INTRODUCTION
Language is the ability to realize and produce speech and convey information, thoughts, feelings, and ideas (McLaughlin, 2011). Numerous disorders and syndromes affect language ability in children. Despite appropriate intelligence quotient (IQ), neurological system, and the presence of an adequate learning environment, specific language impairment (SLI) is defined as difficulty in acquiring oral language (Hewitt, 2002; Talli et al., 2016). Children with SLI typically speak late compared to their same ages of 3 or 5 years; they have restricted vocabulary and brief utterances. Three key markers of SLI are measured by a specialist for domain-specificity: syntax, morphology, and phonology (Van Der Lely, 2005). Although the numbers of affected children are varied across countries, SLI is estimated to be ~7% of English-speaking 5-year-old children in the United States (Evans and Brown, 2015). Evidence implies that untreated speech and language delays can persist in 40%–60% of children (Morgan et al., 2017). Thus, children with SLI are known to carry a high risk of an adverse effect on academic, social, and economic achievements (Bishop et al., 2012).
Among the different 60 detectable chemical elements found in the human body, only about half of these elements are defined to contribute to the structural components of biological molecules such as enzymes, vitamins, and proteins and play an essential role in several life-sustaining processes by mediating critical metabolic reactions (Alqhazo and Rashaid, 2018; Chellan and Sadler, 2015). Therefore, imbalances in the necessary levels of these elements could negatively influence biological processes such as mitochondrial activity, immune regulation, muscle contraction, membrane potential regulation, nerve conduction, macromolecule metabolism, and enzyme activation. These processes correlate with or cause various fatal diseases (Al-fartusie and Mohssan, 2017; Alqhazo and Rashaid, 2018). Toddlers and young children have highly vulnerable brains because of their permeable blood–brain barrier. Thus, the negative impacts of toxic metals on the neurodevelopment of children may explain the SLI etiology (Hsueh et al., 2017). It has been found that elements such as lead (Pb), mercury (Hg), and arsenic (As) have acute toxic effects on the developing brain tissue compared to the adult brain (Hsueh et al., 2017). Hsueh et al. (2017) reported that children exposed to chronic Pb contamination were shown severe behavioral and learning disabilities and low IQ scores. Mallah et al. (2018) published additional data on the role of Pb in initiating atherosclerosis.
Recently, studies have been focused on attempting to understand the relationship between bioelements and their role in disorders such as stuttering, autism spectrum disorder (ASD), and SLI that may affect children’s language ability (Almogren et al., 2013; Alqhazo and Rashaid, 2018; Fi?on et al., 2020; Rashaid et al., 2022). Numerous observational clinical studies showed the significant role of bioelements in the early diagnosis, assessment, and therapy of developmental language disorders (Fi?on et al., 2020; Hsueh et al., 2017; Peñuelas et al., 2019). In addition, some reports demonstrated that many developmental language disorders could be attributed to the early exposure of pregnant women to trace elements and heavy metals at different gestation periods. Its significance reveals that such exposure may trigger excessive damage due to oxidative stress induced by free radical formation (Bocca et al., 2019). Karakis et al. (2021) revealed that increases in Al, As, Cd, Hg, Ni, and Mn have been linked to newborn congenital defects at birth.
Blood concentrations of Cd, Hg, and Pb were measured using inductively coupled mass spectrometry (ICP-MS). Previous studies have shown that children with developmental delay had higher blood Pb levels (Hsieh et al., 2014; Hsueh et al., 2017; Wright et al., 2008). A blood Cd level greater than 1.0 g/l has been linked to an increased risk of developmental delay (Silver et al., 2013). Postnatal Cd exposure has also been linked to learning difficulties in children and cognitive deficits in boys (Rodríguez-Barranco et al., 2014). Both Pb and Cd exert neurotoxic effects on the developing brain of children (Ahmad and Liu, 2020). In addition, it has been reported that heavy metals play a function in developing several neurodevelopmental disorders, such as ASD, and interfere with learning and normal functioning during childhood and adolescence (Long et al., 2019). The abundance of iron (Fe), calcium (Ca), zinc (Zn), iodine (I), magnesium (Mg), molybdenum (Mo), manganese (Mn), and selenium (Se) were lower in ASD hair samples when compared to the neurotypical control group (Blaurock-Busch et al., 2012; Skogheim et al., 2021). Moreover, a study reported that hair and nail samples from ASD have high concentrations of Pb, Cu, and Hg and low levels of Mg and Se. These elemental concentrations were also correlated strongly with the degree of the severity of ASD (Lakshmi Priya and Geetha, 2011).
Despite being a relatively harmless metal, indium (In) can potentially be toxic in particular forms or high doses. This is explained physiologically by the fact that In is a crucial part of bigger biological molecules that can interact with or regulate the levels of many other molecules (Shantanam and Mueller, 2018). In the same context, strontium, the 15th most prevalent element on the earth as it is found in the air, soil, and water, is also connected to pollutants of human activities. It has been demonstrated that some strontium-containing substances boost osteoblasts to form new bone, inhibit osteoclasts, and ultimately stop the absorption of bone (Mirzaee et al., 2020). These data revealed that the optimal physiological concentration range between metal deficiency and toxicity is relatively small and needs to be firmly controlled (Mirzaee et al., 2020).
During the past four decades, the number of children diagnosed with SLI has increased dramatically, and extensive research has been conducted to understand the impact of essential minerals and heavy elements levels on the etiology of SLI (Rashaid et al., 2022; Schwalfenberg and Genuis, 2015). This case-control study aims to examine the levels of five essential minerals, iron (Fe), potassium (K), manganese (Mn), magnesium (Mg), and zinc (Zn), in addition to other nine heavy metals: aluminum (Al), barium (Ba), cadmium (Cd), chromium (Cr), nickel (Ni), lithium (Li), lead (Pb), indium (In), and strontium (Sr), in the scalp hair of Jordanian children with SLI in comparing with their healthy age- and sex-matched fluent control group. The study also examines the correlations between the measured elements.
METHODOLOGY
Study design and data collection
The Institution Review Board at King Abdullah University Hospital (KAUH) (Approval No. 7/558 /2018) approved the procedures of sampling and data collection. The 39 children with SLI (5.46 ± 1.57 years) were recruited from the speech clinic at KAUH in North Jordan between March 2018 and January 2019. The 37 children of the age- and sex-matched fluent control group (5.66 ± 1.66 years) were collected from North Jordan by advertisement, as presented in Table 1. A speech pathologist and child psychiatrist at KAUH medically evaluated the fluent controls. The control group reported healthy, fluent children with no history of voice or speech disorders.
Parents filled out a questionnaire consisting of four sections: demographic section, environmental exposure section, health status, pregnancy period, and maternal status section. Each parent signed consent forms, and all data were confidential by giving identification (ID) codes for each subject.
Hair samples analysis
Essential mineral and heavy element determination is a multifaceted analytical procedure that comprises hair washing, pulverization, acid digestion, and analysis by ICP-MS model (iCAPQ, Thermo Fisher Scientific, Darmstadt, Germany). Subjects should shampoo their hair the same day or the day before sampling. For long hair, a 1 cm diameter lock of long hair close to the scalp was cut from the nape of the neck by clean stainless steel scissors. For subjects with shorter hair, a 2–3 cm diameter lock of short hair was collected by thinning scissors. The hair was stored in a clean paper envelope labeled with an ID number (Alqhazo and Rashaid, 2018; Mallah et al., 2018; Rashaid et al., 2021).
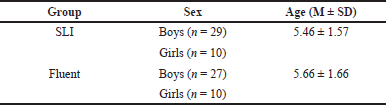 | Table 1. Demographic information of the SLI and fluent groups. [Click here to view] |
Washing and pulverization
Hair samples were cleansed and rinsed in a solution of 0.1% Triton × 100 and vortexed for 10 minutes. The sample was then rinsed with deionized water three times before being washed with acetone and mixed for 10 minutes. After that, samples were dried overnight at 70°C in a drying oven. The dried samples were frozen in liquid nitrogen at −196°C for 45 minutes before being pulverized with a tiny bead beater-16, model 607EUR (BioSpec Products, Bartlesville, OK). The fine powder was then divided into three subsamples of 200 Mg (Alqhazo and Rashaid, 2018; Mallah et al., 2018; Rashaid et al., 2021).
Digestion
The employment of a microwave digestion system [EHOS UP. Milestone S. r.l. Via Fatebenefratelli, Sorisole (BG), Italy] is a robust tool in the disintegration of the hair matrix. For each hair subsample, the fine powder was weighed into subsamples of 200 mg and placed into digestion containers. After that, 9 ml of 69% nitric acid (HNO3) and 2 ml of 30% hydrogen peroxide (H2O2) were added. The vessels were sealed and placed in the microwave digestion system’s rotter. The digested samples were filtered and evaporated on a hot plate at 85°C until the samples were dried.
Sample dilution and standard preparation
After completing the sample dissolution procedure using the microwave digester, all samples were diluted using a 2% HNO3 (w/w) solution. The target dilution factor was 5 by weighing 1 g from the stock sample (0.2 into 10 g) and then leveling the solution up to 5 g by 2% HNO3. The weighing balance used in this process was from Shimadzu (Model: AUX 120, resolution: 0.0001g, maximum mass: 120 g). Calibration curves were constructed using a multielement stock solution of 1,000 ppm of each element. Serial dilutions were done to prepare five different standard concentrations (5, 10, 50, 100, and 500 ppm). The standards were injected into ICP-MS, starting from the lowest concentration to the highest one (Rashaid et al., 2021).
Instrumental analysis and software
The samples were measured randomly by ICP-MS model (iCAPQ, Thermo Fisher Scientific, Darmstadt, Germany). The software is Qtegra (copyright 2013 Thermo Fisher Scientific Inc.) and Chiller: NESLAB ThermoFlex2500, Thermo Scientific (Alqhazo and Rashaid, 2018).
Statistical analyses
Statistical analyses were conducted using Statistical Package for the Social Sciences version 21. Independent sample t-tests were applied to compare concentrations of elements in the hair of ASD and their fluent control groups. Subgroups according to sex were compared to matched fluent controls using t-tests. Pearson’s correlations were calculated to explore the strength and direction of the relationship between elements. The data were presented as mean ± SD (M ± SD); p ≤ 0.05 was considered significant.
RESULTS
As shown in Table 2, the R2 values of all elements were greater than 0.99, which means an excellent linear relationship exists between the intensity and the concentrations. Limit of detections (LODs) were located within the range of 0.001–0.304 ppb. Therefore, the method was considered as a good method for elemental analysis in hair.
The results showed that only the level of Zn was significantly lower in the hair of the SLI group (132.81 ± 35.49 ppm) than in the fluent control group (132.81 ± 35.49 ppm), as shown by the line graph in Figure 1. The outcomes demonstrated that the levels of Al, Cd, In, and Ni were lower in SLI group but not significant. On the other hand, the levels of Ba, Cr, Pd, K, Mg, Li, and Sr were greater in the hair samples of the SLI group than in the fluent control group but also not significantly. Results are presented in Table 3.
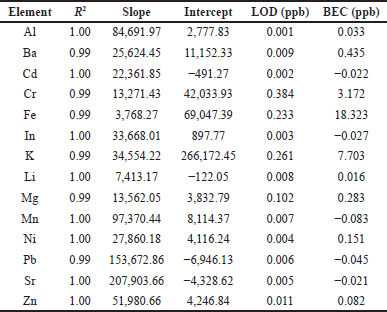 | Table 2. Calibration equation, LOD, and background equivalent concentration (BEC) of the measured elements by ICP-MS. [Click here to view] |
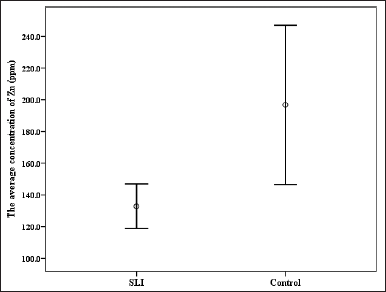 | Figure 1. The mean concentration (ppm) of zinc in SLI and fluent control group. [Click here to view] |
A more clear impact of gender subgroups according to sex was observed. Fluent control girls had significantly (p = 0.04) higher levels of K (M = 90.19, SD = 17.03) than SLI girls (M = 81.11, SD = 9.88) and fluent control boys had significantly (p = 0.003) higher levels of Mg (M = 144. 62, SD = 48.33) than SLI boys (M = 134.17, SD = 34.42). In the SLI group, the correlation coefficients (R2) between the elements are calculated and summarized in Table 4. The coefficients were calculated to evaluate the direction and strength of relationships between the essential minerals and heavy elements. The results indicated that Zn was positively correlated with Mn (R2 = 0.354, p = 0.04). Al was positively correlated with Li (R2 = 0.389, p = 0.05). It was strongly positively correlated with Cd (R2 = 0.998, p < 0.001). Furthermore, Fe and Mn had a strong positive correlation (R2 = 0.818, p < 0.001). Additionally, Mg was strongly positively correlated with Sr (R2 = 0.961, p < 0.001). However, results also revealed that Zn was negatively associated with each of Ba (R2 = −0.392, p = 0.04) and Fe (R2 = −0.423, p = 0.02). In the fluent control group, Al was significantly correlated with Cr (R2 = 0.213, p = 0.04). Mg was positively correlated with K (R2 = 0.323, p = 0.021). Zn was positively correlated with Mg (R2 = 0.314, p = 0.04), whereas Zn was negatively correlated with Mn (R2 = −0.262, p = 0.03).
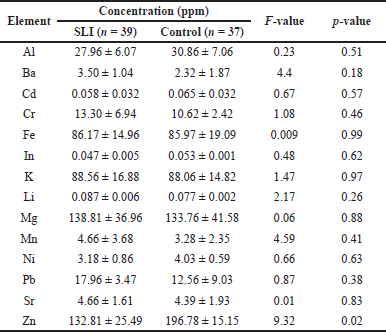 | Table 3. Mean, SD, F-values, and p-values of the tested elements in the SLI and fluent control groups. [Click here to view] |
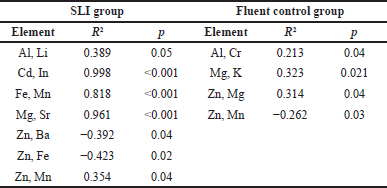 | Table 4. The correlation coefficients (R2) and p-values of tested elements in the SLI group and fluent control group. [Click here to view] |
DISCUSSION
This case-control study is designed to measure the levels of five essential minerals and nine heavy elements in the hair samples of Jordanian children with SLI. Results showed that Zn had a significantly lower concentration in the hair samples of the SLI group compared to the fluent control group. This result agrees with the important role of Zn as a crucial element for all physiological systems, including neural functioning, where it participates in many cellular processes. Zn is the second most prevalent transition metal in the vertebrate host, and it has been discovered that around 10% of host proteins interact with Zn (Kehl-Fie and Skaar, 2010). The data obtained in the current study support our previous results that revealed that both Zn and Cu were deficient in hair samples of children who stutter. Furthermore, the low concentration of Zn was measured in patients with epilepsy (Akyol, 1999) as well as the hair of schizophrenic, ASD, DLD, and depressed cases (Petrilli et al., 2017; Rahman et al., 2009; Rashaid et al., 2022, 2021).
The data also showed that Al, Cd, Ni, and In levels were not significantly lower in the SLI group. These results were in contrast with several published data that assessed the impact of heavy metals on different neurotransmitter diseases. For example, Al was higher in Alzheimer’s disease and other health problems such as dialysis dementia syndrome and downs syndrome (Lukiw et al., 2018). Furthermore, Cd is a heavy metal with a long biological half-life; therefore, its accumulation in the central nervous system and peripheral neuronal systems cause massive toxic effects (Wang and Du, 2013). Additionally, chronic Ni exposure induced behavioral dysfunctions such as depression and biochemical dysfunction.
The studies also showed insignificant levels of Ba, Cr, Pb, K, Mg, Mn, Li, and Sr in SLI. Mg, Mn, and Li were significantly lower in the hair samples of patients with epilepsy and Parkinson’s disease and patients who stutter (Akyol, 1999; Alqhazo and Rashaid, 2018; Forte et al., 2005). These controversial results could be explained because of the differences in the sample sizes. Thus, we recommend using larger sample sizes in future studies.
Zn and Mn are two elements that interact with each other. In the current study, a significant positive relationship was reported. Furthermore, a significant positive correlation was calculated between Fe and Mn. Mn plays an important function in a variety of cellular activities, including lipid, protein, and carbohydrate metabolism, and a cofactor of many enzymes as well as Mn is required by several bacterial proteins (Andreini et al., 2008). Kehl-Fie and Skaar (2010) demonstrated that in addition to Fe, vertebrates sequester Zn and Mn intracellularly and extracellularly to guard against infection (Kehl-Fie and Skaar, 2010). About 500–1,000 species of bacteria live in the human gut, which could draw attention to the role of pathogenic bacteria and the composition of the microbiome of the human body and the role of microflora in the absorbance of elements (Sekirov et al., 2010).
Zn was also negatively correlated with Fe. Our presented results agree with studies that reported high Fe concentrations negatively affect Zn absorption in adults (Kondaiah et al., 2019; Whittaker, 1998). Additionally, Zn was negatively correlated with Ba. The quantity of Ba in foods, including Brazil nuts, seaweed, fish, and some plants and water, is usually not high enough to cause health problems. Ba is only found in nature in combination with other elements. Barium carbonate is generally water-insoluble; therefore, it is toxic to humans because of its solubility in the gastrointestinal tract. While the insoluble compounds of barium are incompetent sources of Ba2+ ion hence, it is nontoxic to humans (McNeill and Isoardi, 2019). For that, it is important to determine which forms of barium compounds are found and their doses for further future research.
CONCLUSION
Hair samples of all samples were cut, washed, dried, physically degraded, hydrolyzed, and analyzed by ICP-MS. Zinc levels were considerably lower in the SLI group’s hair samples compared to the fluent control group. Positive correlations between Zn and Mn, Al and Li, and Fe and Mn were significantly detected in the SLI group. The difference in K levels between fluent control girls and SLI girls and the difference in Mg levels between fluent control boys and SLI boys provided more evidence of the effects of gender. Moreover, the correlation between the selected essential minerals and heavy elements has been detected and discussed to evaluate their impact on the severity of SLI cases. These findings could be helpful in increasing the awareness of regulations and control on exposure to heavy elements during conception. In addition, introduce the blood test of Zn as a potential routine method during pregnancy and for newborn babies that will be helpful in early diagnosis and treatment. Future studies are necessary to investigate the correlations between the elemental content of mothers and their babies at ages less than 4 years that could help understand the cofactors of SLI and other disorders. There are several clinical implications for the outcomes of the current study. Early screening and diagnosis of SLI might be possible by measuring the concentrations of elements in hair. A new treatment practice could be designed by giving children with SLI supplementation of deficient elements or chelation for the toxic elements that may complement the existing speech and language therapy protocols.
Limitations of the study
This study is a descriptive study with a small sample size from North Jordan. In addition to the limited access clinics in the study, enrolling sufficient numbers of children in clinical research is often a significant challenge, as many parents were conservative regarding the children’s participation, especially in developing countries. Therefore, further studies with more subjects from different geographical origins are recommended. The limited financial resources were an obstacle as well. The collection of blood samples from the participants to investigate the correlation between elements in blood and hair and screening more bioelements was costly and undoable at this stage. Therefore, future studies with environmental elemental screens must compare environmental levels with other bio-indicative matrices (i.e., hair, blood, and urine).
ACKNOWLEDGMENTS
The authors would like to express our gratitude to the study participants and their families for allowing us to collect data.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work was supported by the Jordan University of Science and Technology under Grant (number 558-2015).
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
ETHICAL APPROVALS
This study involve experiments on human subjects. King Abdullah university hospital (KAUH) institution review board (IRB), approval No. 10/215/2444, approved the protocol of the study.
DATA AVAILABILITY STATEMENT
Data files are available upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
ADDITIONAL DECLARATION
The article was uploaded to a preprint server before submitting it to the journal, and the authors undertake that they will inform the preprint server once the article gets accepted in this journal.
REFERENCES
Ahmad F, Liu P. (Ascorb)ing pb neurotoxicity in the developing brain. Antioxidants, 2020; 9(12):1–30; https://doi.org/10.3390/antiox9121311
Akyol O. Serum and hair trace element levels in patients with epilepsy and healthy subjects: does the antiepileptic therapy affect the element concentrations of hair? Eur J Neurol, 1999; 6(6):705–9; https://doi.org/10.1046/j.1468-1331.1999.t01-1-660705.x
Al-fartusie FS, Mohssan SN. Essential trace elements and their vital roles in human body. Indian J Adv Chem Sci, 2017; 5(3):127–36; https://doi.org/10.22607/IJACS.2017.503003
Almogren A, Shakoor Z, Almomen A, Hasanato RMW. Levels of heavy metal and trace element among children with autism spectrum disorders. Curr Pediatr Res, 2013; 17(2):79–83.
Alqhazo M, Rashaid AB. The concentrations of bioelements in the hair samples of Jordanian children who stutter. Int J Pediatr Otorhinolaryngol, 2018; 112(September):158–62; https://doi.org/10.1016/j.ijporl.2018.06.045
Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem, 2008; 13(8):1205–18; https://doi.org/10.1007/s00775-008-0404-5
Bishop DVM, Holt G, Line E, McDonald D, McDonald S, Watt H. Parental phonological memory contributes to prediction of outcome of late talkers from 20 months to 4 years: a longitudinal study of precursors of specific language impairment. J Neurodev Disord, 2012; 4(1):1–12; https://doi.org/10.1186/1866-1955-4-3
Blaurock-Busch E, Amin OR, Dessoki HH, Rabah T. Toxic metals and essential elements in hair and severity of symptoms among children with autism. Maedica, 2012; 7(1):38–48. Available via http://www.ncbi.nlm.nih.gov/pubmed/23118818%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3484795
Bocca B, Ruggieri F, Pino A, Rovira J, Calamandrei G, Martínez MÁ, Domingo JL, Alimonti A, Schuhmacher M. Human biomonitoring to evaluate exposure to toxic and essential trace elements during pregnancy. Part A. concentrations in maternal blood, urine and cord blood. Environ Res, 2019; 177(May):108599; https://doi.org/10.1016/j.envres.2019.108599
Chellan P, Sadler PJ. The elements of life and medicines. Philos Trans R Soc A Math Phys Eng Sci, 2015; 373(2037); https://doi.org/10.1098/rsta.2014.0182
Evans JL, Brown TT. Specific language impairment. In Neurobiology of language, Elsevier Inc., Amsterdam, The Netherlands, pp 899–912; https://doi.org/10.1016/B978-0-12-407794-2.00072-9
Fi?on J, Ustymowicz-Farbiszewska J, Krajewska-Ku?ak E. Analysis of lead, arsenic and calcium content in the hair of children with autism spectrum disorder. BMC Public Health, 2020; 20(1):1–8; https://doi.org/10.1186/s12889-020-08496-w
Forte G, Alimonti A, Violante N, Di Gregorio M, Senofonte O, Petrucci F, Sancesario G, Bocca B. Calcium, copper, iron, magnesium, silicon and zinc content of hair in Parkinson’s disease. J Trace Elem Med Biol, 2005; 19(2–3):195–201; https://doi.org/10.1016/j.jtemb.2005.08.003
Hewitt LE. Children with specific language impairment. Laurence B. Leonard. Cambridge, MA: MIT Press, 1998. Pp. 339. Appl Psycholinguist; https://doi.org/10.1017/s0142716402232075
Hsieh RL, Huang YL, Shiue HS, Huang SR, Lin MI, Mu SC, Chung CJ, Hsueh YM. Arsenic methylation capacity and developmental delay in preschool children in Taiwan. Int J Hyg Environ Health, 2014; 217(6):678–86; https://doi.org/10.1016/j.ijheh.2014.02.004
Hsueh YM, Lee CY, Chien SN, Chen WJ, Shiue HS, Huang SR, Lin MI, Mu SC, Hsieh RL. Association of blood heavy metals with developmental delays and health status in children. Sci Rep, 2017; 7(March):1–9; https://doi.org/10.1038/srep43608
Karakis I, Landau D, Gat R, Shemesh N, Tirosh O, Yitshak-Sade M, Sarov B, Novack L. Maternal metal concentration during gestation and pediatric morbidity in children: an exploratory analysis. Environ Health Prev Med, 2021; 26(1):1–11; https://doi.org/10.1186/s12199-021-00963-z
Kehl-Fie TE, Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol, 2010; 14(2):218–24; https://doi.org/10.1016/j.cbpa.2009.11.008
Kondaiah P, Yaduvanshi PS, Sharp PA, Pullakhandam R. Iron and zinc homeostasis and interactions: does enteric zinc excretion cross-talk with intestinal iron absorption? Nutrients, 2019; 11(8); https://doi.org/10.3390/nu11081885
Lakshmi Priya MD, Geetha A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol Trace Elem Res, 2011; 142(2):148–58; https://doi.org/10.1007/s12011-010-8766-2
Long M, Ghisari M, Kjeldsen L, Wielsøe M, Nørgaard-Pedersen B, Mortensen EL, Abdallah MW, Bonefeld-Jørgensen EC. Autism spectrum disorders, endocrine disrupting compounds, and heavy metals in amniotic fluid: a case-control study. Mol Autism, 2019; 10(1):1–19; https://doi.org/10.1186/s13229-018-0253-1
Lukiw WJ, Kruck TPA, Percy ME, Pogue AI, Alexandrov PN, Walsh WJ, Sharfman NM, Jaber VR, Zhao Y, Li W, Bergeron C, Culicchia F, Fang Z, McLachlan DRC. Aluminum in neurological disease - a 36 year multicenter study. J Alzheimer’s Dis Parkinsonism, 2018; 08(06):6–10; https://doi.org/10.4172/2161-0460.1000457
Mallah E, Rayyan WA, Dayyih WA, Al-Majali IS, Qaralleh H, Al-Thunibat OY, Seder N, Bustami M, Qatoosah LA, Arafat T. Analytical and comparative study about the impact of lead homeostasis on cardiovascular disorders in humans. Biomed Pharmacol J, 2018; 11(1):277–84; https://doi.org/10.13005/bpj/1372
McLaughlin MR. Speech and language delay in children. Am Fam Physician, 2011; 83(10):1183–8.
McNeill IR, Isoardi KZ. Barium poisoning: an uncommon cause of severe hypokalemia. Toxicol Commun, 2019; 3(1):88–90; https://doi.org/10.1080/24734306.2019.1691340
Mirzaee M, Semnani S, Roshandel GR, Nejabat M, Hesari Z, Joshaghani H. Strontium and antimony serum levels in healthy individuals living in high- and low-risk areas of esophageal cancer. J Clin Lab Anal, 2020; 34(7); https://doi.org/10.1002/jcla.23269
Morgan A, Ttofari Eecen K, Pezic A, Brommeyer K, Mei C, Eadie P, Reilly S, Dodd B. Who to refer for speech therapy at 4 years of age versus who to “watch and wait”? J Pediatr, 2017; 185:200–4.e1; https://doi.org/10.1016/j.jpeds.2017.02.059
Peñuelas J, Fernández-Martínez M, Ciais P, Jou D, Piao S, Obersteiner M, Vicca S, Janssens IA, Sardans J. The bioelements, the elementome, and the biogeochemical niche. Ecology, 2019; 100(5):1–15; https://doi.org/10.1002/ecy.2652
Petrilli MA, Kranz TM, Kleinhaus K, Joe P, Getz M, Johnson P, Chao MV, Malaspina D. The emerging role for zinc in depression and psychosis. Front Pharmacol, 2017; 8(JUN); https://doi.org/10.3389/fphar.2017.00414
Rahman MA, Azad MAK, Hossain MI, Qusar MMAS, Bari W, Begum F, Huq SMI, Hasnat A. Zinc, manganese, calcium, copper, and cadmium level in scalp hair samples of schizophrenic patients. Biol Trace Elem Res, 2009; 127(2):102–8; https://doi.org/10.1007/s12011-008-8230-8
Rashaid AB, Alqhazo M, Newbury DF, Kanaan H, El-khateeb M, Abukashabeh A, Al-Tamimi F. Evaluation of elements in hair samples of children with developmental language disorder (DLD). Nutr Neurosci, 2022; 0(0):1–10; https://doi.org/10.1080/1028415X.2021.2022068
Rashaid AHB, Nusair SD, Alqhazo MT, Adams JB, Abu-Dalo MA, Bashtawi MA. Heavy metals and trace elements in scalp hair samples of children with severe autism spectrum disorder: a case-control study on Jordanian children. J Trace Elem Med Biol, 2021; 67(May):126790; https://doi.org/10.1016/j.jtemb.2021.126790
Rodríguez-Barranco M, Lacasaña M, Gil F, Lorca A, Alguacil J, Rohlman DS, González-Alzaga B, Molina-Villalba I, Mendoza R, Aguilar-Garduño C. Cadmium exposure and neuropsychological development in school children in southwestern Spain. Environ Res, 2014; 134:66–73; https://doi.org/10.1016/j.envres.2014.06.026
Schwalfenberg GK, Genuis SJ. Vitamin D, essential minerals, and toxic elements: exploring interactions between nutrients and toxicants in clinical medicine. Sci World J, 2015; 2015; https://doi.org/10.1155/2015/318595
Sekirov I, Russell SL, Caetano M Antunes L, Finlay BB. Gut microbiota in health and disease. Physiol Rev, 2010; 90(3):859–904; https://doi.org/10.1152/physrev.00045.2009
Shantanam S, Mueller. Metal toxicity links to Alzheimer’s disease and neuroinflammation. Physiol Behav, 2018; 176(1):139–48; https://doi.org/10.1016/j.jmb.2019.01.018.Metal
Silver MK, Lozoff B, Meeker JD. Blood cadmium is elevated in iron deficient U.S. children: a cross-sectional study. Environ Health A Glob Access Sci Source, 2013; 12(1):1–9; https://doi.org/10.1186/1476-069X-12-117
Skogheim TS, Weyde KVF, Engel SM, Aase H, Surén P, Øie MG, Biele G, Reichborn-Kjennerud T, Caspersen IH, Hornig M, Haug LS, Villanger GD. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ Int, 2021; 152(February); https://doi.org/10.1016/j.envint.2021.106468
Talli I, Sprenger-Charolles L, Stavrakaki S. Specific language impairment and developmental dyslexia: what are the boundaries? Data from Greek children. Res Dev Disabil, 2016; https://doi.org/10.1016/j.ridd.2015.12.014
Van Der Lely HKJ. Domain-specific cognitive systems: insight from grammatical-SLI. Trends Cogn Sci, 2005; 9(2):53–9; https://doi.org/10.1016/j.tics.2004.12.002
Wang B, Du Y. Cadmium and its neurotoxic effects. Oxid Med Cell Longev, 2013; 2013; https://doi.org/10.1155/2013/898034
Whittaker P. 1998. Available via ron and zinc interactions in humans.pdf.
Wright JP, Dietrich KN, Ris MD, Hornung RW, Wessel SD, Lanphear BP, Ho M, Rae MN. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. PLoS Med, 2008; 5(5):0732–9; https://doi.org/10.1371/journal.pmed.0050101