and Application in Bioscience (Ho Chi Minh City, Vietnam). The isolated colonies were maintained as a suspension in 40% (v/v) glycerol, stored at −20°C.
SS162-cultured medium preparation
First, the suspension of Streptomyces sp. SS162 from −20°C preservation was streaked on MPE agar medium (calcium carbonate: 4 g/l; glucose: 20 g/l; sodium chloride: 5 g/l; soybean flour: 5 g/l; agar: 20 g/l), incubating at 30°C within 3–5 days. Next, isolated colonies were picked up and grown in 20 ml of FM3 medium (yeast extract: 5 g/l; starch: 20 g/l; soybean flour: 15 g/l; peptone: 2 g/l; calcium carbonate: 4 g/l; sodium chloride: 18 g/l) for 3 days at 30°C, as a starting inoculum prior to the fermentation process. The fermentation was carried out in 300 ml of FM3 medium supplemented with 5% (v/v) starting inoculum and shaken at 200 rpm for 8 days at 30°C. Then, the biomass was discarded by centrifugation at 10,000 rpm for 10 minutes and the supernatant fluid was stored at −30°C. For cytotoxicity assay, the supernatant fluid was thawed at room temperature and filtered by a sterile 0.22 μm membrane.
Cell line maintenance and growth condition
Cell lines included A549 (human lung carcinoma, ATCC CCL-185), SiHa (human cervical squamous cell carcinoma, ATCC HTB-35), Caov-3 (human ovarian carcinoma, ATCC HTB-75), NCI-N87 (human gastric carcinoma, ATCC CRL-5822), HL-60 (human acute promyelocytic leukemia, ATCC CCL-240), and BJ-5ta (human normal fibroblasts, ATCC CRL-4001). A549 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)-F12 medium (Sigma-Aldrich, Germany), supplemented with 10% (v/v) fetal bovine serum (FBS) (Sigma-Aldrich, Germany). Caov-3 and BJ-5ta cell lines were cultured in DMEM high glucose medium (Sigma-Aldrich, Germany), supplemented with 10% (v/v) FBS. NCI-N87 and HL-60 cell lines were cultured in Roswell Park Memorial Institute 1640 medium (Sigma-Aldrich, Germany), supplemented with 10% (v/v) FBS. SiHa cell line was cultured in Eagle’s minimum essential medium (Sigma-Aldrich, Germany), supplemented with 10% (v/v) FBS (Sigma-Aldrich, Germany). Antibiotic antimycotic solution (100 U/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin B) (Sigma-Aldrich, Germany) was also added to the medium to prevent contamination. All cell lines were cultured in humidified 5% CO2 atmosphere at 37°C in a cell incubator (Esco, Singapore).
Screening of cytotoxicity of SS162-cultured medium on human cancer cell lines
The cytotoxic effects of Streptomyces sp. SS162-cultured medium on human cancer lines was evaluated using 3-(4,5-dimethylthazol-2yl)-2,5-diphenyl tetrazolium-bromide (MTT) assay. First, adherent cells were detached from the culture surface using 0.25% trypsin-0.53 mM Ethylenediamine tetraacetic acid (Sigma-Aldrich, Germany). These cells were centrifuged and the cell density was identified. Then, an amount of 1 × 104 cells was added to each well of a 96-well plate (SPL, Korea). For adherent cells, the cells were left to attach overnight, while the suspension cells were incubated within 1 hour for the reconstitution. After that, the medium was removed and replaced with 95 μl of fresh medium. Next, 5 μl of SS162-cultured medium [ratio 1:20 (v/v)] was added to each well. Nontreated control samples were treated with noncultured FM3 medium. After 48 hours, 5 μl of MTT (5 mg/ml) (Sigma-Aldrich, Germany) was coincubated for 4 hours. Next, 60 μl of lysis buffer [30% (w/v) SDS, 0.03 N HCl] and 90 μl of dimethyl sulfoxide (DMSO) [99.9% (v/v)] were added to each well. The plates were shaken at 850 rpm for 10 minutes, and the optical densities were measured at a wavelength of 550 nm. These experiments were repeated at least in triplicate. The growth inhibition was calculated by the following formula:
Growth inhibition (%) = (1 – (ODTreated – ODBlank)/(ODNon-treated – ODBlank)) × 100.
Evaluation of the anticancer activity of SS162-cultured medium on A549 cell line
Analysis of cell growth and morphology
A549 cells at a density of 1 × 104 cells/well were treated with SS162-cultured medium [ratio 1:20 (v/v)]. Noncultured FM3 medium was used for nontreated control samples. MTT assay was utilized to analyze cell densities of treated and nontreated control samples at different time points of 24, 48, 72, and 96 hours. The morphology of A549 cells treated with SS162-cultured medium was recorded by inverted microscope Nikon Eclipse TiU (Nikon, Japan).
Total RNA extraction and quantitative real-time PCR (qPCR)
A549 cells were treated with SS162-cultured medium and noncultured FM3 medium (nontreated control). Total RNA was collected by using TriSure Kit (Bioline, UK). After that, 500 ng of total RNA was used for reverse transcription with Tetro cDNA Synthesis Kit and Oligo-dT primers (Bioline, UK). Real-time PCR was conducted using SensiFASTTM HRM Kit (Bioline, UK), primers (Phu Sa, Viet Nam), and 1 μl of cDNA template. Primer sequences were referred to previous studies: CDKN1B (F) 5′-AGACTGATCCGTCGGACAGC, (R) 5′-CACAGAACCGGCATTTGGG; TPX2 (F) 5′-ACATCTGAACTACGAAAGCATCC, (R) 5′-GGCTTAACAATGGTACATCCCTTA; CCNE1 (F) 5′-AAATGGCCAAAATCGACAGG, (R) 5′-CGAGGCTTGCACGTTGAGTT; CCND1 (F) 5′-TGAAGGAGACCATCCCCCTG, (R) 5′-TGTTCAATGAAATCGTGCGG; PCNA (F) 5′-TTTGGTGCAGCTCACCCTG, (R) 5′-CGCGTTATCTTCGGCCCTTA; MKI67 (F) 5′-TGTGCCTGCTCGACCCTACA, (R) 5′-TGAAATAGCGATGTGACATGTGCT; ACTB (F) 5′-TCCTGTGGCATCCACGAAACT, (R) 5′-GAAGCATTTGCGGTGGACGAT (Amatori et al., 2017; Lee et al., 2012; Xiang et al., 2012). β-Actin (ACTB) was used as a reference gene. The reactions were performed by LightCycler 96 instrument (Roche Diagnostics, Germany). The following thermal profile was applied: 1 cycle at 95°C for 3 minutess, 40 cycles at 95°C for 5 seconds, 55°C for 10 seconds, and 72°C for 5 seconds. The targeted mRNA expression was normalized to the expression of reference gene β-actin (ACTB) and evaluated in comparison between treated and nontreated samples by the 2−??Ct method. The experiment was conducted in triplicate.
Ethyl acetate (EA) extraction of SS162-cultured medium
The SS162-cultured medium was extracted by EA solvent [ratio 1:1 (v/v)] for 24 hours, which was continually repeated until the color of the solvent remained unchanged. Next, the extract was concentrated and excluded the solvent by the rotary evaporator. The efficiency of EA extraction is 0.047% (0.047 g/100 ml of SS162-cultured medium). Finally, the EA extract was weighed, dissolved in DMSO solvent [99.9% (v/v)], and diluted to gain experimental concentrations (0.2–1,000 μg/ml) for cytotoxicity assay.
Gas chromatography-mass spectroscopy (GC-MS) analysis
The GC-MS analysis was conducted following the protocol of the previous study (Tangjitjaroenkun et al., 2021). The SS162-EA extract was dissolved in acetone solvent and then analyzed in an Agilent Technologies 6890N GC coupled to an Agilent 5973 inert mass selective detector. The GC capillary column was HP-5MS (30 m × 0.25 mm × i.d., 0.25 μm film thickness 0.25 μm). The column temperature was increased from 70°C to 300°C (4°C/minute). The carrier gas was helium, with a column flow rate of 1 ml/minute. The mass selective detector was operated in electron ionization mode at 70 eV with a mass range from 40 to 400 amu. The compounds were identified by comparing them with NIST 14 Library.
Data analysis
Statistical analysis and graphs were performed using GraphPad Prism 8 software (USA). All data were analyzed by unpaired Student’s t-test or one-way ANOVA test. The statistically significant differences were determined at p ≤ 0.05. Data were presented as mean ± SD.
RESULTS
The morphological features of Streptomyces sp. SS162
The morphological features of Streptomyces sp. SS162 grown on MPE agar medium was observed. Specifically, this species developed under the form of colonies with white mycelium, accompanied by undulate, opaque, and dry characteristics (Fig. 1A). The images by scanning electron microscope also revealed that aerial mycelium developed into short, straight to flexuous spore chains, the spore surface is regular and smooth, and the spore length is approximately 1.2–1.5 μm (Fig. 1B).
Cytotoxicity of Streptomyces sp. SS162-cultured medium on different cancer cell lines
The cytotoxicity of SS162-cultured medium against various types of cancer cells was investigated using an MTT assay. The results showed that the growth inhibition percentages induced by SS162-cultured medium after 48 hours of incubation toward the A549 lung carcinoma cell line, SiHa cervical carcinoma cell line, NCI-N87 gastric carcinoma cell line, Caov-3 ovary carcinoma cell line, and HL-60 acute promyelocytic leukemia cell line were 64.63%, 51.73%, 36.73%, 17.66%, and 10.27%, respectively, while that toward BJ-5ta normal fibroblast cell line was 32.62% (Fig. 2A). Notably, the inhibition percentage of SS162-cultured medium on A549 lung cancer cells was two times that of BJ-5ta normal cells proving selective activity of SS162-cultured medium on this cancer cell line. In addition, the cell morphology of treated groups was significantly different from nontreated cells (Fig. 2B). Among the tested cancer cell lines, the inhibitory effect on the A549 cell line presented as the highest; thus, the effect of Streptomyces sp. SS162-cultured medium on A549 cells was favorable for the comprehensive investigation.
Anticancer activity of SS162-cultured medium on A549 cell line
Analysis of A549 cell growth and morphology
The cell density of the treated sample was altered in a time-dependent manner, as evidenced by OD550 values from 0 to 96 hours (Fig. 3A). For treated A549 cells, the OD550 value was unchanged within 48 hours, then increasing by 1.4 and 1.6 times at 72 and 96 hours, respectively (Fig. 3A). On the other hand, for nontreated A549 cells, this value rose by 2.2, 2.8, and 3.2 times at 48, 72, and 96 hours, respectively. The growth-inhibitory percentage increased from 30.25% at 24 hours to 58.01% at 48 hours and then fluctuated at approximately 50% until 96 hours (Fig. 3B).
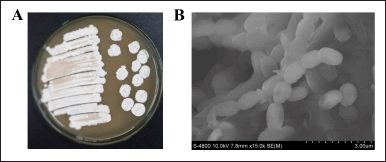 | Figure 1. Morphological features of Streptomyces sp. SS162 on MPE agar medium. (A) SS162’s colony morphology. (B) SS162’s spore-chain morphology was observed with scanning electron microscopy. [Click here to view] |
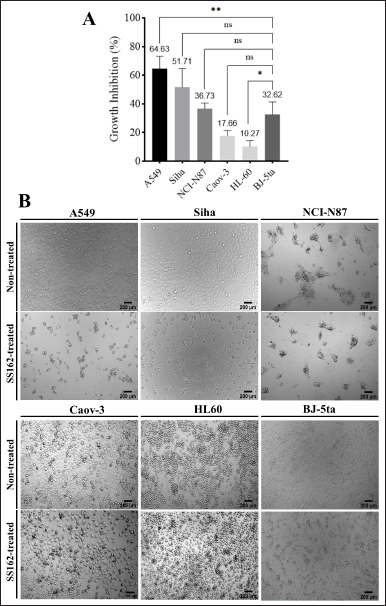 | Figure 2. Cytotoxicity of Streptomyces sp. SS162-cultured medium against different cell lines. (A) Growth inhibition of SS162-cultured medium on cell lines. Data were expressed as mean ± SD of at least three independent experiments. Statistical differences were analyzed by one-way ANOVA (*p ≤ 0.05; **p ≤ 0.01; ns, p > 0.05). (B) Morphological changes of different cell lines induced by SS162-cultured medium. Scale bars: 200 μm. [Click here to view] |
Besides, the image results also showed that cellular morphology changed after being treated with an SS162-cultured medium. The treated A549 cells had changes in their appearance immediately after the exposure. To be more specific, these cells began to round and detach from the culture surface and adjacent cells while maintaining the cell membrane’s intactness (Fig. 3C). In contrast, nontreated A549 cells were adherent on the culture surface with the characteristics of healthy epithelial cells (Fig. 3C). Interestingly, nonadherent round morphology was expressed during the experimental 96-hour period (Fig. 3C). Therefore, the SS162-cultured medium could inhibit the cell proliferation which might be related to the change in interactions between cell and extracellular matrix (ECM).
 | Figure 3. Time-dependent effect of Streptomyces sp. SS162-cultured medium against A549 cell line. (A) Cell densities of treated and nontreated control samples during the time. Data were expressed as mean ± SD of at least three independent experiments. The statistical differences were analyzed by unpaired two-tailed Student’s t-tests (*p ≤ 0.05; **p ≤ 0.01; ns, p > 0.05). (B) The growth-inhibitory percentages of SS162-cultured medium on A549 cells during the time. (C) Optical images of SS162-treated A549 cells at different time points. Scale bars: 200 μm. [Click here to view] |
Effect of SS162-cultured medium on mRNA expression
To identify the underlying molecular mechanism of the SS162-cultured medium on A549 cells’ proliferation, the mRNA expression levels of genes involved in cell cycle progression, such as CDKN1B, TPX2, CCNE1, CCND1, PCNA, and MKI67, were examined (Fig. 4A–F). The results showed that CDKN1B expression in the treated A549 cells was 2.78-fold higher than that in nontreated control samples (Fig. 4A), while there was a decrease in TPX2 mRNA level by 2.75-fold following the treatment (Fig. 4B). Besides, a 1.77-fold decline in MKI67 level of treated samples was also observed despite no statistically significant difference when compared with control samples (Fig. 4F). However, CCNE1, CCND1, and PCNA mRNA levels expressed no significant changes (Fig. 4C–E). In brief, the proliferative inhibition of A549 cells induced by the SS162-cultured medium was related to the upregulation of CDKN1B expression and downregulation of TPX2 expression.
Anticancer effect of EA extract on A549 cell line
To investigate whether bioactive constituents in SS162-cultured medium could concentrate in EA fraction after the extraction, the cytotoxicity of this fraction was evaluated on A549 cells with experimental concentrations ranging from 0.2 to 1,000 μg/ml. As shown in Figure 5A, the effect of the SS162-EA extract was concentration-dependent. At the 1,000 μg/ml concentration, the growth inhibition reached the highest value at 95.4%. The inhibitory percentages decreased following the decline of extract concentration. For concentrations lower than 15.6 μg/ml, the inhibition appeared insignificantly. The 50% growth-inhibitory concentration of SS162-EA extract was 407.38 μg/ml. In the meantime, normal human fibroblasts were less sensitive to SS162-EA extract treatment than A549 cells since the growth-inhibitory percentage for BJ-5ta cells just reached 49.4% at 1,000 μg/ml (Fig. 5A).
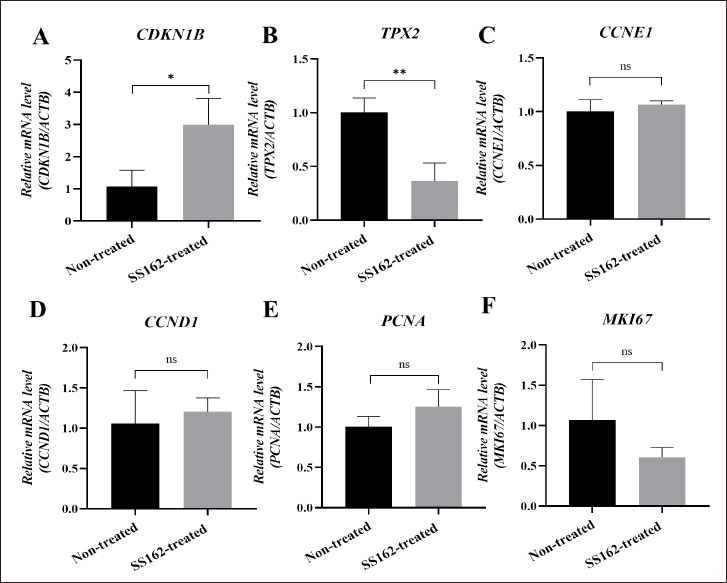 | Figure 4. The effect of Streptomyces sp. SS162-cultured medium on mRNA expression levels in A549 cells. (A–F) Quantitative real-time PCR analysis of genes in treated A549 cells, including CDKN1B, TPX2, CCNE1, CCND1, PCNA, and MKI67. Data were expressed as mean ± SD of three independent experiments. The statistical differences were analyzed by unpaired two-tailed Student’s t-tests (*p ≤ 0.05; **p ≤ 0.01; ns, p > 0.05). [Click here to view] |
Regarding cell morphology, treated cells presented typical features of cell death at the concentration of 1,000 μg/ml. For concentrations of 250 and 500 μg/ml, the cell density exhibited lower than that of the nontreated control sample. However, unlike samples treated with SS162-cultured medium, nonadherent round morphology was not observed in those treated with SS162-EA extract at experimental concentrations. Therefore, it is suggested that different substances present in the SS162-cultured medium might contribute to the anticancer activity of Streptomyces sp. SS162, besides the constituents, is concentrated in SS162-EA extract. To sum up, along with the MTT cytotoxicity assay, the images of cell morphology supported that SS162-EA extract could inhibit the proliferative capacity of A549 cells.
GC-MS analysis of SS162-EA extract
The chemical constituents in the SS162-EA extract were analyzed using the GC-MS method. The GC-MS chromatogram revealed that there were 23 compounds identified in the extract (Fig. 6), accompanied by retention time, molecular formula, molecular weight, and area, which are listed in Table 1. Among them, n-hexadecanoic acid (12.58%), 9,12-octadecadienoic acid (Z,Z)- (67.96%), octadecanoic acid (8.91%), and 3-chrysenamine (1.67%) take the major account in the extract, while the others presented at low percentages (<1%).
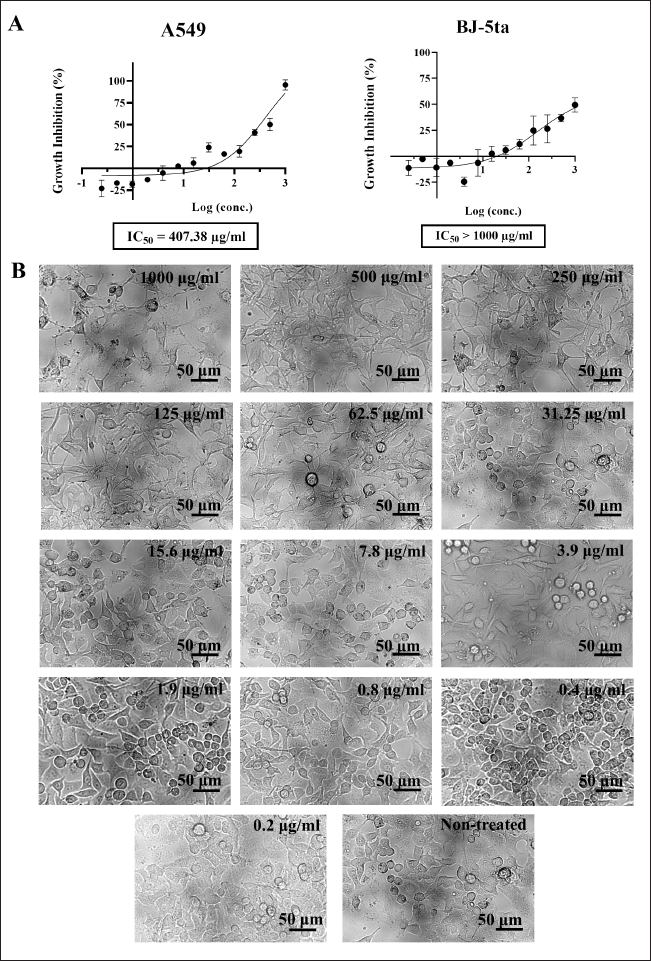 | Figure 5. The cytotoxic effect of SS162-EA extract on A549 cells. (A) The growth-inhibitory percentages of SS162-EA concentrations on A549 cells and BJ-5ta normal fibroblasts. Data were expressed as mean ± SD of three independent experiments. (B) The morphology of A549 cells treated with SS162-EA concentrations. Scale bars: 50 μm. [Click here to view] |
 | Figure 6. GC-MS chromatogram of SS162-EA extract. [Click here to view] |
DISCUSSION
In this study, the preliminary screening indicated anticancer activity of Streptomyces sp. SS162 strain isolated from marine corals, in which SS162-cultured medium displayed inhibitory activity toward different human cancer cell lines. The highest cytotoxic activity was against the A549 lung carcinoma cell line, with growth inhibition of 64.63% after 48 hours of incubation. Meanwhile, SiHa and NCI-N87 suffered moderate cytotoxicity with 51.73% and 36.73% inhibition, respectively. Other cancer cell lines consisting of Caov-3 and HL-60 experienced weak cytotoxic activity (<20%). Notably, the SS162-cultured medium expressed less toxicity to the BJ-5ta normal cell line (32.62%) than A549 cells (64.63%), implying the selective activity on this cancer cell line. A bioactive agent causing toxicity on cancer cells while being unharmed to normal cells is also an important highlight since current chemotherapies usually cause serious side effects on patients despite the remarkable killing effect on tumor cells (Oun et al., 2018). Therefore, the selectivity of tested agents on cancer cells is also an important target for determining promising candidates (Fahmy and Abdel-Tawab, 2021; Tan et al., 2015); thus, Streptomyces sp. SS162 was opted for further investigation.
Regarding the A549 cell line, the SS162-cultured medium caused a proliferative inhibition on these cells during the experimental 96-hour period. After the treatment, the A549 cells became round immediately and detached from adjacent cells and the culture surface while their plasma membrane’s integrity was maintained. Compared to a former study, Kumar et al. (2021) demonstrated the alteration of A549 cells, such as the round morphology, reduction in cell size, and cytoskeleton aggregation after being treated with Streptomyces sp. strain 196’s extract, and these cells were induced apoptotic cell death later (Kumar et al., 2021). In the current study, the SS162-cultured medium impeded the adhesion and proliferation of A549 cells; however, the cells did not experience the induction of apoptosis during experimental time. To the best of our knowledge, this phenomenon has not been reported in other works yet. The adhesion of cells to the ECM through receptors is one of the dominant checkpoints for cell cycle progression to control proliferation, differentiation, and migration (Moreno-Layseca and Streuli, 2014). Integrin, known as a cell-surface receptor, can mediate and interact with ECM proteins to construct cellular morphology and induce the activation of various proliferative signaling pathways, such as PI3K/Akt (Sun et al., 2019), ERK/MAP (Ho et al., 2020), or Rho/ROCK (Streuli and Akhtar, 2009), related to drug resistance of chemotherapeutic agents. It is hypothesized that secondary metabolites presenting in the SS162-cultured medium could affect integrin-ECM interaction, causing the loss of A549 cells’ adhesion and, thereby, affecting cell proliferation afterward.
Our quantiative reverse transcription-PCR data indicated a 2.75-fold decrease in TPX2 mRNA level in treated A549 cells. This is correlated with previous results showing that a decrease in TPX2 expression led to mitotic arrest (Hsu et al., 2017). TPX2 is identified as a prominent interactor triggering the interaction between Aurora A kinase and microtubules of the spindle apparatus during early mitosis (Grover et al., 2012b). Therefore, the correlation between the downregulation of TPX2 mRNA level and mitotic errors would disrupt cell cycle progression and prevent proliferation, which could become a potential therapeutic strategy for cancer treatment (Grover et al., 2012a; Zhang et al., 2018). In this study, the SS162-cultured medium decreased TPX2 mRNA level, which could be related to the proliferative inhibition of A549 lung cancer cells. Interestingly, the antiproliferative capacity of Streptomyces sp. via the downregulation of TPX2 was reported here for the first time. In addition, CDKN1B mRNA expression in A549 cells treated with SS162-cultured medium increased significantly, 2.78-fold higher than in nontreated samples. Cell cycle checkpoint is a prominent mechanism to control cell proliferation, differentiation, and cell death, which depends on the activation of cyclin-dependent kinase families (CDK) (Dickson and Schwartz, 2009). To ensure the cell transition from quiescence to proliferation, the interaction between CDK and cyclin needs to be triggered. In contrast, the proteins blocking this interaction can be listed as CDKN4, CDKN1A, CDKN1B, and CDKN1C functioning as proliferative inhibitors (Ale-Agha et al., 2018). Among them, CDKN1B (p27), a CDK inhibitor of the Kip protein family, involves in the G1/S phase checkpoint. It is demonstrated that the considerable upregulation of CDKN1B could be associated with the growth inhibition of non-small-cell lung cancer cells at the G1/S phase (Leem et al., 2018; Li et al., 2018; Wang et al., 2021). In previous reports on Streptomyces sp., the upregulation of CDKN1B (p27) contributing to anticancer activity was also observed (Cho et al., 2015). Therefore, the SS162-cultured medium also affected A549 cells’ proliferation via the upregulation of the CDKN1B gene besides the decrease in the TPX2 level mentioned above.
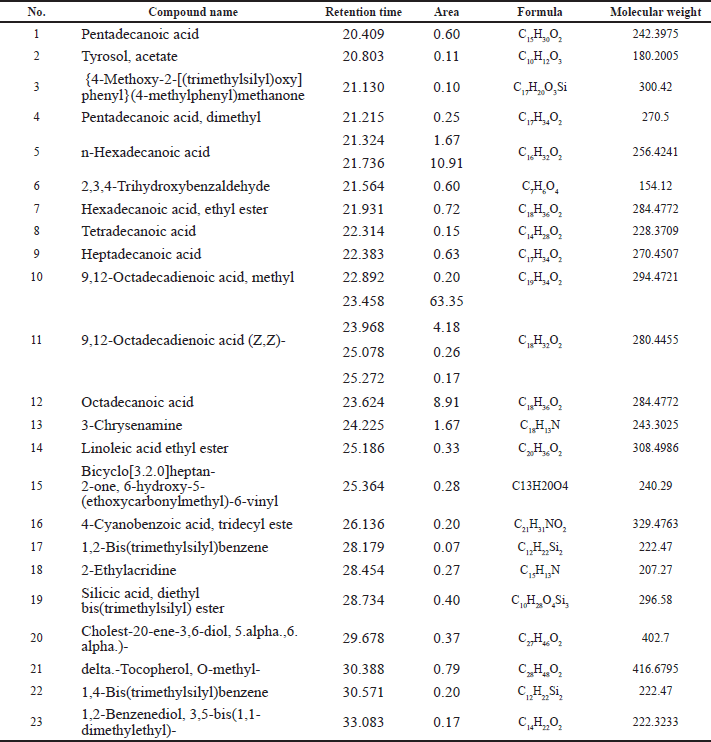 | Table 1. Chemical compounds identified in the SS162-EA extract by GC-MS analysis. [Click here to view] |
The investigation of the SS162-EA extract’s cytotoxicity showed that the IC50 value was 407.38 μg/ml toward the A549 cell line. Regarding previous works evaluating the anticancer activity of Streptomyces sp., the IC50 values of crude extracts were approximately 200–400 μg/ml, to which the value of SS162-EA extract is comparable (Rajivgandhi et al., 2020; Tan et al., 2019, 2015). In addition, importantly, similar to SS162-cultured medium, SS162-EA extract also exhibited a lower toxicity to BJ-5ta human normal fibroblasts than A549 cells, in which calculated selectivity index (SI) (the ratio of IC50 on normal cell line to IC50 on cancer cell line) is greater than 2.5. Compared to other studies, this SI value is higher, implying the potential of Streptomyces sp. SS162’s secondary metabolites presenting in the extract (Fahmy and Abdel-Tawab, 2021; Tan et al., 2015). The GC-MS analysis of SS162-EA extract revealed 23 chemical constituents in SS162-EA extract belonging to saturated fatty acids, unsaturated fatty acids, fatty acid esters, steroids, and phenolic compounds. n-Hexadecanoic acid (12.58%), 9,12-octadecadienoic acid (Z,Z)- (67.96%), octadecanoic acid (8.91%), and 3-chrysenamine (1.67%) present as major compounds in the extract, among which hexadecanoic acid and octadecadienoic acid are familiar constituents which Streptomyces spp. produce when compared with other reports (Al-Dhabi et al., 2020; Mothana et al., 2022; Qi et al., 2019). However, the percentage amount of 9,12-octadecadienoic acid (Z,Z)- in SS162-EA extract was much higher. Regarding the anticancer activity of these compounds, n-hexadecanoic acid was reported on cytotoxicity against HCT-116 and HT-29 colorectal cancer cell lines with IC50 values of 0.8 and 36.04 μg/ml, respectively (Bharath et al., 2021; Ravi and Krishnan, 2017), while 9,12-octadecadienoic acid and 3-chrysenamine have not been investigated yet.
It is also hypothesized that besides the constituents concentrated in the EA fraction, there were other constituents in the SS162-cultured medium contributing to the anticancer activity of Streptomyces sp. SS162. The morphological results supported this hypothesis since SS162-EA extract did not cause a nonadherent round feature on A549 cells which the SS162-cultured medium could induce. In fact, Streptomyces spp. could produce a variety of chemical structures, including peptides, alkaloids, macrolides, lactones, indoles, terpenes, and quinones upon which varied solvents have been used, such as EA, methanol, and hexane, aiming to improve extraction efficiency (Ser et al., 2017; Sivalingam et al., 2019). For that reason, seeking bioactive agents in an SS162-cultured medium could also be continued using other extraction approaches.
CONCLUSION
The genus Streptomyces from the marine environment presents as a promising source of bioactive secondary metabolites. In this research study, it is demonstrated that Streptomyces sp. SS162, derived from marine corals, possesses significant cytotoxic activity against the A549 lung carcinoma cell line while being less toxic to normal cells. More particularly, the SS162-cultured medium could effectively inhibit the proliferation of A549 cells via the upregulation of CDKN1B and the downregulation of TPX2 expression levels. The EA crude extract of SS162-cultured medium also owns antiproliferative capacity on cancer cells with high selectivity (SI >2.5). Therefore, bioactive compounds from Streptomyces sp. SS162 contributing to anticancer activity against lung carcinoma cells need to be identified and isolated in further studies.
ACKNOWLEDGMENTS
The authors thank all members of the Gene Technology and Application group for their great support in this study.
AUTHORS’ CONTRIBUTIONS
THN and TTBN contributed equally to designing experiments, collect data, performing statistical analysis, and writing the manuscript. LTYN carried out experiments and analyzed the database. NHC supervised experiments and reviewed the manuscript. DTPT designed experiments, supervised experiments, and reviewed the manuscript. All authors reviewed and approved the final manuscript for publication.
FINANCIAL SUPPORT
This work was supported by the Faculty of Biology and Biotechnology, VNU-HCM, University of Science, under grant number: SH-CNSH 2022-04.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Al-Dhabi NA, Esmail GA, Ghilan AKM, Arasu MV, Duraipandiyan V. Metabolite profiling of Streptomyces sp. Al-Dhabi-100 isolated from the marine environment in Saudi Arabia with anti-bacterial, anti-tubercular and anti-oxidant potentials. J King Saud Univ Sci, 2020; 32:1628–33.
Ale-Agha N, Goy C, Jakobs P, Spyridopoulos I, Gonnissen S, Dyballa-Rukes N, Aufenvenne K, von Ameln F, Zurek M, Spannbrucker T, Eckermann O, Jakob S, Gorressen S, Abrams M, Grandoch M, Fischer JW, Köhrer K, Deenen R, Unfried K, Altschmied J, Haendeler J. CDKN1B/p27 is localized in mitochondria and improves respiration-dependent processes in the cardiovascular system-new mode of action for caffeine. PLoS Biol, 2018; 16:e2004408.
Amatori S, Persico G, Fanelli M. Real-time quantitative PCR array to study drug-induced changes of gene expression in tumor cell lines. J Cancer Metastatis Treat, 2017; 3:90–9.
Barka EA, Vatsa P, Sanchez L, Gaveau-Vaillant N, Jacquard C, Klenk HP, Clément C, Ouhdouch Y, Wezel GPV. Taxonomy, physiology, and natural products of actinobacteria. Microbiol Mol, 2016; 80:1–43.
Bekiesch P, Zehl M, Domingo-Contreras E, Martín J, Pérez-Victoria I, Reyes F, Kaplan A, Rückert C, Busche T, Kalinowski J, Zotchev SB. Viennamycins: lipopeptides produced by a Streptomyces sp. J Nat Prod, 2020; 83:2381–9.
Benjanuwattra J, Siri-Angkul N, Chattipakorn SC, Chattipakorn N. Doxorubicin and its proarrhythmic effects: a comprehensive review of the evidence from experimental and clinical studies. Pharmacol Res, 2020; 151:104542.
Bharath B, Perinbam K, Devanesan S, AlSalhi MS, Saravanan M. Evaluation of the anticancer potential of hexadecanoic acid from brown algae Turbinaria ornata on HT–29 colon cancer cells. J Mol Struct, 2021; 1235:130229.
Chater KF, Biró S, Lee KJ, Palmer T, Schrempf H. The complex extracellular biology of Streptomyces. FEMS Microbiol Rev, 2010; 34:171–98.
Cho JJ, Chae JI, Kim KH, Cho JH, Jeon YJ, Oh HN, Yoon G, Yoon DY, Cho YS, Cho SS. Manumycin A from a new Streptomyces strain induces endoplasmic reticulum stress-mediated cell death through specificity protein 1 signaling in human oral squamous cell carcinoma. Int J Oncolo, 2015; 47:1954–62.
Dickson MA, Schwartz GK. Development of cell-cycle inhibitors for cancer therapy. Curr Oncol, 2009; 16:36–43.
Fahmy NM, Abdel-Tawab AM. Isolation and characterization of marine sponge–associated Streptomyces sp. NMF6 strain producing secondary metabolite (s) possessing antimicrobial, antioxidant, anticancer, and antiviral activities. J Genet Eng Biotechnol, 2021; 19:1–14.
Fernández-Martínez LT, Borsetto C, Gomez-Escribano JP, Bibb MJ, Al-Bassam MM, Chandra G, Bibb MJ. New insights into chloramphenicol biosynthesis in Streptomyces venezuelae ATCC 10712. Antimicrob Agents Chemother, 2014; 58:7441–50.
Grover A, Singh R, Shandilya A, Priyandoko D, Agrawal V, Bisaria VS, Wadhwa R, Kaul SC, Sundar D. Ashwagandha derived withanone targets TPX2-Aurora A complex: computational and experimental evidence to its anticancer activity. PLoS One, 2012a; 7:e30890.
Grover A, Singh R, Shandilya A, Priyandoko D, Agrawal V, Bisaria VS, Wadhwa R, Kaul SC, Sundar D. Ashwagandha derived withanone targets TPX2-aurora A complex: computational and experimental evidence to its anticancer activity. PLoS One, 2012b; 7:e30890.
Ho Y, Li ZL, Shih YJ, Chen YR, Wang K, Whang-Peng J, Lin HY, Davis PJ. Integrin αvβ3 in the mediating effects of dihydrotestosterone and resveratrol on breast cancer cell proliferation. Int J Mol Sci, 2020; 21:2906.
Hsu CW, Chen YC, Su HH, Huang GJ, Shu CW, Wu TTL, Pan HW. Targeting TPX2 suppresses the tumorigenesis of hepatocellular carcinoma cells resulting in arrested mitotic phase progression and increased genomic instability. J Cancer, 2017; 8:1378–94.
Kumar P, Chauhan A, Kumar M, Kuanr BK, Kundu A, Solanki R, Kapur MK. In vitro and in silico anticancer potential analysis of Streptomyces sp. extract against human lung cancer cell line, A549. 3 Biotech, 2021; 11:254.
Lee N, Hwang S, Lee Y, Cho S, Palsson B, Cho BK. Synthetic biology tools for novel secondary metabolite discovery in Streptomyces. J Microbiol Biotechnol, 2019; 29:667–86.
Lee YC, Tzeng WF, Chiou TJ, Chu ST. MicroRNA-138 suppresses neutrophil gelatinase-associated lipocalin expression and inhibits tumorigenicity. PLoS One, 2012; 7:e52979.
Leem DG, Shin JS, Kim KT, Choi SY, Lee MH, Lee KT. Dammarane-type triterpene ginsenoside-Rg18 inhibits human non-small cell lung cancer A549 cell proliferation via G(1) phase arrest. Oncol Lett, 2018; 15:6043–9.
Li N, Zeng J, Sun F, Tong X, Meng G, Wu C, Ding X, Liu L, Han M, Lu C, Dai F. p27 inhibits CDK6/CCND1 complex formation resulting in cell cycle arrest and inhibition of cell proliferation. Cell Cycle, 2018; 17:2335–48.
Moreno-Layseca P, Streuli CH. Signalling pathways linking integrins with cell cycle progression. Matrix Biol, 2014; 34:144–53.
Mothana AA, Al-Shamahy HA, Mothana RA, Khaled JM, Al-Rehaily AJ, Al-Mahdi AY, Lindequist U. Streptomyces sp. 1S1 isolated from Southern coast of the Red Sea as a renewable natural resource of several bioactive compounds. Saudi Pharm J, 2022; 30:162–71.
Nguyen HT, Pokhrel AR, Nguyen CT, Pham VTT, Dhakal D, Lim HN, Jung HJ, Kim TS, Yamaguchi T, Sohng JK. Streptomyces sp. VN1, a producer of diverse metabolites including non-natural furan-type anticancer compound. Sci Rep, 2020; 10:1756.
Nugraha RY, Faratisha IF, Mardhiyyah K, Ariel DG, Putri FF, Nafisatuzzamrudah, Winarsih S, Sardjono TW, Fitri LE. Antimalarial properties of isoquinoline derivative from Streptomyces hygroscopicus subsp. Hygroscopicus: an in silico approach. BioMed Res Int, 2020; 2020:6135696.
Oun R, Moussa YE, Wheate NJ. The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans, 2018; 47:6645–53.
Petkovi? H, Lukeži? T, Šuškovi? J. Biosynthesis of oxytetracycline by Streptomyces rimosus: past, present and future directions in the development?of tetracycline antibiotics. Food Technol Biotechnol, 2017; 55:3–13.
Qi D, Zou L, Zhou D, Chen Y, Gao Z, Feng R, Zhang M, Li K, Xie J, Wang W. Taxonomy and broad-spectrum antifungal activity of Streptomyces sp. SCA3-4 isolated from rhizosphere soil of Opuntia stricta. Front Microbiol, 2019; 10:1390.
Quach NT, Nguyen QH, Vu THN, Le TTH, Ta TTT, Nguyen TD, Van Doan T, Van Nguyen T, Dang TT, Nguyen XC. Plant-derived bioactive compounds produced by Streptomyces variabilis LCP18 associated with Litsea cubeba (Lour.) Pers as potential target to combat human pathogenic bacteria and human cancer cell lines. Braz J Microbiol, 2021; 52:1215–24.
Quinn GA, Banat AM, Abdelhameed AM, Banat IM. Streptomyces from traditional medicine: sources of new innovations in antibiotic discovery. J Med Microbiol, 2020; 69:1040–8.
Rajivgandhi GN, Ramachandran G, Li JL, Yin L, Manoharan N, Kannan MR, Velanganni AAJ, Alharbi NS, Kadaikunnan S, Khaled JM. Molecular identification and structural detection of anti-cancer compound from marine Streptomyces akiyoshiensis GRG 6 (KY457710) against MCF-7 breast cancer cells. J King Saud Univ Sci, 2020; 32:3463–9.
Ravi L, Krishnan K. Research article cytotoxic potential of n-hexadecanoic acid extracted from Kigelia pinnata leaves. Asian J Cell Biol, 2017; 12:20–7.
Risdian C, Mozef T, Wink J. Biosynthesis of polyketides in Streptomyces. Microorganisms, 2019; 7:124.
Schink JC, Filiaci V, Huang HQ, Tidy J, Winter M, Carter J, Anderson N, Moxley K, Yabuno A, Taylor SE, Kushnir C, Horowitz N, Miller DS. An international randomized phase III trial of pulse actinomycin-D versus multi-day methotrexate for the treatment of low risk gestational trophoblastic neoplasia; NRG/GOG 275. Gynecol Oncol, 2020; 158:354–60.
Ser HL, Tan LTH, Law JWF, Chan KG, Duangjai A, Saokaew S, Pusparajah P, Ab Mutalib NS, Khan TM, Goh BH. Focused review: cytotoxic and antioxidant potentials of mangrove-derived Streptomyces. Front Microbiol, 2017; 8:2065.
Shen B, Du L, Sanchez C, Edwards DJ, Chen M, Murrell JM. The biosynthetic gene cluster for the anticancer drug bleomycin from Streptomyces verticillus ATCC15003 as a model for hybrid peptide-polyketide natural product biosynthesis. J Ind Microbiol Biotechnol, 2001; 27:378–85.
Shen T, Li LM, Xu ZY, Wang YD, Xie WD. Julichrome derivatives and gliotoxin from a soil derived Streptomyces sp. Nat Prod Res, 2021; 35:34–40.
Sivalingam P, Hong K, Pote J, Prabakar K. Extreme environment Streptomyces: potential sources for new antibacterial and anticancer drug leads? Int J Microbiol, 2019; 2019:5283948.
Streuli CH, Akhtar NJBJ. Signal co-operation between integrins and other receptor systems. Biochem J, 2009; 418:491–506.
Sun F, Wang J, Sun Q, Li F, Gao H, Xu L, Zhang J, Sun X, Tian Y, Zhao Q, Shen H, Zhang K, Liu J. Interleukin-8 promotes integrin β3 upregulation and cell invasion through PI3K/Akt pathway in hepatocellular carcinoma. J Exp Clin Cancer Res CR, 2019; 38:449.
Takano H, Toriumi N, Hirata M, Amano T, Ohya T, Shimada R, Kusada H, Amano SI, Matsuda KI, Beppu T. An ABC transporter involved in the control of streptomycin production in Streptomyces griseus. FEMS Microbiol Lett, 2016; 363:fnw149.
Tan LTH, Chan KG, Pusparajah P, Yin WF, Khan TM, Lee LH, Goh BH. Mangrove derived Streptomyces sp. MUM265 as a potential source of antioxidant and anticolon-cancer agents. BMC Microbiol, 2019; 19:1–16.
Tan LTH, Ser HL, Yin WF, Chan KG, Lee LH, Goh BH. Investigation of antioxidative and anticancer potentials of Streptomyces sp. MUM256 isolated from Malaysia mangrove soil. Front Microbiol, 2015; 6:1316.
Tang Y, Lee TS, Khosla C. Engineered biosynthesis of regioselectively modified aromatic polyketides using bimodular polyketide synthases. PLoS Biol, 2004; 2:E31.
Tangjitjaroenkun J, Pluempanupat W, Tangchitcharoenkhul R, Yahayo W, Supabphol R. Antibacterial, antioxidant, cytotoxic effects and GC-MS analysis of mangrove-derived Streptomyces achromogenes TCH4 extract. Arch Biol Sci, 2021; 73:223–35.
Tiran B, Parluk T, Kleinhendler E, Man A, Fomin I, Schwarz Y. Fiberoptic bronchoscopic submucosal injection of mitomycin C for recurrent bening tracheal stenosis: a case series. Isr Med Assoc J IMAJ, 2020; 22:757–60.
Toumatia O, Yekkour A, Goudjal Y, Riba A, Coppel Y, Mathieu F, Sabaou N, Zitouni A. Antifungal properties of an actinomycin D-producing strain, Streptomyces sp. IA1, isolated from a Saharan soil. J Basic Microbiol, 2015; 55:221–8.
Vu HNT, Nguyen DT, Nguyen HQ, Chu HH, Chu SK, Chau MV, Phi QT. Antimicrobial and cytotoxic properties of bioactive metabolites produced by Streptomyces cavourensis YBQ59 isolated from Cinnamomum cassia Prels in Yen Bai Province of Vietnam. Curr Microbiol, 2018; 75:1247–55.
Wang JL, Lan YW, Tsai YT, Chen YC, Staniczek T, Tsou YA, Yen CC, Chen CM. Additive antiproliferative and antiangiogenic effects of metformin and pemetrexed in a non-small-cell lung cancer xenograft model. Front Cell Devel Biol, 2021; 9:688062.
Xiang T, Li L, Yin X, Yuan C, Tan C, Su X, Xiong L, Putti TC, Oberst M, Kelly K, Ren G, Tao Q. The ubiquitin peptidase UCHL1 induces G0/G1 cell cycle arrest and apoptosis through stabilizing p53 and is frequently silenced in breast cancer. PLoS One, 2012; 7:e29783.
Yashiro T, Sakata F, Sekimoto T, Shirai T, Hasebe F, Matsuda K, Kurosawa S, Suzuki S, Nagata K, Kasakura K, Nishiyama M, Nishiyama C. Immunosuppressive effect of a non-proteinogenic amino acid from Streptomyces through inhibiting allogeneic T cell proliferation. Biosci Biotechnol Biochem, 2019; 83:1111–6.
Zhang MY, Liu XX, Li H, Li R, Liu X, Qu YQ. Elevated mRNA levels of AURKA, CDC20 and TPX2 are associated with poor prognosis of smoking related lung adenocarcinoma using bioinformatics analysis. Int J Med Sci, 2018; 15:1676–85.