INTRODUCTION
According to the World Health Organization, cancer is one of the costliest diseases for health systems worldwide and the second leading cause of death. Because of this disease, 10 million deaths were accounted for by 2020, with a high rate of occurrence in low and low-middle-income countries (WHO, 2022). Although current chemotherapy treatments are effective in combating cancer, they can cause various degrees of toxicity, including gastrointestinal and neurological disorders, as well as myelosuppression, among other side effects. These adverse effects often lead to dose limitation and even discontinuation of treatment (McQuade et al., 2014; Rejhová et al., 2018). As cancer statistics continue to rise and the associated toxicity of conventional chemotherapy becomes a greater concern, significant efforts are being made to explore and develop new pharmaceutical agents with chemopreventive potential for cancer.
Among the studies carried out, new alternative therapies against different types of cancer have been developed in the last decades by using natural compounds, their derivatives, and synthetic compounds—that exhibit high anticancer activity. For example, coumarins, hybrids, and conjugates based on this molecule and 5-Fluorouracil are good examples of compounds with anticancer properties (Al-Warhi et al., 2020; Cardona-G et al., 2021; Ramirez-Malule and Cardona-G, 2022). Indeed, several thousands of studies related to cancer treatment are issued every year due to the high rate of deaths caused by this disease. Therefore, it becomes a challenge to have a holistic view of all the studies on this topic due to the large volume of information available in the scientific literature.
Coumarins are a family of benzopyrones widely spread in nature, with reports that show beneficial effects on human health (Heghes et al., 2022). Thus, there are hundreds of studies related to coumarins and coumarin-derivative compounds, including reviews concerning anticancer activity, and structure–activity relationship on coumarin (Küpeli Akkol et al., 2020), the design and synthesis of photo-sensitive probes based on coumarin, and its application in analytical chemistry (Tian et al., 2020), coumarin-based near-infrared fluorogenic probes (Fan et al., 2023), the synthetic strategies to coumarins (Salem et al., 2018), among others. However, a bibliometric analysis of compounds based on coumarins is still lacking. Thus, the aim of this study was to identify and analyze the studies related to coumarins and coumarin hybrids with anticancer activities.
MATERIALS AND METHODS
Database selection and search strategy
Data search and collection were performed in the Scopus database since it is one of the largest databases of peer-reviewed literature worldwide, including medicine, pharmacology, and pharmaceutics. The search equation was designed to explore the studies related to coumarins and their anticancer activities. Thus, the terms “coumarins” and “anticancer activities” was joined by the Boolean operator “AND”. The resulting search equation was queried within the fields of the article title, abstract, and keywords, and the search was refined to articles and reviews (see Ramírez-Malule et al., 2020; Sanchez-Ledesma et al., 2023). The information was retrieved on February 27, 2023, and included the records since 1993.
Search equation: (TITLE-ABS-KEY (coumarin) AND TITLE-ABS-KEY (“anticancer activity”)) AND PUBYEAR > 1992 AND PUBYEAR < 2023 AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “re”)). Timespan: 1993 to 2022.
Data export and bibliometric indicators
The information retrieved for the records during the search were abstract, keywords, and both citation and bibliographical information. Retrieved data were downloaded from Scopus and exported in Excel® format. VOSviewer 1.6.18 was used for visualization and data analysis (van Eck and Waltman, 2010). Five was set as the minimum number of occurrences of a keyword. Forty-three keywords met the threshold after combining the thesaurus terms, leading to seven clusters. Furthermore, the volume and growth of publications, subject areas, co-occurrence keywords network visualization, co-occurrence keywords overlay visualization, top 10 leading institutions and cited papers, were the bibliometric indicators evaluated. Figure 1 shows a flowchart of the methodology used in this study.
RESULTS
Evolution of publishing papers related to coumarins with anticancer activities
A total of 458 records (85.15% and 14.85% research articles and reviews, respectively) related to coumarin compounds and their potential anticancer activity were retrieved from the Scopus database. Figure 2a shows the evolution of published papers related to this topic from 1993 to 2022. A clear increase in number of published papers per year was observed between 2008 and 2015. Then, the issued papers fluctuated around forty documents, excluding 2021 and 2022. The main areas of knowledge of those records were: (i) pharmacology, toxicology, and pharmaceutics, (ii) chemistry, and (iii) biochemistry, genetics, and molecular biology, with 31.4%, 24.0%, and 20.9% of the records, respectively.
Furthermore, institutions from sixty-five countries were involved in the 458 records, where India (32.8%), China (24.0%), and Egypt (12.2%), followed by the United States (7.9%), Saudi Arabia (7.0%), and Poland (4.4%) were the leading countries per number of publications (Fig. 2b). Nevertheless, if the ratio of citations to paper published is considered, Iran ranked first, followed by Italy and the United States (Table 1).
The top 10 institutions by number of publications are depicted in Table 2. Here, the Egyptian institutions ranked first followed by the Indian institutions. The scientific journals with the largest number of publications on the topic are Molecules (24 titles), European Journal of Medicinal Chemistry (23 titles), Anti-Cancer Agents in Medicinal Chemistry (12 titles), Bioorganic and Medicinal Chemistry Letters (11 titles), and Archiv der Pharmazie (10 titles). The 10 most cited titles are displayed in Table 3, from which, 7 titles are reviews and only 3 are research papers.
Bibliometric networks of coumarins with anticancer activities
Figure 3a shows the co-occurrence of the author’s keywords network, which contains seven clusters distinguishable by color-coded. Here, it is worth highlighting the synthesis of hybrid molecules based in coumarins with antibacterial and antiproliferative activity to treat cancer such as lung and breast cancer (green, yellow, red, and light blue clusters), in addition to molecular docking and drug delivery studies (purple, brown, and blue clusters). Besides, those specific topics were where the researchers focused between 2016 and 2020 (Fig. 3b) (43.67% of the records were published in this timespan). For example, studies of molecular docking and breast cancer of hybrid molecules based in coumarins were concentrated in 2019 and 2020 (see yellow nodes in Fig. 3b).
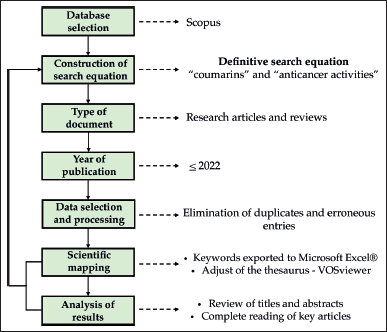 | Figure 1. Methodological flowchart for bibliometric analysis studies. [Click here to view] |
DISCUSSION
Anticancer activity of hybrids based on coumarins
Figure 4 depicts coumarin (1), which is the most basic structure in a vast group of phenolic compounds that consist of an α-pyrone ring fused with a benzene ring. Coumarins belong to a broad family of secondary metabolites that are present in various natural sources including plants, fungi, and microorganisms. However, a lot of coumarins are obtained by several synthetic methods, such as Pechmann reaction, Perkin, or Knoevenagel condensation, but it also sparked the discovery of completely new pathways such as Baylis–Hillman, Horner–Wadsworth-Emmons, Umpolung reaction (Annunziata et al., 2020). This class of compounds shows several pharmacological effects (Salem et al., 2018), including anticancer activity (Al-Warhi et al., 2020; Herrera-R et al., 2018).
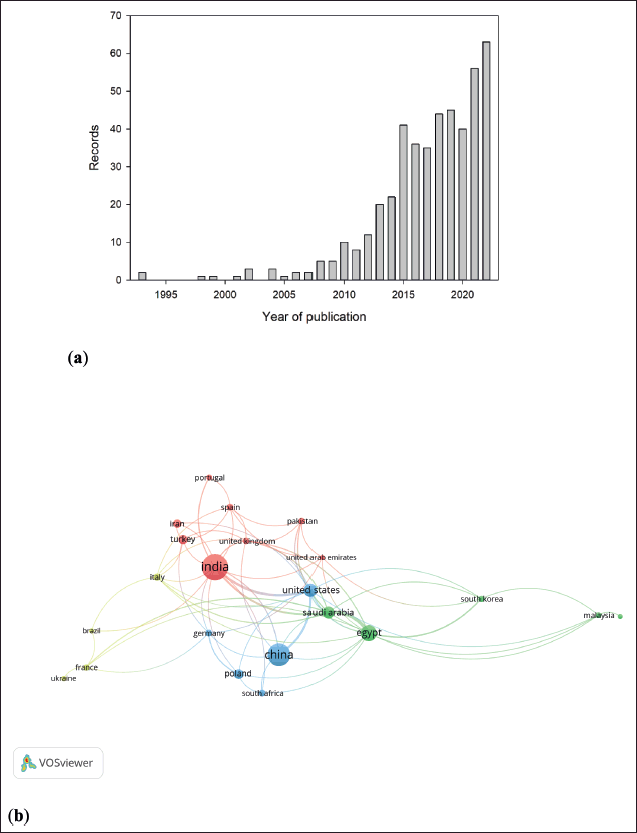 | Figure 2. Evolution of published studies related coumarins with anticancer activities and their countries’ collaboration network. (a) Evolution of published studies from 1993 to 2022. Source: Scopus database. (b) VOSviewer bibliometric map of global collaboration network among countries. [Click here to view] |
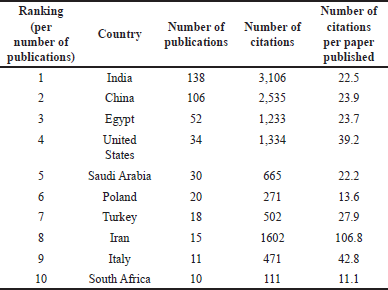 | Table 1. The number of total publications and citations for the top 10 leading countries in studies related to coumarins with anticancer activities between 1993 and 2022. Note: number of citations was retrieved on March 4, 2023. [Click here to view] |
Hybridization of the coumarin nucleus with other moieties (Fig. 4) has resulted in the development of new molecules with improved anticancer activity profiles (Kunal Nepali et al., 2014). Coumarin-pyrazoline hybrids (2) were active against HCT-116 colon cancer cells, with an IC50 of 0.01 µM. The compound with the highest cytotoxicity, 2, was found to have weak enzyme inhibitory activity against PI3K (p110α/p85α) (Amin et al., 2013). Another hybrid, compound (3), a coumarin-monastrol hybrid, inhibited the proliferation of MCF-7 (IC50 = 2.4 µM), T47D (IC50 = 3.1 µM), and MDA-MB-231 (IC50 = 3.9 µM) breast cancer cells (Sashidhara et al., 2013). Coumarin-isatin hybrid (4) was evaluated for its in vitro antitumor activities against various cancer cell lines (HepG2 liver carcinoma, Hela cervical cancer, A549 lung adenocarcinoma, DU145 prostatic cancer, SKOV3 ovarian carcinoma and MCF-7 breast cancer) showing IC50 values ranging from 21.47 to 44.26 µM (Diao et al., 2019). Benzimidazole-coumarin hybrid (5) was screened for in vitro antitumor activity on different cell lines and showed selective inhibition of leukemic cancer cell lines (Paul et al., 2013). Coumarin-chalcone hybrid (6) exhibited around 30-fold greater selectivity towards C33A cervical carcinoma cells over normal fibroblast NIH3T3 cells with an IC50 value of 3.59 μM (Sashidhara et al., 2010).
Stilbene-coumarin hybrids are an emerging class of compounds with promising anticancer activities. Among them, compounds (7) and (8) have shown excellent antiproliferative potency against lung carcinoma H460, with IC50 values of 0.45 and 0.29 µM, respectively (Belluti et al., 2010). Styrylcoumarin (9) has also been evaluated for its antitumor activity against MCF-7 and HCT-28 tumor cell lines, exhibiting varying degrees of growth inhibition with IC50 values of 7.32 and 3.78 µM, respectively (Shen et al., 2013). Compound (10), on the other hand, has demonstrated the highest activity with an IC50 value of 1.01 µM against SW480 cells, by inducing apoptosis through modulation of the tumor-suppressor protein p53. In vivo studies have also shown that this hybrid was able to inhibit the early progression of colon adenocarcinoma (Herrera-R et al., 2020). These findings highlight the potential of coumarin hybrids as potent anticancer agents, possibly by targeting multiple pathways involved in cancer cell growth and survival. The combination of the stilbene and coumarin moieties allows for a wider range of molecular interactions and improved pharmacokinetic properties, as demonstrated by the increased potency and selectivity of the hybrid molecules compared to their parent compounds. Further studies are needed to elucidate the mechanism of action of these compounds and to optimize their pharmacological properties for clinical use.
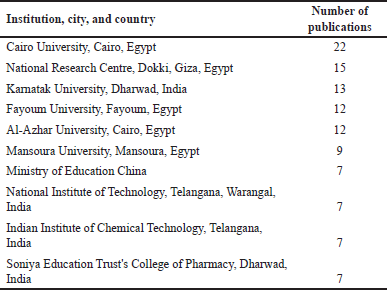 | Table 2. The number of total publications for the top 10 leading institutions in studies related to coumarins with anticancer activities between 1993 and 2022. Note: number of publications was retrieved on March 4, 2023. [Click here to view] |
Molecular docking as a tool for the comprehension of bioactivity molecular mechanisms
Molecular docking studies have emerged as a valuable tool in the identification and optimization of coumarin derivatives as potential anticancer agents. An important number of studies have employed molecular docking simulations to predict the binding modes of coumarin derivatives with various cancer targets as well as to get insights into the molecular mechanisms leading to biological responses observed in vitro. Some selected examples are the following mentioned. Molecular docking simulations were used to investigate the binding affinity of coumarin derivatives against the kinase domain of BCR-ABL, a protein kinase frequently associated with chronic myeloid leukemia. The results suggested that some coumarin derivatives had good binding affinities and could potentially inhibit the activity of BCR-ABL kinase, thus leading to apoptosis in cancer cells and an antiproliferative effect (Subramanian et al., 2017). The binding affinity of coumarin derivatives with the active site of Aurora A kinase, a serine/threonine kinase that is frequently overexpressed in cancer cells, has suggested good binding affinities, leading to antiproliferative effects on cancer cells (Hijjawi et al., 2020). Similarly, molecular docking simulations were used to explore the binding of coumarin derivatives to cyclin-dependent kinase 2 (CDK2), a key target in cancer therapy, showing that several coumarin derivatives had a strong binding affinity to CDK2 and in consequence, antiproliferative activity against cancer cells (Al-Wahaibi et al., 2018). Binding interactions of coumarin derivatives with the active site of topoisomerase IIα, a key enzyme involved in DNA replication and transcription that is frequently overexpressed in cancer cells have been also investigated, revealing high binding affinity, thus potentially acting as topoisomerase inhibitors with anticancer activity (Gomaa et al., 2022).
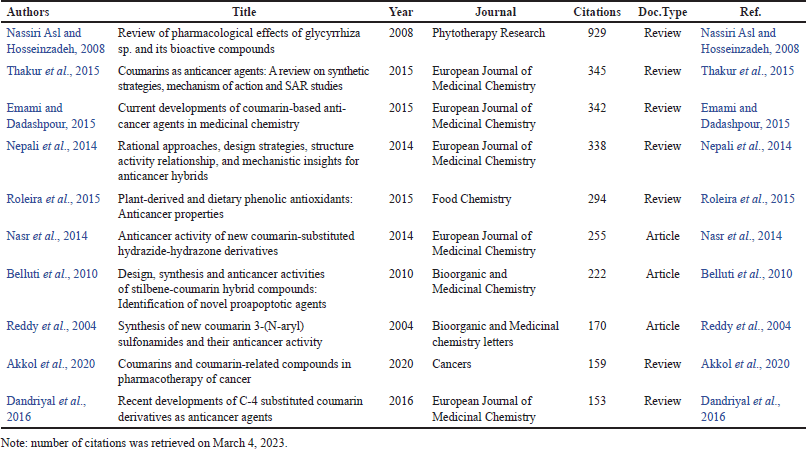 | Table 3. The number of citations for the top 10 leading papers in studies related to coumarins with anticancer activities between 1993 and 2022. [Click here to view] |
Abolibda et al. (2023) evaluated the potential of novel coumarins derivatives as vascular endothelial growth factor receptor (VEGFR-2) inhibitors for the treatment of breast cancer, indicating comparable activities to that of Sorafenib as a reference drug. Triazole-coumarin-glycosyl hybrids and tetrazole hybrid analogs were synthesized, tested, and evaluated via molecular docking for the determination of binding affinity and targeted enzymes. Inhibitory activity against the epidermal growth factor receptor (EGFR), VEGFR-2, and CDK-2/cyclin A2 kinases was compared to reference drugs (El-Sayed et al., 2022). A novel set of the murine double minute (MDM2) inhibitors targeting breast cancer was designed and synthesized using the coumarin ring as a central scaffold and molecular docking study confirmed the interaction of derivative with the key amino acids in the p53 binding pocket of MDM2 protein, inducing down-regulation of MDM2 and anti-apoptosis proteins and up-regulation of p53 and pro-apoptosis proteins leading to cell cycle arrest and induction of apoptosis (Ahmed et al., 2022). A series of coumarin derivatives were synthesized, tested, and assessed via molecular modeling to evaluate the binding affinity and pattern of compounds for antiproliferative and antibacterial activities. The synthesized compounds were found to inhibit VEGFR-2, reduce the migratory potential in human umbilical vein endothelial cells, and induce apoptosis and cell cycle arrest in leukemia cell line HL-60(TB), in addition to antibacterial activity against Kleibsella pneumoniae and inhibited Gram-negative DNA gyrase (Emam et al., 2023).
Drug delivery of compounds based on coumarins
Absorption, Distribution, Metabolism, and Excretion (ADME) studies are crucial for the development of new drug candidates. ADME studies of coumarin derivatives have shown promising pharmacokinetic properties, such as good oral bioavailability, low toxicity, and good metabolic stability. Various drug delivery systems, such as nanoparticles, liposomes, and dendrimers, have been developed to enhance the delivery and bioavailability of coumarin derivatives, as well as to target specific cells or tissues. However, the pharmacokinetic properties of coumarin derivatives can vary depending on their chemical structure, solubility, and formulation, which need to be carefully optimized to maximize their therapeutic potential. Lee et al. (2018) studied the aggregation behavior of a triphenylphosphonium-appended coumarin probe (TPP-C) in (i) pure water and (ii) mixtures of organic solvents and water, and its applications as both mitochondria-targeting probe and drug delivery carrier system. The homogeneous nanoparticles induced J-aggregation and H-aggregation in pure water and mixed solvents, respectively. Interestingly, TPP-C did not show any cytotoxicities in cancer cells and also displayed strong fluorescence caused by the aggregation-induced emission effect with J-aggregation in an aqueous solution. The above is a key aspect since lets visual monitoring and tracking of the drug delivery process, e.g., the anticancer drug doxorubicin (DOX). In addition, the encapsulation of DOX into the TPP-C nanoparticles was evaluated. The authors reported that TPP-C and TPP-C-DOX nanoparticles were selectively accumulated by mitochondria and efficiently delivered to the mitochondria, respectively. Arjmand et al. (2021) also used DOX to design a drug delivery system. The authors developed and synthesized photolabile nanoparticles for the active delivery of an anticancer drug (DOX) with effective light-regulated control over DOX release. Furthermore, Rahimi et al. (2018) designed and synthesized ultraviolet- and pH-responsive iminocoumarin-chitosan nanoparticles via an oil-in-water emulsion system. The authors used the 8-formyl-7-hydroxy-4-methyl coumarin, as a crosslinking agent. The results indicated that chitosan nanoparticles crosslinked by coumarin demonstrate promise as drug nanocarriers for designing drug delivery systems. López et al. (2022) synthesized and characterized two amphiphilic polymer-drug conjugates that combine poly(ethylene glycol) with compounds with anticancer properties such as coumarin and 5-fluorouracil. The conjugates obtained were capable to self-assemble into micelles at high micellar concentrations. In addition, the micelles also permitted the loading of paclitaxel, curcumin, and gemcitabine, indicating a promising co-delivery carrier for synergistic cancer therapy. Specifically, the paclitaxel and curcumin were efficiently loaded, reporting well-controlled in vitro drug release profiles. The authors indicated that the strategy reported is promising for the development of co-delivery carriers for synergistic cancer therapy.
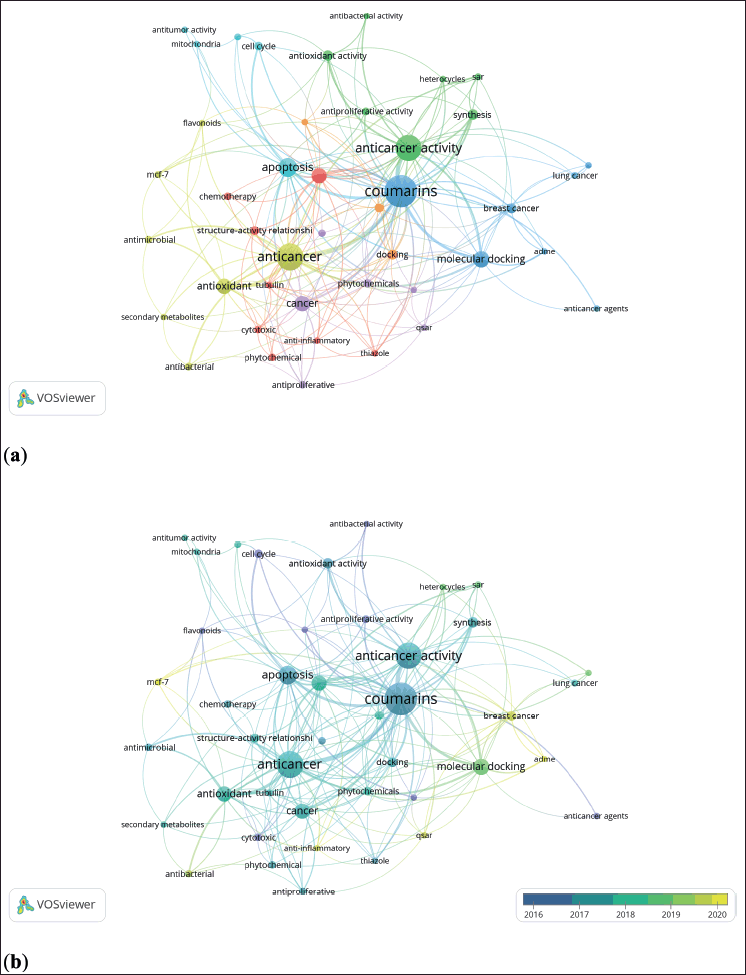 | Figure 3. Bibliometric network of studies related to coumarins with anticancer activities. (a) Research-topic map. (b) Research-topic map with time overlap. Note: minimum number of occurrences of a keyword is five. [Click here to view] |
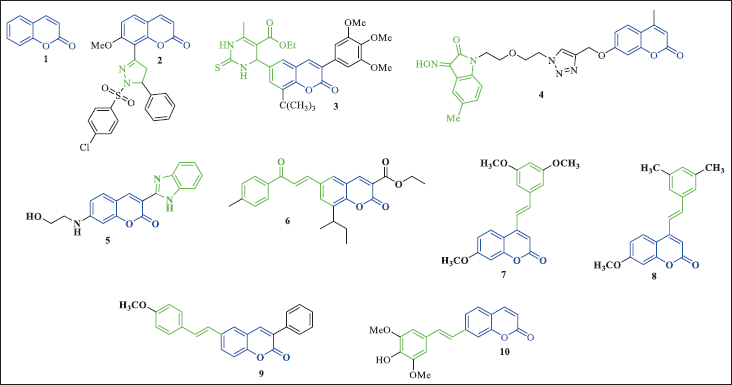 | Figure 4. Coumarin-based compounds. [Click here to view] |
CONCLUSION
The bibliometric networks showed seven clusters, which identified thematic groups where coumarins and coumarins derivatives are key aspects. A clear increase in studies related to coumarins was observed between 2008 and 2022, with (i) pharmacology, toxicology, and pharmaceutics, (ii) chemistry, and (iii) biochemistry, genetics, and molecular biology, as the main areas of this topic. China, India, and Egypt have been the most productive countries in studies related to coumarins and had the strongest collaboration network. However, when the ratio of citations to the number of papers published is considered, Iran, Italy, and the United States were the leading countries. In other words, the studies published from the latter mentioned countries researchers have had the highest academic impact and served as references for other studies that found them pertinent within this field. Synthesis of coumarins hybrids, experimental studies in vitro and in vivo and molecular docking studies emerge as the areas with the most intense research activity about coumarin derivatives and their anticancer properties. The coumarin hybrids have shown potent cytotoxic and antiproliferative activities against various cancer cell lines, and their inhibitory effects on key enzymes such as EGFR, VEGFR-2, and CDK-2/cyclin A2 have been superior to the reference drugs. Furthermore, some of the derivatives are able to induce apoptosis, downregulate anti-apoptotic proteins, and upregulate pro-apoptotic proteins. Molecular docking studies provided insight into the binding affinity between the promising derivatives and their targeted enzymes, supporting their potential as lead compounds for further anticancer drug development. ADME studies displayed promising pharmacokinetic properties, making them suitable candidates for drug delivery.
AUTHOR CONTRIBUTIONS
Conceptualization, W.C.-G. and H.R.; methodology, H.R. and D.G; software, H.R.; validation, W.C.-G, H.R., and D.G.; formal analysis, W.C.-G, H.R., and D.G.; investigation, W.C.-G, H.R., and D.G.; data curation, H.R. and D.G.; writing–original draft preparation, review, and editing, W.C.-G, H.R., and D.G.; visualization, project administration, W.C.-G; funding acquisition, W.C.-G. All authors have read and agreed to the published version of the manuscript.
FINANCIAL SUPPORT
This research was funded by the University of Antioquia and the Ministry of Science MINCIENCIAS, through the Program: NanoBioCáncer 2.0 GAT 2.0. Código: 121092092332, grant: 621-2022, project number 92355.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abolibda TZ, Fathalla M, Farag B, Zaki MEA, Gomha SM. Synthesis and Molecular Docking of Some Novel 3-Thiazolyl-Coumarins as Inhibitors of VEGFR-2 Kinase. Molecules, 2023; 28:001–18. CrossRef
Ahmed EY, Abdel Latif NA, Nasr T, Awad HM, Abdelhafez OM. Design, synthesis, and molecular modeling of coumarin derivatives as MDM2 inhibitors targeting breast cancer. Chem Biol Drug Des, 2022; 99:609–19. CrossRef
Akkol EK, Genç Y, Karpuz B, Sobarzo-Sánchez E, Capasso R. Coumarins and coumarin-related com-pounds in pharmacotherapy of cancer. Cancers, 2020; 12:001–25. CrossRef
Al-Wahaibi LH, Abu-Melha HM, Ibrahim DA. Synthesis of novel 1,2,4-triazolyl coumarin derivatives as potential anticancer agents. J Chem, 2018; 5201374:001–8. CrossRef
Al-Warhi T, Sabt A, Elkaeed EB, Eldehna WM. Recent advancements of coumarin-based anticancer agents: an up-to-date review. Bioorg Chem, 2020; 103(104163):001–15.
Amin KM, Eissa AAM, Abou-Seri SM, Awadallah FM, Hassan GS. Synthesis and biological evaluation of novel coumarin–pyrazoline hybrids endowed with phenylsulfonyl moiety as antitumor agents. Eur J Med Chem, 2013; 60:187–98. CrossRef
Annunziata F, Pinna C, Dallavalle S, Tamborini L, Pinto A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int J Mol Sci, 2020; 21(4618):001–81. CrossRef
Arjmand F, Salami-Kalajahi M, Roghani-Mamaqani H. Preparation of photolabile nanoparticles by coumarin-based crosslinker for drug delivery under light irradiation. J Phys Chem Solids. 2021; 154(110102):001–10. CrossRef
Belluti Federica, Fontana G, Bo LD, Carenini N, Giommarelli C, Zunino F. Design, synthesis and anti-cancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapoptotic agents. Bioorg Med Chem, 2010; 18:3543–50. CrossRef
Cardona-G W, Herrera-R A, Castrillón-L W, Ramírez-Malule H. Chemistry and anticancer activity of hybrid molecules and derivatives based on 5-fluorouracil. Curr Med Chem, 2021; 28(27):5551–601. CrossRef
Dandriyal J, Singla R, Kumar M, Jaitak V. Recent developments of C-4 substituted coumarin derivatives as anticancer agents. Eur J Med Chem, 2016; 119:141–68. CrossRef
Diao Q, Guo H, Wang G. Design, synthesis, and In Vitro anticancer activities of diethylene glycol tethered Isatin-1,2,3-triazole-coumarin Hybrids. J Heterocycl Chem. 2019;56:1667–1671. CrossRef
van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics, 2010; 84:523–38. CrossRef
El-Sayed WA, Alminderej FM, Mounier MM, Nossier ES, Saleh SM, Kassem AF. Novel 1,2,3-triazole-coumarin hybrid glycosides and their tetrazolyl analogues: design, anticancer evaluation and molecular docking targeting EGFR, VEGFR-2 and CDK-2. Molecules, 2022; 27(2047):001–27. CrossRef
Emam SH, Hassan RA, Osman EO, Hamed MIA, Abdou AM, Kandil MM. Coumarin derivatives with potential anticancer and antibacterial activity: design, synthesis, VEGFR-2 and DNA gyrase inhibition, and in silico studies. Drug Dev Res, 2023; 84:433–57. CrossRef
Emami S, Dadashpour S. Current developments of coumarin-based anti-cancer agents in medicinal chemistry. Eur J Med Chem, 2015; 102:611–30. CrossRef
Fan Y, Wu Y, Hou J, Wang P, Peng X, Ge G. Coumarin-based near-infrared fluorogenic probes: Recent advances, challenges and future perspectives. Coord Chem Rev, 2023; 480(215020):001–17. CrossRef
Gomaa MS, Ali IAI, el Enany G, el Ashry ESH, el Rayes SM, Fathalla W. Facile sythesis of some coumarin derivatives and their cytotoxicity through VEGFR2 and topoisomerase II inhibition. Molecules, 2022; 27(8279):001–16. CrossRef
Heghes SC, Vostinaru O, Mogosan C, Miere D, Iuga CA, Filip L. Safety profile of nutraceuticals rich in coumarins: an update. Front Pharmacol, 2022; 13:001–9. CrossRef
Herrera-RA, Castrillón W, Otero E, Ruiz E, Carda M, Agut R. Synthesis and antiproliferative activity of 3- and 7-styrylcoumarins. Med Chem Res, 2018; 27:1893–905. CrossRef
Herrera-RA, Naranjo TW, Maldonado ME, Moreno-QG, Yepes A, Cardona-G W. Styrylcoumarin 7-SC2 induces apoptosis in SW480 human colon adenocarcinoma cells and inhibits azoxymethane-induced aberrant crypt foci formation in BALB/c mice. Med Chem Res, 2020; 29:377–95. CrossRef
Hijjawi MS, Abutayeh RF, Taha MO. Structure-based discovery and bioactivity evaluation of novel aurora-a kinase inhibitors as anticancer agents via docking-based comparative intermolecular contacts analysis (dbCICA). Molecules, 2020; 25(6003):001–29. CrossRef
Küpeli Akkol E, Genç Y, Karpuz B, Sobarzo-Sánchez E, Capasso R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers, 2020; 12(1959):001–025. CrossRef
Lee JH, Kim KY, Jin H, Baek YE, Choi Y, Jung SH, Lee SS, Bae J, Jung JH. Self-assembled coumarin nanoparticle in aqueous solution as selective mitochondrial-targeting drug delivery system. ACS Appl Mater Interfaces, 2018; 10:3380–91.
López S, Rodríguez-López J, García MT, Rodríguez JF, Pérez-Ortiz JM, Ramos MJ, Ramos MJ, Gracia I. Self-assembled coumarin- and 5-fluorouracil-PEG micelles as multifunctional drug delivery systems. J Drug Deliv Sci Technol, 2022; 74(103582):001–12. CrossRef
McQuade RM, Bornstein JC, Nurgali K. Anti-colorectal cancer chemotherapy-induced diarrhoea: current treatments and side-effects. Int J Clin Med, 2014; 05:393–406. CrossRef
Nasr T, Bondock S, Youns M. Anticancer activity of new coumarin substituted hydrazide-hydrazone derivatives. Eur J Med Chem, 2014; 76:539–48. CrossRef
Nassiri Asl M, Hosseinzadeh H. Review of pharmacological effects of glycyrrhiza sp. and its bioactive compounds. Phytother Res, 2008; 22:709–24. CrossRef
Nepali Kunal, Sharma S, Sharma M, Bedi PMS, Dhar KL. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur J Med Chem, 2014; 77:422–87. CrossRef
Paul K, Bindal S, Luxami V. Synthesis of new conjugated coumarin–benzimidazole hybrids and their anticancer activity. Bioorg Med Chem Lett, 2013; 23:3667–72. CrossRef
Rahimi S, Khoee S, Ghandi M. Development of photo and pH dual crosslinked coumarin-containing chitosan nanoparticles for controlled drug release. Carbohydr Polym, 2018; 201:236–45. CrossRef
Ramirez-Malule H, Cardona-G W. Bibliometric analysis of recent research on 5-Fluorouracil (2015–2020). J Appl Pharm Sci, 2022; 12:070–7.
Ramírez-Malule H, Quiñones-Murillo DH, Manotas-Duque D. Emerging contaminants as global environmental hazards. A bibliometric analysis. Emerg Contam. 2020; 6:179–93. CrossRef
Reddy NS, Mallireddigari MR, Cosenza S, Gumireddy K, Bell SC, Reddy EP, Reddy MR. Synthesis of new coumarin 3-(N-aryl) sulfonamides and their anticancer activity. Bioorg Med Chem Lett, 2004; 14:4093–7. CrossRef
Rejhová A, Opattová A, ?umová A, Slíva D, Vodi?ka P. Natural compounds and combination therapy in colorectal cancer treatment. Eur J Med Chem, 2018; 144:582–94. CrossRef
Roleira FMF, Tavares-Da-Silva EJ, Varela CL, Costa SC, Silva T, Garrido J, Borges F. Plant derived and die-tary phenolic antioxidants: anticancer properties. Food Chem, 2015; 183:235–58. CrossRef
Salem MA, Helal MH, Gouda MA, Ammar YA, El-Gaby MSA, Abbas SY. An overview on synthetic strategies to coumarins. Synth Commun. 2018; 48:1534–50.
Sanchez-Ledesma LM, Ramírez-Malule H, Rodríguez-Victoria JA. Volatile fatty acids production by acidogenic fermentation of wastewater: a Bibliometric analysis. Sustainability, 2023; 15(2370):001–24. CrossRef
Sashidhara K V., Avula SR, Sharma K, Palnati GR, Bathula SR. Discovery of coumarin–monastrol hy-brid as potential antibreast tumor-specific agent. Eur J Med Chem, 2013; 60:120?7. CrossRef
Sashidhara K V., Kumar A, Kumar M, Sarkar J, Sinha S. Synthesis and in vitro evaluation of novel coumarin–chalcone hybrids as potential anticancer agents. Bioorg Med Chem Lett, 2010; 20:7205–11. CrossRef
Shen W, Mao J, Sun J, Sun M, Zhang C. Synthesis and biological evaluation of resveratrol-coumarin hybrid compounds as potential antitumor agents. Med Chem Res, 2013; 22:1630?40. CrossRef
Subramanian V, Muhammad SA, Sundaram K, Ravi S. Novel thiadiazole derivatives as Bcr-Abl tyrosine kinase inhibitors. J Appl Pharm Sci, 2017; 7:068–76.
Thakur A, Singla R, Jaitak V. Coumarins as anticancer agents: a review on synthetic strategies, mecha-nism of action and SAR studies. Eur J Med Chem. 2015; 101:476–95. CrossRef
Tian G, Zhang Z, Li H, Li D, Wang X, Qin C. Design, synthesis and application in analytical chemis-try of photo-sensitive probes based on coumarin. Crit Rev Anal Chem, 2020; 51:001–17. CrossRef
World Health Organization. 2022. Cancer. [Online] Available via https://www.who.int/news-room/fact-sheets/detail/cancer (Accessed 28 February 2023).