INTRODUCTION
Medicinal plants have efficacy as medicine in healing and preventing disease. One of the plants used by the people of West Sumatra, Indonesia, as medicine, is myrtle (Rhodomyrtus tomentosa). Traditionally, this plant has been used as a medicine to treat wounds, scabies, headaches, stomachaches, and diarrhea, preventing bleeding and infection after childbirth (WHO, 1989). Based on previous research, the R. tomentosa extract has a potent growth-inhibiting effect against pathogenic bacteria such as Escherichia coli, Staphylococcus aureus, Shigella dysenteriae, and Salmonella typhi (Dachryanus et al., 2002; Salni et al., 2002; Salni and Marisa, 2019; Sinulingga et al., 2018).
Some microorganisms, such as bacteria and fungi, live as endophytes and inhabit the interior of plants. According to Petrini (1991), they colonize healthy tissue at some point in their life cycle without causing significant damage. Endophytic fungi that inhabit healthy living tissue without causing side effects also produce certain biological activities of secondary metabolites structurally unique (Strobel, 2003). To colonize the host and nutrition, it can synthesize metabolites for competence with co-occurring endophytes, hosts, and pathogens (Strobel and Daisy, 2003). There are approximately 1.5 million species of fungi on Earth, 1 million of which are endophytic. They primarily inhabit the interiors of plants’ stems, leaves, flowers, and seeds, which can be transferred in horizontal or vertical directions (Bacon and White, 2000; Hartley and Gange, 2009). They produce potent antifungal, antiviral, antibacterial, cytotoxic, and immunosuppressive metabolisms (Ruma et al., 2013).
This research continues our investigation of natural products from several mangrove plants and marine sponges (Artasasta et al., 2017; Aminah et al., 2020; Handayani et al., 2016, 2018, 2019, 2020a, 2021; Handayani and Aminah, 2017; Handayani and Artasasta, 2017; Sandrawati et al., 2020). From the marine sponge-derived fungus Cochliobolus geniculatus WR12, radicinin was obtained. This compound shows cytotoxic activity with IC50 values of 60.68; 30.89; and 87.89 μg/ml against WiDr, T47D, and Hela cells but is not toxic to Vero cells (IC50 value of 607.31 μg/ml). Radicinin showed higher cytotoxic activity against T47D cell cultures (IC50 = 25.01 ppm) than doxorubicin (IC50 = 33.49 ppm). This compound showed a minimum inhibitory concentration (MIC) of 125 μg/disc against methicillin-resistant S. aureus (Handayani et al., 2020b). From fungus Fusarium proliferatum AED3, derived from the mangrove plant Ardisia elliptica, beauvericin was obtained. The cytotoxic activity of the compounds was tested using the 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide test on T47D cancer cells with an IC50 value of 112.2 μg/ml (Handayani et al., 2021).
In this study, we isolated endophytic fungi from the medicinal plant R. tomentosa and evaluated their potential for antimicrobial activity. Based on literature studies, research related to the endophytic fungi of R. tomentosa or the antimicrobial potential of this microorganism is still very limited.
MATERIALS AND METHODS
Identification of sample material
The material used was ±100 g of leaves, bark, and roots of R. tomentosa obtained from the Bukik Nago Sungkai, Lambung Bukit Village, Padang, West Sumatra, Indonesia, (Geographic coordinates: °56’57.3’’ S 100°21’15.4’’ E). Plant identification was carried out at the Andalas University Herbarium, Department of Biology, Faculty of Mathematics and Natural Sciences, Andalas University, Padang, West Sumatra, Indonesia. The letter number for plant authentication was 119/K-ID/ANDA/III/2021, and the voucher number was HF0012021.
Isolation of endophytic fungi from leaves, bark, and roots of R. tomentosa
The surfaces of the leaves, bark, and roots were surface-sterilized by rinsing under running water for 10 minutes, followed dipped in 70% ethanol for 1–2 minutes. The samples were then rinsed again with ethanol 70% for 30 seconds after being soaked in bleach solvent (NaOCl 5.3%) for 5 minutes. To stop the sterilization with EtOH, dry the tissue with a sterile cotton cloth or rinse it with sterile artificial seawater (Kjer et al., 2010).
Isolation by direct planting
Cleavage surface attached to the agar medium. Incubation was carried out at 25°C (room temperature) for 3 days. The agar dish was labeled, parafilm-sealed, and stored at 20°C–25°C. Cultures are maintained at 20°C–25°C under daylight. 2–3 days after the experiment’s start, fungal growth from the cut segments starts and lasts until 14 days later (Kjer et al., 2010).
Isolation by pouring method
The sample was dispersed in aqua dest sterile for about 10 g until the volume on Erlenmeyer reached 100 ml. The mixture was then diluted until it contained 10−6 ml. One milliliter of the sample was poured aseptically onto sabouraud dextrose agar (SDA) medium in the Petri dish. The plate was incubated at 25°C–27°C for 5–7 days (Kjer et al., 2010).
Pure fungi isolate cultivation in rice medium
Pure Fungi isolate in a Petri dish was sliced 1 × 1 cm, then cultured in rice medium and incubated for 4–6 weeks at room temperature. Maximum growth of the fungi occurs when the rice is completely covered by the fungi isolate (Kjer et al., 2010).
Extraction of the secondary metabolites
Pure fungus isolates that have been further cultivated were extracted by maceration with ethyl acetate (EtOAc) at a ratio of 1:1 for 24 hours and evaporated using a rotary evaporator to produce fungus extract. The extract is used for antimicrobial screening (Kjer et al., 2010).
Screening for antimicrobial activity
The disk diffusion method was used to screen the EtOAc extracts of endophytic fungi for antimicrobial activity against several pathogenic microbes: S. aureus the American Type Culture Collection (ATCC) 25923, E. coli ATCC25922, and clinical isolate Candida albicans. Nutrient Agar (Merck®) and SDA (Merck®) containing a microbial suspension were added to Petri dishes (0.5 and 0.7 McFarland for bacteria and fungus, respectively). Positive controls against bacterial and fungal pathogens were performed using chloramphenicol (Oxoid®) and nystatin disk. A paper disk was used as a negative control containing 20 μl of dimethyl sulfoxide (DMSO). The plate of fungi was incubated at 25°C–27°C for 48 hours and 37°C for 24 hours for bacteria. The extract was diluted to a concentration of 5% with DMSO. The clear zone on the disk indicates the fungal extract’s bacterial and fungal inhibition activity (Bauer et al., 1959). Testing for antimicrobial activity was done in triplicates. For each fungus extract with an inhibition zone larger than 10 mm, the mean diameter of the inhibition zone and the standard deviation were computed.
Determination of the secondary metabolite content
EtOAc extracts of endophytic fungi with an inhibition zone >10 mm were screened for secondary metabolites. As previously described, the phytochemical examination was conducted to determine the extract’s terpenoid, alkaloid, phenolic, and steroid content (Handayani et al., 2019; Tiwari et al., 2011)
Microscopic examination
Endophytic fungi showing antibacterial activity were identified using a microscope with 2000 × magnification. The fungus specimen was placed on an object glass. Then lactophenol was drizzled and combined. Glass cover was placed over the specimen. The morphology of the specimens was examined after the results of molecular identification were obtained.
Molecular identification
DNA extraction
Three endophytic fungal strains (RTKB3, RTKB4, and RTKB7) with the most significant antibacterial activity were identified using DNA amplification and sequencing of the internal transcribed spacer (ITS) region, as per the molecular biological protocol. The DNA extraction was performed using a modified Atashpaz et al. (2010) approach. After the fungus had grown for 24 hours in the SDA medium, its cell mass was moved to the microtube with 500 μl of lysis buffer. At 4°C and 18,000 rpm, the mixture was spun in a centrifuge for 5 minutes. The filtrate was removed, and 50 μl of TE buffer pH 8.0 was used to dissolve the DNA in the pellet.
Amplification by polymerase chain reactions (PCRs) and the sequence of the ITS-coding gene
A KOD FX Neo machine from Toyobo, Japan, was employed for PCR amplification. ITS1 (F 5’- TCC GTA GGT GAA CCT GCG G-3’) and primer ITS4 (R 5’- TCC TCC GCT TAT TGA TAT GC-3’) were used in this PCR, which was conducted following the PCR master mix protocol. The following is the PCR amplification reaction: One cycle at 95°C for 5 minutes, then 35 cycles with steps of denaturation at 95°C for 30 seconds, annealing at 55°C for 1 minute, and extension at 72°C for 1 minute, followed by one cycle at 72°C for 6 minutes, were used in the reaction (Ferrer et al., 2001). The products were sent to Malaysia. The DNA sequences were aligned pairwise on the National Center for Biotechnology Information (NCBI) and MycoBank databases using Basic Local Alignment Search Tool (BLAST). The phylogenetic tree was created in Molecular Evolutionary Genetics Analysis 7.0 using the neighbor-joining method and a bootstrap value of 1,000 replications (Kumar et al., 2016).
RESULT AND DISCUSSION
Rhodomyrtus tomentosa plants belong to the Myrtaceae family. It is a kind of wild plant with woody trees. In the open plains, they are almost as tall as adults (they can get up to 4 m). The leaves are rigid, about 5–7 cm long and 2–3.5 broad cm, oval in shape, the tip from blunt to sharp, at the top glossy green and grayer below. The leaves can be seen in Figure 1a. The flowers are single or in groups (clusters) of 2–3 flowers, 2.5–3 cm in diameter, pink to purple with many stamens and odorless. The flower of R. tomentosa can be seen in Figure 1b. The fruit is an oval-shaped berry, about 1–1.5 cm long and about 1 cm wide, with persistent calyx. The young fruit is green and tastes tart (bitter), while the ripe fruit is red–purple to black and tastes sweet—hairy fruit skin like velvet. A persistent calyx is at the top of the fruit (Fig. 1c) (Burkill, 1993).
Thirteen fungal isolates were obtained from R. tomentosa (Aiton) Hassk based on their different morphological characteristics and coded according to the organ of their host plant (coded as RTA1-3, RTKB1-7, and RTD1) (Fig. 2). The weight of EtOAc extract ranged from 1 to 6.2 g (Table 1). The antimicrobial activity of EtOAc extracts of individual fungal isolates was tested. The results of antibacterial activity screening of fungus extract with a concentration of 5% against S. aureus, E. coli, and C albicans are presented in Table 2. The EtOAc extract that had the highest antibacterial activity against the growth of S. aureus and E. coli was RTKB7 isolate with an average inhibition zone diameter of 19.90 ± 0.34 and 18.61 ± 0.87 mm, respectively. The RTKB3 isolate had an average inhibition zone diameter of 11.29 ± 1.17 and 10.69 ± 0.56 mm. In contrast, the RTKB4 isolate had an average inhibition zone diameter of 9.71 ± 0.56 and 10.15 ± 0.61 mm (Table 3, Fig. 3). According to the diameter of the zone inhibition, Davis and Stout (1971) divided inhibition strength into four categories: very strong (20 mm), strong (10–20 mm), medium (6–10 mm), and weak (6 mm). All extracts tested negative for antifungal activity against C. albicans. It is suspected that this is due to the inability of the EtOAc extract to penetrate the cell wall of the C. albicans because this fungus has a complex cell wall, 100–400 nm thick. The sterol membrane in the cell wall plays a vital role as an antifungal target (Brunton, 2006). Thus, endophytic fungi extracts generally have a small or negative inhibition zone against C. albicans.
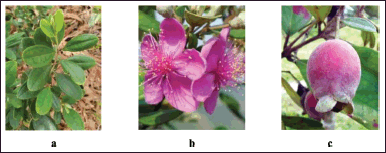 | Figure 1. The pictures of (a) leaves, (b) flowers, and (c) fruit of R. tomentosa (Aiton) Hassk. [Click here to view] |
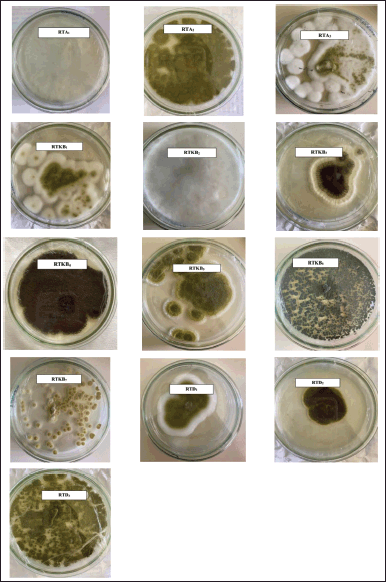 | Figure 2. The pictures of isolated fungal endophytes from R. tomentosa (Aiton) Hassk growing on SDA. [Click here to view] |
Based on the test results for the content of secondary metabolites, the EtOAc extract of selected fungus from the stem bark of R. tomentosa (Aiton Hassk) contains flavonoid, steroid, and terpenoid (Table 4). Furthermore, fungus isolates of RTKB3, RTKB4, and RTKB7, which have antibacterial activity, were identified macroscopically, microscopically, and molecularly. The identification of fungi requires both morphological and molecular methods. Morphologically, fungi were classified according to their colony, color, spore size, texture, shape, growth rate, and conidial attachment (Domsch et al., 2007). The fungus RTKB3 had brown, rough, round conidia ranging in size from 3.5–4 μm and an inconspicuous white mycelium at the colony’s edge (Fig. 2). The fungus isolate of RTKB4 microscopically had round-shaped vesicles with diameters ranging from 17.52 to 23.4 μm. The conidia are globose with a diameter ranging from 3.5 to 4.5 μm. The conidiophores are long, cylindrical, and colorless (hyaline) (Fig. 4).
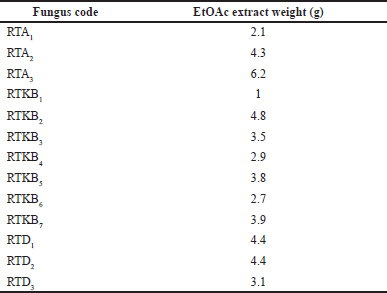 | Table 1. EtOAc extract weight (g) of endophytic fungi from R. tomentosa (Aiton) Hassk. [Click here to view] |
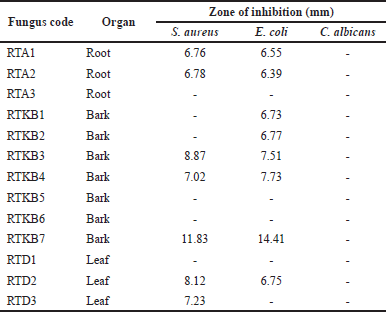 | Table 2. Antimicrobial activity of endophytic fungi from R. tomentosa (Aiton) Hassk against pathogenic microbes. [Click here to view] |
 | Table 3. Antibacterial activity of fungus isolate RTKB3, RTKB4, and RTKB7. [Click here to view] |
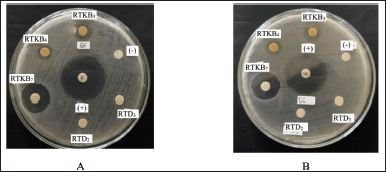 | Figure 3. The agar plate pictures of the inhibition zone of fungal isolates RTKB3, RTKB4, and RTKB7 extract against the growth of (A) S. aureus and (B) E. coli. [Click here to view] |
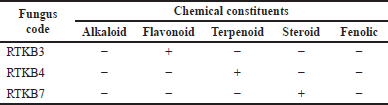 | Table 4. Phytochemical screening of EtOAc extracts of RTKB3, RTKB4, and RTKB7. [Click here to view] |
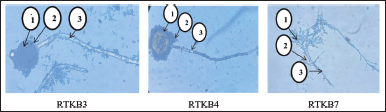 | Figure 4. The microscopic pictures of fungal isolate RTKB3 (1. Vesicle, 2. Conidia, 3. Conidiophore), RTKB4 (1. Vesicle, 2. Conidia, 3. Conidiophore), and RTKB7 (1. Conidia, 2. Sterigmata 3. Conidiophore) [Click here to view] |
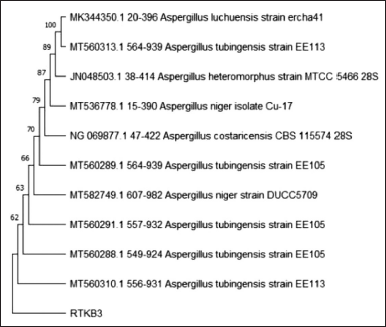 | Figure 5. The phylogenetic tree inferred using the neighbor-joining method of ITS sequence of fungus RTKB3 derived from R. tomentosa (Aiton) Hassk and its allied taxa. [Click here to view] |
The fungus RTKB7 has oval-shaped conidia of varying sizes, septate hyphae, branched conidiophores, and fialids at the ends. Some of them have chlamydospores (Fig. 4) (Domsch et al., 2007; Samson et al., 1988).
The molecular identification was performed at the Indonesian Center for Biodiversity and Biotechnology. A BLAST scan in NCBI-GenBank and MycoBank revealed that isolate RTKB3 and Aspergillus heteromorphus strain MTCC 5466 28S have a 99.25 percent identity. Isolate RTKB4 and RTKB7 were 100% identic with Aspergillus niger isolate DGGE gel band def2 and Paecilomyces subglobosus strain Centraalbureau Voor Schimmelcultures 125145, respectively (Figs. 5–7). The sequence data of RTKB3, RTKB4, and RTKB7 were submitted to GenBank with accession no OQ592160, OQ592159, and OQ592158, respectively. Microscopic observations of fungi have been carried out to complete the results of molecular identification.
Endophytic fungi were reported can produce secondary metabolites of various groups, such as aliphatic metabolites, alkaloids, flavonoids, glycosides, lactones, phenyl propanoids, quinones, steroids, terpenoids, and xanthones (Zhang et al., 2006). Aspergillus heteromorphus has been reported to produce gallic acid with bioactivities such as antibacterial, antiviral, analgesic, and antioxidant (Bakheet et al., 2014; Prasad et al., 2011).
Sadananda et al. (2011) reported A. niger and Alternaria alternata (Host Tabebula argentea) produces lapachol, anticancer agent. Aspergillus niger PN2, an endophytic fungus isolated from Taxus baccata produced lovastatin (Raghunath et al., 2012). Wei et al. (2022) obtained five compounds from A. niger xj spore powder. These compounds are ergosterol (1), β-sitosterol (2), 5 -pentadecylresorcinol (3), 5-hydroxymethyl-2-furancarboxylic acid (4), and succinimide (5). All compounds showed antibacterial activity against three plant pathogens; Agrobacterium tumefaciens T-37, Erwinia carotovora EC-1, and Ralstonia solanacearum RS-2. Compound 4 showed the strongest antibacterial activity against the tested bacteria, and RS-2 was the most sensitive to compound 4, showing the lowest MIC of 15.56 μg/ml.
Little is known about a chemical constituent produced by P. subglobosus. However, secondary metabolites produced by Paecilomyces vary greatly, including terpenoids, alkaloids, sterols, fatty acids, polyketides, peptides, and others (Salem et al., 2022). One of the most representative metabolites of this fungus is paeciloquinones. This compound is a tyrosine kinase inhibitor with various biological activities such as antimicrobial, antiviral, insecticidal, and cytotoxic (Sun et al., 2004).
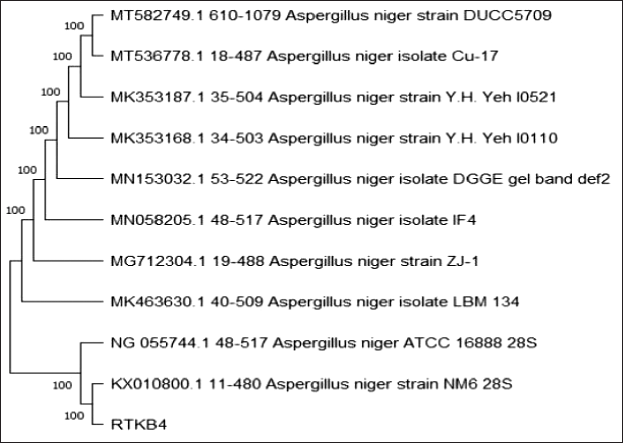 | Figure 6. The phylogenetic tree inferred using the neighbor-joining method of ITS sequence of fungus RTKB4 derived from R. tomentosa (Aiton) Hassk and its allied taxa. [Click here to view] |
 | Figure 7. The phylogenetic tree inferred using the neighbor-joining method of ITS sequence of fungus RTKB7 derived from R. tomentosa (Aiton) Hassk and its allied taxa. [Click here to view] |
CONCLUSION
Endophytic fungi have been isolated from the rose myrtle’s roots, barks, and leaves (R. tomentosa (Ait) Hassk). This study found three endophytic fungi capable of producing antibacterial compounds: Aspergillus heteromorphic, A. niger, and P. subglobosus. Advanced research of these findings and ongoing efforts to purify, identify, and optimize fermentation is necessary. Due to their capacity to synthesize the same compound originating from their host plant, endophytic fungi isolated from plants have drawn numerous researchers’ attention in basic and applied research.
ACKNOWLEDGEMENT
We appreciate the financial support BOPTN of Andalas University, Padang, Indonesia, provided for the project titled Penelitian Dasar Unggulan Klaster Riset Publikasi Guru Besar (PDU-KRP1GB-Unand), No. T/6/UN.16.17/PT.01.03/KO-PDU-KRP1GB-Unand/2022.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICT OF INTERESTS
The authors declare that they do not have any conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aminah I, Putra AE, Arbain D, and Handayani D. Antibacterial potential of fungi derived extracts of marine sponge Acanthostrongylophora ingens. AACL Bioflux, 2020; 13(2):1118.
Artasasta MA, Yanwirasti, Djamaan A, Handayani D. Cytotoxic activity screening of ethyl acetate fungal extracts derived from the marine sponge Neopetrosia chaliniformis AR-01. J App Pharm Sci, 2017; 7(12):174–8.
Atashpaz S, Khani S, Barzegari A, Barar J, Vahed S, Azarbaijani R, Omidi Y. A robust universal method for extraction of genomic DNA from bacterial species. Microbiology, 2010; 79(4):538–42. CrossRef
Bacon CW, White J. Microbial Endophytes. CRC press, Marcel Dekker, New York, 2000. CrossRef
Bauer AW. Single-disk antibiotic-sensitivity testing of staphylococci. AMA Arch Intern Med, 1959; 104(2):208. CrossRef
Bakheet MS, Soltan S, Gadalla A, Haredy HH, Shakoor MA. Antioxidants (Vitamin E and Gallic Acid) as valuable protective factors against myocardial infarction. Basic Res J Med Clin Sci, 2014; 11:109–22.
Brunton LL. The Pharmacological basis of therapeutics. 11th edition, USA, The McGraw Hill, New York, 2006.
Burkill IH. A dictionary of the economic products of the Malay Peninsula. 3rd Printing, Publication Unit, Ministry of Agriculture, Kuala Lumpur, Malaysia, vol. 1, pp 1–1240, 1993.
Davis WW, Stout TR. Disc plate method of microbiological antibiotic assay. J Microbiol, 1971; 22(4):659–65. CrossRef
Domsch K, Gams W, Anderson T. In: Gams W (ed.). 2nd edition, Compendium of soil fungi, IHW-Verlag, Eching, Germany, 2007.
Ferrer C, Colom F, Frases S, Mulet E, Abad JL, Alió JL. Detection and identification of fungal pathogens by PCR and by ITS2 and 5.8S ribosomal DNA typing in ocular infections. J Clin Microb, 2001; 39(8):2873–9. CrossRef
Handayani D, Ornando R, Rustini. Antimicrobial activity screening of symbiotic fungi from marine sponge Petrosia nigrans collected from south coast of West Sumatera, Indonesia. Int J Pharmacogn Phytochem Res, 2016; 8(4):623–6.
Handayani D, Artasasta MA. Antibacterial and cytotoxic activities screening of symbiotic fungi extract isolated from marine sponge Neopetrosia chaliniformis AR-01. J App Pharm Sci, 2017; 7(05):066–9.
Handayani D, Ananda N, Artasasta MA, Rustini R, Fadriyanti O, Tallei TE. Antimicrobial activity screening of endophytic fungi extracts isolated from brown algae Padina sp. J App Pharm Sci, 2019; 9(3):9–13. CrossRef
Handayani D, Aminah I. Antibacterial and cytotoxic activities of ethyl acetate extract of symbiotic fungi from West Sumatra marine sponge Acanthrongylophora ingens. J App Pharm Sci, 2017; 7(2):237–40.
Handayani D, Rasyid W, Rustini, Tallei TE, Hertiani T. Cytotoxic activity screening of fungal extracts derived from the West Sumatran marine sponge Haliclona fascigera to several human cell lines: Hela, WiDr, T47D, and Vero. J Appl Pharm Sci, 2018; 8(1):55–8.
Handayani D, Artasasta MA, Safirna N, Ayuni DF, Tallei TE, Hertiani T. Fungal isolates from marine sponge Chelonaplysilla sp.: diversity, antimicrobial and cytotoxic activities. Biodiversitas, 2020a; 21(5):1954; doi:10.13057/biodiv/d210523 CrossRef
Handayani D, Putri RA, Ismed F, Hertiani T, Ariantari NP and Proksch P. Bioactive metabolite from marine sponge derived fungus Cochliobolus geniculatus WR12, Rasayan J. Chem, 2020b; 13(1), 417–422; doi:10.31788/RJC.2020.1315517 CrossRef
Handayani D, Mulia P, Andayani R, Wahyuni FS, and Ariantari NP. Secondary metabolite from manggrove endophytic fungus Fusarium proliferatum AED3, rasayan J. Chem, 2021:150–5; doi: 10.31788/RJC.2021.1456447 CrossRef
Handayani D, Artasasta MA, Mutia D, Atikah N, Rustini, Tallei TE. Antimicrobial and cytotoxic activities screening fungal secondary metabolites isolated from marine sponge Callyspongia sp. AACL Bioflux, 2021; 14(1):249.
Hartley SE, Gange AC. Impacts of plant symbiotic fungi on insect herbivores: mutualism in a multitrophic context. Annu. Rev. Entomol, 2009; 54:323–342. CrossRef
Kjer J, Debbab A, Aly AH, Proksch P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat Protoc, 2010; 5(3):479–90. CrossRef
Petrini O. Fungal endophytes of tree leaves. Microb Ecol Leaves, 1991; 179–97; doi: 10.1007/978-1-4612-3168-4_9 CrossRef
Prasad D, Gupta RK, Venkataratnam GS. Kamini NR, and Gowthaman MK. Utilization of bahera fruits for production of tannase and Gallic Acid by Aspergillus Heteromorphus MTCC 5466 and synthesis of Propyl gallate thereof. Global J Biotechnol Biochem Res, 2011; 6(3):119–128.
Raghunath R, Radhakrishna A, Angayarkanni J, Palaniswamy M. Production and cytotoxicity studies of lovastatin from Aspergillus niger PN2, an endophytic fungi isolatedfrom Taxus baccata. Int J Appl Biol Pharm Tech, 2012; 3(3):342–52.
Ruma K, Sunil K, Prakash H. Antioxidant, anti-inflammatory, antimicrobial and cytotoxic properties of fungal endophytes from Garcinia species. Int J Pharm Sci, 2013; 5:889–897.
Sadananda T, Nirupama R, Chaithra K, Govindappa M, Chandrappa C, Raghavendra B. Antimicrobial and antioxidant activities of endophytes from tabebuia argentea and identification of anticancer agent (lapachol). J Med Plants Res, 2011; 5:3643–52.
Salem SH, El-Maraghy SS, Abdel-Mallek AY, Abdel-Rahman MAA, Hassanein EHM, Al-Bedak OA, Abd El-Aziz FEA. The antimicrobial, antibiofilm, and wound healing properties of ethyl acetate crude extract of an endophytic fungus Paecilomyces sp. (AUMC 15510) in earthworm model. Sci Rep, 2022; 12:19239; doi:10.1038/s41598-022-23831-4 CrossRef
Salni S, Marisa H. Evaluation on antibacterial activity of karamunting leaf extract (Rhodomyrtus tomentosa(Ait) Hassk) with various solvents to Shigella dysenteriae and Salmonella typhi, Mal J Fund Appl Sci, 2019; 15(5):671–4. CrossRef
Salni D, Sargent MV, Skelton BW, Soediro I, Sutisna M, White AH, Yulinah E. Rhodomyrtone, an antibiotic from Rhodomyrtus tomentosa. Aust J of Chem, 2002; 55(3):229–32 CrossRef
Samson RA, Evans HC and Latge JP. Atlas of entomopathogenic fungi. Springer-Verlag, New York, NY, 1988. CrossRef
Sandrawati N, Hati SP, Yunita F, Putra AE, Ismed F, Tallei TE, Hertiani T, Handayani D. Antimicrobial and cytotoxic activities of marine sponge-derived fungal extracts isolated from Dactylospongia sp. J Appl Pharm Sci, 2020; 10(04):028–33. CrossRef
Sinulingga SE, Hasibuan PAZ, and Suryanto D. Antibacterial activity of karamunting (Rhodomyrthus tomentosa) leaf extract and fractions. Asian J Pharm Clin Res, 2018; 11(3):163–5. CrossRef
Strobel GA. Endophytes as sources of bioactive products. Microbes Infect, 2003; 5(6):535–44. CrossRef
Strobel GA, Daisy B, Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev, 2003; 67:491–502. CrossRef
Sun MH, Liu XZ. Effects of Paecilomyces lilacinus M-14 fermentation filtrate on the affinity between soybean cyst nematode and soybean root. Acta Phytopathol Sin, 2004; 34:376–9.
Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci, 2011; 1(1):98–106.
Zhang HW, Song YC, Tan RX. Biology and chemistry of endophytes. Nat Prod Rep, 2006; 23:753–71. CrossRef
Wei L, Zhang Q, Xie A, Xiao Y, Guo K, Mu S, Xie Y, Li Z, He T. Isolation of bioactive compounds, antibacterial activity, and action mechanism of spore powder from Aspergillus niger xj. Front Microbiol, 2022; 13:934857; doi: 10.3389/fmicb.2022.934857 CrossRef
WHO. Medicinal plants in Vietnam. Institute of Materia Medica, Hanoi, Vietnam, 1989.