INTRODUCTION
Natural products (NPs) have a long history in pharmacotherapy, especially in the management of cancer and infectious disturbances (Carroll et al., 2023; Chen et al., 2023). Naturally occurring bioactive chemicals have become an essential source of drugs since they have been used to treat a variety of diseases (Abdel-Aziz et al., 2018; Elkhouly et al., 2021a, 2021b; Mohammed et al., 2019; Yang et al., 2023a, 2023b; Yeung et al., 2018). Scientists are dealing with various illnesses in our community owing to reestablished circumference and life manner. Several researchers are working on the different emerging diseases to understand and cure them by using various chemical and natural preparations; however, still, numerous topics are untouched due to inferior knowledge and technical tools (Aditi et al., 2017; Bode et al., 2002; El-Wakil et al., 2022). NPs have been utilized for the remediation of several ailments and diseases since ancient times (Dias et al., 2012; El-Demerdash et al., 2012; Ghareeb et al., 2023; Holland and Carroll, 2023; Mohammed et al., 2022; Okasha et al., 2022; Sayed et al., 2022). The earliest records of NPs used were from 8000 BC. Most of the evidences for the earliest use of NPs for medication comes from archaeologists who have explored some ancient sites such as caves (Dias et al., 2012). The Egyptian Ebers Papyrus (2900 BC) documents up to 700 plant-based drugs to create prescriptions, ointments, potions, inhalers, and pills to cure certain conditions. Opium, cannabis, and linseed oil were used (Cragg and Newman, 2005). The Chinese materia medica (1100 BC) (Wu Shi Er Bing Fang, comprises 52 prescriptions), Shennong Herbal (~100 BC, 365 drugs), and the Tang Herbal (659 AD, 850 drugs) are documented archives of the utilization of NPs (Cragg and Newman, 2005). The Greek physician Hippocrates, (460–370 BCE), the father of modern medicine and possibly the most recognized name in medicine, was born in Greece (Porter, 1998). In the 8th century, the Arabs were the first to privately own pharmacies. The poet, philosopher, pharmacist, and physician “Avicenna” participated much in the disciplines of pharmacy and medicine within works like the Canon Medicine (Cragg and Newman, 2005). Taken together, the current review aims to explore the chemical and biological aspects of some marine algae and marine sponges collected in the Red Sea, in order to build a comprehensive and intensive vision of what has been discovered in such an environment, which is considered a strategic treasure for obtaining medicines from natural sources.
MARINE NPS (MNPS)
Recently, the marine ecosystem has attracted many attentions as it contains diverse types of marine organisms including sponges, algae, microbes, tunicates, soft corals, mollusks, seaweeds, and sea cucumbers, among others. Also, it is a rich and promising source of bioactive compounds as well as other medicinal, nutritional, and pharmacological potentials (Carroll et al., 2022; El-Demerdash et al., 2020a; Ghareeb et al., 2020; Holland and Carroll, 2023; Ibrahim et al., 2021). Moreover, the marine environment is characterized by its chemical diversity and biomedical value highlighting its massive potential as a vital source of therapeutic agents (Carroll et al., 2020, 2021; El-Demerdash et al., 2020b, 2021; Liang et al., 2023). In the same context, a diverse array of chemical compounds has been isolated or identified from different marine organisms such as alkaloids (Moriou et al., 2021; Tempone et al., 2021), anthraquinones (Chen et al., 2022), peptides (Ghareeb et al., 2020), polysaccharides (Ghareeb et al., 2020), polyketides (Ghareeb et al., 2020), and terpenes (Chen et al., 2022). Various extracts of marine organisms and/or their pure isolates exhibited a broad spectrum of bioactivities like antimicrobial (Krome et al., 2022; Liang et al., 2023), antioxidant (Catarino et al., 2023; Hamed et al., 2020), anti-Gyr-B enzyme (Agour et al., 2022), antiallergic (Xie et al., 2017), antibiofilm (Cepas et al., 2019), anticancer (Agena et al., 2023; Panggabean et al., 2022), anti-inflammatory (Ghareeb et al., 2020; Rocha et al., 2022), anticoagulant (Qin et al., 2023), antiparasitic (Mostafa et al., 2022), antiallergic (Chen et al., 2023), and antiaging (Yang et al., 2023a, 2023b). The marine environment is considered a strategic treasure and a huge warehouse for the production of bioactive compounds, which act as lead compounds in the pharmaceutical industry. Recently, Ghareeb et al. (2020) stated that numerous marine-derived molecules are still under preclinical trials (Phase III, Phase II, and Phase I) for the medication of cancer, inflammation, Alzheimer’s disease, and wound healing. On the other side, several marine-derived drugs gained the Food and Drug Administration’s agreement like cytarabine, trabectedin, eribulin mesylate, and brentuximab vedotin 63 for cancer treatment, while Keyhole Limpet hemocyanin, vidarabine, ziconotide were approved for viral pain and hypertriglyceridemia treatments, respectively (Ghareeb et al., 2020). As a part of our continued program to identify pharmacologically active MNPs, herein, we provide a concise update about the chemistry and biomedical potentialities of selected marine organisms, with emphasis on those collected from the Red Sea coastal area. A list of 74 compounds was reported and tabulated alongside their therapeutic activities wherever applicable.
MATERIALS AND METHODS
In our current study, the subsequent databases and search engines have been used to get the peer-reviewed articles: the Marin-Lit database “The Royal Society of Chemistry,” Google Scholar, MDPI, Science Direct, SciFinder, and PubChem. The search keywords are “Natural products (NPs), Marine natural products (MNPs), Approved marine drugs, chemical and biological profiles of Thalassia hemprichii, Siphonochalina siphonella, Latrunculia magnifica, and Crella (Grayella) cyathophora.” The search covers the period 1980–2023. The selection of topics relied on articles that give a general overview of marine organisms, including chemical and biological profiles, and then the search was focused in depth on the organisms under study from the chemical and biological aspects. Also, ChemOffice was also utilized to draw the chemical skeletons. Additionally, Excel was used to draw graphs.
CHEMISTRY AND BIOLOGICAL IMPORTANCE OF SELECTED SEAGRASS AND MARINE SPONGES COLLECTED FROM THE RED SEA
In this manuscript, we present up-to-date insights about chemical and biological diversifications of selected marine organisms, with a focus on those collected from the Red Sea coastal areas. For the handling of this documentation, all isolated MNPs are tabulated where they have been recovered along with their recorded biological potentialities whenever possible.
Seagrass T. hemprichii: secondary metabolites and their bioactivities
Herein, a comprehensive outline of chemical ingredients isolated and/or identified from the seagrass T. hemprichii is reported. The detected compounds were categorized as sulphated flavonoids, flavonoid glycosides, sterols, sterol glycosides, phenolic acids, carotenoids, nitrogen compounds, and benzophenones. Additionally, the available pertinent bioactivities of the isolated compounds are mentioned whenever applicable. Ten compounds comprising diosmetin 7-O-β-glucosyl-2″-sulphate (Thalassiolin D) (1), diosmetin7-O-β-glucoside (2), apigenin 6,8-C-β-diglucoside (3), kaempferol 3-O-(6″-O-p-coumaroyl)-β-glucoside (4), β-stigmasterol (5), β-stigmasterol 3-O-β-glucoside (6), p-hydroxy-benzoic acid (7), 4,4′-dihydroxybenzophenone (8), octopamine (9), and diadinoxanthin (10) were obtained from the methanolic extract of seagrass T. hemprichii obtained from the Saudi Red Sea coast. Thalassiolin D exhibited in vitro antiviral hepatitis C virus (HCV) protease effect with a half-maximal inhibitory concentration (IC50) equal to 16 μM (Hawas and Abou El-Kassem, 2017) (Fig. 1).
Additionally, further phenolic constituents including isoscutellarein 7-O-β-xylopyranoside-2’’-O-sulfate (11), isoscutellarein 7-O-β-xylopyranoside (12), isoscutellarein (13), caffeic acid (14), and rosmarinic acid (15) were recovered from the methanolic extract of the seagrass T. hemprichii obtained from the south Marsa Alam coast, Egypt. Isoscutellarein 7-O-β-xylopyranoside-2’’-O-sulfate (11) showed a strong antibacterial effect with minimum inhibitory concentration (MIC) values of 2.5 and 1.5 μg/ml against Bacillus subtilis and Pseudomonas aeruginosa, respectively (Hawas, 2014) (Fig. 2).
Chemical investigations of the ethanol extract of seagrass T. hemprichii obtained from South China led to the isolation of 11 compounds including (S)-methoxy-(3,5-dimethoxy-4-hydroxyphenyl)ethanediol (16), 3,4,5-trihydroxybenzoic acid (17), 4-hydroxybenzoic acid (18), (E)-3-(4-methoxyphenyl)-2-propenoic acid (19), caffeic acid (20), chicoric acid (21), syringin (22), 5-hydroxy-3’,4’,7-trimethoxyflavone (23), 4’-hydroxy-3’,5,7-trimethoxyflavone (24), thalassiolin A (25), and thalassiolin B (26) (Qi et al., 2012) (Fig. 3).
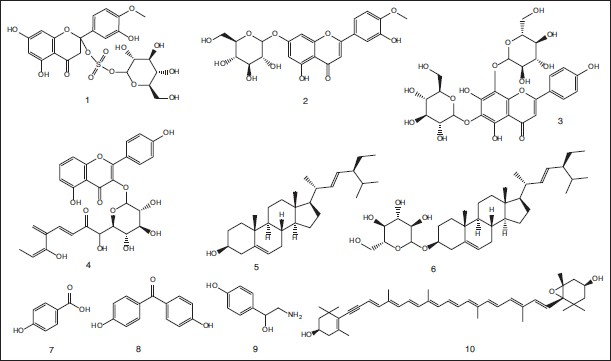 | Figure 1. Chemical structures of flavonoidal, phenolic acid, sterols, amine and carotenoid compounds (1–10) isolated from the methanolic extract of seagrass T. hemprichii obtained from the Saudi Red Sea coast. [Click here to view] |
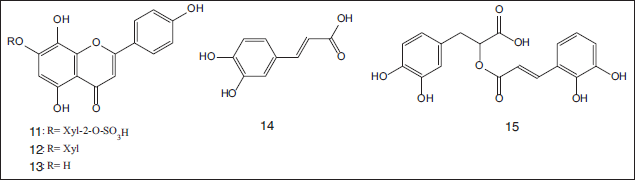 | Figure 2. Chemical structures of flavonoidal and phenolic acid compounds (11–15) isolated from the methanolic extract of seagrass T. hemprichii obtained from the south Marsa Alam coast, Egypt. [Click here to view] |
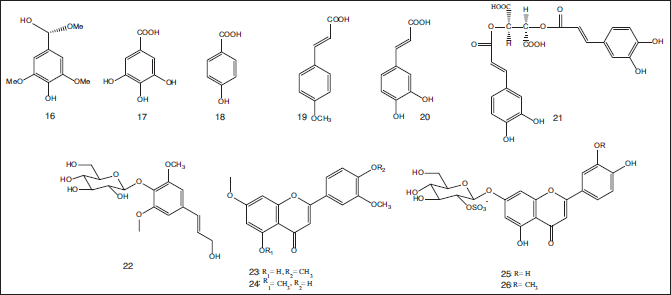 | Figure 3. Chemical structures of flavonoidal, phenolic acid, and phenolic derivative compounds (17–26) isolated from the ethanol extract of seagrass T. hemprichii obtained from South China. [Click here to view] |
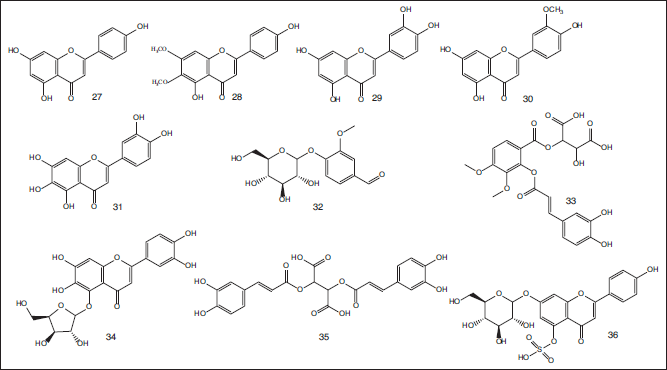 | Figure 4. Chemical structures of flavonoidal and phenolic derivative compounds (27–36) identified in the ethanol extract of the seagrass T. hemprichii collected from the Red Sea, Egypt. [Click here to view] |
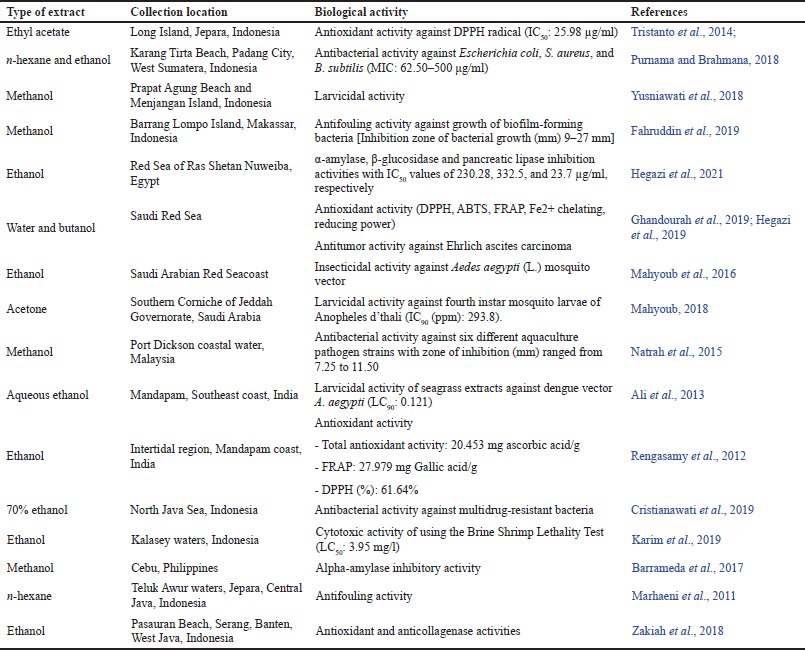 | Table 1. Some reported bioactivities of seagrass T. hemprichii extracts. [Click here to view] |
Diadinoxanthin (10) (a carotenoids pigment) was detected by high-performance liquid chromatography technique in the seagrass T. hemprichii sample collected from Menjangan Kecil Waters, Karimunjawa Islands, Indonesia (Nugraheni et al., 2010). Moreover, chemical profiling of the ethanol extract of the seagrass T. hemprichii collected from the Red Sea of Ras Shetan, Nuweiba, Egypt, led to isolation of flavonoid aglycones namely apigenin (27), isoscutellarein (13), cirsimaritin (28), luteolin (29), chrysoeriol (30), and 6-hydroxyl luteolin (31). In the same context, UPLC-HRMS/MS analysis of the desired extract led to tentative identification of about 144 secondary metabolites among them are vanillin-O-glucoside (32), O-caffeoyl-O-hydroxyl dimethoxy benzoyl tartaric acid (33), luteolin 7-O-glucoside sulphate (Thalassioline A) (25), 6-hydroxyl luteolin-O-xyloside (34), di-O-caffeoyl tartaric acid (35), chrysoeriol 7-O-glycoside sulphate (Thalassioline B) (26), and apigenine7-O-glucoside sulphate (Thalassioline C) (36) (Hegazi et al., 2021) (Fig. 4).
Biological activities of seagrass T. hemprichii extracts
Previous reports revealed that different extracts and fractions obtained from the seagrass T. hemprichii showed numerous biological activities like antimicrobial (Supaphon et al., 2013), antioxidant (Tristanto et al., 2014; Ulfa et al., 2014), cytotoxicity (Dewi et al., 2012), antidiabetic (Jayaprakash et al., 2017), and larvicidal (Yusniawati et al., 2018). Herein, we listed some reported biological activities of some extracts obtained from seagrass T. hemprichii (Table 1).
Marine sponge S. siphonella (Callyspongia siphonella)
Marine sponge S. siphonella: secondary metabolites and their bioactivities
Phytochemically, different extracts from the marine sponge S. siphonella were investigated for their phytoconstituents using chromatographic and spectroscopic tools. Numerous classes of secondary metabolites were detected in such extracts including polyacetylene amides (Ki et al., 2021), steroids (Alam et al., 2020), triterpenes (Carmely and Kashman, 1983), polyacetylene alcohols (Ki et al., 2019), and brominated oxindole alkaloids (El-Hawary et al., 2019). Three diacetylenic amides (Siphonellamide A–C) (37–39), one monoacetylenic amide (Siphonellamide D) (40), one fatty amide (Siphonellamide E) (41), alkamide, N-[2-(1H-indol-3-yl)ethyl]hexadecanamide (42), and callyspongamide A (43) were isolated from the chloroform-soluble fraction of S. siphonella collected from the reefs southwest of Magawish Island, Hurghada, Egypt. Siphonellamide A and B showed cytotoxic actions with IC50 values varying from 9.4 to 34.1 μM, while Siphonellamide E (41) showed cytotoxic effect against HeLa cells with an IC50 value of 78.4 μM. Callyspongamide A exhibited a medium cytotoxic effect versus HeLa, MCF-7, and A549 cell lines (Ki et al., 2020) (Fig. 5).
A steroid (Siphonocholin) (44) was isolated from an aqueous ethanol extract of S. siphonella obtained from Sharm Obhur (Jeddah, Saudi Arabian red seacoast). The compound exhibited anti-QS and antibiofilm activity against some bacterial pathogens including Chromobacterium violaceum, P. aeruginosa, Methicillin-resistant Staphylococcus aureus and Acinetobacter baumannii with MIC values varying from 64 to 256 μg/ml (Alam et al., 2020). Eight squalene-derived triterpenes were isolated from petroleum ether extract of marine sponge S. siphonella collected from Naima in the Gulf of Eilat, Red Sea. The isolated compounds have been identified as Sipholenol-A (45), Sipholenone-A (46), Sipholenone-B (47), Sipholenone-C (48), Sipholenol-B (49), Sipholenol-C (50), Sipholenol-D (51), and Sipholenol-E (52) (Carmely and Kashman, 1983) (Fig. 6).
Additionally, five triterpenes were isolated from CH2Cl2-MeOH extract (1:1) of the marine sponge S. siphonella obtained from Sharm Obhur, Jeddah, Saudi Arabia. These compounds were characterized as Neviotine-A (53), Neviotine?C (54), sipholenol?A (55), sipholenone?A (56), and sipholenol?L (57). Compounds 53–54 and 56 were evaluated against MCF?7, PC?3, and A549 cell lines and showed antiproliferative activities with IC50 in the range of 7.9–87 μM (Angawi et al., 2014) (Fig. 7).
Ki et al. (2019) reported the isolation of five polyacetylenic alcohols from the chloroform-soluble fraction of the marine sponge S. siphonella, obtained from southwest of Magawish Island, Hurghada, Egypt. The isolated compounds were identified as siphonellanol A (58), siphonellanol B (59), siphonellanol C (60), dehydroisophonochalynol (61), and siphonchalynol (62). Siphonellanols A-C exhibited mild cytotoxic effects against HeLa, MCF-7, and A549 with IC50 values ranging from 25.9 to 69.2 μM (Ki et al., 2019). Additionally, two brominated oxindole alkaloids namely 5-bromo trisindoline (63) and 6-bromo trisindoline (64) were isolated from the marine sponge Callyspongia siphonella collected from Hurghada, Red Sea Coast, Egypt. These compounds exhibited antibacterial effects versus S. aureus with MIC values of 8 and 4 μg/ml and B. subtilis with MIC values of 16 and 4 μg/ml, respectively. Also, they showed mild biofilm inhibitory effects in P. aeruginosa (49.32% and 41.76% inhibition), respectively. Moreover, they showed a mild in vitro antitrypanosomal effect (13.47 and 10.27 μM), respectively, and a strong cytotoxic activity was recorded against various human cancer cell lines (El-Hawary et al., 2019). Callysterol (ergosta-5,11-dien-3β-ol) (65), a sterol, was separated from the marine sponge C. siphonella obtained from the Red Sea, Egypt. It showed anti-inflammatory activity using the rat-hind paw edema assay (Youssef et al., 2010) (Fig. 8).
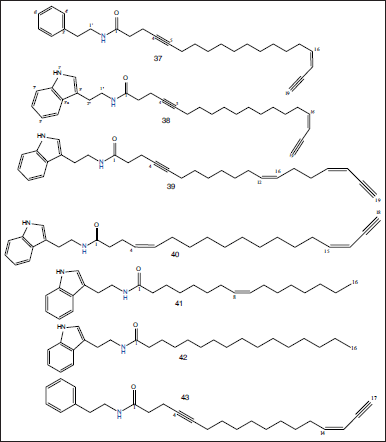 | Figure 5. Chemical structures of mono and diacetylenic amide compounds (37–43) isolated from the chloroform-soluble fraction of S. siphonella collected from the reefs southwest of Magawish Island, Hurghada, Egypt. [Click here to view] |
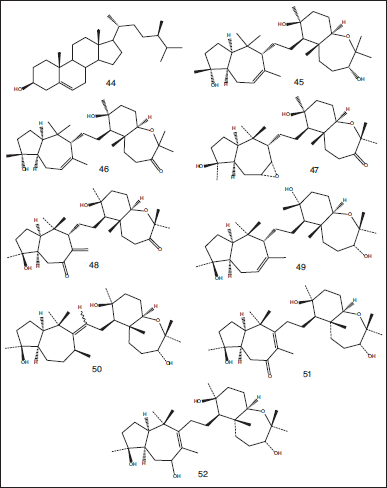 | Figure 6. Chemical structures of steroidal compound (44) isolated from aqueous ethanol extract of S. siphonella obtained from Sharm Obhur (Jeddah, Saudi Arabian red seacoast) and squalene-derived triterpene compounds (44–52) isolated from petroleum ether extract of marine sponge S. siphonella collected from Naima in the Gulf of Eilat, Red Sea. [Click here to view] |
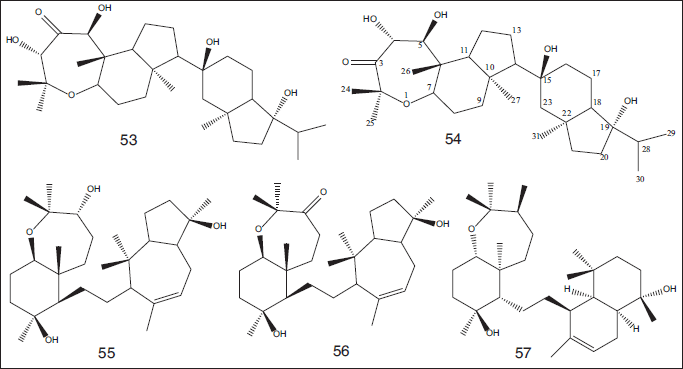 | Figure 7. Chemical structures of triterpene compounds (53–57) isolated from CH2Cl2-MeOH extract (1:1) of the marine sponge S. siphonella obtained from Sharm Obhur, Jeddah, Saudi Arabia. [Click here to view] |
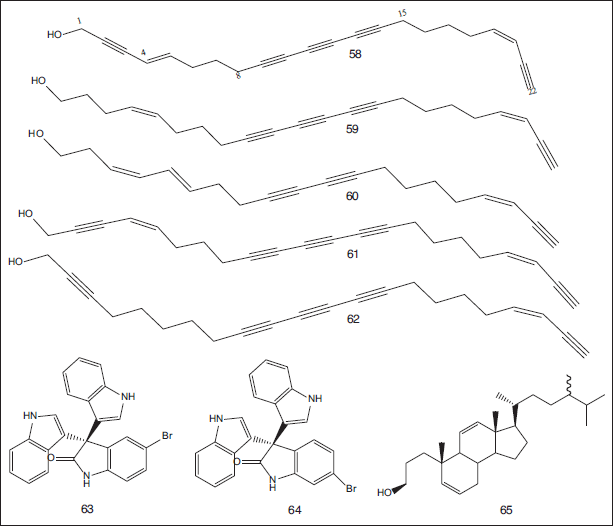 | Figure 8. Chemical structures of polyacetylenic alcohols compounds (58–62) isolated from the chloroform-soluble fraction of the marine sponge S. siphonella, obtained from southwest of Magawish Island, Hurghada, Egypt; brominated oxindole alkaloids compounds (63, 64) isolated from the marine sponge C. siphonella collected from Hurghada, Red Sea Coast, Egypt; and a sterol compound (65) separated from the marine sponge C. siphonella obtained from the Red Sea, Egypt. [Click here to view] |
 | Table 2. Summarized list of the reported compounds (Source, location of organism, and available bioactivity). [Click here to view] |
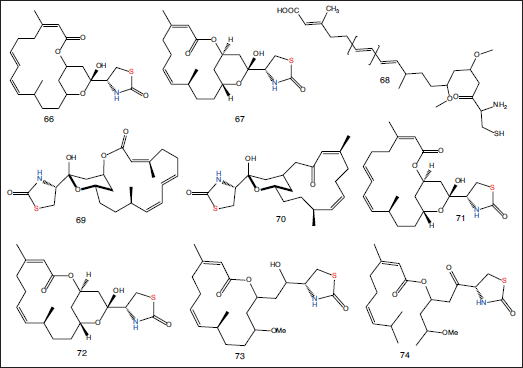 | Figure 9. Chemical structures of macrolide compounds (66–68) separated from the Red Sea sponge L. magnifica; compounds (69, 70) isolated from petroleum ether extract of L. magnifica collected from Gulf of Eilat; and compounds (71–74) separated from the Red Sea sponge L. magnifica. [Click here to view] |
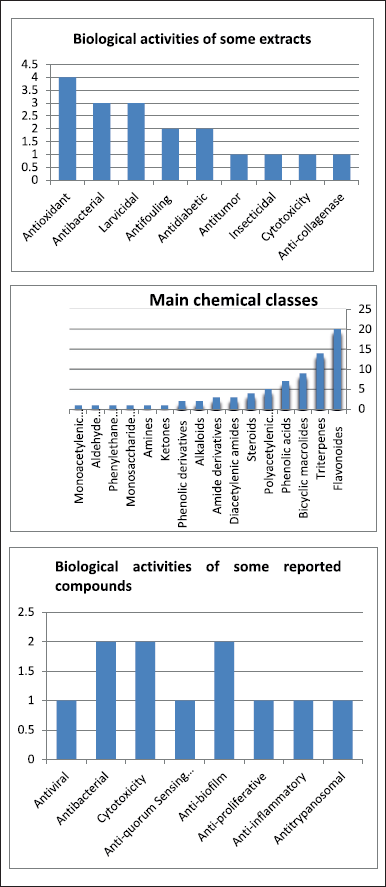 | Figure 10. Reported biological activities for compounds isolated seaweed and marine sponge collected from Red Sea marine organisms. [Click here to view] |
Marine sponge L. magnifica (Negombata magnifica)
Marine sponge L. magnifica: secondary metabolites and their bioactivities
Three toxins, namely latrunculin A–C (66–68), were separated from the Red Sea sponge L. magnifica (Kashman et al., 1980). Two latrunculins A (69) and B (70) were isolated from petroleum ether extract of L. magnifica collected from the Gulf of Eilat (Groweiss et al., 1983). Latrunculins A–D (71–74) were separated from the Red Sea sponge L. magnifica (Kashman et al., 1985) (Fig. 9). All the reported compounds are listed in Table 2.
Marine sponge C. (Grayella) cyathophora
Marine sponge C. (Grayella) cyathophora: secondary metabolites and their bioactivities
Reviewing the literature indicates that there are inadequate previous studies related to the chemical characterization of various extracts of C. (Grayella) cyathophora.
Biological and pharmacological activities of marine sponge C. (Grayella) cyathophora extracts
The aqueous ethanol extract of marine sponge C. (Grayella) cyathophora collected from the Gulf of Aqaba, Red Sea, Egypt, showed cytotoxic activity to Vero cells with hepatitis A virus with a MIC value of 2.929 μg/ml. The extract showed antibacterial activity against P. aeruginosa. Also, it showed antioxidant activity with IC50 value of 748 μg/ml. Moreover, the anti-inflammatory activity was 89.91% (El-Damhougy et al., 2017).
To sum up, the biological activities of some extracts and the main chemical classes as well as the biological activities of some reported compounds are summarized in Figure 10.
CONCLUSION
One of the distinguishing characteristics of marine-derived compounds is the diversity of their chemical structural, biological and pharmacological applications, which makes it a promising dais for drug discovery from natural sources. This review highlights an up-to-date comprehensive survey regarding the phytochemical and biological aspects of seaweed and marine sponges. Additionally, this review delivers a sign that numerous chemical ingredients have been isolated or identified from seagrass (T. hemprichii) and marine sponges (S. siphonella, L. magnifica, and C. (Grayella) cyathophora). The dominant compounds in T. hemprichii are phenolic compounds, while the dominant compounds in marine sponges are amides, alkaloids, terpenes, and steroids. Some of the reported compounds showed a broad spectrum of biological activities including antiviral, antibacterial, cytotoxicity, anti-quorum sensing (anti-QS), antibiofilm, anti-proliferative, anti-inflammatory, and antitrypanosomal activities. Moreover, it was noted that both types of chemical components and their biological properties are affected by the habitats of these organisms. These marine organisms are considered the most attractive biological targets and deserve more biological exploration due to the biological activities demonstrated by their chemical components as well as their various extracts. Moreover, this review sheds light on the enormous correlation between the chemical entities and the biological activities of marine-derived fungi. To sum up, these findings are likely to be used in the development of the pharmaceutical industry.
ACKNOWLEDGMENTS
All authors are immensely thankful to their institutions for the unlimited support (1) Faculty of Pharmacy, Cairo University, Egypt; (2) Theodor Bilharz Research Institute, Egypt; and (3) National Research Centre, Egypt.
LIST OF ABBREVIATIONS
NPs: Natural products; MNPs: Marine natural products; IC50: The half-maximal inhibitory concentration; MIC: Minimum inhibitory concentration; HeLa: Immortal cell line; MCF-7: Breast cancer cell line; A549: Adenocarcinomic human alveolar basal epithelial cells; DPPH: 2,2-diphenyl-1-picrylhydrazyl; ABTS: 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid; FRAP: Ferric reducing antioxidant power; LC50: Lethal concentration 50; Anti-QS: Anti-quorum sensing; PC?3: Human prostate cancer cell Line; HCV: Hepatitis C virus.
AUTHOR CONTRIBUTION
S.E.A.A., M.A.G., and A.A.H. wrote the main manuscript text. M.A.G. and E.M. prepared Figures 1–10 and Tables 1 and 2. M.A.G. and A.A.H. revised the manuscript. S.E. supervised the work. All authors reviewed the manuscript.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTERESTS
The authors declare that they have no known competing commercial interests or personal relationships that could have appeared to influence the work reported in this paper.
ETHICAL APPROVALS
This study does not include conducting any experiments on humans or animals.
DATA AVAILABILITY
All data generated or analyzed during this study are included in this published article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdel-Aziz MS, Ghareeb MA, Saad AM, Refahy LA, Hamed AA. Chromatographic isolation and structural elucidation of secondary metabolites from the soil-inhabiting fungus Aspergillus fumigatus 3T-EGY. Acta Chromatogr, 2018; 30:243–49; doi:10.1556/1326.2017.00329
Aditi M, Arjun V, Gayathri M. A brief review on medicinal plants from South India, endophytes and their antidiabetes properties. Int J Curr Res, 2017; 9:1–4; doi:10.7324/IJCRR.2017.9201
Agena R, Cortés-Sánchez AJ, Hernández-Sánchez H, Jaramillo-Flores ME. Pro-apoptotic activity of bioactive compounds from seaweeds: promising sources for developing novel anticancer drugs. Mar Drugs, 2023; 21:182; doi:10.3390/md21030182
Agour MA, Hamed AA, Ghareeb MA, Abdel-Hamid EAA, Ibrahim MK. Bioactive secondary metabolites from marine Actinomyces sp. AW6 with an evaluation of ADME?related physicochemical properties. Arch Microbiol, 2022; 204:537; doi:10.1007/s00203-022-03092-5
Alam P, Alqahtani AS, Husain FM, Rehman MT, Alajmi MF, Noman OM, El Gamal AA, Al-Massarani SM, Khan MS. Siphonocholin isolated from red sea sponge Siphonochalina siphonella attenuates quorum sensing controlled virulence and biofilm formation. Saudi Pharm J, 2020; 28:1383–91; doi:10.1016/j.jsps.2020.09.002
Ali MS, Ravikumar S, Beula JM. Larvicidal potential of seagrass extracts against dengue vector Aedes aegypti (Insecta: Diptera: Culicidae). Int J Pharm Bio Sci, 2013; 4:62–7.
Angawi RF, Saqer E, Abdel-Lateff A, Badria FA, Ayyad SN. Cytotoxic neviotane triterpene-type from the red sea sponge Siphonochalina siphonella. Pharmacogn Mag, 2014; 10:334–41; doi:10.4103/0973-1296.133292
Barrameda DNM, Cabonita KCD, Canora RP, Corcino RCB, Dy JC, Ebo VMB, Loren L, Romuga CJKJ, Sanchez EHA, Siguan MKT, Tabada SAW. In vitro determination of alpha-amylase inhibitory activity of Turtle Sea grass (Thalassia hemprichii) methanol extract on starch solution via Colorimetric Spectrophotometer. MD Thesis, School of Medicine-University of Cebu-Banilad Campus, Cebu, Philippines, 2017.
Bode B, Bethe B, Hofs R, Zeek A. Big effects from small changes: possible ways to explore nature’s chemical diversity. ChemBioChem, 2002; 3:619–27; doi:10.2174/157339981666620040809058
Carmely S, Kashman Y. The Sipholanes: A novel group of triterpenes from the marine sponge Siphon ochalina siphonella. J Org Chem, 1983; 48:3517–25; doi:10.1021/jo00168a029
Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR. Marine natural products. Nat Prod Rep, 2020; 37:175; doi:10.1039/c9np00069k
Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR. Marine natural products. Nat Prod Rep, 2021; 38:362; doi:10.1039/d0np00089b
Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR. Marine natural products. Nat Prod Rep, 2022; 39:1122; doi:10.1039/d1np00076d
Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR. Marine natural products. Nat Prod Rep, 2023; 40:275–325; doi:10.1039/D2NP00083K
Catarino MD, Silva-Reis R, Chouh A, Silva S, Braga SS, Silva AMS, Cardoso SM. Applications of antioxidant secondary metabolites of Sargassum spp. Mar Drugs, 2023; 21:172; doi:10.3390/md21030172
Cepas V, López Y, Gabasa Y, Martins CB, Ferreira JD, Correia MJ, Santos LMA, Oliveira F, Ramos V, Reis M, Castelo-Branco R, Morais J, Vasconcelos V, Probert I, Guilloud E, Mehiri M, Soto SM. Inhibition of bacterial and fungal biofilm formation by 675 extracts from microalgae and cyanobacteria. Antibiotics, 2019; 8:77; doi:10.3390/antibiotics8020077
Chen N, Zhang S, Javeed A, Jian C, Liu Y, Sun J, Wu S, Fu P, Han B. Structures and anti-allergic activities of natural products from marine organisms. Mar Drugs, 2023; 21:152; doi:10.3390/md21030152
Chen Z-H, Guo Y-W, Li X-W. Recent advances on marine mollusk-derived natural products: chemistry, chemical ecology and therapeutical potential. Nat Prod Rep, 2022; 39; doi:10.1039/d2np00021k
Chen Z-H, Guo Y-W, Li X-W. Recent advances on marine mollusk-derived natural products: chemistry, chemical ecology and therapeutical potential. Nat Prod Rep, 2023; 40:509–56; doi:10.1039/D2NP00021K
Cragg GM, Newman DJ. Biodiversity: a continuing source of novel drug leads. Pure Appl Chem, 2005; 77:7–24; doi:10.1351/pac200577010007
Cristianawati O, Sibero MT, Ayuningrum D, Nuryadi H, Syafitri E, Riniarsih I, Radjasa OK. Screening of antibacterial activity of seagrass-associated bacteria from the North Java Sea, Indonesia against multidrug-resistant bacteria. AACL Bioflux, 2019; 12:1054–64.
Dewi CSU, Soedharma D, Kawaroe M. Phytochemical compound and toxicity of seagrass Enhalus acoroides and Thalassia hemprichii from Pramuka Island, DKI Jakarta). J Penyuluh Perikan Kelaut, 2012; 3:23–7; doi:10.24319/jtpk.3.23-27
Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites, 2012; 2:303–36; doi:10.3390/metabo2020303
El-Damhougy KA, El-Naggar HA, Ibrahim HA, Bashar MA, Abou-Senna FM. Biological activities of some marine sponge extracts from Aqaba Gulf, Red Sea, Egypt. Int J Fish Aquat Stud, 2017; 5:652–9.
El-Demerdash A, Dawidar AM, Keshk EM, Abdel-Mogib M. Gingerdione from the rhizomes of Curcuma longa. Chem Nat Compd, 2012; 48:646–8; doi:10.1007/s10600-012-0333-y
El-Demerdash A, Ermolenko L, Gros E, Retailleau P, Thanh BN, Gauvin-Bialecki A, Al-Mourabit A. Short-cut bio-inspired synthesis of tricyclic guanidinic motifs of crambescidins and batzelladines marine alkaloids. Eur J Org Chem, 2020a; 35:5677–84; doi:10.1002/ejoc.202000744
El-Demerdash A, Hassan A, Abd El-Aziz TM, Stockand JD, Arafa RK. Marine brominated tyrosine alkaloids as promising inhibitors of SARS-CoV-2. Molecules, 2021; 26:6171; doi:10.3390/molecules26206171
El-Demerdash A, Petek S, Debitus C, Al-Mourabit A. Crambescidin acid from the French Polynesian Monanchora sp. marine sponge. Chem Nat Compd, 2020b; 56:1180–2; doi:10.1007/s10600-020-03262-1
El-Hawary SS, Sayed AM, Mohammed R, Hassan HM, Rateb ME, Amin E, Mohammed TA, El-Mesery M, Bin Muhsinah A, Alsayari A, Wajant H, Anany MA, Abdelmohsen UR. Bioactive brominated oxindole alkaloids from the red sea sponge Callyspongia siphonella. Mar Drugs, 2019; 17:465; doi:10.3390/md17080465
Elkhouly HI, Hamed AA, El Hosainy AM, Ghareeb MA, Sidkey NM. Bioactive secondary metabolite from endophytic Aspergillus Tubenginses ASH4 isolated from Hyoscyamus muticus: antimicrobial, antibiofilm, antioxidant and anticancer activity. Pharmacogn J, 2021a; 13:434–42; doi:10.5530/pj.2021.13.55
Elkhouly HI, Sidkey NM, Ghareeb MA, El Hosainy AM, Hamed AA. Bioactive secondary metabolites from endophytic Aspergillus terreus AH1 isolated from Ipomoea carnea growing in Egypt. Egypt J Chem, 2021b; 64:7511–20; doi:10.21608/EJCHEM.2021.85908.4161
El-Wakil ES, El-Shazly MAM, El-Ashkar AM, Aboushousha T, Ghareeb MA. Chemical profiling of Verbena officinalis and assessment of its anticryptosporidial activity in experimentally infected immunocompromised mice. Arab J Chem, 2022; 15:103945; doi:10.1016/j.arabjc.2022.103945
Fahruddin F, Johannes E, Dwyana Z. Antifouling potential of Thalassia hemprichii extract against growth of biofilm-forming bacteria. Sci Asia, 2019; 45:21–7; doi:10.2306/scienceasia1513-1874.2019.45.021
Ghandourah, M, Hawas, UW, Abou El-Kassem, LT, Bamkhrama, M, Taie, HAA. Antioxidant and antitumor metabolites of Saudi Red Sea seagrasses Halodule uninervis and Thalassia hemprichii. Lett Org Chem, 2019; 16:50–8; doi:10.2174/1570178615666180525110832
Ghareeb MA, Sobeh M, Aboushousha T, Esmat M, Mohammed HS, El-Wakil ES. Polyphenolic profile of Herniaria hemistemon aerial parts extract and assessment of its anti-cryptosporidiosis in a murine model: in silico supported in vivo study. Pharmaceutics, 2023; 15:415; doi:10.3390/pharmaceutics15020415
Ghareeb MA, Tammam MA, El-Demerdash A, Atanasov AG. Insights about clinically approved and preclinically investigated marine natural products. Curr Res Biotechnol, 2020; 2:88–102; doi:10.1016/j.crbiot.2020.09.001
Groweiss A, Shmueli U, Kashman Y. Marine toxins of Latrunculia magnifica. J Org Chem, 1983; 48:3512–16; doi:10.1021/jo00168a028
Hamed AA, Soldatou S, Qader MM, Arjunan S, Miranda KJ, Casolari F, Pavesi C, Diyaolu OA, Thissera B, Eshelli M, Belbahri L, Luptakova L, Ibrahim NA, Abdel-Aziz MS, Eid BM, Ghareeb MA, Rateb ME, Ebel R. Screening fungal endophytes derived from under-explored Egyptian marine habitats for antimicrobial and antioxidant properties in factionalised textiles. Microorganisms, 2020; 8:1617; doi:10.3390/microorganisms8101617
Hawas UW, Abou El-Kassem LT. Thalassiolin D: a new flavone O-glucoside sulphate from the seagrass Thalassia hemprichii. Nat Prod Res, 2017; 31:2369–74; doi:10.1080/14786419.2017.1308367
Hawas UW. A new 8-hydroxy flavone O-xyloside sulfate and antibacterial activity from the Egyptian seagrass Thalassia hemprichii. Chem Nat Compd, 2014; 50:629–32; doi:10.1007/s10600-014-1040-7
Hegazi NM, Saad HH, Marzouk MM, Abdel Rahman MF, El Bishbishy MH, Zayed A, Ulber R, Ezzat SM. Molecular networking leveraging the secondary metabolomes space of Halophila stipulaceae (Forsk.) Aschers. and Thalassia hemprichii (Ehrenb. ex Solms) Asch. in tandem with their chemosystematics and antidiabetic potentials. Mar Drugs, 2021; 19:279. https://doi.org/10.3390/md19050279
Holland DC, Carroll AR. Marine indole alkaloid diversity and bioactivity. What do we know and what are we missing? Nat Prod Rep, 2023; doi:10.1039/D2NP00085G
Ibrahim AM, Hamed AA, Ghareeb MA. Marine, freshwater, and terrestrial snails as models in the biomedical applications. Egypt J Aquat Biol Fish, 2021; 25:23–38; doi:10.21608/EJABF.2021.172142
Jayaprakash B, Veeraiyan S, Gunaseelan S, Purushothaman Y, Raja G. Antidiabetic activity of Thalassia hemprichii (Seagrass) in Alloxan induced diabetic mice. IOSR J Pharm Biol Sci, 2017; 12:24–33.
Karim FY, Kawung NJ, Wagey BT. Toxicity test of a species of Thalassia hemprichii from Kalasey waters using the brine shrimp lethality method. J Pesisir dan Laut Tropis, 2019; 7:265–70; doi:10.35800/jplt.7.3.2019.26017
Kashman Y, Groweiss A, Lidor R, Blasberger D, Carmely S. Latrunculins: NMR study, two new toxins and a synthetic approach. Tetrahedron, 1985; 41:1905–14; doi:10.1016/S0040-4020(01)96553-6
Kashman Y, Groweiss A, Shmueli U. Latrunculin, a new 2-thiazolidinone macrolide from the marine sponge Latrunculia magnifica. Tetrahedron Lett, 1980; 21:3629–32; doi:10.1016/0040-4039(80)80255-3
Ki D, El-Desoky AH, Kodama T, Wong CP, Abdel Ghani M, El-Beih AA, Mizuguchi M, Morit H. New cytotoxic polyacetylene amides from the Egyptian marine sponge Siphonochalina siphonella. Fitoterapia, 2021; 142:104511; doi:10.1016/j.fitote.2020.104511
Ki D, El-Desoky AH, Wong CP, Abdel-Ghani M, El-Beih AA, Mizuguchi M, Morita H. New cytotoxic polyacetylene alcohols from the Egyptian marine sponge Siphonochalina siphonella. J Nat Med, 2019; 74:409–14; doi:10.1007/s11418-019-01377-6
Ki DW, El-Desoky AH, Wong CP, Abdel-Ghani M, Ahmed A, Mizuguchi M, Morita H. New cytotoxic polyacetylene alcohols from the Egyptian marine sponge Siphonochalina siphonella. J Nat Med, 2020; 74:409–14; doi:10.1007/s11418-019-01377-6
Krome AK, Becker T, Kehraus S, Schiefer A, Gütschow M, Chaverra-Muñoz L, Hüttel S, Jansen R, Stadler M, Ehrens A, Pogorevc D, Müller R, Hübner MP, Hesterkamp T, Pfarr K, Hoerauf A, Wagner KG, König GM. Corallopyronin A: antimicrobial discovery to preclinical development. Nat Prod Rep, 2022; 38; doi:10.1039/d2np00012a
Liang J, She J, Fu J, Wang J, Ye Y, Yang B, Liu Y, Zhou X, Tao H. Advances in natural products from the marine-sponge-associated microorganisms with antimicrobial activity in the last decade. Mar Drugs, 2023; 21:236; https://doi.org/10.3390/md21040236
Mahyoub JA, Hawas UW, Al-Ghamdi KM, Aljameeli MME, Shaher FM, Bamakhrama MA. The biological effects of some marine extracts against Aedes aegypti (L.) mosquito vector of the dengue fever in Jeddah Governorate, Saudi Arabia. J Pure Appl Microbiol, 2016; 10:1949.
Mahyoub JA. Mosquito larvicidal activity of seaweed extracts against Anopheles d’thali with reference to its side effects on aquatic nontraget organisms. Int J Mosq Res, 2018; 5:34–8.
Marhaeni B, Radjasa OK, Khoeri MM, Sabdono A, Bengen DG, Sudoy H. Antifouling activity of bacterial symbionts of seagrasses against marine biofilm-forming bacteria. J Environ Protect, 2011; 2:1245–9; doi:10.4236/jep.2011.29143
Mohammed HS, Abdel-Aziz MM, Abu-baker MS, Saad AM, Mohamed MA, Ghareeb MA. Antibacterial and potential antidiabetic activities of flavone C-glycosides isolated from Beta vulgaris subspecies cicla L. var. flavescens (Amaranthaceae) cultivated in Egypt. Curr Pharm Biotechnol, 2019; 20:595–604; doi:10.2174/1389201020666190613161212
Mohammed HS, Ghareeb MA, Aboushousha T, Heikal EA, Abu El wafa SA. An appraisal of Luffa aegyptiaca extract and its isolated triterpenoidal saponins in Trichinella spiralis murine models. Arab J Chem, 2022; 15:104258; doi:10.1016/j.arabjc.2022.104258
Moriou C, Lacroix D, Petek S, El-Demerdash A, Trepos R, Leu TM, Florean C, Diederich M, Hellio C, Debitus C, Al-Mourabit A. Bioactive bromotyrosine derivatives from the pacific marine sponge Suberea clavata (Pulitzer-Finali, 1982). Mar Drugs, 2021; 19:143; doi:10.3390/md19030143
Mostafa O, Al-Shehri M, Moustaf M. Promising antiparasitic agents from marine sponges. Saudi J Biol Sci, 2022; 29:217–27; doi:10.1016/j.sjbs.2021.08.068
Natrah FMI, Harah ZM, Sidik BJ, Izzatul NMS, Syahidah A. Antibacterial activities of selected seaweed and seagrass from Port Dickson Coastal water against different aquaculture pathogens. Sains Malays, 2015; 44:1269–73; doi:10.17576/jsm-2015-4409-08
Nugraheni SA, Khoeri MM, Kusmita L, Widyastuti Y, Radjasa OK. Characterization of carotenoid pigments from bacterial symbionts of seagrass Thalassia hemprichii. J Coast Dev, 2010; 14:51–60.
Okasha H, Aboushousha T, Coimbra MA, Cardoso SM, Ghareeb MA. Metabolite profiling of Alocasia gigantea leaf extract and its potential anticancer effect through autophagy in hepatocellular carcinoma. Molecules, 2022; 27:8504; doi:10.3390/molecules27238504
Panggabean JA, Adiguna SP, Murniasih T, Rahmawati SI, Bayu A, Putra MY. Structure-activity relationship of cytotoxic natural products from Indonesian marine sponges. Rev Bras Farmacogn, 2022; 32:12–38; doi:10.1007/s43450-021-00195-w
Porter R. The greatest benefit to mankind: a medical history of humanity 1946-2002 (1st American ed.). W.W. Norton, New York, NY, 1998.
Purnama AA, Brahmana DM. Antibacterial bioactivity seagrass Thalassia hemprichii and Enhalus acoroides. J Biol Univ Andalas, 2018; 6:2303–162.
Qi SH, Huang LS, He F, Zhang S, Dong JD. Phytochemical and chemotaxonomic investigation of seagrass Thalassia hemprichii (Ehrenb.) Aschers (Hydrocharitaceae). Biochem Syst Ecol, 2012; 43:128–31; doi:10.1016/j.bse.2012.03.006
Qin L, Yang Y, Mao W. Anticoagulant property of a sulfated polysaccharide with unique structural characteristics from the green alga Chaetomorpha aerea. Mar Drugs, 2023; 21:88; doi:10.3390/md21020088
Rengasamy RRK, Rajasekaran A, Micheline AG, Perumal A. Antioxidant activity of seagrasses of the Mandapam coast. India Pharm Biol, 2012; 50:182–7; doi:10.3109/13880209.2011.591807
Rocha DHA, Pinto DCGA, Silva AMS. Macroalgae specialized metabolites: evidence for their anti-Inflammatory health benefits. Mar Drugs, 2022; 20:789; doi:10.3390/md20120789
Sayed AM, El-Hawary SS, Abdelmohsen UR, Ghareeb MA. Antiproliferative potential of Physalis peruviana-derived magnolin against pancreatic cancer: a comprehensive in vitro and in silico study. Food Funct, 2022; 13:11733; doi:10.1039/d2fo01915a
Supaphon P, Phongpaichit S, Rukachaisirikul V, Sakayaroj J. Antimicrobial potential of endophytic fungi derived from three seagrass species: Cymodocea serrulata, Halophila ovalis and Thalassia hemprichii. PLoS One, 2013; 8:e72520; doi:10.1371/journal.pone.0072520
Tempone AG, Pieper P, Borborema SET, Thevenard F, Lago JHG, Croft SL, Anderson EA. Marine alkaloids as bioactive agents against protozoal neglected tropical diseases and malaria. Nat Prod Rep, 2021; 38:2214; doi:10.1039/d0np00078g
Tristanto R, Putri MA, Situmorang AP, Suryanti S. Optimalization use of seagrass leafs Thalassia hemprichii as natural antioxidant source. Indones Fish Res J, 2014; 10:26–9; doi:10.14710/ijfst.10.1.26-29
Ulfa FS, Anggo AD, Romadhon. Test of potential activities of antioxidant with multilevel extraction methods on seagrass dugong (Thalassia hemprichii) of Jepara waters. Indone J Fish Sci Technol, 2014; 10:26–9.
Xie CL, Liu Q, Xia JM, Gao Y, Yang Q, Shao ZZ, Liu G, Yang XW. Anti-allergic compounds from the deep-sea-derived actinomycete Nesterenkonia flava MCCC 1K00610. Mar Drugs, 2017; 15:71; doi:10.3390/md15030071
Yang H, Zhang Q, Zhang B, Zhao Y, Wang N. Potential active marine peptides as anti-aging drugs or drug candidates. Mar Drugs, 2023a; 21:144; doi:10.3390/md21030144
Yang Z, Liu J, Wei S, Deng J, Feng X, Liu S, Liu M. A novel strategy for bioactive natural products targeting NLRP3 inflammasome in Alzheimer’s disease. Front Pharmacol, 2023b; 13:1077222; doi:10.3389/fphar.2022.1077222
Yeung AWK, El-Demerdash A, Berindan-Neagoe I, Atanasov AG, Ho YS. Molecular responses of cancers by natural products: modifications of autophagy revealed by literature analysis. Crit Rev Oncog, 2018; 23:347–70; doi:10.1615/CritRevOncog.2018027566
Youssef DTA, Ibrahim AK, Khalifa SI, Mesbah MK, Mayer AMS, van Soest RWM. New anti-inflammatory Sterols from the Red Sea Sponges Scalarispongia aqabaensis and Callyspongia siphonella. Nat Prod Commun, 2010; 5:27–31; doi:10.1177/1934578X1000500107
Yusniawati Y, Yasman Y, Handayani W. Bioactivity of Thalassia hemprichii (Hydrocharitaceae) methanolic extract from West Bali National Park as Aedes aegypti larvacide. AIP Conf Proc, 2018; 2023:020130; doi:10.1063/1.5064127
Zakiah K, Anwar E, Nurhayati T. In-vitro evaluation of antioxidant activity and anti-collagenase activity of Thalassia hempricii as a potent ingredients for anti-wrinkle cosmetics. Pharmacogn J, 2018; 10:778–82; doi:10.5530/pj.2018.4.131