INTRODUCTION
Marine microorganisms have been reported as one of the sources of compounds developed and used as drug agents, considering 75% of living organisms come from the marine environment (Saeed et al., 2021; Stincone and Brandelli, 2020). Previous reports supported the successful development of drugs from the marine environment has a success rate four times higher than other naturally derived compounds (Sigwart et al., 2021). Marine endophytic fungi are microorganisms that have produced potential compounds for drugs (El-Bondkly et al., 2021). Endophytic fungi have been discovered and isolated from various marine habitats, including sponges, mangroves, algae, and seagrasses, that have antifungal, anti-inflammatory, anticancer, antibacterial, antiviral, antiparasitic, immunosuppressive, and antioxidant activities (El-Bondkly et al., 2021).
Algal and sponges have much diversity and are important ecosystems in marine environments and even the world (Amelia et al., 2022; McCoy and Kamenos, 2015). Algae and sponges have benefited marine biotas such as fish and shellfish (Amelia et al., 2022; McCoy and Kamenos, 2015). In addition, algae and coral have long been used by humans in various fields, including the fishing industry, health, coastal protection, and tourism (Berdalet et al., 2016; Eastwood et al., 2017; Hoegh-Guldberg et al., 2007). For microorganisms, algae and sponges become ideal symbiosis places (Van de Water et al., 2018). Several studies have revealed communication between fungi and their symbiotic place, including algae and sponges (Zuluaga-Montero et al., 2010). However, the detailed symbiotic interactions between them are still not widely studied (Van de Water et al., 2018). The fungi isolated from algae or sponges are quite varied species-wise and produce quite various activities of secondary metabolites (Chen et al., 2022; Gao and Zhang, 2022).
A wide variety of algae-associated fungi and sponge-associated fungi has been isolated, producing new and known compounds (Chen et al., 2022; Gao and Zhang, 2022). The compounds have been reported to have biological activity, and some of them have become lead compounds to be developed and used in clinical applications (Saeed et al., 2021). In this review, we presented the diversity of sources, chemistries, and bioactivities of new compounds isolated from algae-associated and sponge-associated fungi gathered from the literature from 2017 to 2021. It covers 339 secondary metabolites focusing on the classification of compounds as fungi-producing, host-associated, and bioactive, and a comparison between algae-associated and sponge-associated fungi was made. Zhang et al. (2020) reviewed the diversity of bioactivity and structure of 571 metabolites from sponge-associated fungi and gathered the literature from 2010 to 2018. In another review in 2022, Gao and Zhang (2022) reported a total of 196 new metabolites isolated from algae-associated fungi covering from 2016 to 2021, including the chemical diversity and biological activities.
Algae-associated fungi
This review reported a total of 169 new secondary metabolites discovered from algae-associated fungi. According to structure, all the secondary metabolites can be classified as terpenes, alkaloids, aromatics, polyketides, lactones, and other compounds, which are dominated by terpenes 60% (Fig. 1A). Based on producing fungal strains, the secondary metabolites were isolated from various fungal species: Trichoderma sp., Aspergillus sp., and Penicillium sp. reflected predominant producers of the secondary metabolites, which were calculated for 54%, 12%, and 7%, respectively. On the other hand, the remaining secondary metabolites are scattered across 11 strains of fungal species, including the rare genera, such as Chondrostereum sp., Alternaria sp., Nemania sp., Talaromyces sp., Acremonium sp., Eurotium sp., Pestalotiopsis sp., Stereum sp., Acrostalagmus sp., Pyrenochaetopsis sp., and Halosphaeriaceae sp. (Fig. 1B).
Although the fungi are greatly disparate in terrestrial and marine environments, marine-derived fungi dominate fungal diversity. Environmental diversity, such as alterations in temperature, pressure, light, and mineral composition, such as salt levels, may affect the diversity of fungi inhabiting marine environments. Several studies have shown that the diversity of fungi varies according to their host genus. In this review, the variegated species to their algae-host genus were obtained from 21 algae. As depicted in Figure 2A, 18%, 15%, and 8% of these fungi are derived from Rhodomela sp., Gracilaria sp., and Sargassum sp., respectively, and others were isolated from Laurencia sp., Chondria sp., Grateloupia sp., Chondrus sp., Pterocladiella sp., Ulva lactuca, Laminaria sp., Asparagopsis sp., Lomentaria sp., Coelarthrum sp., Mastophora rosea, Padina sp., Undaria pinnatifida, Ceramium japonicum, Codium fragile, Fucus vesiculosus, Enteromorpha prolifera, and Symphyocladia latiuscula. Three of these algae are green algae, namely, U. lactuca, C. fragile, and E. prolifera, whereas the others are red and brown algae, and the red algae dominate as a source of fungi. The red and brown algae possess a high diversity of fungi that are the most important hosts of fungi from the marine. In contrast, the diversity of fungi from green algal is lower, which may be because of the comparatively short life cycle of green algae.
In addition to their classification as chemical, fungi-producing, and host-associated, these new secondary metabolites from algae-associated fungi possess exceptional biological activities. A total of 90 compounds were reported to have biological activities in which anti-marine-phytoplankton, antibacterial, and cytotoxicity activities are dominating capabilities in these new secondary metabolites with 21%, 11%, and 7%, respectively. Other activities include antimicroalgal, antioxidant, antifungal, anti-inflammatory, insecticidal, angiotensin-converting enzyme (ACE) inhibitory, acetylcholinesterase (AChE) inhibitory, and antiangiogenic (Fig. 2B).
Terpenes
Terpenes are a class of compounds that are widely produced by fungi and have diverse biological activities. Terpenes are reported to have antibacterial, antifungal, and antiviral activities. Some terpenes show a broad spectrum of antibacterial activities. In this report, terpenes are the most widely isolated group of compounds from fungi symbiotic with algae, with as many as 101 compounds. A total of 78 compounds were reported isolated from the fungus Trichoderma spp., and others were isolated from Alternaria alternata, Aspergillus sydowii, Chondrostereum sp., Nemania bipapillata, and Penicillium chrysogenum. Based on biological activity, they are reported to have antibacterial, antifungal, antimicroalgal, anti-inflammation, antioxidant activities, and cytotoxicity and inhibition of marine phytoplankton. In this part, detailed descriptions of classified terpenes are provided below.
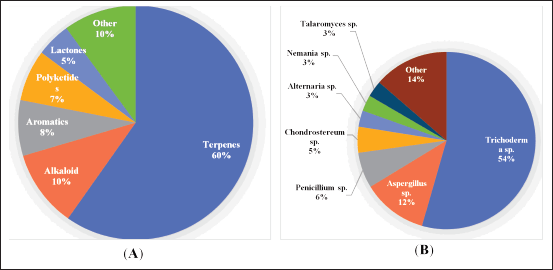 | Figure 1. Distributions of new compounds produced algae-associated and their activities. Distribution of the secondary metabolites corresponding to the chemical structure (A) and fungi-producing (B). [Click here to view] |
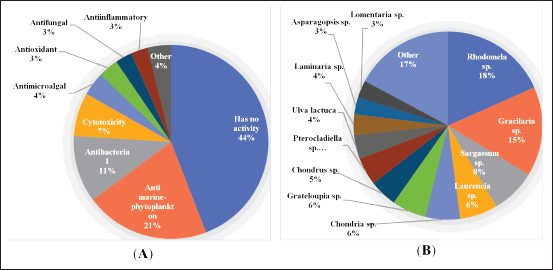 | Figure 2. Distributions of new compounds produced algae-associated and their activities. Distribution of the secondary metabolites corresponding to the biological activity (A) and algae-associated (B). [Click here to view] |
Sesquiterpenes
In this report, as many as 78 sesquiterpenes were reported to have been isolated from algae-associated fungi, with as many as 60 compounds isolated from the fungus Trichoderma spp. Sixteen compounds from the Trichoderma spp. were isolated from red alga Rhodomela confervoides, namely, 14-O-methyl CAF-603 (1), 14-O-methyltrichocarotin G (2), cadin-4-en-11-ol (3), cycloner-3-en -7,11-diol (4), isoepicyclonerodiol oxide (5), methylhydroheptelidate (6), norepicyclonerodiol oxide (7), trichobisabolin M (8) and N (9), and trichocadinin H-N (10 – 16). Compounds 1, 2, 4, 6, 7, and 10–16 showed inhibition of the marine phytoplankton, and compounds 8 and 9 have antibacterial activities with minimal inhibitory concentration (MIC) values of 13 to 50 μg/ml (Liu et al., 2021; Song et al., 2020; Zou et al., 2021). Other reported 15 isolated compounds from the Trichoderma spp., obtained from Gracilaria sp, namely, trichocadinin A (17), trichocarotin A-H (18 – 25), 4-cadinen-11,12-dio (26), 4-cadinen-11,13-diol (27), and trichobisabolin I-L (28–31) (Fig. 3). Compounds 17, 19–21, and 24–31 showed inhibition of the marine phytoplankton (Shi et al., 2018; Song et al., 2019a, 2019b).
The Chondria tenuissima-associated fungi Trichoderma brevicompactum ADL-9-2 produced ten sesquiterpenes, namely, trichocuparin A-B (32–33) and trichodermarin G-N (34–41). Compounds 34–36, 39, and 40 showed antifungal and antimicroalgal activities, and compounds 37 and 38 showed antifungal activities (Shi et al., 2020). Furthermore, eight compounds have been isolated from Trichoderma spp., isolated from red algae Chondrus ocellatus, trichobisabolin A-H (42–49), of which compounds 45 and 49 showed inhibition of marine phytoplankton (Shi et al., 2019). The other 11 sesquiterpenes were isolated from 4 strains of Trichoderma spp. obtained from Laminaria japonica, Grateloupia sp., Laurencia okamurai, and Sargassum sp., namely, 10-cycloneren-3,5,7- triol (50), 11-methoxy-9-cycloneren-3,7-diol (51), 8-acoren-3,11-diol (52), methyl 3,7-dihydroxy-15-cycloneranate (53), 4-hydroxyepicyclonerodiol oxide (54), 5-hydroxyepicyclonerodiol oxide (55), trichodermol chlorohydrin (56), (10E)-isocyclonerotriol (57), (10Z)-isocyclonerotriol (58), 12-nor-11-acetoxybisabolen-3,6,7-triol (59), and bisabolan-1,10,11-triol (60) (Fig. 3). Compounds 50–53 showed inhibition of the marine phytoplankton, and compounds 56, 59, and 60 have antibacterial activity (Liu et al., 2020; Ma et al., 2021; Song et al., 2018a, 2018b).
Eighteen other sesquiterpenes were isolated from Chondrostereum sp. NTOU4203, N. bipapillata (AT-05), P. chrysogenum LD-201812, A. sydowii EN-434*, and Stereum sp. OUPS-124D-4, were obtained from the algae Pterocladiella capillacea, Asparagopsis taxiformis, Grateloupia turuturu, S. latiuscula, and U. pinnatifida, respectively. Namely, chondroterpene A-H (61–68), (+)-(2R,4S,5R,8S)-4-deacetyl-5-hydroxy-botryenalol (69), (+)-(2R,4R,5R,8S)-4-deacetyl-5-hydroxy-botryenalol (70), (+)-(2R,4S,5R,8R)-4-deacetyl-botryenalol (71), nemenonediol A – B (72–73), (2′R)-stachyline B (74), (2′R)-westerdijkin A (75), (7S,8S)-8-hydroxysydowic acid (76), (± )-(7 R*,10 R*)-10-hydroxysydowic acid (77), and dihydro-1,5-secovibralactone (78) (Fig. 3). Compound 61 showed inhibition of nitric oxide production in murine BV-2 microglial cells, and 76 inhibited AChE and butyrylcholinesterase (BuChE). Furthermore, compound 75 revealed selective cytotoxicity against the HepG2 cell line (IC50 = 22.0 μM) (Hsiao et al., 2017; Hu et al., 2020; Jiang et al., 2020; Medina et al., 2019; Yamada et al., 2018).
Diterpenes
Six diterpenes were reported to be isolated from Trichoderma asperellum A-YMD-9-2 and Trichoderma harzianum X?5 isolated from the red algae Gracilaria verrucosa and brown algae L. japonica, respectively, namely, trichaspside C-E (79–81), 3S-hydroxyharzianone (82), 3R-hydroxy-9R,10R-dihydroharzianone (83), and 11R-methoxy-5,9,13-proharzitrien-3-ol (84) (Fig. 4). All the compounds showed inhibition of the marine phytoplankton (Song et al., 2018a, 2019b).
Meroterpenoids
Seventeen meroterpenoids were reported to have been isolated from two strains of fungi, Alternaria sp. and Trichoderma sp., which were isolated from Lomentaria hakodatensis, R. confervoides, and Sargassum sp., the compounds were sesteralterin (85), tricycloalterfurene A–D (86–89), trichobisabolin Q–Z (90–99), (7S)-1-hydroxy-3-p-menthen-9-oic acid (100), and (7R)-1-hydroxy-3-p-menthen-9-oic acid (101) (Fig. 5). Compounds 85 and 86 showed inhibition of the marine phytoplankton (Shi et al., 2017; Song et al., 2018b; Zou et al., 2021).
Alkaloids
Alkaloids are a substantial and structurally diverse group of natural and liable for beneficial biological activities. Alkaloids have enthusiastically contributed to the development of drugs in various aspects, including synthesis, structural alteration, and substructures, which remain the focus of much research. The alkaloids were reported as compounds isolated from fungi in marine habitats. The unique complexity of structure causes the biological activity of alkaloids from marine fungi to be quite varied and strong. In this report, 18 alkaloids have been reported from algae-associated fungi along with their biological activities. Penicillium sp. strain KMM 4672 isolated from Padina sp. produced four diketopiperazine alkaloids, namely citriperazine A-D (102–105), which have cytotoxic activity in human prostate cell lines (Yurchenko et al., 2020). Li et al. (2021) reported that three diketopiperazines alkaloids were isolated from Aspergillus creber EN-602, obtained from the red algae R. confervoides, namely, 3-hydroxyprotuboxepin K (106), 3,15-dehydroprotuboxepin K (107), and versiamide A (108) (Fig. 6). Compound 106 had ACE inhibitory activity (IC50 = 22.4 μM and 107 and 108 had antimicrobial activity in the various aquatic bacteria (MIC = from 8 to 64 μg/ml).
In other reports, the Sargassum-associated fungi T. asperellum cf44-8 and Eurotium cristatum EN-220 were produced of diketopiperazine, oxazole, and piperazine alkaloid, namely, methylcordysinin A (109), 4-oxazolepropanoic acid (110), N-(40-hydroxyprenyl)-cyclo(alanyltryptophyl) (111), isovariecolorin I (112), 30-hydroxyechinulin (113), and 29-hydroxyechinulin (114), respectively. Compound 111 had the potential as an insecticidal agent and antioxidant, and 112 and 113 had the potential as insecticides (Du et al., 2017; Song et al., 2018b). Three indole diketopiperazine alkaloids and two diketopiperazine alkaloids were reported to have been isolated from Acrostalagmus luteoalbus TK-43 and Aspergillus versicolor OUCMDZ-2739, associated with the algae C. fragile and E. prolifera, respectively, namely (+)-acrozine A (115), acrozine B (116), acrozine C (117), 3-[6-(2-methylpropyl)-2-oxo-1H-pyrazin-3-yl]-propenamide (118), and (±)-brevianamide X (119) (Fig. 6). Compounds 115 and 116 show anti-AChE and antibacterial activity, respectively (Cao et al., 2019; Liu et al., 2019).
Aromatics compounds
Five aromatic anthraquinone compounds and three aromatic phenol compounds have been reported to be isolated from Aspergillus terreus EN-539 and Talaromyces islandicus EN-501, respectively, obtained from the algae L. okamurai, namely, 8-hydroxyconiothyrinone B (120), 8,11-dihydroxyconiothyrinone (121), 4R,8-dihydroxyconiothyrione B (122), 4S,8-dihydroxyconiothyrinone B (123), 4S,8-dihydroxy -10-O-methyldendryol E (124), and terreprenphenol A-C (125 – 127). All compounds (120–127) have been reported to have antibacterial activity, and compounds 121–124 had antibacterial and antioxidant activities (Li et al., 2017, 2019). Five other aromatic compounds were isolated from Pestalotiopsis neglecta SCSIO41403 and P. chrysogenum AD-1541 isolated from the algae Coelarthrum sp. and G. turuturu, including three carboxylic acids and two benzophenone, namely, pestallic acids F-G (128–129), neopestalone (130), and chryxanthone A-B (131 – 132), respectively (Fig. 7). Compound 131 had cytotoxic activity in five human tumor cell lines, A549, BT-549, HeLa, HepG2, and MCF-7 (IC50 = 41.7, 20.4, 23.5, 33.6, and 46.4 μM, respectively). Meanwhile, compound 132 has cytotoxic activity in the two human tumor cell lines, A549 and THP-1 (IC50 = 20.4 and 41.1 μM, respectively) (Wang et al., 2020; Zhao et al., 2018).
Lactones
Eight lactones, including three decalinoylspirotetramic acid derivatives, two diketomorpholines, carboxylic acid, isocoumarin, and steroid lactones, were isolated from Pyrenochaetopsis sp. FVE-001, Aspergillus alabamensis EN-548, P. neglecta SCSIO41405, Trichoderma citrinoviride A-WH-20-5, and Trichoderma atroviride RR-dl-3− 11, associated with F. vesiculosus, C. japonicum, Coelarthrum sp., L. okamurai, and R. confervoides, respectively: namely, pyrenosetin A-C (133–135), 4-epi-seco-shornephine A carboxylic acid (136), 4-epi-seco-shornephine A methyl ester (137), pestalotiopyrone N (138), trichophenol A9 (139), and 4-(p-hydroxyphenethoxy)demethylincisterol A3 (140) (Fig. 8). Compounds 133 and 134 had cytotoxic activity in the A-375 cell line (IC50 = 2.8 and 6.3 μM, respectively). Compound 136 had antibacterial activity on five aquatic bacteria, Escherichia coli, Ed. ictaluri, M. luteus, and V. alginolyticus (MIC = 64, 32, 32, and 64 μg/ml, respectively). Meanwhile, compound 137 had antibacterial activity on the three aquatic bacteria E. coli, Ed. ictaluri, and M. luteus (MIC = 16, 64, and 64 μg/ml, respectively) (Fan et al., 2020; Liu et al., 2020, 2021; Wang et al., 2020; Yang et al., 2018).
Polyketides
Twelve polyketides have been reported to be isolated in marine algae-associated fungi. Seven were highly oxygenated polyketides isolated from Aspergillus giganteus NTU967 collected from the green alga U. lactuca, namely, aspergilsmin A–G (141–147). Another five, including two azaphilone polyketides and one each of macrodiolide, lauric acid, and pentaketide polyketide, were isolated from Penicillium sclerotiorum, Halosphaeriaceae sp. OUPS-135D-4, T. atroviride RR-dl-3−12, and P. chrysogenum LD-201810, derived from Grateloupia sp., Sargassum thunbergii, R. confervoides, and G. turuturu, respectively: namely, 8a-epi-eupenicilazaphilone C (148), 8a-epi-hypocrellone A (149), halosmysin A (150), methyl 3,5-dihydroxydodecanoate (151), and penilactonol A (152) (Fig. 9). Compound 143 was reported to have cytotoxic activity in hepatocellular carcinoma and prostate cancer cells (SK-Hep-1 and PC-3, IC50 = 2.7–7.3 μM) and potential activity as an antiangiogenic. The 149 had selective toxicity in the neuroblastoma SH-SY5Y cell line and selective inhibition of TNF-α-induced NFκB phosphorylation. Compound 150 showed potent cytotoxic activity against three leukemia cell lines (murine P388, human HL-60, and murine L1210, IC50 = 2.2–11.7 μM) (Chen et al., 2020; Jiang et al., 2020; Liu et al., 2021; Wang et al., 2021a; Yamada et al., 2020).
 | Figure 3. Structures of 1–78. [Click here to view] |
Other compounds
Seventeen other compounds have been reported to be isolated from the fungus T. asperellum A-YMD-9-2, Acremonium sp. NTU492, Stereum sp. OUPS-124D-1, T. asperellum cf44-6, and A. alabamensis EN-549, derived from G. verrucosa, M. rosea, U. pinnatifida, Sargassum sp., and C. japonicum, respectively. The compounds included seven cycloneranes, namely, 3,7,11-trihydroxycycloneran-10-one (153), cycloneran-3,7,10,11-tetraol (154), cycloneran-3,7,11-triol (155), 11,12,15-trinorcycloneran-3,7,10-triol (156), 7,10S-epoxycycloneran-3,15-diol (157), 7,10R-epoxycycloneran-3,15-diol (158), and (10Z)-15-acetoxy-10-cycloneren-3,7-diol (159); four peptides, namely, acrepeptin A-D (160–163); three carboxylic acids, namely, sterepinic acids A-C (164–166); two trichodenones, namely, dechlorotrichodenone C (167) and 3-hydroxytrichodenone C (168); and one steroid, namely, 28-acetoxy-12β,15α,25-trihydroxyergosta-4,6,8(14),22-tetraen-3-one (169) (Fig. 10). Compounds 153–159 and 167–168 have anti-marine-phytoplankton. Meanwhile, compounds 167–169 have antibacterial activity (Hsiao et al., 2020; Song et al., 2018b, 2019c; Yamada et al., 2018; Yang et al., 2018).
Sponge-associated fungi
In recent years, an augmentative number of studies highlighted that many active secondary metabolites from sponges are of microorganism origin due to similar chemical structures found in terrestrial microorganisms. Of many marine organisms, sponges are considered the most prolific source of therapeutic compounds as these animals harbor many secondary metabolites, many of which are worthwhile for human health. Sponge-associated fungi are a group of microorganisms found to produce secondary metabolites.
In this report, 170 new secondary metabolites were reported to have been isolated from various marine sponge-associated fungi and were found in 18 genera. Most of the new secondary metabolites found in sponge-associated fungi are produced by Aspergillus sp. (32%), while Penicillium sp. and Talaromyces sp. account for about 11% and 10% of the new secondary metabolites reported. The rest were found from the fungi Setosphaeria sp., Pestalotiopsis sp., Acremonium sp., Alternaria sp., Didymellaceae sp., Cymostachys sp., Eupenicillium sp., Cladosporium sp., Fusarium sp., Trichoderma sp. Arthrinium sp, Pleosporales sp., Ascomycota sp., Daldinia eschscholtzii sp., and Neosartorya fennelliae sp. (Fig. 11A). The fungi were derived from three sponges: Callyspongia sp. (29%), Phakellia fusca (16%), and Axinella cannabina (9%). The rest were derived from Xestospongia testudinaria, Plakortis simplex, Aaptos, Isopod, Haliclona sp., Paratetilla sp., Agelas oroides, N. chaliniformis, Petrosia sp., Stylissa sp., Chalinidae, Epipolasis sp., Mycale sp., Phyllospongia foliascens, Reniochalina sp., Sarcotragus muscarum, Stelletta sp., Aka coralliphaga, and Hymeniacidon perleve (Fig. 11B).
The new secondary metabolites produced by sponge-associated fungi are a diverse array of structures observed. The majority of the new secondary metabolites are lactones (19%), polyketides (18%), alkaloids (16%), and aromatics (16%) (Fig. 12A). In addition to the diverse structures, the new secondary metabolites derived from sponge-associated fungi exhibit diverse and pronounced biological activities. Antimicrobial activity and cytotoxicity are the most prominent activities, as indicated by 12 and 11% of the new secondary metabolites. Other activities include IL-6 immune-suppressive, antifungal, anti-inflammatory, NF-α immune-suppressive, antioxidant, alpha-glucosidase inhibitors, anti-BChE, anti-Aβ fibrillization, anti-Mycobacterium tuberculosis, and antiviral effects (Fig. 12B).
 | Figure 4. Structures of 79–84. [Click here to view] |
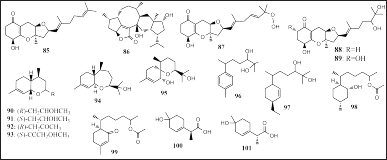 | Figure 5. Structures of 85–101. [Click here to view] |
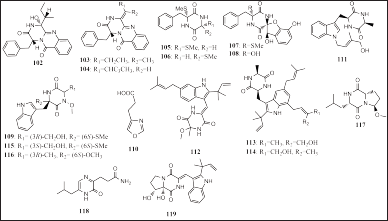 | Figure 6. Structures of 102–119. [Click here to view] |
Alkaloids
Twenty-nine alkaloids have been reported to be isolated from sponge-associated fungi Alternaria sp., Aspergillus sp., and Penicillium sp., derived from the sponges Callyspongia sp., Epipolasis sp., Isopods, and P. fusca, respectively. Twelve alkaloids were reported to be piperazine alkaloids, namely, pyranamides A-D (170–173), secopyranamide C (174), protuboxepins F-J (175 – 179), and solitumines A-B (180–181). Nine pyrrolidine alkaloids included preussins C– I (182–188) and (11R)/(11S)-preussins J–K (189–190). Four indole alkaloids included candidusin D (191) and solitumidines A-C (192–194). Three thiazole alkaloids included altenusinoides A– B (195–196) and methyl 2-(6-hydroxybenzothiazol-4-yl) acetate (197) (Fig. 13). Compounds 176, 182, and 191 have cytotoxic activity. Meanwhile, 183–190 have been reported to have IL-6 immune-suppressive activity (IC50 = 22, 8.2, 9.9, 0.11. 14, 0.19, 2.3, and 16 μM, respectively) (Buttachon et al., 2018; Chen et al., 2018; Gu et al., 2018a; Luo et al., 2019a; Rodríguez et al., 2020).
Aromatic
Twenty-eight aromatic compounds have been reported to be isolated from sponge-associated fungi consisting of phenol, benzofuran, anthraquinone, epimer, chromone, amino acid, anthracenedione, and ether. Thirteen phenols were isolated from Didymellaceae sp. SCSIO F46, Alternaria sp. SCSIO41014, A. sydowii J05B-7F-4, and Ascomycota sp. VK12 derived from the sponge Callyspongia sp., Stelletta sp., and unidentified sponges, respectively, namely, 1-hydroxy-6-methyl-11-methoxy-8-hydroxymethylxanthone (198), 7-(2-hydroxyphenyl) butane-7,8,9-triol (199), coleophomones E and F (200 and 201), diorcinols F and L (202 and 203), boric acid E (204), alternariphent A (205), β-d-glucopyranosyl aspergillusene A (206), and (3R)-(3′,5′-dihydroxyphenyl)butan-2-one (207) (Fig. 14). Compound 203 was reported to have promising cytotoxic activity against four tumor cell lines (Huh-7, HeLa, DU145, and HL60, IC50 = 5.7–9.6 μM), and 204 had promising COX-2 inhibitory activity (IC50 = 3.3 μM). Compound 207 displayed cytotoxic activity on three human cancer cell lines (HepG2, MCF-7, and SK-Mel2, IC50 = 65.9, 57.7, and 96.5 μM) and has promising NO inhibitory activity against lipopolysaccharide (LPS)-stimulated BV2 cells (IC50 = 76.5 μM) (Liu et al., 2017; Pang et al., 2018c; Quang et al., 2021; Tian et al., 2018). Wang et al. (2017, 2020, 2021a, 2021b) reported six phenol benzofurans isolated from the marine sponge-associated fungus Cymostachys sp. NBUF082 was obtained from the Aaptos sponge, namely, cymopolyphenols A–F (208–213), with compounds 208 and 210–213 being weakly antimicrobial (MIC =16–64 μg/ml).
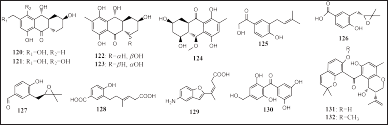 | Figure 7. Structures of 120–132. [Click here to view] |
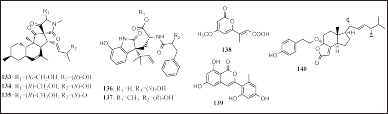 | Figure 8. Structures of 133–140. [Click here to view] |
 | Figure 9. Structures of the 141–152. [Click here to view] |
 | Figure 10. Structures of 143–169. [Click here to view] |
Two reports from different research teams, Artasasta et al. (2021) and Sibero et al. (2019) have successfully isolated five anthraquinones from fungal fermentation. Three anthraquinones were isolated from N. chaliniformis-associated fungi Aspergillus nomius NC06 and two anthraquinones were isolated from Xestospongia sp.-associated fungi Fusarium sp. KJMT.FP.4.3, namely, oxisterigmatocystin J–L (214 – 216) and karimunones A and B (217 and 218), respectively. Compounds 214 and 215 had cytotoxic activity against HT29 colon cancer cells (IC50 = 6.28, and 15.14 μM). Meanwhile, compound 218 had antibacterial activity on resistant bacteria (Salmonella enterica ser. Typhi, MIC = 125 μg/ml). Seven other aromatic compounds, consisting of two epimers, two chromones, one amino acid, one aromatic, and one anthracenedione, were isolated from P. fusca-associated fungi Pestalotiopsis heterocornis XWS03F09, Haliclona-associated fungi Aspergillus sp. LS57, Haliclona-associated fungi D. eschscholtzii KJMT FP 4.1, Isopod-associated Penicillium solitum IS1?A f, Stylissa flabelliformis-associated fungi Talaromyces stipitatus KUFA 0207, and A. oroides-associated fungi Penicillium canescens 4.14.6a, respectively: namely, heterocornol O and P (219 and 220), aspergilluone A (221), karimanone (222), solitumidine D (223), bis(1,4,5-trihydroxy-7-methylanthraquinone) (224), and diphenyl ether (225) (Fig. 14). Compounds 219 and 220 had cytotoxic activities on four cancer cell lines (BGC-823, Ichikawa, HepG2, and 7860, IC50 = 22.1–54.3 μM). Compound 221 had anti-M. tuberculosis (MIC = 32 μg/ml, in vitro) and antibacterial activity on three bacterial [Staphylococcus aureus (ATCC 6,538), Bacillus subtilis (JCM 1,465) and E. coli (JCM 1,649), MIC = 64, 8, 128 μg/ml, respectively]. Compound 222 showed antibacterial activity on multidrug-resistant bacterial S. enterica ser. Typhi (MIC = 125 μg/ml) (Artasasta et al., 2021; Frank et al., 2019; Lei et al., 2019; Liu et al., 2017, 2021; Noinart et al., 2017; Pang et al., 2018c; Quang et al., 2021; Rodríguez et al., 2020; Sibero et al., 2019, 2020; Tian et al., 2018; Wang et al., 2021b).
Lactones
Thirty-three lactones were reported from various marine sponge-associated fungi, including fifteen isolated from Talaromyces rugulosus derived from A. cannabina and ten compounds from Setosphaeria sp. SCSIO41009 was obtained from Callyspongia sp., namely, lactone acid n-butyl ester (226), 4-methoxylactone acid n-butyl ester (227), lactone diacid 7-O-n-butyl ester (228), lactone diacid (229), (3S)-cis-resorcylide (230), (3S,7S)-7-hydroxyresorcylide (231), (3S,7R)-7-hydroxyresorcylide (232), (3S,7S)-7-methoxyresorcylide 9 (233), (3S,7R)-7-methoxyresorcylide (234), (3S,7S)-7-O-n-butylresorcylide (235), (3S,7R)-7-O-n-butylresorcylide (236), talarodilactone A and B (237 and 238), thalumarin A and B (239 and 240), setosphalide A and B (241 and 242), 5-O-desmethylcolletotrialide (243), (S)-colletotrialide (244), exserolide I–K (245–247), 5-hydroxy-3-methoxy-5-methyl-4-butylfuran-2(5H)- one (248), and botryorhodine I and J (249 and 250), respectively (Fig. 15). Compounds 237 and 238 have potential cytotoxicity in the murine lymphoma (L5178Y cell line, IC50 = 3.9 and 1.3 μM, respectively), and 249 and 250 have antifungal activities on two fungal pathogens (Colletotrichum asianum and Colletotrichum acutatum MIC = 0.16, 0.63, of 0.31, and 0.63 mg/ml, respectively) (Küppers et al., 2017; Pang et al., 2018d).
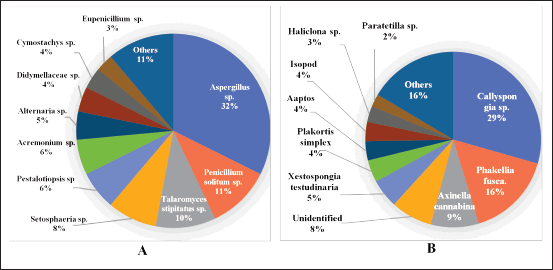 | Figure 11. Distributions of sponge-associated new compounds. Distribution of the fungi-producing (A) and sponge-associated (B) secondary metabolites. [Click here to view] |
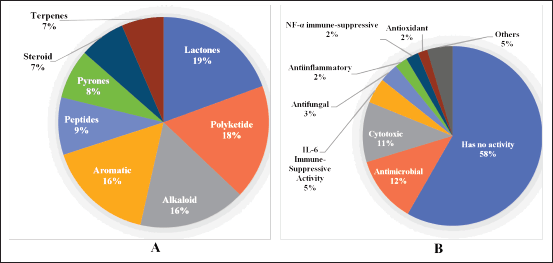 | Figure 12. Distributions of sponge-associated new compounds. Distribution of the secondary metabolites corresponding to the chemical structure (A) and biological activity (B). [Click here to view] |
The other eight lactones were isolated from the sponge-associated fungus Alternaria sp. SCSIO41014, A. terreus, Cladosporium sp. SCSIO41010, N. fennelliae KUFA 0811, and P. heterocornis derived from the sponges Callyspongia sp., A. coralliphaga, and P. fusca, namely, nordihydroaltenuenes A (251), isoochracinate A (252), asperteretal D (253), asperteretal E (254), (3R)-3-(2-hydroxypropyl)-6,8-dihydroxy-3,4-dihydroisocoumarin (255), paecilin E (256), and pestaloisocoumarin A and B (257 and 258) (Fig. 15). The 253 and 254 had a potential for inhibition of a-glucosidase (IC50 = 8.65 to 20.3 mM) and 256–258 have antibacterial effects on Gram-positive bacteria (MIC = 25 to 100 μg/ml).
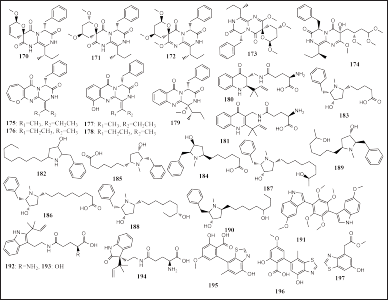 | Figure 13. Structures of 170–197. [Click here to view] |
Peptides
Eleven peptides were reported from sponge-associated including eight compounds from P. fusca-associated fungi Acremonium persicinum F10, namely, acremonpeptide E and F (259 and 260), Al (III)-acremonpeptide E and F (261 and 262), aselacin D (263), Fe (III)-acremonpeptide E and F (264 and 265), and Ga (III)-acremonpeptide E (266). Seven compounds were isolated from Aspergillus derived from sponges Petrosia sp., Reniochalina sp., and Callyspongia sp., namely, petrosamides A–C (267–269), sclerotiotide L (270), violaceomide A (271), and aspergillamides C and D (272 and 273) (Fig. 16). Compounds 267–269 expressed inhibition of pancreatic lipase (IC50 = 7.6, 1.8, and 0.5 μM, respectively) and compound 271 inhibition of IL-10 expression induced of LPS on THP-1 cells (Li and Li, 2021; Liu et al., 2018; Luo et al., 2019b; Tang et al., 2020).
Polyketides
Thirty new polyketides have been isolated from sponge-associated fungi, including nine compounds from Aspergillus sp., eight compounds from Penicillium sp., and six compounds from Pestalotiopsis sp. The fungi were obtained from X. testudinaria, Stelletta sp., Paratetilla sp., A. oroides, P. fusca, and unidentified sponges: namely, (+)1-O-demethylvariecolorquinones A (274), eurobenzophenone A–C (275–277), euroxanthone A and B (278 and 279), aspergchromone A and B (280 and 281), diorcinolic acid (282), sclerotiorin A–D (283–286), bromophilone A and B (287 and 288), penicitrinone G (289), erubescensoic acid (290), pestalotiopol A–D (291–294), and heterocornol M and N (295 and 296), respectively (Fig. 17). The 276 and 278 showed inhibition of NF-κB and NO production in SW480 and BV2 microglia cells, respectively, induced by LPS. Compounds 289–290 have antibacterial activity and 291–292 have cytotoxic activities against BGC-823, SMMC-7721, Ichikawa, and 7,860 human cancer cell lines (IC50 = 16.5 to 52.1 μM). Meanwhile, 295 showed cytotoxic activities against HepG2 and BGC-823 human cancer cell lines (IC50 = 20.4 and 61.1 μM) (Du et al., 2018; Frank et al., 2019; Jia et al., 2019; Kumla et al., 2019; Lei et al., 2019, 2020; Liu et al., 2017; Sabdaningsih et al., 2020; Wang et al., 2017).
Seven other polyketides were produced by the fungus Trichoderma sp. SCSIO41004, Alternaria sp. SCSIO41014, and Pleosporales sp. NBUF144 derived from Callyspongia sp. and Chalinidae, namely, 5,7-dihydroxy-3-methyl -2-(2-oxopropyl)naphthalene-1,4-dione (297), 7-acetyl-1,3,6-trihydroxyanthracene-9,10- dione (298), trichbenzoisochromen A (299), altertoxin VII (300), butyl xanalterate (301), 2′-hydroxy bisdechlorogeodin (302), and globosuxanthone F (303) (Fig. 17). Compounds 300 and 303 exerted cytotoxic activities; the 300 was cytotoxic against three tumor cell lines (K562, SGC-7901, and BEL-7402, IC50 = 26.58, 8.75, and 13.11 μg/ml) and the 303 had strong cytotoxicity against human acute lymphatic leukemia cells (CCRF-CEM, IC50 = 0.46 μM) (Pang et al., 2018a, 2018c; Zhou et al., 2021).
Pyrones
Thirteen pyrones group compounds have been isolated from sponge-associated fungi, Aspergillus flocculosus, Aspergillus niger, Fusarium lateritium 2016F18-1, P. chrysogenum 14XS09-2, Penicillium erubescens KUFA 0220, and Setosphaeria sp., SCSIO41009 obtained from the sponges Stylissa sp., Haliclona sp., P. foliascens, P. simplex, and Callyspongia sp., namely, ochraceopone F (304), nipyrone A-C (305–307),13-dehydroxy-1,11-deacetylpyripyropene A (308), 1-deacetylpyripyropene A (309), 1 (310), 2 (311), SPF-3059-26, and setosphapyrone A-D (313–316) (Fig. 18). Compounds 305 and 306 showed antibacterial activites against pathogenic bacteria (S. aureus, E. coli, B. subtilis MRSA, and M. tuberculosis, MIC = 32–128 μM), compound 307 had strong antibacterial activity (S. aureus and B. subtilis, MIC = 8 and 16 μM), and compound 312 had antibacterial in the E. coli ATCC 25922 (MIC = 64 mg/l) (Cao et al., 2017; Ding et al., 2019; Kumla et al., 2019; Pang et al., 2018d; Shin et al., 2018).
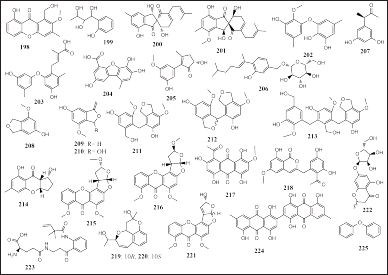 | Figure 14. Structures of 198–225. [Click here to view] |
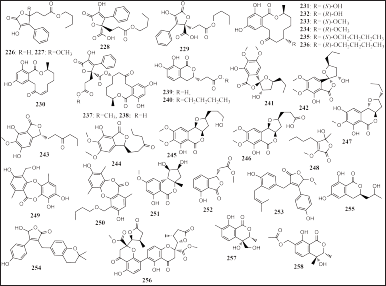 | Figure 15. Structures of 226–258. [Click here to view] |
Steroids
Twelve steroids have been reported to be isolated from various sponge-associated fungi Cladosporium sp. SCSIO41009, Aspergillus sp. LS116, T. stipitatus KUFA 0207, and Aspergillus fumigatus HNMF0047 derived from Callyspongia sp., Haliclona sp., S. flabelliformis, and unidentified sponges, respectively, namely, 16-O-deacetylhelvolic acid 21,16-lactone (317), 6-O-propionyl-6,16-O-dideacetylhelvolic acid 21,16-lactone (318), 1,2-dihydro-6,16-O-dideacetylhelvolic acid 21,16-lactone (319), 1,2-dihydro-16-O-deacetylhelvolic acid 21,16-lactone (320), 16-O-propionyl-16-O-deacetylhelvolic acid (321), 6-O-propionyl-6-O-deacetylhelvolic acid (322), and 24-epi-6β,16β-diacetoxy-25-hydroxy-3,7-dioxo-29-nordammara-1,17(20)-diene-21,24-lactone (323), and three compounds have been confirmed as oxygenated steroids produced by the SCSIO41008 strain, namely, cladosporisteroid A–C (324–326).
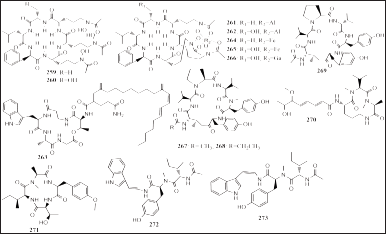 | Figure 16. Structures of 259–273. [Click here to view] |
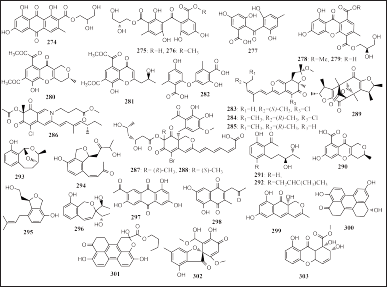 | Figure 17. Structures of 274–303. [Click here to view] |
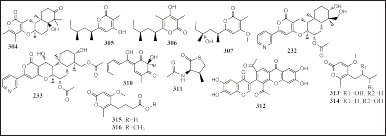 | Figure 18. Structures of 304–316. [Click here to view] |
Two other steroids produced by strains LS116 and KUFA 0207, namely, aspergillsteroid (327) and tharosterone (328), respectively (Fig. 19). In terms of the biological activity of all alkaloids, compounds 321, 322, and 327 had antibacterial activity, while compound 325 had antiviral activity, and 327, 328, and 325 had stronger activity against S. agalactiae (MIC = 16, 2, and 8 μg/ml, respectively) and 325 against V. harveyi (MIC =16 μg/ml) and 325 has activity against H3N2 (IC50 = 16.2 μM) (Kong et al., 2018; Noinart et al., 2017; Pang et al., 2018b; Xu et al., 2020).
Terpenes
Eleven terpenes, including meroterpenoids and sesquiterpenes isolated from sponge-associated fungi, including two meroterpenoids from Arthrinium sp., four meroterpenoids from Eupenicillium sp. 6A-9, two sesquiterpenes from A. persicinum KUFA 1007, and others from P. heterocornis and Trichoderma sp. HPQJ-34, were obtained from the sponges S. muscarum, P. simplex, Mycale sp., P. fusca, and H. perleve, namely, spiroarthrinol A and B (329 and 330), 1-methoxy-hydropreaustinoid A1 (331), 22-deoxy-10-oxominiolutelide B (332), eupeniacetal A and B (333 and 334), hydroberkeleyone B (335), acremine S and T (336 and 337), isopolisin B (338), and 5-hydroxycyclopeni cillone (339) (Fig. 20). Compounds 331 and 333–335 have immune-suppressive activity (TNF-α, 22.6, 43.1, 28.5, and 42.3 μM, respectively). The 336 and 337 have AChE and BuChE inhibitory activity (% inhibition at 6.6 μM = 10.42, 14.08, 30.71, and 10.53, respectively). Compound 357 has antibacterial activity against S. aureus and B. subtilis (MIC = 25 to 100 μg/ml) and 339 has antioxidative, anti-Aβ fibrillization, and neuroprotective activities (Alves et al., 2019; Elissawy et al., 2017; Fang et al., 2017; Gu et al., 2018b; Lei et al., 2017).
Comparative of algae-associated and sponge-associated fungi
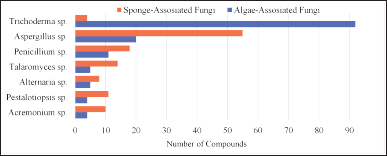
Algae- and sponge-associated fungi are the two groups of endophytic fungi found to produce new secondary metabolites. As shown in Figure 21, Trichoderma is described as the greatly predominant producers of new secondary metabolites from algae-associated fungi, while Aspergillus sp. is predominant in sponge-associated fungi. Penicillium sp. and T. stipitatus sp. are fungi that constantly produce new secondary metabolites. The remaining new secondary metabolites were produced by 28 fungi. Although fungi are highly disparate in terrestrial and marine habitats, marine-derived fungi possess more diversity. As noted before, drivers of nutrition, heat, air pressure, and light may affect the diversity of marine-derived fungi. Moreover, several reviews have indicated that the diversity of marine-derived fungi varied according to their host genus. In this review, the fungal producers of new secondary metabolites were explored from 21 algae and 23 sponges.
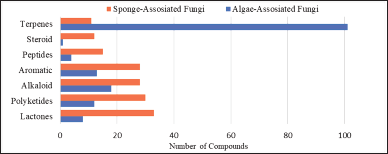
In this review, new secondary metabolites produced by algae- and sponge-derived fungi possessed a high structural diversity. A total of 339 new secondary metabolites were isolated from algae-derived fungi and sponge-derived fungi, which are categorized into terpenes, alkaloids, aromatics, polyketides, lactones, peptides, and steroids. Terpenoids produced the most algae-associated fungi and the least sponge-associated fungi, while lactones, polyketides, alkaloids, and aromatics were produced equally by sponge-associated fungi, as represented in Figure 22.
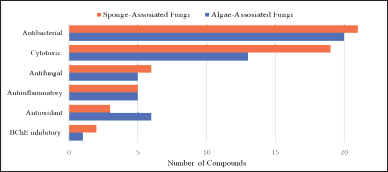
In addition to the high structural diversity, the new secondary metabolites derived from algae-associated fungi and sponge-associated fungi show diverse and apparent biological activities. As represented in Figure 23, 173 (34%) new secondary metabolites isolated from algae-associated fungi and sponge-associated fungi are found to possess considerable biological activities, of which 73 new secondary metabolites exhibit potent antibacterial and cytotoxicity activity. Moreover, some of the new secondary metabolites are shown to have one or more varieties of biological activities and are shown to have moderate to potent bioactivities. These strong bioactivities create some of the new secondary metabolite preferable candidates for developing new drugs, agrochemicals, and lead compounds in the future.
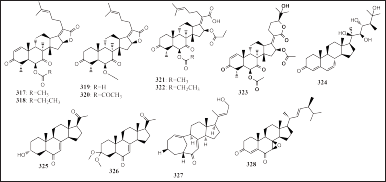 | Figure 19. Structures of 317–328. [Click here to view] |
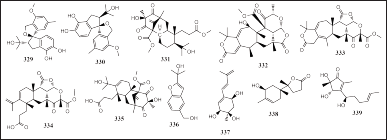 | Figure 20. Structures of 329–339. [Click here to view] |
 | Figure 21. Comparison of fungal producers of new secondary metabolites. [Click here to view] |
CONCLUSION
Marine algae and sponges are exceptional fungal sources for producing new secondary metabolites. From 2017 to 2021, natural product research into algae-derived fungi and sponge-derived fungi led to 339 new secondary metabolites. These new secondary metabolites were observed to have significantly varied in structure and various bioactivities. These new secondary metabolites have great potential in treating diseases. However, the bioactivities of these new secondary metabolites were exclusively in vitro tested; thus, there is more interest in in vivo studies for the molecular mechanism. Furthermore, there is great potential in discovering and developing new compounds to be used as led compounds derived from algae-associated and sponge-associated fungi.
 | Figure 22. Comparison of secondary metabolites corresponding to the chemical structure. [Click here to view] |
 | Figure 23. Comparison of biological activities isolated from algae-associated fungi and sponge-associated fungi. [Click here to view] |
ACKNOWLEDGMENTS
This report was funded by the Department of Pharmacy, Faculty of Health Science, University of Muhammadiyah Mataram, Mataram.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
There are no conflicts of interest to report.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Alves AJS, Pereira JA, Dethoup T, Cravo S, Mistry S, Silva AMS, Pinto MMM, Kijjoa A. A new meroterpene, a new benzofuran derivative and other constituents from cultures of the marine sponge-associated fungus Acremonium persicinum KUFA 1007 and their anticholinesterase activities. Mar Drugs, 2019; 17:379–92.
Amelia TSM, Suaberon FAC, Vad J, Fahmi ADM, Saludes JP, Bhubalan K. Recent advances of marine sponge-associated microorganisms as a source of commercially viable natural products. Mar Biotechnol, 2022; 24:492–512.
Artasasta MA, Yanwirasti Y, Taher M, Djamaan A, Ariantari NP, Edrada-Ebel RA, Handayani D. Apoptotic activity of new oxisterigmatocystin derivatives from the marine-derived fungus Aspergillus nomius NC06. Mar Drugs, 2021; 19:631–41.
Berdalet E, Fleming LE, Gowen R, Davidson K, Hess P, Backer LC, Moore SK, Hoagland P, Enevoldsen H. Marine harmful algal blooms, human health and wellbeing: challenges and opportunities in the 21st century. J Mar Biol Assoc UK, 2016; 96:61–91.
Buttachon S, Ramos AA, Inácio Â, Dethoup T, Gales L, Lee M, Costa PM, Silva AMS, Sekeroglu N, Rocha E, Pinto MMM, Pereira JA, Kijjoa A. Bis-indolyl benzenoids, hydroxypyrrolidine derivatives and other constituents from cultures of the marine sponge-associated fungus Aspergillus candidus KUFA0062. Mar Drugs, 2018; 16:119–41.
Cao J, Li XM, Meng LH, Konuklugil B, Li X, Li HL, Wang BG. Isolation and characterization of three pairs of indolediketopiperazine enantiomers containing infrequent N-methoxy substitution from the marine algal-derived endophytic fungus Acrostalagmus luteoalbus TK-43. Bioorg Chem, 2019; 90:103030–7.
Cao QX, Wei JH, Deng R, Feng GK, Zhu XF, Lan WJ, Li HJ. Two new pyripyropenes from the marine fungus Fusarium lateritium 2016F18-1. Chem Biodivers, 2017; 14:e1600298–304.
Chen Y, Chen R, Xu J, Tian Y, Xu J, Liu Y. Two new altenusin/thiazole hybrids and a new benzothiazole derivative from the marine sponge-derived fungus Alternaria sp. SCSIOS02F49. Molecules, 2018; 23:2844–52.
Chen Y, Pang X, He Y, Lin X, Zhou X, Liu Y, Yang B. Secondary metabolites from coral-associated fungi: source, chemistry and bioactivities. J Fungi, 2022; 8:1043–87.
Chen JJ, Wang SW, Chiang YR, Pang KL, Kuo YH, Shih TY, Lee TH. Highly oxygenated constituents from a marine alga-derived fungus Aspergillus giganteus NTU967. Mar Drugs, 2020; 18:303–13.
Ding L, Ren L, Li S, Song J, Han Z, He S, Xu S. Production of new antibacterial 4-hydroxy-α-pyrones by a marine fungus Aspergillus niger cultivated in solid medium. Mar Drugs, 2019; 17:344–62.
Du FY, Li X, Li XM, Zhu LW, Wang BG. Indolediketopiperazine alkaloids from Eurotium cristatum en-220, an endophytic fungus isolated from the marine alga Sargassum thunbergii. Mar Drugs, 2017; 15:24–34.
Du X, Liu D, Huang J, Zhang C, Proksch P, Lin W. Polyketide derivatives from the sponge associated fungus Aspergillus europaeus with antioxidant and NO inhibitory activities. Fitoterapia, 2018; 130:190–7.
Eastwood EK, Clary DG, Melnick DJ. Coral reef health and management on the verge of a tourism boom: a case study from Miches, Dominican Republic. Ocean Coast Manag, 2017; 138:192–204.
El-Bondkly EAM, El-Bondkly AAM, El-Bondkly AAM. Marine endophytic fungal metabolites: a whole new world of pharmaceutical therapy exploration. Heliyon, 2021; 7:e06362–77.
Elissawy AM, Ebada SS, Ashour ML, Özkaya FC, Ebrahim W, Singab AB, Proksch P. Spiroarthrinols a and B, two novel meroterpenoids isolated from the sponge- derived fungus Arthrinium sp. Phytochem Lett, 2017; 20:246–51.
Fan B, Dewapriya P, Li F, Blümel M, Tasdemir D. Pyrenosetins A–C, new decalinoylspirotetramic acid derivatives isolated by bioactivity-based molecular networking from the seaweed-derived fungus Pyrenochaetopsis sp. FVE-001. Mar Drugs, 2020; 18:47–63.
Fang F, Zhao J, Ding L, Huang C, Naman CB, He S, Wu B, Zhu P, Luo Q, Gerwick WH, Yan X, Wang Q, Zhang Z, Cui W. 5-hydroxycyclopenicillone, a new β-amyloid fibrillization inhibitor from a sponge-derived fungus Trichoderma sp. HPQJ-34. Mar Drugs, 2017; 15:260–72.
Frank M, Hartmann R, Plenker M, Mándi A, Kurtán T, Özkaya FC, Müller WEG, Kassack MU, Hamacher A, Lin W, Liu Z, Proksch P. Brominated azaphilones from the sponge-associated fungus Penicillium canescens Strain 4.14.6a. J Nat Prod, 2019; 82:2159–66.
Gao LW, Zhang P. An update on chemistry and bioactivities of secondary metabolites from the marine algal-derived endophytic fungi. Phytochem Rev, 2022; 2:2049–87.
Gu BB, Jiao FR, Wu W, Jiao W, Li L, Sun F, Wang SP, Yang F, Lin HW. Preussins with inhibition of IL-6 expression from Aspergillus flocculosus 16D-1, a fungus isolated from the marine sponge Phakellia fusca. J Nat Prod, 2018a; 81:2275–81.
Gu BB, Wu W, Liu LY, Tang J, Zeng YJ, Wang SP, Sun FP, Li L, Yang F, Lin HW. 3,5-Dimethylorsellinic acid derived meroterpenoids from Eupenicillium sp. 6A-9, fungus isolated from the marine sponge Plakortis simplex. Eur J Org Chem, 2018b; 2018:48–59.
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME. Coral reefs under rapid climate change and ocean acidification. Science, 2007; 318:1737–42.
Hsiao G, Chi WC, Pang KL, Chen JJ, Kuo YH, Wang YK, Cha HJ, Chou SC, Lee TH. Hirsutane-type sesquiterpenes with inhibitory activity of microglial nitric oxide production from the red alga-derived fungus Chondrostereum sp. NTOU4196. J Nat Prod, 2017; 80:1615–22.
Hsiao G, Wang SW, Chiang YR, Chi WC, Kuo YH, Phong DA, Chen CY, Lee TH. Anti-inflammatory effects of peptides from a marine algicolous fungus Acremonium sp. NTU492 in BV-2 microglial cells. J Food Drug Anal, 2020; 28:283–91.
Hu X, Li X, Meng L, Wang B. Antioxidant bisabolane-type sesquiterpenoids from algal-derived fungus Aspergillus sydowii EN-434. J Ocean Limnol, 2020; 38:1532–6.
Jia Q, Du Y, Wang C, Wang Y, Zhu T, Zhu W. Azaphilones from the marine sponge-derived fungus Penicillium sclerotiorum OUCMDZ-3839. Mar Drugs, 2019; 17:260–82.
Jiang LL, Tang JX, Bo YH, Li YZ, Feng T, Zhu HW, Yu X, Zhang XX, Zhang JL, Wang W. Cytotoxic secondary metabolites isolated from the marine alga-associated fungus Penicillium chrysogenum LD-201810. Mar Drugs, 2020; 18:276–86.
Kong FD, Huang XL, Ma QY, Xie QY, Wang P, Chen PW, Zhou LM, Yuan JZ, Dai HF, Luo DQ, Zhao YX. Helvolic acid derivatives with antibacterial activities against Streptococcus agalactiae from the marine-derived fungus Aspergillus fumigatus HNMF0047. J Nat Prod, 2018; 81:1869–76.
Kumla D, Dethoup T, Gales L, Pereira JA, Freitas-Silva J, Costa PM, Silva AMS, Pinto MMM, Kijjoa A. Erubescensoic acid, a new polyketide and a Xanthonopyrone SPF-3059-26 from the culture of the marine sponge-associated fungus Penicillium erubescens KUFA 0220 and antibacterial activity evaluation of some of its constituents. Molecules, 2019; 24:208–18.
Küppers L, Ebrahim W, El-Neketi M, Özkaya FC, Mándi A, Kurtán T, Orfali RS, Müller WEG, Hartmann R, Lin W, Song W, Liu Z, Proksch P. Lactones from the sponge-derived fungus Talaromyces rugulosus. Mar Drugs, 2017; 15:359–75.
Lei H, Lei J, Zhou X, Hu M, Niu H, Song C, Chen S, Liu Y, Zhang D. Cytotoxic polyketides from the marine sponge-derived fungus Pestalotiopsis heterocornis XWS03F09. Molecules, 2019; 24:2655–63.
Lei H, Lin X, Han L, Ma J, Ma Q, Zhong J, Liu Y, Sun T, Wang J, Huang X. New metabolites and bioactive chlorinated benzophenone derivatives produced by a marine-derived fungus Pestalotiopsis heterocornis. Mar Drugs, 2017; 15:69–79.
Lei H, Zhang D, Ding N, Chen S, Song C, Luo Y, Fu X, Bi X, Niu H. New cytotoxic natural products from the marine sponge-derived fungus Pestalotiopsis sp. by epigenetic modification. RSC Adv, 2020; 10:37982–8.
Li Y, Li Z. Cyclopeptide derivatives from the sponge-derived fungus Acremonium persicinum F10. Mar Drugs, 2021; 19:537–51.
Li HL, Li XM, Li X, Wang CY, Liu H, Kassack MU, Meng MH, Wang BG. Antioxidant hydroanthraquinones from the marine algal-derived endophytic fungus Talaromyces islandicus EN-501. J Nat Prod, 2017; 80:162–8.
Li HL, Li XM, Yang SQ, Meng LH, Li X, Wang BG. Prenylated phenol and benzofuran derivatives from Aspergillus terreus EN-539, an endophytic fungus derived from marine red alga Laurencia okamurai. Mar Drugs, 2019; 17:605–13.
Li HL, Yang SQ, Li XM, Li X, Wang BG. Structurally diverse alkaloids produced by Aspergillus creber EN-602, an endophytic fungus obtained from the marine red alga Rhodomela confervoides. Bioorg Chem, 2021; 110:104822.
Liu J, Gu B, Yang L, Yang F, Lin H. New anti-inflammatory cyclopeptides from a sponge-derived fungus Aspergillus violaceofuscus. Front Chem, 2018; 6:226–34.
Liu Y, Ding L, He J, Zhang Z, Deng Y, He S, Yan X. A new antibacterial chromone from a marine sponge-associated fungus Aspergillus sp. LS57. Fitoterapia, 2021; 154:105004.
Liu XH, Hou XL, Song YP, Wang BG, Ji NY. Cyclonerane sesquiterpenes and an isocoumarin derivative from the marine-alga-endophytic fungus Trichoderma citrinoviride A-WH-20-3. Fitoterapia, 2020; 141:104469.
Liu XH, Song YP, Wang BG, Ji NY. Sesquiterpenes and lipids from the algicolous fungus Trichoderma atroviride RR-dl-3-9. Phytochem Lett, 2021; 45:6–12.
Liu S, Wang H, Su M, Hwang GJ, Hong J, Jung JH. New metabolites from the sponge-derived fungus Aspergillus sydowii J05B-7F-4. Nat Prod Res, 2017; 31:1682–6.
Liu W, Wang L, Wang B, Xu Y, Zhu G, Lan M, Zhu W, Sun K. Diketopiperazine and diphenylether derivatives from marine algae-derived Aspergillus versicolor OUCMDZ-2738 by epigenetic activation. Mar Drugs, 2019; 17:6–18.
Luo X, Chen C, Tao H, Lin X, Yang B, Zhou X, Liu Y. Structurally diverse diketopiperazine alkaloids from the marine-derived fungus Aspergillus versicolor SCSIO 41016. Org Chem Front, 2019a; 6:736–40.
Luo XW, Lin Y, Lu YJ, Zhou XF, Liu YH. Peptides and polyketides isolated from the marine sponge-derived fungus Aspergillus terreus SCSIO 41008. Chin J Nat Med, 2019b; 17:149–54.
Ma XY, Song YP, Shi ZZ, Ji NY. Three sesquiterpenes from the marine-alga-epiphytic fungus Trichoderma hamatum Z36-7. Phytochem Lett, 2021; 43:98–102.
McCoy SJ, Kamenos NA. Coralline algae (Rhodophyta) in a changing world: integrating ecological, physiological, and geochemical responses to global change. J Phycol, 2015; 51:6–24.
Medina RP, Araujo AR, Batista JM, Cardoso CL, Seidl C, Vilela AFL, Domingos HV, Costa-Lotufo LV, Andersen RJ, Silva DHS. Botryane terpenoids produced by Nemania bipapillata, an endophytic fungus isolated from red alga Asparagopsis taxiformis—Falkenbergia stage. Sci Rep, 2019; 9:12318.
Noinart J, Buttachon S, Dethoup T, Gales L, Pereira JA, Urbatzka R, Freitas S, Lee M, Silva AMS, Pinto MMM, Vasconcelos V, Kijjoa A. A new ergosterol analog, a new bis-anthraquinone and anti-obesity activity of anthraquinones from the marine sponge-associated fungus Talaromyces stipitatus KUFA 0207. Mar Drugs, 2017; 15:139–51.
Pang X, Lin X, Tian Y, Liang R, Wang J, Yang B, Zhou X, Kaliyaperumal K, Luo X, Tu Z, Liu Y. Three new polyketides from the marine sponge-derived fungus Trichoderma sp. SCSIO41004. Nat Prod Res, 2018a; 32:105–11.
Pang X, Lin X, Wang J, Liang R, Tian Y, Salendra L, Luo X, Zhou X, Yang B, Tu Z, Liu Y. Three new highly oxygenated sterols and one new dihydroisocoumarin from the marine sponge-derived fungus Cladosporium sp. SCSIO41007. Steroids, 2018b; 129:41–6.
Pang X, Lin X, Wang P, Zhou X, Yang B, Wang J, Liu Y. Perylenequione derivatives with anticancer activities isolated from the marine sponge-derived fungus, Alternaria sp. SCSIO41014. Mar Drugs, 2018c; 16:280–93.
Pang X, Lin X, Yang J, Zhou X, Yang B, Wang J, Liu Y. Spiro-phthalides and isocoumarins isolated from the marine-sponge-derived fungus Setosphaeria sp. SCSIO41009. J Nat Prod, 2018d; 81:1860–8.
Quang TH, Phong NV, Hanh TTH, Cuong NX, Ngan NTT, Oh H, Nam NH, Minh CV. Cytotoxic and immunomodulatory phenol derivatives from a marine sponge-derived fungus Ascomycota sp. VK12. Nat Prod Res, 2021; 35:5153–9.
Rodríguez JPG, Bernardi DI, Gubiani JR, Magalhães de Oliveira J, Morais-Urano RP, Bertonha AF, Bandeira KF, Bulla JIQ, Sette LD, Ferreira AG, Batista Jr JM, de Souza Silva T, Dos Santos RA, Martins CHG, Lira SP, da Cunha MG, Trivella DBB, Grazzia N, Gomes NES, Gadelha F, Miguel DC, Cauz ACG, Brocchi M, Berlinck RGS. Water-soluble glutamic acid derivatives produced in culture by Penicillium solitum IS1-A from King George Island, Maritime Antarctica. J Nat Prod, 2020; 83:55–65.
Sabdaningsih A, Liu Y, Mettal U, Heep J, Riyanti, Wang L, Cristianawati O, Nuryadi H, Sibero MT, Marner M, Radjasa OK, Sabdono A, Trianto A, Schäberle TF. A new citrinin derivative from the Indonesian marine sponge-associated fungus Penicillium citrinum. Mar Drugs, 2020; 18:227–38.
Saeed AFUH, Su J, Ouyang S. Marine-derived drugs: recent advances in cancer therapy and immune signaling. Biomed Pharmacother, 2021; 134:111091–200.
Shi ZZ, Fang ST, Miao FP, Yin XL, Ji NY. Trichocarotins A–H and trichocadinin A, nine sesquiterpenes from the marine-alga-epiphytic fungus Trichoderma virens. Bioorg Chem, 2018; 81:319–25.
Shi ZZ, Liu XH, Li XN, Ji NY. Antifungal and antimicroalgal trichothecene sesquiterpenes from the marine algicolous fungus Trichoderma brevicompactum A-DL-9-2. J Agric Food Chem, 2020; 68:15440–8.
Shi ZZ, Miao FP, Fang ST, Liu XH, Yin XL, Ji NY. Sesteralterin and tricycloalterfurenes A–D: terpenes with rarely occurring frameworks from the marine-alga-epiphytic fungus Alternaria alternata k21-1. J Nat Prod, 2017; 80:2524–9.
Shi ZZ, Miao FP, Fang ST, Yin XL, Ji NY. Trichobisabolins A-H, eight new bisabolane derivatives from the marine-alga-epiphytic fungus Trichoderma asperellum Y6–2. Fitoterapia, 2019; 134:372–7.
Shin HJ, Choi BK, Trinh PTH, Lee HS, Kang JS, Van TTT, Lee HS, Lee JS, Lee YJ, Lee J. Suppression of RANKL-induced osteoclastogenesis by the metabolites from the marine fungus Aspergillus flocculosus isolated from a Sponge Stylissa sp. Mar Drugs, 2018; 16:14–23.
Sibero MT, Zhou T, Fukaya K, Urabe D, Radjasa OKK, Sabdono A, Trianto A, Igarashi Y. Two new aromatic polyketides from a sponge-derived Fusarium. Beilstein J Org Chem, 2019; 15:2941–7.
Sibero MT, Zhou T, Igarashi Y, Radjasa OK, Sabdono A, Trianto A, Bachtiarini TU, Bahry MS. Chromanone-type compounds from marine sponge-derived Daldinia eschscholtzii KJMT FP 4.1. J App Pharm Sci, 2020; 10:001–7.
Sigwart JD, Blasiak R, Jaspars M, Jouffray JB, Tasdemir D. Unlocking the potential of marine biodiscovery. Nat Prod Rep, 2021; 38:1235–42.
Song YP, Fang ST, Miao FP, Yin XL, Ji NY. Diterpenes and sesquiterpenes from the marine algicolous fungus Trichoderma harzianum X-5. J Nat Prod, 2018a; 81:2553–9.
Song YP, Miao FP, Fang ST, Yin XL, Ji NY. Halogenated and nonhalogenated metabolites from the marine-alga-endophytic fungus Trichoderma asperellum cf44-2. Mar Drugs, 2018b; 16:266–75.
Song YP, Miao FP, Liang XR, Yin XL, Ji NY. Harziane and cadinane terpenoids from the alga-endophytic fungus Trichoderma asperellum A-YMD-9-2. Phytochem Lett, 2019a; 32:38–41.
Song YP, Miao FP, Liu XH, Yin XL, Ji NY. Seven chromanoid norbisabolane derivatives from the marine-alga-endophytic fungus Trichoderma asperellum A-YMD-9-2. Fitoterapia, 2019b; 135:107–13.
Song YP, Miao FP, Liu XH, Yin XL, Ji NY. Cyclonerane derivatives from the algicolous endophytic fungus Trichoderma asperellum A-YMD-9-2. Mar Drugs, 2019c; 17:252–61.
Song YP, Shi XS, Wang BG, Ji NY. Cadinane and carotane derivatives from the marine algicolous fungus Trichoderma virens RR-dl-6-8. Fitoterapia, 2020; 146:104715.
Stincone P, Brandelli A. Marine bacteria as source of antimicrobial compounds. Crit Rev Biotechnol, 2020; 40:306–19.
Tang WZ, Liu JT, Hu Q, He RJ, Guan XQ, Ge GB, Han H, Yang F, Lin HW. Pancreatic lipase inhibitory cyclohexapeptides from the marine sponge-derived fungus Aspergillus sp. 151304. J Nat Prod, 2020; 83:2287–93.
Tian Y, Lin X, Zhou X, Liu Y. Phenol derivatives from the sponge-derived fungus Didymellaceae sp. SCSIO F46. Front Chem, 2018; 6:536–44.
Van de Water JAJM, Allemand D, Ferrier-Pagès C. Host-microbe interactions in octocoral holobionts - recent advances and perspectives. Microbiome, 2018; 6:64–92.
Wang HC, Ke TY, Ko YC, Lin JJ, Chang JS, Cheng YB. Anti-inflammatory azaphilones from the edible alga-derived fungus Penicillium sclerotiorum. Mar Drugs, 2021a; 19:529–40.
Wang Y, Lin XP, Ju ZR, Liao XJ, Huang XJ, Zhang C, Zhao BX, Xu SH. Aspergchromones A and B, two new polyketides from the marine sponge-associated fungus Aspergillus sp. SCSIO XWS03F03. J Asian Nat Prod Res, 2017; 19:684–90.
Wang J, Peng Q, Yao X, Liu Y, Zhou X. New pestallic acids and diphenylketone derivatives from the marine alga-derived endophytic fungus Pestalotiopsis neglecta SCSIO41403. J Antibiot, 2020; 73:585–8.
Wang T, Zhou J, Zou J, Shi Y, Zhou W, Shao P, Yu T, Cui W, Li X, Wu X, Ye J, Yan X, Naman CB, Lazaro JEH, He S. Discovery of cymopolyphenols A–F from a marine mesophotic zone aaptos sponge-associated fungus Cymostachys sp. NBUF082. Front Microbiol, 2021b; 12:638610–24.
Xu P, Ding L, Wei J, Li Q, Gui M, He X, Su D, He S, Jin H. A new aquatic pathogen inhibitor produced by the marine fungus Aspergillus sp. LS116. Aquaculture, 2020; 520:734670–6.
Yamada T, Kogure H, Kataoka M, Kikuchi T, Hirano T. Halosmysin A, a novel 14-membered macrodiolide isolated from the marine-algae-derived fungus Halosphaeriaceae sp. Mar Drugs, 2020; 18:320–9.
Yamada T, Matsuda M, Seki M, Hirose M, Kikuchi T. Sterepinic acids A–C, new carboxylic acids produced by a marine alga-derived fungus. Molecules, 2018; 23:1336–46.
Yang SQ, Li XM, Li X, Chi LP, Wang BG. Two new diketomorpholine derivatives and a new highly conjugated ergostane-type steroid from the marine algal-derived endophytic fungus Aspergillus alabamensis EN-547. Mar Drugs, 2018; 16:114–23.
Yurchenko AN, Berdyshev DV, Smetanina OF, Ivanets EV, Zhuravleva OI, Rasin AB, Khudyakova YV, Popov RS, Dyshlovoy SA, von Amsberg G, Afiyatullov SS. Citriperazines A-D produced by a marine algae-derived fungus Penicillium sp. KMM 4672. Nat Prod Res, 2020; 34:1118–23.
Zhang B, Zhang T, Xu J, Lu J, Qiu P, Wang T, Ding L. Marine sponge-associated fungi as potential novel bioactive natural product sources for drug discovery: a review. Mini Rev Med Chem, 2020; 20:1966–2010.
Zhao DL, Yuan XL, Du YM, Zhang ZF, Zhang P. Benzophenone derivatives from an algal-endophytic isolate of Penicillium chrysogenum and their cytotoxicity. Molecules, 2018; 23:3378–86.
Zhou J, Zhang H, Ye J, Wu X, Wang W, Lin H, Yan X, Lazaro JEH, Wang T, Naman CB, He S. Cytotoxic polyketide metabolites from a marine mesophotic zone chalinidae sponge-associated fungus Pleosporales sp. NBUF144. Mar Drugs, 2021; 19:186–95.
Zou JX, Song YP, Liu XH, Li XN, Ji NY. Bisabolane, cadinane, and cyclonerane sesquiterpenes from an algicolous strain of Trichoderma asperelloides. Bioorg Chem, 2021; 115:105223.
Zuluaga-Montero A, Toledo-Hernández C, Rodríguez JA, Sabat AM, Bayman P. Spatial variation in fungal communities isolated from healthy and diseased sea fans Gorgonia ventalina and seawater. Aquat Biol, 2010; 8:151–60.