INTRODUCTION
All of the systems and functions of the human body’s organs are tightly regulated by the brain. Therefore, care must be conducted throughout all stages of life to afford a healthy brain from the beginning of human life (Wang et al., 2020a, 2020b). Good care is necessary to provide for the proper development of all parts of the brain. Proper development is necessary as a way to control brain deterioration during the aging process.
Without it, brain deterioration may cause neurodegenerative problems. Neurodegenerative diseases include Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and Huntington’s disease (HD). Examples of brain degeneration include cognitive impairment and memory loss-related illnesses of vital brain functions. Many factors contribute to the progression of the diseases, while genetic mutations and worsening environments play significant roles. An unhealthy lifestyle worsens the condition (Wang et al., 2020a, 2020b). Keeping an aging brain sharp is necessary since, nowadays, human beings live in a situation where their lifespan is increasing. Slowing down brain aging occurs in the healthy brain. Mild cognitive impairment in the early stages of memory loss or other cognitive ability loss happens in individuals who maintain the ability to perform most activities in their daily lives.
Data from the Indonesian Ministry of Health showed an increasing trend of neurodegenerative problems among elderly people, such as dementia, in the era of an increasing number of elderly people in 2035. The World Health Organization stated that dementia will become a serious issue as the number will triple in 2050 (Immanuel and Natalia, 2021).
This article presents an overview of the neuroprotective properties of traditional Indonesian medicinal plants. Since its invention, original medicine manuscripts have provided various information on the health benefits of herbs for brain health. People in the community learn about the neuroprotective activity of various herbs from previous generations.
Cabe Puyang is one of the famous Indonesian manuscripts authored by Mardisiswojo and Harsono (1968). The manuscript described the health benefits of various medicinal herbs, including for brain health. Herbs used against different kinds of health problems related to the central nervous system (CNS) are grouped into herbs to combat spasms, stimulants, mental illness, vertigo, migraine, and weakness of the nervous system. Since traditional medicines have been proven to have health benefits empirically, it is important to consider their application continually. Nowadays, through advanced and modern research methods, it is becoming easier to understand the pharmacological action of herbs for brain health. Modern methods help to explore their active ingredients and mechanisms of action, metabolism, distribution, and secretion. Recent studies on some medicinal herbs from Indonesia for neuroprotection will be discussed.
Pathology of neurodegenerative diseases
In neurodegenerative diseases, protein aggregation, mitochondrial failure, glutamate toxicity, calcium load, proteolytic stress, oxidative stress, neuroinflammation, and aging are all diseases on the molecular and cellular level that destroy neurons (Gan et al., 2018; Kiaei, 2013). Amyloidoses, tauopathies, α-synucleinopathies, and TDP-43 proteinopathies are the most frequent neurodegenerative disorders (Dugger and Dickson, 2017).
Amyloidosis is the deposition of abnormally folded proteins in tissues. The proteins are then aggregated into insoluble fibrils and could cause organ damage (Picken, 2020). In neurodegenerative diseases, amyloid-like filamentous aggregates are predominantly located in the cytoplasm of neurons and glia. The most prevalent kind of amyloidosis is known as β-amyloid (Aβ), and it is not connected to the human brain. In addition to being an indicator of AD, it is also a sign of many other neurodegenerative diseases in older people, especially in those who have apolipoprotein E4, which is the main genetic risk factor for AD (Dugger and Dickson, 2017). A β, τ, and α-syn have overlapping protein abnormalities in a number of neurodegenerative diseases, as can be seen in Figure 1.
Tauopathy is a neurodegenerative disease defined by the buildup of dysfunctional tau protein-paired helical filaments. It is a typical morphology of protein disorder in AD. The accumulation is aggregated into neurofibrillary or gliofibrillary tangles in the human brain, and tau tangles can be seen microscopically in stained brain samples. Characteristics of the neuropathological phenotype of tauopathies are based on some factors, including anatomical areas, cell types, and the isoform of tau in the pathological deposits. New things about the disease, have already been found, like the fact that it could spread from cell to cell, which helps develop treatments (Kovacs, 2018).
Synucleinopathies are neurodegenerative diseases produced by α-synuclein aggregates. It is a 140-amino acid presynaptic protein that may play a role in synaptic vesicle trafficking. α-synuclein was formerly recognized as the primary ingredient of Lewy bodies, a neuronal inclusion observed in certain clinical disorders, such as PD. Lewy bodies appear mainly within neurons. Multiple system atrophy refers to the accumulation of α-synuclein found within oligodendrocytes. Accumulation of α -synuclein is also found in neurites, namely Lewy neuritis (Dugger and Dickson, 2017).
TDP-43 proteinopathy is the aberrant formation of inclusion bodies in the cytoplasm, nucleus, and cell processes by a nuclear protein. It is the predominant constituent of neuronal inclusions in ALS and is also identified in 25% to 50% of AD cases, primarily in limbic distribution (Dugger and Dickson, 2017). These diseases attack specific neuronal clusters with vulnerable neurons. Complex anatomical features, such as long-distance brain projections and complex synaptic connections, cause the vulnerability. Intricate neuronal structures are susceptible to metabolic shortages, necessitating a substantial amount of energy to sustain their complex operations. Since mitochondria generate the majority of the chemical energy required to power the cell’s metabolic activities, it must exert more effort. Consequently, the production of adenosine triphosphate increases, leading to a rise in reactive oxygen species (ROS) creation in mitochondria. The degradation of the mitochondrial quality control and respiratory chain enzymes results in metabolic stress. Then, transporter-induced glutamate receptor dysfunction and excessive excitatory postsynaptic neurons will ensue. The subsequent accumulation of calcium within the mitochondria and endoplasmic reticulum induced metabolic stress in neuronal cells. In addition to metabolic stress, energy-hungry neurons may be more susceptible than normal neurons to various toxic chemicals, protein aggregations, and glutamate excitotoxicity. Therefore, it is necessary to restore metabolic function to protect vulnerable neurons against neurodegeneration (Muddapu et al., 2020).
Current natural-based medicines for neuroprotection
Many studies show that using chemical substances in modern medicine gives rise to cognitive impairments and neurodegenerative diseases associated with metabolic diseases. This is the main reason people use natural medicines, including herbal ones, to minimize possible side effects and environmental pollution. Thus, it also helps control the increasing prevalence of nervous system disorders. A recent study demonstrates the potential efficacy of herbs in the treatment of various neurodegenerative diseases, including AD and stroke. The empirical knowledge of using some herbs nowadays has already been supported by scientific explanations for neuroprotection, such as Ginkgo biloba and Panax ginseng. Such an explanation includes the pharmacological actions of the bioactive compounds for neuroprotection.
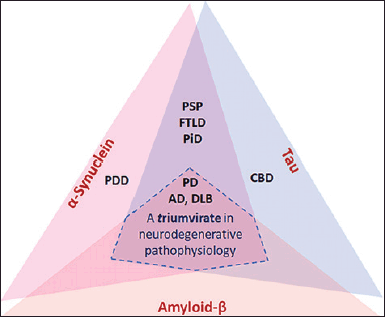 | Figure 1. Aβ, tau, and α-syn have overlapping protein abnormalities in a number of neurodegenerative diseases (Sengupta and Kayed, 2022). [Click here to view] |
The antioxidant, anti-inflammatory, and antiapoptotic characteristics of certain herbs used in treating AD were the subject of a study. The study discovered that the bioactive compounds in herbs work on multiple targets, known as “multitarget intervention.” It includes Aβ protection, induced oxidative stress, amyloid-secretion inhibition, and apoptosis. In this regard, it could be agreed that herbal medicines may have the potency to overcome the limitations of conventional medicine (More et al., 2013).
METHODS
Plants with neuroprotective potency were selected from Google’s page using the keyword “herbs neuroprotective” and were regrouped for special plants found in Indonesia. A database of the selected plants was developed by ScienceDirect (sciencedirect.com). The selection of articles was based on some criteria:
a. Year of publication: 2011–2021
b. Article type: both research and review articles
c. Published in Q1 Scopus-indexed journals
d. Topic of selected articles should be related to the objective of the review: Neuroprotective activity of Indonesian herbal medicines
e. The selected herbs were then reselected in accordance with herbs used for CNS in the Cabe Puyang Manuscript. Table 1 describes local and scientific names, health benefits, and parts used of the herbs against CNS health problems in the Cabe Puyang Manuscript. There were 13 herbs used against spasms, 31 herbs for stimulants, 7 for mental illnesses, 27 for vertigo, and 19 herbs for the weakness of the nervous system (Mardisiswojo and Harsono, 1968).
RESULTS
Table 1 details the scientific names, health benefits, and constituents of the herbs used to treat CNS-related diseases in the Cabe Puyang Manuscript (Mardisiswojo and Harsono, 1968). There were 13 herbs used against spasms, 31 for stimulants, 7 for mental illnesses, 27 for vertigo, and 19 for weakness of the nervous system.
Table 2 lists 31 herbs used as neuroprotective agents in Indonesia. It was selected from the preliminary selection with certain criteria already determined.
Twelve herbs were reselected from the preliminary selections. Reselection was based on the herbs used for the CNS in the Cabe Puyang Manuscript (Mardisiswojo and Harsono, 1968). Table 3 describes the scientific names and local names of the reselected herbs.
Zingiber officinale (Jahe)
The plant Z. officinale Roscoe is the scientific name for ginger, known as “jahe” in Indonesia. Due to its pungent aroma, nutritional benefits, and pharmacological capabilities, ginger has been utilized as a food, spice, dietary supplement, and flavoring agent and in traditional medical remedies. Numerous beneficial characteristics, such as physiological and pharmacological action at the molecular level in terms of neuroprotection, were discovered (Kiyama, 2020). Ginger contains sesquiterpenes (elemene, farnesene, and zerumbone), diarylheptanoids (cineole, citral, limonene, and pinenes), phenolics (gingerols, [6]-shogaol, [6]-paradol, zingerone, and curcumin). The memory-protecting effects of gingerol, shogaol, and borneol have been tested and found to be highly effective. Other chemicals in ginger are involved in biological processes such as apoptosis, cell cycle/deoxyribonucleic acid (DNA) damage, chromatin/epigenetic regulation, cytoskeletal regulation and adhesion, immunology, and inflammation. They exert their effects through distinct signaling pathways associated with cell activities and functions, such as autophagy, cellular metabolism, and mitogen-activated protein kinase (Kiyama, 2020; Talebi et al., 2021).
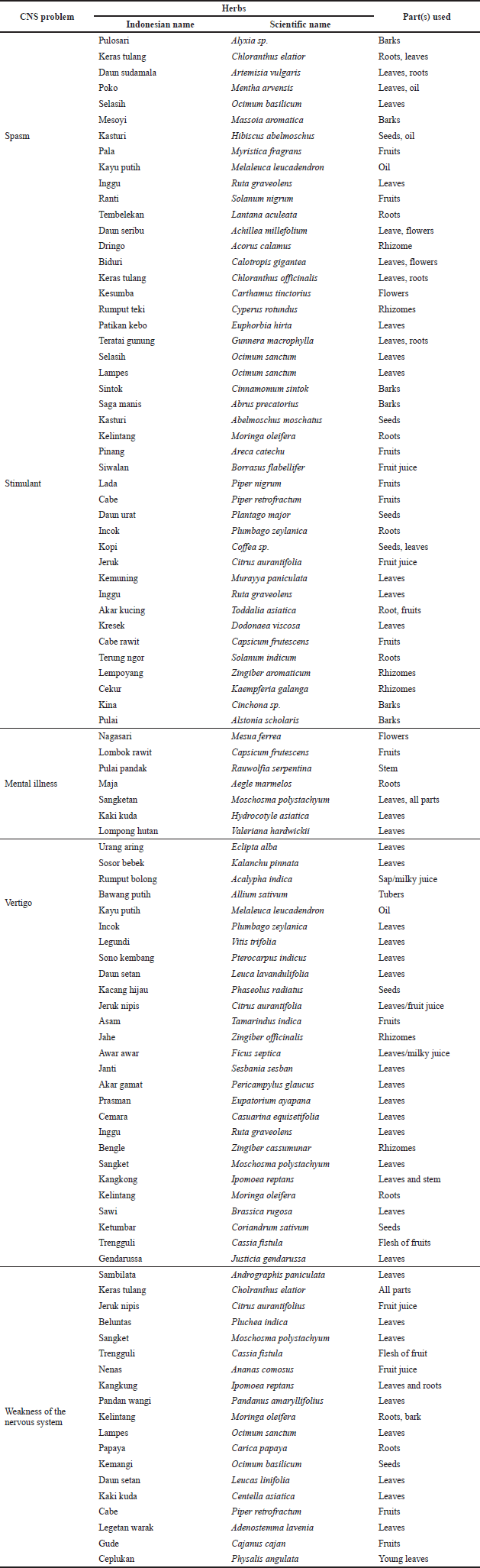 | Table 1. Herbs used against CNS disorders in Cabe Puyang manuscript. [Click here to view] |
Estrogen, specifically phytoestrogens, is one of the most important bioactive compounds in nature. Both the molecular processes and detection techniques of estrogen have been explored. Ginger active ingredients stimulate the molecular pathways of estrogen activities, such as cancer chemoprevention and treatment of menopausal syndromes, polycystic ovary syndrome, prostatic hyperplasia, endometriosis, and AD (Kiyama, 2020). Oral administration of ginger was also shown to reduce spinal neuroinflammation, lowering spared nerve injury (SNI)-induced neuropathic pain symptoms, suggesting ginger as a unique and attractive alternative for the treatment of neuropathic pain (Borgonetti et al., 2020).
Centella asiatica (Pegagan)
Centella asiatica (L.) Urban, famously known worldwide as gotu kola, has been used traditionally in many Asian countries, including China and India. However, in Indonesia, it is usually called by the local name “pegagan.” It is a member of the Apiaceae family, and its synonyms are Hydrocotyle asiatica and Hydrocotyle erecta. Common English names are Indian Pennyworth and Asian Pennyworth (Orhan, 2012).
Empirically, the leaves are used as a salad in some parts of Indonesia, popularly named pegagan. The plant grows abundantly, and the leaves are believed by the Acehnese ethnic group in North Sumatra, Indonesia, to be a memory enhancer. Villagers of the Sundanese ethnic group in West Java Province of Indonesia have used the raw leaves as a vegetable.
Early evidence of its use for health attracts researchers to further understand its pharmacological action. External application has been proven for its skin ulcer and wound healing properties. The most famous benefit of an internal application is related to the application in the Ayurvedic tradition as a memory booster, preventing cognitive deficits and improving brain function (Gray et al., 2017).
Centella asiatica contains many secondary metabolites, such as essential oils and phenolic compounds like epicatechin, catechin, quercetin, kaempferol, and related glycosides. It is famous for its high content of pentacyclic triterpenoids. The saponins are asiaticosides and madecassosides, while their aglycones are asiatic acid and madecassic acid. Essential oils are also present in the aerial parts of the herb and contain a high level of sesquiterpenes and monoterpenoids (Gray et al., 2017).
Centella asiatica and its chemical constituents could be used to treat several neurological disorders. Research has demonstrated its efficacy against neuroinflammatory activities, oxidative stress, mitochondrial malfunction, and disruption in brain-derived neurotrophic factors (BDNF) for treating AD and PD.
Neuroinflammation occurs in AD and PD. Cytokines as proinflammatory mediators are closely related to neurodegenerative lesions manifested in AD (Alasmari et al., 2018). As the source of the buildup of Aβ-containing neuritic plaques and neurofibrillary tangles, the lesion altered the expression and metabolism of amyloid precursor protein (Yuan et al., 2020). Centella asiatica showed its neuroprotective effect in the context of Aβ toxicity. The result of in vitro test using neuroblastoma cells showed that aqueous extract could protect the cells from Aβ-induced cytotoxicity (Gray et al., 2017).
A study on the oxidative and antioxidant effects of C. asiatica showed its radical scavenging activity. This is very important since increased production of ROS can directly affect neuronal synaptic activity and neurotransmission and cause cognitive dysfunction. A study has found that the terpenoid contents of C. asiatica increased free radical scavengers superoxide dismutase (SOD) and glutathione peroxidase (GPX) and helped in reducing the level of ROS (Chintapanti et al., 2018; Gray et al., 2017; Tönnies and Trushina, 2017; Welbat et al., 2018).
The activity of triterpenoids in C. asiatica in reducing ROS helps prevent mitochondrial dysfunction. This is significant because ROS activates the cell death signaling pathway. Myocardial dysfunction is closely linked to AD and PD (Gray et al., 2017; Nataraj et al., 2017a; Onyango et al., 2017).
The asiatic acid and asiaticoside contents of C. asiatica showed an increasing effect on the BDNF content. BDNF helps in the maintenance of neuron survival and neurotransmitter regulation (Boondam et al., 2019; Chintapanti et al., 2018; Gopi and Arambakkam, 2017; Nataraj et al., 2017b). Neurodegenerative diseases cause a reduction in the concentration of this factor (Giacobbo et al., 2019)
Allium sativum (Bawang Putih)
Garlic is used widely in Indonesian households for daily needs. There is a growing demand for the consumption of garlic, but unfortunately, the production has shown a downward trend (Hadianto et al., 2019). As a member of the Alliaceae family, its scientific name is A. sativum and popularly known as “bawang putih,” its Indonesian local name.
 | Table 2. Names of selected herbs with neuroprotective activity. [Click here to view] |
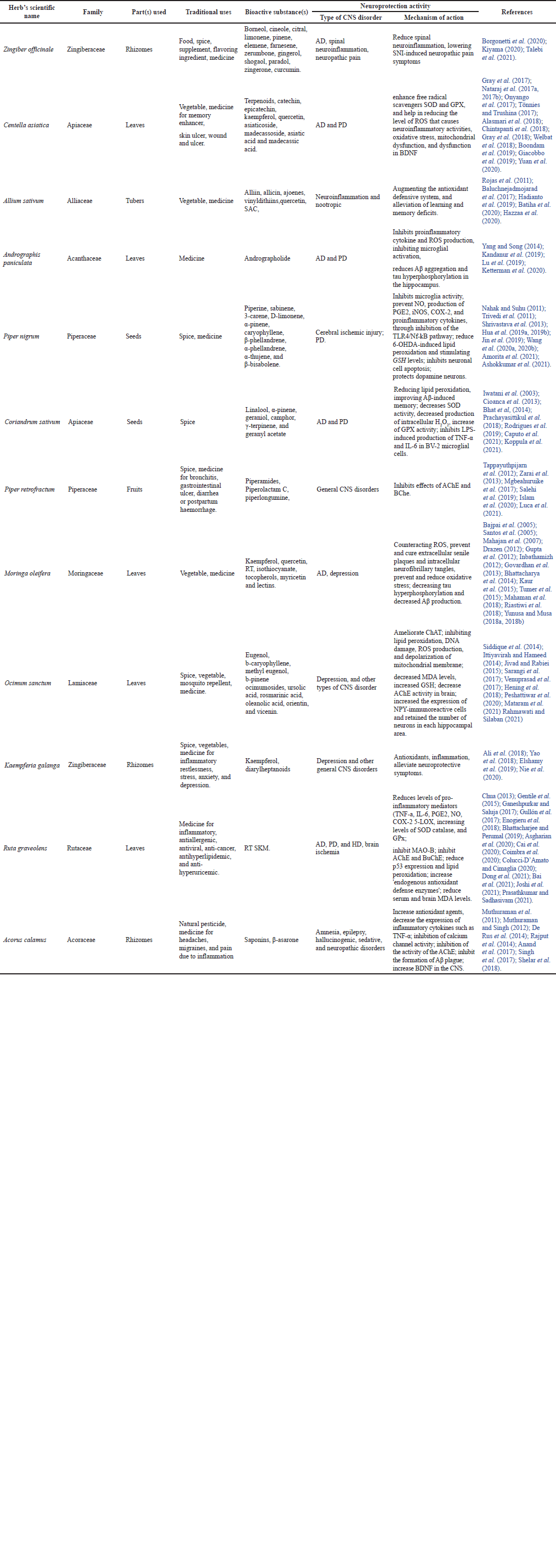 | Table 3. Herbs with neuroprotection activity. [Click here to view] |
Garlic is aromatic, and research reported its several biological properties, including its neuroprotective potency. The presence of sulfur-containing phytoconstituents such as alliin, allicin, ajoenes, vinyldithiins, and flavonoids such as quercetin is responsible for its therapeutic properties (Batiha et al., 2020). The main active compound that contributes to its neuroprotective activity is S-allylcysteine (SAC) (Rojas et al., 2011). SAC is the most abundant organosulphur compound derived from garlic. SAC is water-soluble and known for having high antioxidant capacity, besides its anti-inflammatory and nootropic properties. Its antioxidant capacity effectively augment the antioxidant defensive system in streptozotocin-diabetic rats. This demonstrated the effectiveness of SAC in reducing learning and memory deficiencies (Baluchnejadmojarad et al., 2017).
Garlic powder has also been studied for its efficacy against monosodium glutamate- (MSG-) induced neurotoxicity. It is indicated that the enhancement of antioxidant activity and reduction of lipid peroxidation of A. sativum can prevent MSG-induced neurotoxicity and improve short-term memory (Hazzaa et al., 2020)
Andrographis paniculata (Sambiloto)
Andrographis paniculata, also known as King of Bitters, grows well from mainland India and China to Southeast Asia, such as Thailand, Malaysia, and Indonesia. In Indonesia, it is known locally as “sambiloto” and has been part of an ingredient in Indonesian traditional medicine. As a member of the Acanthaceae family, the plant is widely utilized for various ailments, and this is due to its antioxidant, anti-inflammatory, and anti- or proapoptosis activities. The main active compounds that play major roles in preventing and curing diseases are flavonoids and andrographolides. Andrographolides are a labdane diterpenoid that can cross the blood-brain barrier, and this triggered various studies to reveal the action at different brain regions.
Andrographolide, a major compound, is known to have antioxidant, immunomodulatory, antihyperglycemic, antimicrobial, antiviral, anticancer, anti-inflammatory, and neuroprotective activities (Kandanur et al., 2019; Ketterman et al., 2020; Lu et al., 2019). The emergence of activity is thought to be due to the presence of pharmacophore groups on the C-3, C-14, and C-19 chains and double bonds at C-12/13 and C-8/17, which have the potential to provide activities (Kandanur et al., 2019). The presence of these pharmacophore groups ultimately makes andrographolide a compound that has a neuroprotective potential equivalent to phenolic compounds (Yang and Song, 2014).
Studies on the efficacy of andrographolide in combating AD showed some mechanisms. One of them is the reduction of Aβ aggregation and tau hyperphosphorylation in the hippocampus of aged degus (Lindsay et al., 2020). Aggregation and deposition of Aβ usually accompany microglia-mediated neuroinflammation in AD pathogenesis. The aggregation itself triggers the generation of proinflammatory chemicals via microglial hyperactivation. This is the cycle of neuronal injury and degeneration; hence, neuroinflammatory response inhibition becomes a treatment method for AD.
PD is a neurodegenerative disease of the CNS, and the specific mechanism of the disease’s origin or progression is not entirely understood. However, evidence indicates that neuroinflammatory response in the brain is a crucial mechanism in the etiology of PD. Since overactivation of microglia results in the generation of proinflammatory mediators, suppression of microglia has been shown to protect neurons from damage in vitro and in vivo models of PD. Previous research has demonstrated that andrographolide inhibits the generation of proinflammatory cytokines and ROS. Therefore, andrographolide may alleviate PD symptoms by reducing microglial activation (Lu et al., 2019).
Piper nigrum (Lada Hitam)
Black pepper (Piper nigrum) is a member of the Piperaceae family and is known as the King of Spices (Ashokkumar et al., 2021). It is native to India, and Indonesia is one of the world’s largest pepper-producing countries. It also is the second-largest area of pepper cultivation (Amorita et al., 2021).
The Javanese ethnic of Indonesia named it “merica,” while the ethnics of the Sumatra Island of Indonesia named it “lada.” The plant has a round fruit and single seed with a spicy and sharp taste. In some countries, such as India and China, pepper is considered to have medicinal properties. The chemical constituents are alkaloids, phenols, tannins, coumarins, saponins, flavonoids, glycosides, and essential oils (Nahak and Suhu, 2011; Trivedi et al., 2011).
The major constituents of essential oil of black pepper are 3-carene, sabinene, D-limonene, caryophyllene, α-pinene, α-phellandrene, β-phellandrene, β-bisabolene, and α-thujene. Piperine, the main active ingredient of the fruit, is an alkaloid that gives a characteristic pungent sensory effect to the pepper. It has been reported of its medicinal properties, such as antidepressive, anti-inflammatory, antioxidant, and antiepileptic (Wang et al., 2017). It also has antiplatelet aggregation and antiatherogenic properties (Hua et al., 2019a, 2019b, 2019c).
Piperine also has the potential for neuroprotection. Some research to explore further its cerebral brain functioning is being conducted (Ashokkumar et al., 2021). An investigation into the effects of oral piperine treatment on cerebral ischemia injury in rats revealed a decrease in the area of cerebral injury, an improvement in behavioral impairment, and a decrease in the severity of cellular damage following permanent middle cerebral artery occlusion injury. This demonstrates that piperine is effective in providing neuroprotection by regulating apoptotic processes. Globally, ischemic stroke contributes to long-term impairment and morbidity, making neuroprotection crucial (Wang et al., 2017). Black pepper can inhibit microglia activity, prevent nitric oxide (NO), production of prostaglandin E2, inducible NO synthase, cyclooxygenase-2, and proinflammatory cytokines, through inhibition of the Toll-like receptor 4/nuclear factor-kB pathway to prevent cognitive and neurodegenerative disorders (Jin et al., 2019).
Piperine possesses antioxidant properties via inhibiting 6-hydroxydopamine (6-OHDA)-induced lipid peroxidation and boosting glutathione (GSH) levels. In addition, it suppresses neuronal cell death, exhibiting a protective effect via antiapoptotic and anti-inflammatory pathways in 6-OHDA- induced PD (Shrivastava et al., 2013). Piperine also has a cytoprotective effect, nuclear factor-erythroid factor 2-related factor 2 activation, and upregulation of related phase II antioxidant enzymes, such as heme oxygenase-1 and NAD(P)H quinone oxidoreductase 1 and protects dopamine neurons, thereby attenuating PD (Wang et al., 2020a, 2020b).
Coriandrum sativum (Ketumbar)
Coriandrum sativum (Apiaceae) is known as coriander or Chinese parsley. In Indonesia, its local name is “ketumbar,” and the fruit is mixed with other ingredients in preparing local dishes. Local people are seldom aware of the medicinal value of the fruit and other parts of the plant. The plant is adapted from the Mediterranean area and is acclimatized in many countries with a temperate climate.
The main constituents of essential oil of the plant are α-pinene, linalool, geraniol, γ-terpinene, camphor, and geranyl acetate (Cioanca et al., 2013). The seeds contain lipids, vitamins, and minerals, such as potassium, phosphorus, calcium, magnesium, zinc, and sodium (Bhat et al., 2014; Iwatani et al., 2003). The bioactive chemicals of coriander showed a variety of biological activities, including antioxidant, neuroprotective, and anti-inflammatory effects.
Linalool is the primary component of seeds and has been shown to influence various important pathogenic pathways. It is found that the neuroprotective property of coriander mainly relates to the potent antioxidant activity of coriander. It includes neuroprotective, antioxidant, analgesic, anti-inflammatory, anticancer, anxiolytic, anticonvulsant, hypnotic, and antidiabetic (Prachayasittikul et al., 2018).
Linalool can be useful as a neuroprotective by reducing lipid peroxidation, oxidative damage, anti-inflammatory, antidepressant, and anxiolytic in the presence of interactions of GABA receptors (Prachayasittikul et al., 2018). Coriander can improve locomotor, balance, and coordination and reduce mercury-induced oxidative stress (Rodrigues et al., 2019).
Coriander is useful in improving Aβ-induced memory by attenuating oxidative stress. Linalool decreased SOD activity and decreased the production of intracellular H2O2 with a simultaneous increase of GPX activity. This could decrease the stimulation of lipid peroxidation and protein oxidation, implying that coriander possesses strong antioxidant properties (Cioanca et al., 2013). Coriander treatment inhibited lipopolysaccharides (LPS)-induced production of TNF-α and IL-6 in BV-2 microglial cells in a concentration-dependent manner, indicating that C. sativum fruit extract might be beneficial in delaying the progression of neuroinflammation and oxidative stress-mediated neurodegeneration seen in PD (Koppula et al., 2021).
Linalool was able to counteract the reduction of mitochondrial dehydrogenase activity and the increase in intracellular ROS production and caspase-3 activation induced by Aβ1-42 oligomers. Linalool can interact with N-methyl-d-aspartate receptors and inhibit acetylcholine release. It modulates some voltage-gated calcium channel subtypes and their electrophysiological properties. Such modulation and modification exert anxiolytic and neuroprotective effects on linalool as a natural product of potential interest in treating AD (Caputo et al., 2021).
Piper retrofractum (Cabe Jawa)
Piper retrofractum is one of the members of the genus Piper of the Piperaceae family. The Indonesians named it “cabe Jawa” or “cabe Jamu.” Empirically, it is used mainly as one of the important ingredients in traditional Indonesian drinks and is believed to give a warming sensation. Its elongated-shaped fruits are believed to have some medicinal benefits and are traditionally used for bronchitis, gastrointestinal ulcers, diarrhea, or postpartum hemorrhage (Mgbeahuruike et al., 2017; Salehi et al., 2019). Studies revealed that spices from the family Piperaceae are rich in bioactive compounds, such as piperamides, lignans, flavonoids, and essential oils (Yadav et al., 2020). Therefore, they can show multiple pharmacological actions with antioxidant, anti-inflammatory, and neuroprotective effects (Salehi et al., 2019; Yadav et al., 2020).
It is known from a previous study that the total flavonoid content of P. retrofractum is high compared to green and black peppers (Zarai et al., 2013). Piperolactam C and piperlongumine were solely found in P. retrofractum, and the study also revealed the total antioxidant potency of the fruit (Luca et al., 2021). The neuroprotective of P. retrofractum is closely related to its moderate anti-butyrylcholinesterase (BChe) effect of Piper spices, with values ranging from 0.60 mg galanthamine equivalents (GALAE)/g in P. retrofractum to 3.11 mg GALAE/g in black pepper (Luca et al., 2021).
The anticholinesterase (AChE) inhibitor of Piper sp. has rarely been reported. Another study showed that the methanolic extract from fruits of P. nigrum and P. retrofractum showed IC50 values of 11.133 and 14.08 μg/ml against AChE inhibitor, respectively (Tappayuthpijarn et al., 2012).
Moringa oleifera (Kelor)
Moringa (M. oleifera) is a member of the Moringaceae family, a native plant from Africa and Asia. It is cultivated for its nutritious pods, edible leaves, and flowers and has also been known for its health benefits. In Indonesia, its local name is kelor, and it has been used as a vegetable. But, up untill now, people have never realized its health benefits as a functional food (Riastiwi et al., 2018).
Research that has been conducted revealed its richness in bioactive compounds, which include flavonoids, vitamins, carotenoids, polyphenols, glucosinolates, isothiocyanates, tannins, and saponins. Such richness may explain its various health benefits, which have been proven by its pharmacological actions (Vergara-Jimenez et al., 2017).
The antioxidant potential of different parts of M. oleifera is chiefly ascribed to the presence of ascorbic acid, β-carotene (Mahajan et al., 2007), kaempferol, rutin (RT), quercetin, and isothiocyanate from leaves (Bajpai et al., 2005; Gupta et al., 2012; Tumer et al., 2015); tocopherols, lectins, and myricetin from seeds (Govardhan et al., 2013; Mahajan et al., 2007; Santos et al., 2005); phytosterols and from flowers (Inbathamizh, 2012). Its antioxidant property could explain its pharmacological action against neurological disorders, and this is because of its ability to counteract ROS. However, a previous study showed the potency of M. oleifera in preventing and curing extracellular senile plaques and intracellular neurofibrillary tangles found in neurological disorders such as AD. According to the study, hyperhomocysteinemia induced AD-like pathology in rats. Homocysteine is one of the major risk factors for AD (Drazen, 2012). The result showed the ability of the extract to prevent and reduce oxidative stress, which helps prevent cognitive impairment, and decrease neurodegeneration, such as decreased tau hyperphosphorylation and Aβ production (Mahaman et al., 2018).
Kaur et al. (2015) conducted current research on the antidepressant effects of M. oleifera on laboratory rats. The antidepressant properties of the alcoholic extract of M. oleifera were coupled with fluoxetine (an antidepressant), and the result was a reduction in the rats’ depressive condition. It was also recognized that the extract behaved similarly to the family of drugs known as selective serotonin reuptake inhibitors. Similar evidence of the antidepressant action of M. oleifera extract has been documented elsewhere (Bhattacharya et al., 2014; Yunusa and Musa, 2018a, 2018b).
Ocimum sanctum (Kemangi)
Ocimum sanctum L. belongs to the Lamiaceae family. The plant was originally introduced in India and has now spread worldwide, including Indonesia. In Indonesia, O. sanctum is known by the name “kemangi.”
Some pharmacological actions of the plants are antimicrobial, antidiabetic, antistress, hepatoprotective, anticarcinogenic, anti-inflammatory, immunomodulatory, radioprotective, analgesic, cardioprotective, antioxidant, and mosquito repellent (Rahmawati and Silaban, 2021; Siddique et al., 2014). Its neuroprotective effect was known through the activity of O. sanctum hydroalcoholic extract against H2O2 in inducing neuronal cell damage through its antioxidative defense mechanism (Venuprasad et al., 2017).
A previous study has been conducted to evaluate the protective effect of O. sanctum extract (OSE) against trimethyltin (TMT) challenge by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide, reduction assay in cultured human embryonic kidney-293 (HEK-293) cell, and excessive ROS formation induced by TMT caused cell death (Gunasekar et al., 2001). It is already described that the choline acetyltransferase (ChAT) enzyme also plays a crucial role in neuroprotection in the cells.
Pretreatment with OSE is capable of ameliorating ChAT. By preventing lipid peroxidation, DNA damage, ROS generation, and mitochondrial membrane depolarization, OSE can preserve cell viability (Venuprasad et al., 2017). Several studies have demonstrated the neuroprotective and antistress properties of polyphenols and flavonoids, which are the principal bioactive components of herbal extracts. OSE has a neuroprotective impact by improving cell viability and sustaining ChAT expression in HEK-293 cells as an in vitro model of neurodegenerative disorders, as determined by Hening et al. (2018).
The major bioactive components of O. sanctum are ursolic acid, flavonoids, rosmarinic acid, and tannins. Essential oil contents are eugenol, methyl eugenol, β-pinene, β-caryophyllene, and ocimumosides. Flavonoids include ursolic acid, oleanolic acid, orientin, and vicenin (Sarangi et al., 2017; Venuprasad et al., 2017). Flavonoid, phenolic, and fatty acid metabolites in O. sanctum exhibit antioxidant effects (Venuprasad et al., 2017).
Ursolic acid in O. sanctum has shown antioxidant effect through significantly decreased methane dicarboxylic aldehyde (MDA) levels, increased GSH (Jivad and Rabiei, 2015; Sarangi et al., 2017), protection of TH positive neurons from degeneration, and significantly obviated the complex I inhibition and promoted mitochondrial biogenesis (Ittiyavirah and Hameed, 2014; Peshattiwar et al., 2020). Other activities of O. sanctum were decreased AChE activity in rat brains (Peng et al., 2021), increased expression of neuropeptide Y (NPY) immunoreactive cells, and retained the number of neurons in each hippocampal area (Mataram et al., 2021).
Kaempferia galanga (Kencur)
Kaempferia galanga is a member of the Zingiberaceae family and is named “kencur” among Indonesians. It grows easily and has been used through generations as a spice in daily dishes and for health purposes. The research found its medicinal benefits, including inflammatory restlessness, stress, anxiety, and depression (Elshamy et al., 2019; Yao et al., 2018).
One of the active chemicals of K. galanga is kaempferol, a type of flavonoid with anti-inflammatory, antioxidant, and neuroprotective properties (Nie et al., 2020). A methanol extract of K. galanga rhizomes showed antioxidant activity in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay and NO radical scavenging assays in a concentration-dependent manner. The antioxidant activity was demonstrated through its essential oil extracts, which have considerable DPPH radical scavenging activity (Ali et al., 2018). Compared to indomethacin, which has anti-inflammatory properties, the chloroform, cyclohexane, and ethyl acetate extracts with diarylheptanoids obtained from K. galanga demonstrated a significant reduction of LPS-induced NO in macrophage RAW 264.7 cells. Kaempferia galanga extracts and purified components have also alleviated neuroprotective symptoms (Elshamy et al., 2019; Yao et al., 2018).
Ruta graveolens (Inggu)
Inggu is the Indonesian name for the Rutaceae species R. graveolens, and the name in English is Rue. It has been used as a part of traditional medicines in Indonesia, especially by people of the Central Javanese Javanese ethnic group.
Skimmianine (SKM, 83) is a quinoline alkaloid isolated from R. graveolens L. Compound 83 (5 mg/kg, i.p.) exhibited potential edema inhibition in a carrageenan-induced acute illness model. Its efficacy was superior to that of the positive control diclofenac as it significantly decreased levels of proinflammatory mediators (TNF-a, IL-6, PGE2, and NO), as well as COX-2 and 5-LOX activity; antioxidant effect demonstrated by elevated levels of SOD, catalase, and GPx; decreased levels of thiobarbituric acid reactive substances and nitrites (Bai et al., 2021; Prasathkumar and Sadhasivam, 2021).
One of the bioactive flavonoid compounds, RT, has been identified and isolated from the plant extract (Gullón et al., 2017; Prasathkumar and Sadhasivam, 2021). RT-rich plants have been used in traditional medicine for centuries in the form of a drink or food. Because of its versatile nature, RT is now a well-known constituent of more than 130 registered medicinal preparations (Chua, 2013). RT, also known as rutoside, QE-3-rutinoside, and vitamin P, is a dietary polyphenolic bioflavonoid extracted from fruits, and many Chinese medical herbs, such as Fagopyrum tataricum (tartary buckwheat), Flos Sophorae (Huai Mi), and R. graveolens (Yun Xiang) various pharmacological activities, including antioxidant, antiallergenic, anti-inflammatory, anticarcinogenic, antiviral, and superoxide radical potential (Enogieru et al., 2018), antiapoptotic, cardioprotective, vasoprotective, antihyperlipidemic, nephroprotective, antihyperuricemic, neuroprotective, hepatoprotective, and anticancer properties have been reported for RT (Cai et al., 2020; Dong et al., 2021; Joshi et al., 2021).
The neuronal activity of R. graveolens and its key RT metabolites are not restricted to a single function but encompass a wide range of actions. Moreover, some are especially significant because they can interfere with pathogenic pathways implicated in neurodegenerative mechanisms’ start and/or progression, such as those in PD, AD, and HD. Ruta graveolens inhibits monoamine oxidase B (MAO-B) in PD, according to research examining the plant’s impact on neurodegenerative disorders (Bhattacharjee and Perumal, 2019; Coimbra et al., 2020).
Previous studies have reported that the successful application of R. graveolens in an in vivo murine DA model as an acetylcholinesterase (AChE) inhibitor (Colucci-D’Amato and Cimaglia, 2020) and BuChE inhibitor is the hexane extract of R. graveolens at a concentration of 400 g/ml. This extract had a dose-dependent inhibitory activity, and inhibition was higher than 50% at a concentration of 100 g/ml, the lowest concentration used (Bhattacharjee and Perumal, 2019; Coimbra et al., 2020). RT has demonstrated neuroprotective properties against brain ischemia. RT administration reduces “ischemic neuronal apoptosis” as a result of decreased p53 expression and lipid peroxidation and increased “endogenous antioxidant defense enzymes.” RT inhibits proinflammatory cytokine activity by decreasing microglial TNF-a and IL-1b production. As indicated by the suppression of oligomeric b-amyloid cytotoxicity, this action appears to be effective in treating AD (Ganeshpurkar and Saluja, 2017). Regular intake of natural food sources, such as groats or buckwheat noodles, has been reported to be potentially effective in protecting against neuronal loss in AD (Enogieru et al., 2018).
Among different medicinal plants, the highest clinical evidence was observed for R. graveolens, encouraging its potential efficacy in MS symptoms (Coimbra et al., 2020). Extracts of R. graveolens and RT, due to their strong antioxidant activity, increased serum and brain antioxidant capacities and reduced serum and brain MDA levels (Asgharian et al., 2020).
Research showed the activity of the aqueous extract of R. graveolens in inducing death in different glioblastoma cell lines (U87MG, C6, and U138). Glioblastoma multiforme is a brain tumor with a poor prognosis (Gentile et al., 2015).
Acorus calamus (Dringo)
Acorus calamus of the Araceae family, known in Indonesia as jeringau or dringu, is a plant widely used for various purposes, such as beverages and as nutritious ingredients. Traditionally, it is used to treat headaches, migraines, and pain due to inflammation in Indian and Southeast Asian medical systems, including in Indonesia (Anand et al., 2017; Muthuraman et al., 2011; Muthuraman and Singh, 2012). The activities were due to the contents found in the plant, including flavonoids, tannins, saponins, polyphenols, and essential oils. Research supports its utilization due to its potency to reduce blood lipid levels and in neuropharmacology for amnesia, epilepsy, hallucinogenic, sedative, and neuropathic disorders (De Rus et al., 2014; Muthuraman et al., 2011).
The mechanism of action on the neuropharmacological activity is due to antioxidant, anti-inflammatory, and calcium ion inhibition activities of the chemical content of the plant. The contents included saponin and asarone compound groups (De Rus et al., 2014; Muthuraman and Singh, 2012). Saponins and β-asarone may act as antioxidant agents, decreasing the expression of inflammatory cytokines such as TNF-α and inhibiting calcium channel activity (Muthuraman et al., 2011; Rajput et al., 2014; Singh et al., 2017). Another mechanism is the inhibition of the activity of the acetylcholinesterase (AChE) enzyme, which indirectly inhibits the formation of Aβ plague (Anand et al., 2017; Rajput et al., 2014). Acorus calamus is able to increase BDNF in the CNS responsible for improving memory (Shelar et al., 2018). However, A. calamus needs special attention to the β-asarone known to show a good natural pesticide. Therefore, A. calamus is categorized as a toxic plant (Rajput et al., 2014; Singh et al., 2017).
DISCUSSION
Indonesia is blessed with huge biodiversity, which is the main reason behind the application of herbs for health purposes in the daily lives of people in the community. Almost all the herbs for neuroprotection that have been selected and included in the Cabe Puyang Manuscript are popular and have been used as spices in traditional dishes, vegetables, and beverages and in traditional medicine. Historically, most of the herbs originated from various parts of the world, including South East Asian regions, but nowadays, they are grown abundantly in many parts of Indonesia. This indicated the existence of cultural exchanges among traders from many parts of Asian countries, including China, Thailand, Myanmar, and Malaysia. The exchange of culture covers a vast area, including health issues. Historically, exchange on the application of traditional oriental medicine in China and India, for example, existed and could be traced back to some original manuscripts of Indonesia. It included various forms of herbal medicine.
Rhizomes of the Zingiberaceae family, Zingiber officinalis and K. galanga, grow in many parts of the country, while certain areas produce better quality. Research has shown that they act as antioxidants and anti-inflammatory agents, among other things. Ginger constituents exert neurological effects through specific signaling pathways associated with cell functions. The essential oil content of K. galanga showed its neuroprotection activity through antioxidant action. Both are popular spices in local dishes and traditional drinks. Wedang jahe, the local name for ginger tea, is believed by some to help with energy enhancement, while its action as a neuroprotector is not known. Kaempferia galanga is popular and often used to help cure coughs that cause hoarse voices. This is the reason for their regular usage among people in the community. From the empirical use supported by scientific data, regular usage is the most important prerequisite to enjoying the health benefits of herbs.
Centella asiatica is one of the adaptogenic agents, and research proved its pharmacological activity as a memory enhancer. Leaves are eaten as a daily salad in the western part of Java Island, Indonesia. People from the northern part of Sumatera Island use it as a vegetable and believe its action to be a memory enhancer.
Andrographis paniculata is an adaptogenic agent. It tastes bitter and has been used as an ingredient in a traditional drink, famously named paitan drink. Paitan means bitter in the Javanese language. People believe that drinking can help control blood sugar and cholesterol. Because research has shown that it promotes brain health, drinking it regularly should be encouraged. Andrographolide, as the major compound, showed antioxidant, anti-inflammatory, and neuroprotective activities.
Piper retrofractum grows well in some parts of the country, such as Madura Island in East Java Province, and is a very popular spice. It is often used as an ingredient in some traditional drinks, such as cabe lempuyang drink, which is effective against many health problems, especially during recovery from illnesses. Regular use of the drink may help in improving brain performance and controlling the degeneration of the brain caused by its richness in bioactive compounds.
Allium sativum, P. nigrum, and coriander are part of daily Indonesian dishes. Coriander has a strong oxidative property. The piperine content of P. nigrum and the SAC content of A. sativum are strong neuroprotective agents. Therefore, their usage supports their benefits in controlling brain health.
Moringa oleifera and O. sanctum are well-known vegetables used by people, especially in rural areas. They often eat the leaves of O. sanctum without understanding its health benefits. People who live in big cities just take a small amount of O. sanctum as a salad. The study found flavonoids and polyphenols as their main active compounds and proved the neuroprotective and antistress activities of the leaves. Nowadays, the leaves of M. oleifera are especially popular among people who live in urban areas. They know its high nutrient content, including vitamin C. The research found its antioxidant property that could explain its pharmacological action against neurological problems. As they grow well in the surrounding areas, people should be encouraged to consume both leaves as vegetables to maintain brain health.
Acorus calamus is a special herb among Javanese people, especially in Central Java. Traditionally, they believe the magical power of the rhizome can throw away evil spirits. But, research indicates its antianxiety and antistress properties. Saponin and β-asarone groups showed the antioxidant and anti-inflammatory properties of the rhizome, which explain its effectiveness in preventing memory loss.
Ruta graveolens is not popular. Its local name is “inggu.” Research is being conducted to find the pharmacological activity, such as against hepatitis viruses. However, recent research indicates the action of extract and RT, its active compound, in enhancing memory function and learning capability in animal brain cells.
CONCLUSION
Some herbs have been included in the traditional medicine system of Indonesia to be used as neuroprotectors. The Cabe Puyang Manuscript of Indonesian Traditional Medicine categorized the herbs into those used for different health problems related to the CNS. They are grouped into herbs to combat spasms, stimulants, mental illness (insane), vertigo, migraines, and weakness of the nervous system. All of the selected herbs used as neuroprotectors in the Indonesian system of medicine were mentioned in the Cabe Puyang Manuscript. In this review, all of them showed a strong indication of their action as antioxidative and anti-inflammatory in brain tissues. Both actions are examples of the pathologies of neurodegeneration. The actions help in understanding their health benefits for the brain’s health through the synergistic action of the chemical contents of the herbs. From such findings, it is important to encourage people to use them naturally throughout their lives. More research should be encouraged to understand the other actions of the herbs related to neuroprotective actions, such as against protein aggregations in the brain.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research was supported and funded by Hibah Mandat Article Review Penelitian Internal Universitas Airlangga 2021.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Alasmari F, Alshammari MA, Alasmari AF, Alanazi WA, Alhazzani K. Neuroinflammatory cytokines induce amyloid beta neurotoxicity through modulating amyloid precursor protein levels/metabolism. Biomed Res Int, 2018; 2018; doi:10.1155/2018/3087475
Ali H, Yesmin R, Satter MA, Habib R, Yeasmin T. Antioxidant and antineoplastic activities of methanolic extract of Kaempferia galanga Linn. Rhizome against Ehrlich ascites carcinoma cells. J King Saud Univ Sci, 2018; 30(3):386–92; doi:10.1016/j.jksus.2017.05.009
Amorita C, Daryanto AS, Sahara. Competitiveness analysis of Indonesian pepper in international market. Int J Res Rev, 2021; 8(5):38–52; doi:10.52403/ijrr.20210507
Anand A, Patience AA, Sharma N, Khurana N. The present and future of pharmacotherapy of Alzheimer’s disease: a comprehensive review. Eur J Pharmacol, 2017; 815(October):364–75; doi:10.1016/j.ejphar.2017.09.043
Asgharian S, Hojjati MR, Ahrari M, Bijad E, Deris F, Lorigooini Z. Ruta graveolens and rutin, as its major compound: investigating their effect on spatial memory and passive avoidance memory in rats. Pharm Biol, 2020; 58(1):447–53; doi:10.1080/13880209.2020.1762669
Ashokkumar K, Murugan M, Dhanya MK, Pandian A, Warkentin TD. Phytochemistry and therapeutic potential of black pepper [Piper nigrum (L.)] essential oil and piperine: a review. Clin Phytosci, 2021; 7(1); doi:10.1186/s40816-021-00292-2
Bai R, Yao C, Zhong Z, Ge J, Bai Z, Ye X, Xie T, Xie Y. Discovery of natural anti-inflammatory alkaloids: potential leads for the drug discovery for the treatment of inflammation. Eur J Med Chem, 2021; 213:113165; doi:10.1016/j.ejmech.2021.113165
Bajpai M, Pande A, Tewari SK, Prakash D. Phenolic contents and antioxidant activity of some food and medicinal plants. Int J Food Sci Nutr, 2005; 56(4):287–91. doi:10.1080/09637480500146606
Baluchnejadmojarad T, Kiasalari Z, Afshin-Majd S, Ghasemi Z, Roghani M. S-allyl cysteine ameliorates cognitive deficits in streptozotocin-diabetic rats via suppression of oxidative stress, inflammation, and acetylcholinesterase. Eur J Pharmacol, 2017; 794(November 2016):69–76; doi:10.1016/j.ejphar.2016.11.033
Batiha EG, Beshbishy AM, Wasef LG, Elewa YHA, Al-Sagan AA, Abd El-Hack ME, Taha AE, Abd-Elhakim YM, Prasad DH. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): a review. Nutrients, 2020; 12(3):1–21; doi: 10.3390/nu12030872
Bhat S, Kaushal P, Kaur M, Sharma HK. Coriander (Coriandrum sativum L.): processing, nutritional and functional aspects. Afr J Plant Sci, 2014; 8(1):25–33; doi:10.5897/ajps2013.1118
Bhattacharjee M, Perumal E. Potential plant-derived catecholaminergic activity enhancers for neuropharmacological approaches: a review. Phytomedicine, 2019; 55(February 2018):148–64. doi:10.1016/j.phymed.2018.07.010
Bhattacharya A, Kumar S, Mishra S, Sahu P, Agrawal D, Behera R. Antipyretic effect of ethanolic extract of Moringa oleifera leaves on albino rats. Tanta Med J, 2014; 42(2):74; doi:10.4103/1110-1415.137810
Boondam Y, Songvut P, Tantisira MH, Tapechum S, Tilokskulchai K, Pakaprot N. Inverted U-shaped response of a standardized extract of Centella asiatica (ECa 233) on memory enhancement. Sci Rep, 2019; 9(1):1–11; doi:10.1038/s41598-019-44867-z
Borgonetti V, Governa P, Biagi M, Pellati F, Galeotti N. Zingiber officinale Roscoe rhizome extract alleviates neuropathic pain by inhibiting neuroinflammation in mice. Phytomedicine, 2020; 78(February 2020):153307; doi:10.1016/j.phymed.2020.153307
Cai Y, Zheng Q, Sun R, Wu J, Li X, Liu R. Recent progress in the study of Artemisiae Scopariae herba (Yin Chen), a promising medicinal herb for liver diseases. Biomed Pharmacother, 2020; 130(June):110513; doi:10.1016/j.biopha.2020.110513
Caputo L, Piccialli I, Ciccone R, de Caprariis P, Massa A, De Feo V, Pannaccione A. Lavender and coriander essential oils and their main component linalool exert a protective effect against amyloid-β neurotoxicity. Phyther Res, 2021; 35(1):486–93; doi:10.1002/ptr.6827
Chintapanti S, Pratap RK, Sreenivasula RP. Behavioral and neurochemical consequences of perinatal exposure to lead in adult male Wistar rats: protective effect by Centella asiatica. Environ Sci Pollut Res, 2018; 25(13):13173–85; doi:10.1007/s11356-018-1500-x
Chua LS. A review on plant-based rutin extraction methods and its pharmacological activities. J Ethnopharmacol, 2013; 150(3):805–17; doi:10.1016/j.jep.2013.10.036
Cioanca O, Hritcu L, Mihasan M, Hancianu M. Cognitive-enhancing and antioxidant activities of inhaled coriander volatile oil in amyloid β(1-42) rat model of Alzheimer’s disease. Physiol Behav, 2013; 120:193–202; doi:10.1016/j.physbeh.2013.08.006
Coimbra AT, Ferreira S, Duarte AP. Genus Ruta: a natural source of high value products with biological and pharmacological properties. J Ethnopharmacol, 2020; 260(April):113076; doi:10.1016/j.jep.2020.113076
Colucci-D’Amato L, Cimaglia G. Ruta graveolens as a potential source of neuroactive compounds to promote and restore neural functions. J Tradit Complement Med, 2020; 10(3):309–14; doi:10.1016/j.jtcme.2020.05.002
De Rus JA, Subedi R, Ghimire SK, Rochet JC. Nepalese traditional medicine and symptoms related to Parkinsons disease and other disorders: patterns of the usage of plant resources along the Himalayan altitudinal range. J Ethnopharmacol, 2014; 153(1):178–89; doi:10.1016/j.jep.2014.02.016
Dong J, Zhang L, Xu N, Zhou S, Song Y, Yang Q, Liu Y, Yang Y, Ai X. Rutin reduces the pathogenicity of Streptococcus agalactiae to tilapia by inhibiting the activity of sortase A. Aquaculture, 2021; 530(July 2019):735743; doi:10.1016/j.aquaculture.2020.735743
Drazen JM. Transparency for clinical trials—the TEST act. N Engl J Med, 2012; 367(9):863–4; doi:10.1056/nejme1209433
Dugger BN, Dickson DW. Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol, 2017; 9(7); doi:10.1101/cshperspect.a028035
Elshamy AI, Mohamed TA, Essa AF, Abd-El Gawad AM, Alqahtani AS, Shahat AA, Yoneyama T, Farrag ARH, Noji M, El-Seedi HR, Umeyama A, Paré PW, Hegazy MF. Recent advances in Kaempferia phytochemistry and biological activity: a comprehensive review. Nutrients, 2019; 11; doi:10.3390/nu11102396
Enogieru AB, Omoruyi SI, Hiss DC, Ekpo OE. Potential antiparkinsonian agents derived from South African medicinal plants. J Herb Med, 2018; 13(May):1–7; doi:10.1016/j.hermed.2018.06.001
Gan L, Cookson MR, Petrucelli L, La Spada AR. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat Neurosci, 2018; 21(10):1300–9; https//doi.org/101038/s41593-018-0237-7.
Ganeshpurkar A, Saluja AK. The pharmacological potential of Rutin. Saudi Pharm J, 2017; 25(2):149–64; doi:10.1016/j.jsps.2016.04.025
Gentile MT, Ciniglia C, Reccia MG, Volpicelli F, Gatti, M, Thellung S, Florio T, Melone MAB, D’Amato LC. Ruta graveolens L. induces death of glioblastoma cells and neural progenitors, but not of neurons, via ERK 1/2 and AKT activation. PLoS One, 2015; 10(3):1–17; doi:10.1371/journal.pone.0118864
Giacobbo BL, Doorduin J, Klein H, Dierckx R, Bromberg E, de Vries EFJ. Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol Neurobiol, 2019; 56(1):3295–312.
Gopi M, Arambakkam JV. Asiaticoside: attenuation of rotenone induced oxidative burden in a rat model of hemiparkinsonism by maintaining the phosphoinositide-mediated synaptic integrity. Pharmacol Biochem Behav, 2017; 155:1–15; doi:10.1016/j.pbb.2017.02.005
Govardhan SRS, Negi PS, Radha C. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. J Funct Foods, 2013; 5(4):1883–91; doi:10.1016/j.jff.2013.09.009.
Gray NE, Magana AA, Lak P, Wright KM, Quinn J, Stevens JF, Maier CS, Soumyanath A. Centella asiatica——phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem Rev, 2018; 17(1):161–94; doi:10.1007/s11101-017-9528-y.Centella
Gray NE, Zweig JA, Matthews DG, Caruso M, Quinn JF, Soumyanath A. Centella asiatica attenuates mitochondrial dysfunction and oxidative stress in Aβ-exposed hippocampal neurons. Oxid Med Cell Longev, 2017; 2017; doi:10.1155/2017/7023091
Gullón B, Lú-Chau TA, Moreira MT, Lema JM, Eibes G. Rutin: a review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci Technol, 2017; 67:220–35; doi:10.1016/j.tifs.2017.07.008
Gunasekar P, Li L, Prabhakaran K, Eybl V, Borowitz JL, Isom GE. Mechanisms of the apoptotic and necrotic actions of trimethyltin in cerebellar granule cells. Toxicol Sci, 2001; 64(1):83–9.
Gupta R, Mathur M, Bajaj VK, Katariya P, Yadav S, Kamal R, Gupta RS. Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J Diabetes, 2012; 4(2):164–71; doi:10.1111/j.1753-0407.2011.00173.x
Hadianto A, Amanda D, Asogiyan PK. An analysis of garlic self-sufficiency in Indonesia. Sos Dan Ekonomi Pertan, 2019; 13(1):25–4.
Hazzaa SM, Abdelaziz SAM, Eldaim MAA, Abdel-Daim MM, Elgarawany GE. Neuroprotective potential of Allium sativum against monosodium glutamate-induced excitotoxicity: impact on short-term memory, gliosis, and oxidative stress. Nutrients, 2020; 12(4); doi:10.3390/nu12041028
Hening P, Mataram MBA, Wijayanti N, Kusindarta DL, Wihadmadyatami H. The neuroprotective effect of Ocimum sanctum Linn. ethanolic extract on human embryonic kidney-293 cells as in vitro model of neurodegenerative disease. Vet World, 2018; 11(9):1237–43; doi:10.14202/vetworld.2018.1237-1243
Hua S, Liu J, Zhang Y, Li J, Zhang X, Dong L, Zhao Y, Fu X. Piperine as a neuroprotective functional component in rats with cerebral ischemic injury. Food Sci Nutr, 2019a; 7(11):3443–51; doi:10.1002/fsn3.1185
Hua S, Wang B, Chen R, Zhang Y, Zhang Y, Li T, Dong L, Fu X. Neuroprotective effect of dichloromethane extraction from Piper nigrum L. and Piper longum L. on permanent focal cerebral ischemia injury in rats. J Stroke Cerebrovasc Dis, 2019b; 28(3):751–60.
Hua S, Wang B, Chen R, Zhang Y, Zhang Y, Li T, Dong L, Fu X. Neuroprotective effect of dichloromethane extraction from Piper nigrum L. and Piper longum L. on permanent focal cerebral ischemia injury in rats. J Stroke Cerebrovasc Dis, 2019c; 28(3):751–60.
Immanuel J, Natalia EC. Strategi kampanye Alzheimer Indonesia #janganmaklumdenganpikun dalam membangun kesadaran akan isu demensia. Profesi Humas J Ilm Ilmu Hub Masy, 2021; 6(1):67; doi:10.24198/prh.v6i1.28296
Inbathamizh LPE. Gas chromatography-mass spectrometric analyses of methanol extract of Moringa oleifera flowers. Int J Chem Anal Sci, 2012; 2:1394–7.
Islam M, Hasan J, Snigdha H, Ali S, Sharifi-Rad J, Martorell M, Mubarak MS. Chemical profile, traditional uses, and biological activities of Piper chaba Hunter: a review. J Ethnopharmacol, 2020; 257(112853):1–10; doi: 10.1016/j.jep.2020.112853
Ittiyavirah SP, Hameed J. Herbs treating Parkinson’s disease. Biomed Aging Pathol, 2014; 4(4):369–76; doi:10.1016/j.biomag.2014.08.003
Iwatani Y, Arcot J, Shrestha AK. Determination of folate contents in vegetables. Asia Pac J Clin Nutr, 2003; 10(4):37–48; doi: 10.1016/S0889-1575(02)00159-X
Jin X, Liu MY, Zhang DF, Zhong X, Du K, Qian P, Gao H, Wei MJ. Natural products as a potential modulator of microglial polarization in neurodegenerative diseases. Pharmacol Res, 2019; 145(77); doi:10.1016/j.phrs.2019.104253
Jivad N, Rabiei Z. Review on herbal medicine on brain ischemia and reperfusion. Asian Pac J Trop Biomed, 2015; 5(10):789–95; doi:10.1016/j.apjtb.2015.07.015
Joshi M, Shankar R, Pathak K, Yadav R. Polycystic ovarian syndrome: a review covering phytoconstituents for its outstrip management. Pharmacol Res Mod Chinese Med, 2021; 1:100011; doi:10.1016/j.prmcm.2021.100011
Kandanur SGS, Tamang N, Golakoti NR, Nanduri S. Andrographolide: a natural product template for the generation of structurally and biologically diverse diterpenes. Eur J Med Chem, 2019; 176:513–33; doi:10.1016/j.ejmech.2019.05.022
Kaur G, Invally M, Sanzagiri R, Buttar HS. Evaluation of the antidepressant activity of Moringa oleifera alone and in combination with fluoxetine. J Ayurveda Integr Med, 2015; 6(4):273–9; doi:10.4103/0975-9476.172384
Ketterman AJ, Wongtrakul J, Saisawang C. Phytochemical andrographolide modulates NF-κB and JNK in human neuroblastoma SH-SY5Y cells, a cell model for Parkinson’s disease. Heliyon, 2020; 6(6):e04121; doi:https://doi.org/10.1016/j.heliyon.2020.e04121
Kiaei M. New hopes and challenges for treatment of neurodegenerative disorders: great opportunities for young neuroscientists. Basic Clin Neurosci, 2013; 4(1):3–4.
Kiyama R. Nutritional implications of ginger: chemistry, biological activities and signaling pathways. J Nutr Biochem, 2020; 86:108486; doi:10.1016/j.jnutbio.2020.108486
Koppula S, Alluri R, Kopalli SR. Coriandrum sativum attenuates microglia mediated neuroinflammation and Mptp-induced behavioral and oxidative changes in Parkinson’s disease mouse model. EXCLI J, 2021; 20:835–50; doi:10.17179/excli2021-3668
Kovacs GG. Tauopathies. Handb Clin Neurol, 2018; 145:355–68; doi:10.1016/B978-0-12-802395-2.00025-0
Lindsay CB, Zolezzi JM, Rivera DS, Cisternas P, Bozinovic F, Inestrosa NC. Andrographolide reduces neuroinflammation and oxidative stress in aged Octodon degus. Mol Neurobiol, 2020; 57(2):1131–45; doi:10.1007/s12035-019-01784-6
Lu J, Ma Y, Wu J, Huang H, Wang X, Chen Z, Chen J, He H, Huang C. A review for the neuroprotective effects of andrographolide in the central nervous system. Biomed Pharmacother, 2019; 117(June):109078; doi:10.1016/j.biopha.2019.109078
Luca SV, Gawe?-B?ben K, Strz?pek-Gomó?ka M, Czech K, Trifan A, Zengin G, Korona-Glowniak I, Minceva M, Gertsch J, Skalicka-Wo?niak K. Insights into the phytochemical and multifunctional biological profile of spices from the genus Piper. Antioxidants, 2021; 10(10); doi:10.3390/antiox10101642
Mahajan SG, Mali RG, Mehta AA. Effect of Moringa oleifera Lam. seed extract on toluene diisocyanate-induced immune-mediated inflammatory responses in rats. J Immunotoxicol, 2007; 4(2):85–96; doi:10.1080/15476910701337472
Mahaman YAR, Huang F, Wu M, Wang Y, Wei Z, Bao J, Salissou MTM, Ke D, Wang Q, Liu R, Wang JZ, Zhang B, Chen D, Wang X. Moringa oleifera alleviates homocysteine-induced Alzheimer’s disease-like pathology and cognitive impairments. J Alzheimer’s Dis, 2018; 63(3):1141–59; doi:10.3233/JAD-180091
Mardisiswojo S, Harsono R. Cabe Puyang Warisan Nenek Moyang, Jilid II. PT Karya Wreda, Jakarta, Indonesia, 1968.
Mataram MBA, Hening P, Harjanti FN, Karnati S, Wasityastuti W, Nugrahaningsih DAA, Kusindarta DL, Wihadmadyatami H. The neuroprotective effect of ethanolic extract Ocimum sanctum Linn. in the regulation of neuronal density in hippocampus areas as a central autobiography memory on the rat model of Alzheimer’s disease. J Chem Neuroanat, 2021; 111(November):101885; doi:10.1016/j.jchemneu.2020.101885
Mgbeahuruike EE, Yrjönen T, Vuorela H, Holm Y. Bioactive compounds from medicinal plants: focus on Piper species. S Afr J Bot, 2017; 112:54–69; doi:10.1016/j.sajb.2017.05.007
More S, Kumar H, Kang, Song S, Lee, Choi D. Advances in neuroprotective ingredients of medicinal herbs by using cellular and animal models of Parkinson’s disease. Evid Based Complement Altern Med, 2013; 2013:1–15; doi: 10.1155/2013/957875
Muddapu V, Dharshini, Chakravarthy V, Gromiha M. Neurodegenerative diseases–is metabolic deficiency the root cause? Front Neurosci, 2020; 14(213):1–19; doi: 10.3389/fnins.2020.00213
Muthuraman A, Singh N. Neuroprotective effect of saponin rich extract of Acorus calamus L. in rat model of chronic constriction injury (CCI) of sciatic nerve-induced neuropathic pain. J Ethnopharmacol, 2012; 142(3):723–31; doi:10.1016/j.jep.2012.05.049
Muthuraman A, Singh N, Jaggi AS. Protective effect of Acorus calamus L. in rat model of vincristine induced painful neuropathy: an evidence of anti-inflammatory and anti-oxidative activity. Food Chem Toxicol, 2011; 49(10):2557–63; doi:10.1016/j.fct.2011.06.069
Nahak G, Sahu RK. Phytochemical evaluation and antioxidant activity of Piper cubeba and Piper nigrum. J Appl Pharm Sci, 2011; 1(8):153–7.
Nataraj J, Manivasagam T, Justin Thenmozhi A, Essa MM. Neuroprotective effect of asiatic acid on rotenone-induced mitochondrial dysfunction and oxidative stress-mediated apoptosis in differentiated SH-SYS5Y cells. Nutr Neurosci, 2017a; 20(6):351–9; doi:10.1080/1028415X.2015.1135559
Nataraj J, Manivasagam T, Justin Thenmozhi A, Essa MM. Neurotrophic effect of asiatic acid, a triterpene of Centella asiatica against chronic 1-Methyl 4-Phenyl 1, 2, 3, 6-tetrahydropyridine hydrochloride/probenecid mouse model of Parkinson’s disease: the role of MAPK, PI3K-Akt-GSK3β and mTOR signalling pathwa. Neurochem Res, 2017b; 42(5):1354–65; doi:10.1007/s11064-017-2183-2
Nie F, Zhang W, Cui Q, Fu Y, Li H, Zhang J. Kaempferol promotes proliferation and osteogenic differentiation of periodontal ligament stem cells via Wnt/β-catenin signaling pathway. Life Sci, 2020; 258:118143; doi:10.1016/j.lfs.2020.118143
Onyango IG, Khan SM, Bennett Jr JP. Mitochondria in the pathophysiology of Alzheimer ’ s and Parkinson ’ s diseases. Front Biosci Landmark, 2017; (4):854–72.
Orhan IE. Centella asiatica (L.) Urban: from traditional medicine to modern medicine with neuroprotective potential. Evid Based Complementary Altern Med, 2012; 2012:1–8; doi: 10.1155/2012/946259
Peng Y, Tao H, Wang S, Xiao J, Wang Y, Su H. Dietary intervention with edible medicinal plants and derived products for prevention of Alzheimer’s disease: a compendium of time-tested strategy. J Funct Foods, 2021; 81:104463.
Peshattiwar V, Muke S, Kaikini A, Bagle S, Dighe V, Sathaye S. Mechanistic evaluation of ursolic acid against rotenone induced Parkinson’s disease– emphasizing the role of mitochondrial biogenesis. Brain Res Bull, 2020; 160(March):150–61; doi:10.1016/j.brainresbull.2020.03.003
Picken MM. The pathology of amyloidosis in classification: a review. Acta Haematol, 2020; 143(4):322–34; doi:10.1159/000506696
Prachayasittikul V, Prachayasittikul S, Ruchirawat S, Prachayasittikul V. Coriander (Coriandrum sativum): a promising functional food toward the well-being. Food Res Int, 2018; 105:305–23; doi:10.1016/j.foodres.2017.11.019
Prasathkumar M, Sadhasivam S. Chitosan/hyaluronic acid/alginate and an assorted polymers loaded with honey, plant, and marine compounds for progressive wound healing—know-how. Int J Biol Macromol, 2021; 186(July):656–85; doi:10.1016/j.ijbiomac.2021.07.067
Rahmawati F, Silaban H. Bioactivity of Kemangi leaves (Ocimum sanctum) and Ruku leaves (Ocimum tenuiflorum). Int J Heal Sci Res, 2021; 11(5):379–91; doi:10.52403/ijhsr.20210558
Rajput SB, Tonge MB, Karuppayil SM. An overview on traditional uses and pharmacological profile of Acorus calamus Linn. (Sweet flag) and other Acorus species. Phytomedicine, 2014; 21(3):268–76; doi:10.1016/j.phymed.2013.09.020
Riastiwi I, Damayanto IPGP, Ridwan R, Handayani T, Leksonowati A. Moringa oleifera distribution in Java and Lesser Sunda Islands which is attributed with annual rainfall. Biosaintifika J Biol Biol Educ, 2018; 10(3):613–21; doi:10.15294/biosaintifika.v10i3.16115.
Rodrigues KE, de Oliveira FR, Barbosa B, Paraense RSO, Bannwart CM, Pinheiro BG, de Santana Botelho A, Muto NA, do Amarante CB, Hamoy M, de Matos Macchi B, do Socorro Ferraz Maia C, do Prado AF, do Nascimento JLM. Aqueous Coriandrum sativum L. extract promotes neuroprotection against motor changes and oxidative damage in rat progeny after maternal exposure to methylmercury. Food Chem Toxicol, 2019; 133(110755):1–40; doi: 10.1016/j.fct.2019.110755
Rojas P, Serrano-García N, Medina-Campos ON, Pedraza-Chaverri J, Maldonado PD, Ruiz-Sánchez E. S-Allylcysteine, a garlic compound, protects against oxidative stress in 1-methyl-4-phenylpyridinium-induced parkinsonism in mice. J Nutr Biochem, 2011; 22(10):937–44; doi:10.1016/j.jnutbio.2010.08.005
Salehi B, Zakaria ZA, Gyawali R, Ibrahim SA, Rajkovic J, Shinwari ZK, Khan T, Sharifi-Rad J, Ozleyen A, Turkdonmez E, Valussi M, Tumer TB, Fidalgo LM, Martorell M, Setzer WN. Piper species: a comprehensive review on their phytochemistry, biological activities and applications. Molecules, 2019; 24; doi:10.3390/molecules24071364
Santos AFS, Argolo ACC, Coelho LCBB, Paiva PMG. Detection of water soluble lectin and antioxidant component from Moringa oleifera seeds. Water Res, 2005; 39(6):975–80; doi:10.1016/j.watres.2004.12.016
Sarangi SC, Joshi D, Kumar R, Kaleekal T, Gupta YK. Pharmacokinetic and pharmacodynamic interaction of hydroalcoholic extract of Ocimum sanctum with valproate. Epilepsy Behav, 2017; 75:203–9; doi:10.1016/j.yebeh.2017.08.018
Sengupta U, Kayed R. Amyloid β, tau, and α-synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog Neurobiol, 2022; 214:102270.
Shelar M, Nanaware S, Arulmozhi S, Lohidasan S, Mahadik K. Validation of ethnopharmacology of ayurvedic sarasvata ghrita and comparative evaluation of its neuroprotective effect with modern alcoholic and lipid based extracts in β-amyloid induced memory impairment. J Ethnopharmacol, 2018; 219:182–94; doi:10.1016/j.jep.2018.02.032
Shrivastava P, Vaibhav K, Tabassum R, Khan A, Ishrat T, Khan MM, Ahmad A, Islam F, Safhi MM, Islam F. Anti-apoptotic and anti-inflammatory effect of piperine on 6-OHDA induced Parkinson’s rat model. J Nutr Biochem, 2013; 24(4):680–7; doi:10.1016/j.jnutbio.2012.03.018
Siddique YH, Faisal M, Naz F, Jyoti S, Rahul. Role of Ocimum sanctum leaf extract on dietary supplementation in the transgenic Drosophila model of Parkinson’s disease. Chin J Nat Med, 2014; 12(10):777–81; doi:10.1016/S1875-5364(14)60118-7
Singh H, Bhushan S, Arora R, Singh Buttar H, Arora S, Singh B. Alternative treatment strategies for neuropathic pain: role of Indian medicinal plants and compounds of plant origin-A review. Biomed Pharmacother, 2017; 92:634–50; doi:10.1016/j.biopha.2017.05.079
Talebi M, ?lgün S, Ebrahimi V, Talebi M, Farkhondeh T, Ebrahimi H, Samarghandian S. Zingiber officinale ameliorates Alzheimer’s disease and cognitive impairments: lessons from preclinical studies. Biomed Pharmacother, 2021; 133; doi:10.1016/j.biopha.2020.111088
Tappayuthpijarn P, Sattaponpan C, Sakpakdeecharoen I, Ittharat A. Cholinesterase inhibitory and antioxidant activities of Thai traditional remedies potentially used for Alzheimer’S disease. Rev Clin Pharmacol Drug Ther, 2012; 10(2):105; doi:10.17816/rcf102105
Tönnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimer’s Dis, 2017; 57(4):1105–21; doi:10.3233/JAD-161088
Trivedi MN, Khemani A, Vachhani UD, Santani CPS, Santani DD. Pharmacognostic, phytochemical analysis and antimicrobial activity of two piper species. Int J Compr Pharm, 2011; 02(May):5–8.
Tumer TB, Rojas-Silva P, Poulev A, Raskin I, Waterman C. Direct and indirect antioxidant activity of polyphenol- and isothiocyanate-enriched fractions from moringa oleifera. J Agric Food Chem, 2015; 63(5):1505–13; doi:10.1021/jf505014n
Venuprasad MP, Kandikattu HK, Razack S, Amruta N, Khanum F. Chemical composition of Ocimum sanctum by LC-ESI–MS/MS analysis and its protective effects against smoke induced lung and neuronal tissue damage in rats. Biomed Pharmacother, 2017; 91:1–12; doi:10.1016/j.biopha.2017.04.011
Vergara-Jimenez M, Almatrafi MM, Fernandez ML. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants, 2017; 6(4):91.
Wang L, Cai X, Shi M, Xue L, Kuang S, Xu R, Qi W, Li Y, Ma X, Zhang R, Hong F, Ye H, Chen H. Identification and optimization of piperine analogues as neuroprotective agents for the treatment of Parkinson’s disease via the activation of Nrf2/keap1 pathway. Eur J Med Chem, 2020a; 199:1–21; doi:10.1016/j.ejmech.2020.112385
Wang Y, Pan Y, Li H. What is brain health and why is it important? BMJ, 2020b; 371:m3683; doi:10.1136/bmj.m3683
Wang, B, Zhang, Y, Huang, J, Li T, Fu, X. Anti-inflammatory activity and chemical composition of dichloromethane extract from Piper nigrum and Piper longum on permanent focal cerebral ischemia injury in rats. Rev Bras Farmacogn, 2017; 27(3):369–74; doi: 10.1016/j.bjp.2017.02.003
Welbat JU, Chaisawang P, Pannangrong W, Wigmore P. Neuroprotective properties of asiatic acid against 5-fluorouracil chemotherapy in the hippocampus in an adult rat model. Nutrients, 2018; 10(8):2–11; doi:10.3390/nu10081053
Yadav V, Krishnan A, Vohora D. A systematic review on Piper longum L.: bridging traditional knowledge and pharmacological evidence for future translational research. J Ethnopharmacol, 2020; 247(112255):112–225; doi: 10.1016/j.jep.2019.112255
Yang EJ, Song KS. Andrographolide, a major component of Andrographis paniculata leaves, has the neuroprotective effects on glutamate-induced HT22 cell death. J Funct Foods, 2014; 9(1):162–72; doi:10.1016/j.jff.2014.04.023
Yao F, Huang Y, Wang Y, He X. Anti-inflammatory diarylheptanoids and phenolics from the rhizomes of kencur (Kaempferia galanga L.). Ind Crops Prod, 2018; 125(December 2017):454–61; doi:10.1016/j.indcrop.2018.09.026
Yuan Z, Yidan Z, Jian Z, Xiangjian Z, Guofeng Y. Molecular mechanism of autophagy: its role in the therapy of Alzheimer’s disease. Curr Neuropharmacol, 2020; (10.2174/1570159x18666200114163636).
Yunusa S, Musa A. Ethyl-acetate and aqueous fractions of Moringa oleifera Lam (Moringaceae) leaf extract possess antidepressant activity in mice. Afr J Pharmacol, 2018a; 7(1):1–6; http://journals.uonbi.ac.ke/ajpt/article/view/1692
Yunusa S, Musa A. Evaluation of antidepressant effect of ethanol extract and chloroform fraction of Moringa oleifera Lam. (Moringaceae) leaf in mice. J Drug Res Dev, 2018b; 4(1); doi:10.16966/2470-1009.140
Zarai Z, Boujelbene E, Ben Salem N, Gargouri Y, Sayari A. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT Food Sci Technol, 2013; 50(2):634–41; doi:10.1016/j.lwt.2012.07.036