INTRODUCTION
Microdesmis keayana J.Leonard and Microdesmis puberula Hook.f. ex Planch. are two major species out of about 11 species found in the genus Microdesmis, Pandaceae (van Welzen, 2011). The plants are well spread in the tropical and subtropical African regions, including Ghana, Congo Republic, Ivory Coast, Nigeria, Sierra Leone (Royal Botanical Garden Kew, 2022), Burundi, Gabon, and Rwanda (Dounias, 2008). Both species are comparable in their morphology and medicinal uses, and in some regions, are confused as similar species (Alvarez Crus, 2008; Dounias, 2008). Besides their medicinal uses, the plants are important leafy vegetables with essential nutritional content eaten by some tribes as chew sticks by locals (Dounias, 2008) and browse plants for animals (Esonu et al., 2004; Okon et al., 2018; Umoh et al., 2004).
Local names of M. keayana and M. puberula vary throughout Africa due to ethnocultural diversity in the continent. Some M. keayana local African names include Sonoufoko (West Africa), Idi-apata, Aringo, Igi-ope (Yoruba), Mkpiri, Kpirimbo (Igbo), Amama, Erankpata (Esan), Ntanebit (Efik), Akpalata, Ingolongolo (Bayelsa), kawa (Boki), Babében evela (Diola), Gbihi, Kondgu (Kono), Kpendeile (Kissi), Bulon (Sherbro), and Efima (Anyi-Ndenye). Microdesmis puberula local African names include Sonoufoko (West Africa), Esunsun, Idi-apata, Aringo, Igi-ope, Igi ori apata (Yoruba), Mkpiri, Mbugbo, Kpirimbo (Igbo), amama, erankpata (Esan), Ntanebit (Efik), Akpalata, Ingolongolo (Bayelsa), Ofema (Ashanti), Nikee (Wonegizi), Dikota (Congo), and Mokula (Mbendjele BaYaka) (Akpanyung et al., 2013; Ariwaodo et al., 2012; Burkill, 1997; Etuk et al., 2020; Idu et al., 2009; Ihinmikaiye et al., 2021; Komlaga et al., 2015; Kpadehyea et al., 2022; Malan and Neuba, 2011; Salali et al., 2016; Uzodimma, 2013).
All parts of the studied species, including roots, leaves, stems, fruits, and whole plants, are used by locals for traditional medicinal purposes, including erectile dysfunction and infertility, malaria, skin and intestinal infections, pains, diabetes, diarrhea, and tumors. Their extensive ethnomedicinal utility could be due to their exceptional pharmacological activities, which include antimicrobial, antioxidant, antisickling, analgesic, aphrodisiac, antistress, analgesic, and antimalarial (Bouquet and Debray, 1974; Egunyomi et al., 2009, Okany et al., 2012; Roumy et al., 2008; Zamblé et al., 2006a, 2006b). Medicinal plants’ recent global acceptance and popularity due to their safe, low cost, easy accessibility, and effectiveness (Ogunmefun, 2018; Sofowora et al., 2013) makes M. keayana and M. puberula potential source of bioactive compounds for drug discovery. Although some in vitro and in vivo pharmacological studies of the plants have been reported by several researchers, there is a need for more pharmacologic and clinical studies to prove its efficacy and safety, and support its use in traditional medicine.
Microdesmis keayana and M. puberula share several similarities ranging from their ethnobotanical description, distribution, uses, and phytochemical constituents (Dounias, 2008; Roumy et al., 2008). An ethnobotanical survey in Southwestern Nigeria carried out at the early stage of this research revealed that the local names, “Idi-apata” and “Aringo” in Yoruba, are used mutually for both species among many traditional medicine practitioners and botanists and, thus, informed the need to report both species in this review article.
The present review is aimed to offer a firsthand compilation and databank of ethnopharmacology, phytochemistry, and biological activities of the plants, creating quick access information for future research on the plants.
METHODOLOGY
Relevant literature in this review was accessed from several electronic bibliographic databases, which include PubMed, Medline, Google, Google Scholar, Research Gate, Royal Botanical Garden Kew, JSTOR, The Plant List, and Academia, using several search terms, such as Microdesmis, M. puberula, M. keayana, chemical constituents, and ethnopharmacology of Microdesmis species. The search terms yielded more than 100 publications accessible online. The scientific names of the plants were validated using Royal Botanical Garden Kew, JSTOR, and The Plant List online websites.
Botanical description and distribution of M. keayana and M. puberula
Both Microdesmis species are nearly similar in morphology, making their identification difficult (Fig. 1). (Dounias, 2008). The two species are either short trees or shrubs that are dioecious, growing up to 6 m in height, with stems measuring up to about 8 cm in diameter. Their leaves are alternate and simple with about 4 mm long stipules. The petioles are generally 4–12 mm long with elliptical-oblong or ovate blades and asymmetrical bases looking cuneate to round with an acute and somewhat acuminate apex and finely toothed margin that is almost entire. Flowers are unisexual with green, short-hairy calyx, petals that are pink-orange, nearly circular to ovate-oblong, and short-hairy in the upper half; female flowers have superior ovaries. Fruits of both species are ovoid drupe-shaped, measuring 10–12 × 9–11 mm. They are usually smooth when fresh but appear wrinkled when hard and appear shiny and red (one to two seeded). Seeds are primarily ovate, compacted, and rounded seedlings with epigeal growth (Alvarez Crus, 2008; Baker, 1913; Burkill, 1997; Schmeizer and Gurib-Fakim, 2008; van Welzen, 2011) (Fig. 1).
Microdesmis keayana and M. puberula are small woody trees found in temperate regions of Africa (Fig. 2). They are the most widely distributed among the nine species in the genus Microdesmis found in Africa (Royal Botanical Garden Kew, 2022; van Welzen, 2011) (Fig. 2).
ETHNOMEDICINAL USES
Generally, almost all parts of M. keayana and M. puberula, such as fruits, leaves, leaf twigs, stem bark, root, and whole plants, are used for ethnomedicinal purposes. The plants have a vast range of traditional medicinal applications that include preparations, such as decoctions and paste for treating erectile dysfunction, pains, wound healing, and infections, respectively. Microdesmis puberula is used as a browse plant for cattle and goats (Esonu et al., 2004; Okon et al., 2017). The traditional use of the plants, their local names, and methods of preparation are shown summarized in Table 1.
CHEMICAL CONSTITUENTS
Previous investigations on Microdesmis species revealed the presence of polyamine alkaloids, such as spermine and spermidine derivatives as well as quinolones in M. keayana and M. puberula (Roumy et al., 2008; Zamblé et al., 2006a, 2006b). The leaves and roots were reported to possess important phytochemicals, such as alkaloids, flavonoids, saponins, steroids, tannins, and terpenoids (Akpanyung et al., 2013; Gbadamosi and Oloyede, 2014; Odesanmi et al., 2012; Okon et al., 2017). Coumarins and anthraquinones were found to be present in the roots and leaves of M. keayana by Acheampong et al. (2018), while Akpanyung et al. (2013) found reducing sugars in the roots of the plant. Studies to evaluate the nutritional and quantitative phytochemical properties of M. puberula revealed that the leaf contained alkaloids, saponins, cardiac glycosides, terpenes, and nutrients like carbohydrates, crude proteins, minerals, e.g., zinc, iron, calcium, potassium, magnesium, and phosphate alongside with other vitamins (A, B1, B2, and C) (Esonu et al., 2004; Okon et al., 2018; Umoh et al., 2004; Uwemedimo et al., 2018). Studies by Abakedi and Asuquo (2016), Abakedi (2017), and Abakedi and Sunday (2021) revealed that M. puberula leaf and root extracts inhibited the corrosion of aluminum in an acidic medium, which was predicted to be due to the presence of heteroatoms like nitrogen, oxygen, and sulfur in alkaloids, terpenes, and anthraquinones.
 | Figure 1. Pictures of M. keayana (A) West African Plants (2023) and M. puberula (B1-2) Flora of the World (2015). [Click here to view] |
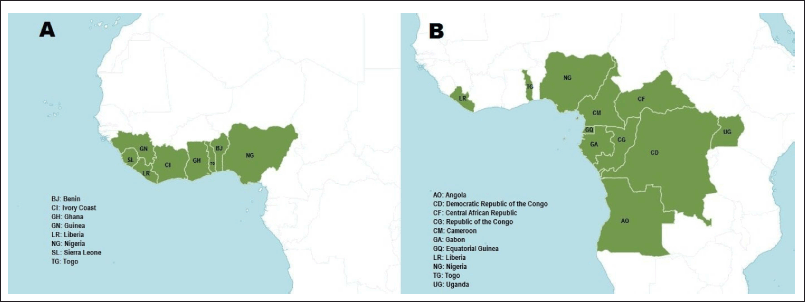 | Figure 2. Map showing the geographical distribution of M. keayana (A) and M. puberula (B) (Royal Botanical Garden Kew). [Click here to view] |
Three spermidine alkaloids were isolated from the methanol root extract of M. keayana by Zamble et al. (2006a, 2006b); N5, N10-di(p-coumaroyl)-N1-feruloylspermidine (Keayanidine A)(1), N5-(p-coumaroyl)-N1, N10-diferulosylspermidine (Keayanidine B)(2), and N1, N5, N10-triferuloylspermidine (Keayanidine C)(3). In another study by Zamble et al. (2007) on the hydromethanolic root extract of M. keayana, two new compounds: xanthoquininamide (6-hydroxyquinoline-4-carboxamide) (4) and N5-(p-coumaroyl)-N1, N14-diferuloyl spermine (keayanine) (5) were isolated, the latter being a spermine derivative. In addition, Roumy et al. (2008) reported the isolation of three spermines: N1, N5, N15-tris(p-coumaroyl) spermine (Keayanaine B) (6), N1-feruloyl-N5, N15-di(p-coumaroyl) spermine (keayanaine C) (7), and N1, N5, N15-tris(feruloyl) spermine (keayanine D) (8) from the hydromethanolic extracts of M. keayana and M. puberula roots. Roumy et al. (2008), in the same study, isolated four compounds: keayanidines A, B, and C, and keayanine A (previously isolated in M. keayana root) from the root of M. puberula. The isolated chemical compounds with their structures and classes are summarized in Figure 3.
PHARMACOLOGICAL ACTIVITIES
The various pharmacological activities of M. keayana and M. puberula include aphrodisiac, antimicrobial, antioxidant, analgesic properties, antiplasmodial, and antistress activities. (Acheampong et al., 2018; Bawo et al., 2020; Cagri-Mehmetoglu et al., 2017; Okany et al., 2012; Vonthron-Sénécheau et al., 2003; Zamblé et al., 2006a, 2006b; 2008 and 2009) (Table 2). Previous studies on the plants revealed several overlaps in their pharmacological activities (Table 3).
Fertility and aphrodisiac properties
Plant products that have antioxidant and sexually potentiating properties can be used as treatment choices for infertility in males (Muanya and Odukoya, 2008; Zamblé et al., 2009). The effect of M. keayana aqueous root extract on vasorelaxant and hypotensive activity in normotensive rabbits and guinea pig aorta strips using the organ bath was investigated by Zamblé et al. (2006a, 2006b). It was revealed that M. keayana root increased endothelial nitric oxide synthetase 3 (eNos) messenger ribonucleic acid (mRNA) (an important enzyme that synthesizes nitric oxide (NO), an essential mediator of erectile function) levels and NO production and also reversed oxidative stress due to its antioxidant properties. The result showed a positive correlation in its sexual behavioral functions, which supports the use of M. keayana use in ethnomedicine for the management of erection problems. Several studies have shown the expression and increased activity of eNOS during erection, which results in increased NO production (Li et al., 2019; Wen et al., 2011). Zamblé et al. (2008) further studied the sexual behavioral actions of aqueous root extract and two compounds keayanidine B and keayanine isolated from M. keayana, on male albino rats. The aqueous extract and pure isolated alkaloids were administered orally at doses of 150 and 3 mg/kg, respectively, to male albino rats. Microdesmis keayana root extract significantly increased mounting frequency, decreased mount latency, and increased intromission and ejaculatory frequencies after 1 hour 15 minutes and 3 hour 15 minutes. Zamblé et al. (2009) in a study to explore the pharmacologic basis surrounding the ethnomedicinal use of M. keayana for treating erection dysfunction, evaluated two compounds keayanidine B and keayanine (from M. keayana root extract) for their potential to induce vasodilation in isolated aortic rings of rats. Keayanidine B and keayanine, administered in ranging concentrations of 1.10−9–3.10−4 M, were found to cause a relaxed contraction induced by phenylephrine with IC50 of 23.3 ± 1.3 μM/l for keayanidine B and 27.5 ± 2.4 μM/l for keayanine in a dose-dependent fashion. The results showed that the vasodilating properties of the two isolated alkaloids were caused by their ability to increase eNos mRNA and NO levels.
Muanya and Odukoya (2008) investigated the fertility effect of M. keayana ethanol root extract and other plant root extracts commonly used in South West Nigeria for treating erectile dysfunction and boosting sperm count and libido in males. Lipid peroxidation was used as an index to evaluate the aphrodisiac properties of these plants. The lipid peroxidation activity of the plants was assayed by measuring malondialdehyde levels in the homogenate of raw and cooked fish. The findings indicated that M. keayana root extract significantly decreased lipid peroxidation due to its antioxidant properties (Muanya and Odukoya, 2008).
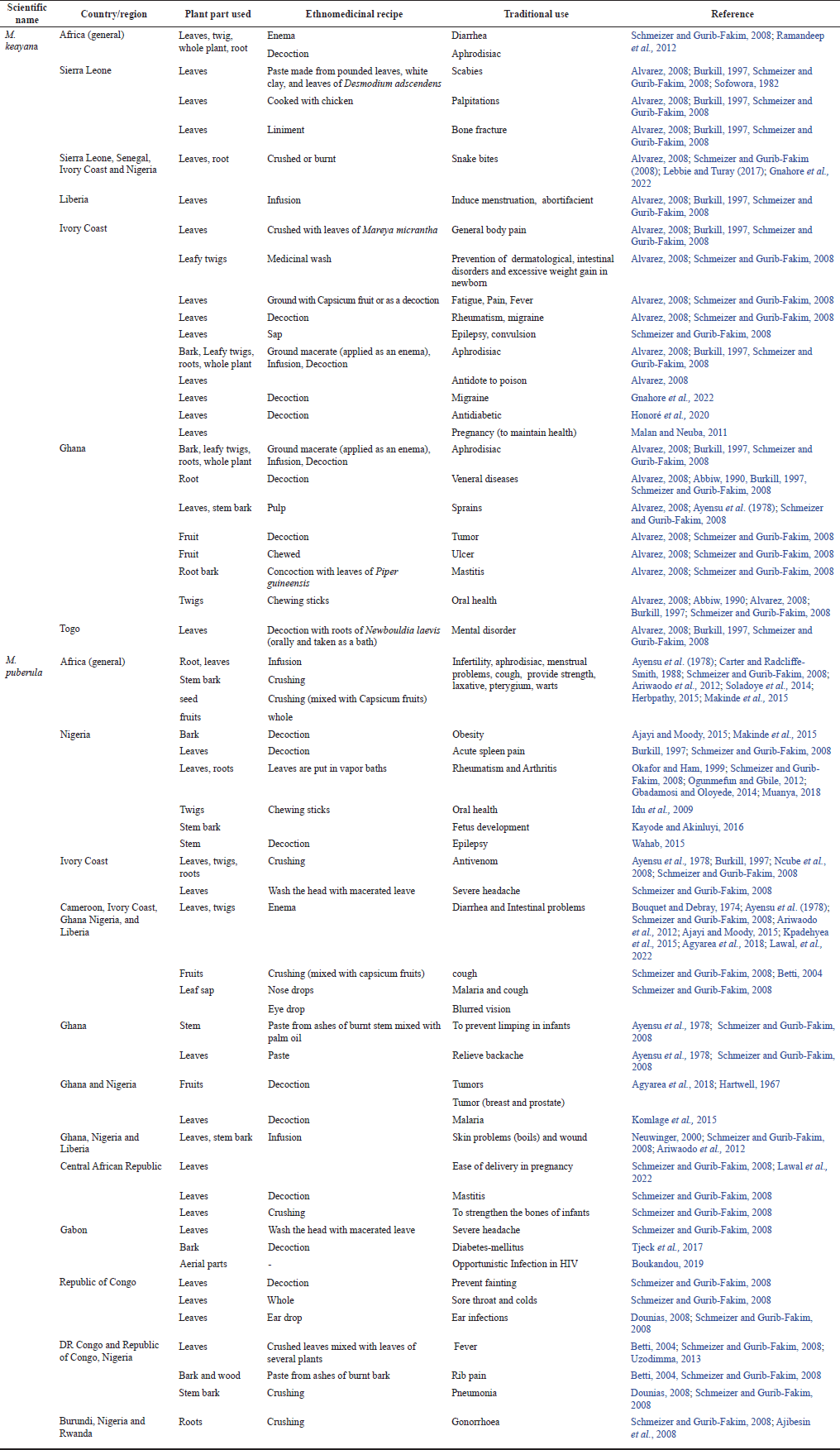 | Table 1. Ethnomedicinal uses of M. keayana and M. puberula. [Click here to view] |
The aphrodisiac potential of the two Microdesmis species needs more exploration as some commercial herbal products in the market have M. keayana as one of the ingredients (Barlowesherbalelixirs, 2020). The evidence above supports the use of M. keayana as an aphrodisiac in folklore. However, no scientific discoveries existed to support the traditional use of M. puberula as an aphrodisiac or fertility enhancer. This scientific gap indicates the need for more pharmacological research on both plants, especially on fractions and isolated compounds.
Antimalarial activities
Malaria is an endemic disease that affects more than 3.5 billion people worldwide, with higher mortality rates in Africa (Snow and Omumbo, et al., 2006). It is transmitted by Plasmodium sp. majorly Plasmodium falciparum (Bawo et al., 2020; WHO, 2022). Microdesmis keayana is used traditionally for treating malaria. This ethnomedicinal claim was confirmed when the antiplasmodial and cytotoxic activity of M. keayana methylene chloride leaves extract was evaluated alongside three Ivorian plants (Vonthron-Sénécheau et al., 2003). Four of the extracts were tested on K1 chloroquine-resistant P. falciparum strain by in vitro microculture radioisotope technique, which uses the uptake of [3H]hypoxanthine by parasites as an indicator of viability. The results showed that M. keayana methylene chloride and methanol leaf extract were able to inhibit P. falciparum growth by inhibiting the uptake of [3H]hypoxanthine with IC50 values of 12.2 μg/ml and >20 μg/ml for methylene chloride and methanol extracts, respectively (Vonthron-Sénécheau et al., 2003). Zirihi et al. (2005) studied the antiplasmodial and cytotoxicity of M. keayana ethanol root extract and 32 other West African plants against the chloroquine-resistant FcB1/Colombia strain of P. falciparum by in vitro models. The finding showed M. keayana extract was inactive against P. falciparum with IC50 values >50 g/ml. The larvicidal activity of M. puberula hexane leaf extracts, along with two other plants, was evaluated using biolarvicidal bioassay protocols, and Dipex pesticide (1 ppm) was used as a positive control (Bawo et al., 2020). The result showed a significant increase in the mortality rate of mosquito larvae, with the highest and lowest mortality rate at 70 and 10 ppm, respectively. Similarly, the hexane leaf extracts also had a biolarvicidal effect at an LC50 value of 32.83 ppm.
The report above shows some disparity in the in vitro antiplasmodial results of M. keayana. This necessitates the need for more scientific investigation on other solvent fractions and pharmacological screening techniques to validate the traditional usage of plants in curing malaria. Also, further research is encouraged on the isolation of bioactive compounds with antiplasmodial activity as a scaffold for new drug development for malaria treatment and to better understand the mechanism of action.
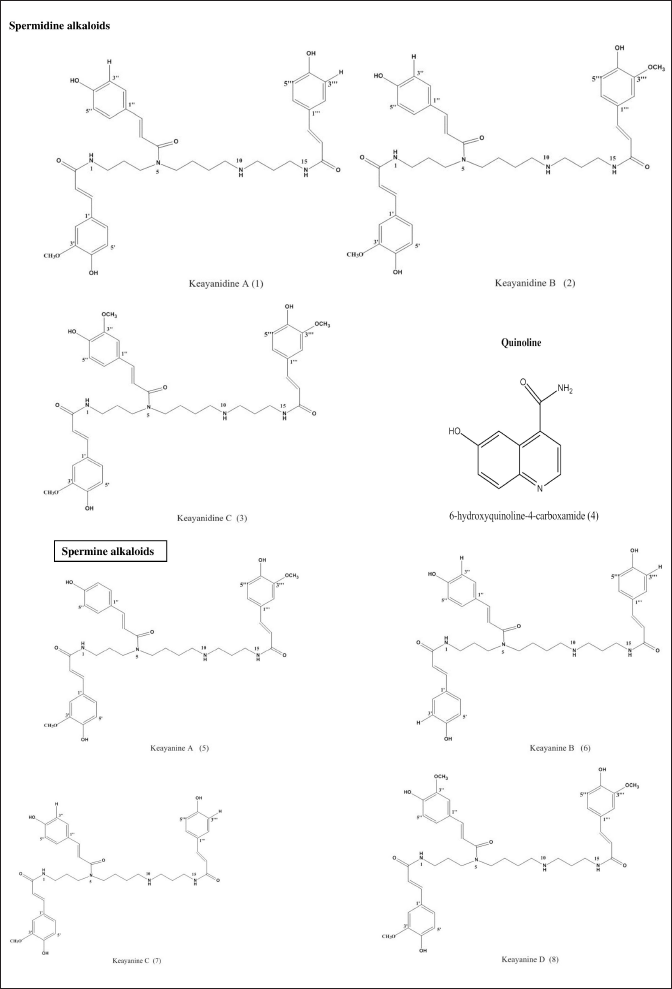 | Figure 3. Chemical structures and names of compounds reported from M. keayana and M. puberula. [Click here to view] |
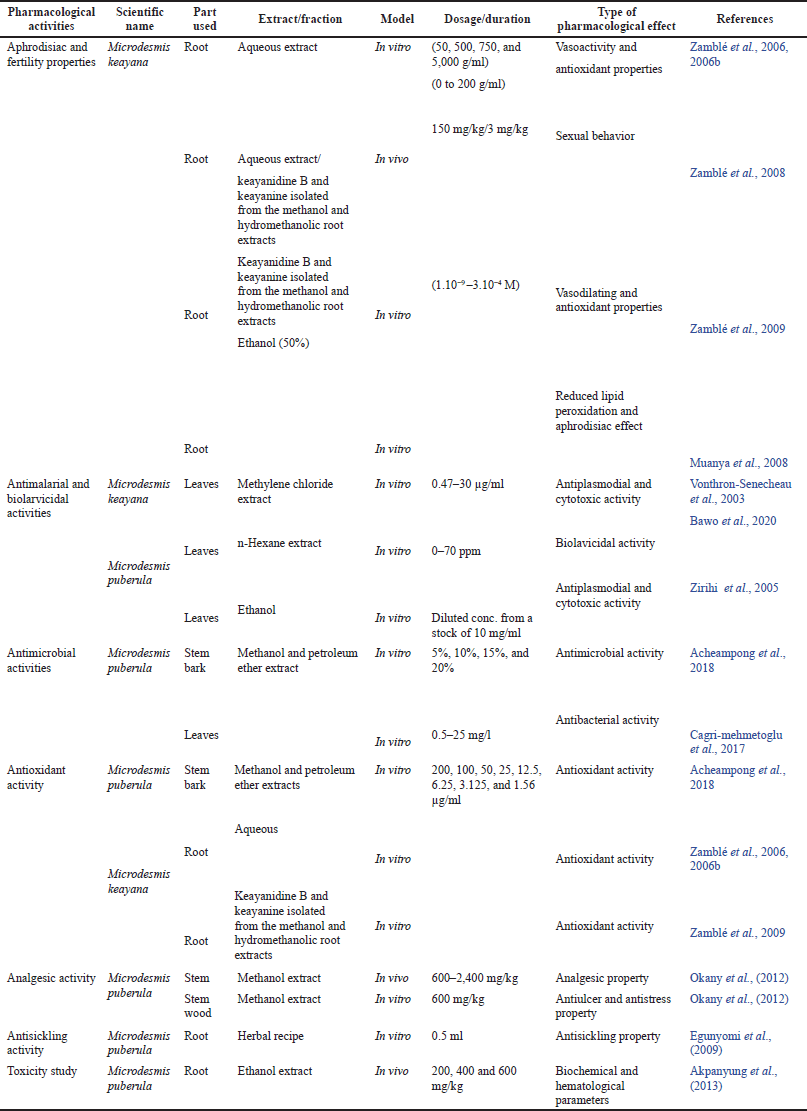 | Table 2. Pharmacological activities of M. keayana and M. puberula. [Click here to view] |
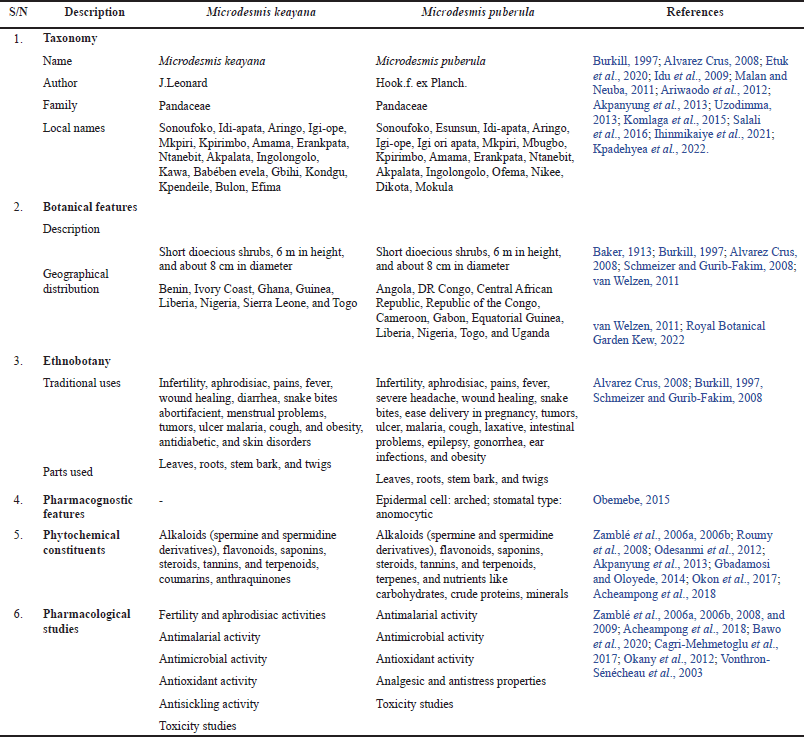 | Table 3. Summary of the similarities and differences between M. keayana and M. puberula. [Click here to view] |
Antimicrobial activity
Acheampong et al. (2018) assessed the antimicrobial activity of methanol and petroleum ether stem extracts of M. puberula by the agar diffusion method against designated microorganisms were used, such as Salmonella typhi, Bacillus subtilis, Pseudomonas aeruginosa, Candida albicans, Klebsiella pneumonia, Streptococcus pyogenes, Enterococcus faecalis, Staphylococcus aureus, Neisseria gonorrhoeae, and Escherichia coli. The methanol extract inhibited the growth of Gram-positive and Gram-negative bacteria in the agar diffusion test at 12–16 ppm, while the petroleum ether extract exhibited no antimicrobial activity, both having minimum inhibitory concentration value of 6.25–12.5 and 50–200 mg/ml, respectively. Also, the antibacterial properties of M. puberula leaf extract and three other medicinal plants in Nigeria (Hypoestes verticillaris, Icacina trichantha, and Enterolobium cyclocarpum) were evaluated by Cagri-Mehmetoglu et al. (2017). Microdesmis puberula extract inhibited the growth of S. aureus and E. sakkai with an inhibition zone of 8 mm.
The use of M. keayana and M. puberula in treating infections and skin diseases in ethnomedicine is yet to be extensively proven scientifically. The above report shows they are active against designated microorganisms. Possible isolation of bioactive lead compounds with antibacterial and antifungal activities is vital and encouraged.
Antioxidant activity
Antioxidants are important defense mechanisms of the body against the deleterious effect of free radicals, such as reactive oxygen species, involved in the development of several disease conditions (Agarwal and Prabakaran, 2005). Previous studies have shown that M. keayana and M. puberula are natural antioxidant reservoirs (Acheampong et al., 2018, Zamblé et al., 2006a, 2006b). Acheampong et al. (2018) investigated the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging properties and total antioxidant capacity of M. puberula methanol and petroleum ether stem bark extracts. The results showed both extracts possess DPPH radical scavenging activity with IC50 values of 1.1 and 1.2 μg/ml, respectively. The methanol extract proved more potent than the petroleum ether extract with a total antioxidant capacity of 21.75 mg ascorbic acid equivalent/gram of dry extract and 96.11 mg ascorbic acid equivalent/gram of dry extract for petroleum ether at the lowest extract concentration of 1.56 μg/ml. Studies by Zamblé et al. (2006a, 2006b) on the antioxidant activity of M. keayana aqueous root extract on superoxide anion, hydrogen peroxide (H2O2), hypochlorous acid (HOCl), and hydroxyl radical (HO•) revealed that the extract had a very significant dose-dependent radical scavenging activities in two systems (cellular and noncellular) against superoxide radical-anion with IC50 of 34.29 ± 2.384 and 19.46 ± 1.90 g/ml for noncellular and cellular systems, respectively. It also showed substantial scavenging activities against H2O2, HO•, and HOCl with IC50 of 49.75 ± 0.25, 57.8 ± 0.75, and 63.5 ± 0.5 g/ml, respectively (Zamblé et al., 2006a, 2006b). In another study, Zamblé et al. (2009) investigated DPPH radical scavenging, O2•-, and H2O2 antioxidant activity of keayanidine B and keayanine isolated from the root of M. keayana. Keayanidine B and keayanine showed strong antioxidant effects against DPPH with IC50 values of 33.0 ± 0.7 and 30.2 ± 0.9 μM/l, respectively, and against superoxide anion and H2O2 with IC50 varying from 16.2 ± 0.4 to 20.2 ± 0.7 μM/l in the cell-free system and from 13.2 ± 0.7 to 16.3 ± 0.8 μM/l in the cellular system (Zamblé et al., 2009). The antioxidant activities of the root and stem bark extracts of M. keayana and M. puberula could be responsible for the pharmacological activity of the plants, which further gives credence to their use in folklore as fertility enhancers, aphrodisiacs and in the treatment of other disease conditions triggered by reactive oxygen species (Zamblé et al., 2006a, 2006b and 2009). Further research is crucial to isolate antioxidant phytochemicals from the various parts of the plants.
Analgesic and antistress properties
Microdesmis keayana and M. puberula are used in ethnomedicine as pain relievers (Alvarez Crus, 2008; Ayensu, 1978; Betti, 2004; Muanya, 2018; Schmeizer and Gurib-Fakim, 2008). The analgesic property of M. puberula methanol stem wood extract was investigated by Okany et al. (2012) using standard analgesic models like the acetic acid writhing and the hot plate analgesic tests. It was revealed that the extract significantly ameliorated both neurogenic and inflammatory pain dose-dependently at 600–2,400 mg/kg. Okany et al. (2012) employed the forced swimming test and immobilization stress-induced ulcer protocol to evaluate the antistress properties of M. puberula methanol stem wood extract. The results showed that the duration of immobility was significantly decreased by the extract at the dose of 600 mg/kg and also a reduced ulcer index in the stressed rats’ group treated with the extract.
Antisickling activity
The antisickling activities of two herbal recipes were evaluated by Egunyomi et al. (2009). The first recipe consisted of M. keayana methanol root extract and 27 other plants, while the second recipe consisted of seven plant extracts without M. keayana, p-hydroxybenzoic acid, and normal saline were used as controls. The two herbal recipes demonstrated antisickling activity against sickled erythrocytes. The first ethnobotanical recipe containing M. keayana inhibited red blood cell (RBC) sickling with 63.4% inhibition, while the second herbal recipe had a percentage inhibition of 78.2% at 180 minutes incubation (Egunyomi et al., 2009). More scientific work is required to validate the antisickling property of the plant and the possible isolation of bioactive compounds as new agents against sickle cell disease.
Toxicity studies
Investigations of the acute toxicity profile of M. keayana and M. puberula were carried out in several toxicity studies to determine the safety of extracts of both plants. Okany et al. (2012) revealed that M. puberula has a safety profile of 15 g/kg when administered orally with an LD50 of 1,412.5 mg/kg. An acute toxicity study of M. puberula by oral administration on albino rats for 14 days revealed that the plant has a wide safety margin with an LD50 of more than 5,000 mg/kg (Akpanyung et al., 2013). Aspartate transaminase and alanine transferase levels of rats in the treatment groups were reduced when compared with the control group. No toxic effect on the liver and kidney was obtained, and hematological parameters like packed cell volume, hemoglobin, mean corpuscular volume, mean corpuscular hemoglobin concentration, and RBC were not significantly elevated. In contrast, the serum lipid profile of low-density lipoprotein and triglycerides were significantly elevated with reduced high-density lipoprotein levels. Uwemedimo et al. (2018) investigated the acute toxicity of the leaf extract of M. puberula using albino mice by intraperitoneal (i.p.) route using the method of Lorke. The mean lethal dose (LD50) of the extract was estimated to be 2,872.28 mg/kg, which proposes that ingestion as a leaf meal may not be detrimental to livestock and humans. Microdesmis keayana aqueous root extract following oral administration caused no death or toxicity at a dose of 2 g/kg body weight in albino rats (Zamblé et al., 2008). Acute and subacute toxicity studies on M. keayana and M. puberula have shown substantial safety and acceptability on all investigated parameters, supporting their widespread use in ethnomedicine.
CONCLUSION
This review, for the first time, has provided a compendium of information on the ethnopharmacological and phytochemical properties of M. keayana and M. puberula. The plants possess an untapped reservoir of phytochemicals as leads for drug discovery and development. There is a need for substantial studies for a proper understanding of the taxonomy and pharmacognostic similarities and differences of both species. The different plant parts, such as root, bark, and leaf, have close characteristics in terms of morphology, distribution, and overlapping medicinal uses. Some biological activities reported on M. keayana include antimicrobial, toxicity, antioxidant, antisickling, analgesic, aphrodisiac, and antimalarial, whereas in M. puberula, they comprise antibacterial, toxicity, antioxidant, antistress, analgesic, and antimalarial activities have been reported. Despite the pharmacological studies reported for both Microdesmis species, several ethnomedicinal claims need scientific data for validation and a better understanding of their mechanism of pharmacological actions, especially at the molecular level. Phytochemical investigations on the two plants have yielded about eight chemical compounds, the majority belonging to the groups of spermine and spermidine alkaloids. Most of the studies on M. keayana and M. puberula were on crude extracts, which has created a gap for further research, particularly on the fractions and the isolated compounds. Clinical studies on both species are encouraged to establish the dose, efficacy, and safety of human subjects in managing disease conditions, as challenges such as dose and dosage optimization have continuously posed major drawbacks in herbal medicine use. Acute and subacute toxicity reports on both species revealed that they are safe and nontoxic, as higher doses of the extracts elicited no adverse effects in experimental animals.
ACKNOWLEDGMENTS
The authors are very grateful to Prof. M. N. Femi-Oyewo (Provost, College of Pharmacy, ABUAD) and Prof. P. O. Erah, who assisted in proofreading the manuscript.
List of abbreviations: eNos, endothelial nitric oxide synthase; mRNA, messenger ribonucleic acid, RBC, red blood cell.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The publication of this research work was funded by Afe Babalola University research grant.
CONFLICTS OF INTEREST
No conflicts of interest were declared by the authors in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abakedi OU. Aluminium corrosion inhibition by Microdesmis puberula leaf extract in 2 M hydrochloric acid solution. Int J Innov Sci Eng, 2017; 5(3):6–14.
Abakedi OU, Asuquo JE. Corrosion inhibition of mild steel in 1M H2SO4 solution by Microdesmis puberula leaf extract. Am Chem Sci J, 2016; 16(1):1–8. CrossRef
Abakedi OU, Sunday MV. Potential of Microdesmis puberula root extract as an eco-friendly inhibitor for aluminium corrosion in 2 M HCl solution. IOSR J Appl Chem, 2021; 14(5):15–21.
Abbiw DK, 1990. Useful plants of Ghana: West African uses of wild and cultivated plants. Intermediate Technology Publications, Royal Botanic Gardens, Kew, London, UK. CrossRef
Acheampong A, Amankwaa LT, Afriyie IO, Baah KA. Antioxidant and antimicrobial activity of the methanol and petroleum ether extracts of the stem of Microdesmis puberula. Pharm Chem J, 2018; 5(1):38–48.
Agarwal A, Prabakaran SA. Mechanism, measurement, and prevention of oxidative stress in male reproductive physiology. Indian J Exp Biol, 2005; 43:963–74.
Agyarea C, Spieglerc V, Asaseb A, Scholzc M, Hempeld G, Hense A. An ethnopharmacological survey of medicinal plants traditionally used for cancer treatment in the Ashanti region, Ghana. J Ethnopharmacol, 2018; 212:137–52. CrossRef
Ajayi TO, Moody JO. Ethnobotanical survey of plants used in the management of obesity in Ibadan, South-Western Nigeria. Niger J Pharm Res, 2015; 11(1):22–31.
Ajibesin KK, Ekpo BA, Bala DN, Essien EE, Adesanya SA. Ethnobotanical survey of Akwa Ibom state of Nigeria. J Ethnopharmacol, 2008; 115:387–408. CrossRef
Akpanyung EO, Ita SO, Opara KA, Davies KG, Ndem JI, Uwah AF. Phytochemical screening and effect of ethanol root extract of Microdesmis puberula on some haematological and biochemical parameters in normal male albino Wistar rats. J Med Plant Res, 2013; 7(31):2338–42. CrossRef
Alvarez Crus NS. Microdesmis keayana J.Léonard. 2008. [Online]. Available via https://uses.plantnet-project.org/e/index.php?title=Microdesmis_keayana_(PROTA)&mobileaction=toggle_view_desktop (Accessed 17 November 2022).
Ariwaodo JO, Chukwuma EC, Adeniji KA. Some medicinal plant species of Asamagbe Stream Bank Vegetation, Forestry Research Institute of Nigeria, Ibadan. J Plant People Appl Res, 2012; 10:541–9.
Ayensu ES. Medicinal plants of West Africa. Reference Publications Inc, Algonac, MI, 1978.
Baker JA. Flora of tropical Africa. Royal Botanical Gardens Kew, London, UK, 1913.
Barlowesherbalelixirs. Microdesmis keayana root powder—450 mg capsules. 2020. [Online]. Available via https://barlowesherbalelixirs.com/microdesmis-keayana-root-powder-450mg-capsules (Accessed 22 November 2022).
Bawo DDS, Oyedeji AA, Solomon PB, Briyai FO, Jasper F, Abowei N. Assessment of the larvicidal efficacy of the hexane-leaf-extracts of selected tropical plant species. Budapest Int J Exact Sci, 2020; 2(2):136–40. CrossRef
Betti JL. An ethnobotanical study of medicinal plants among the Baka pygmies in the Dja biosphere reserve, Cameroon. Afr Study Monogr, 2004; 25(1):1–27.
Boukandou MMM. Investigation of plants used in Gabonese traditional medicine for the treatment of opportunistic infections caused by HIV. Ph.D Dissertation. [Online]. 2019. Available via http://hdl.handle.net/11602/1416 (Accessed 22 November 2022).
Bouquet A, Debray M. Plantes médicinales de la Côte d’Ivoire. O.R.S.T.O.M. L’Office de la Recherche Scientifique et Technique Outre-Mer, Paris, France, 1974.
Burkill HM. The useful plants of west tropical Africa. 2nd edition, Royal Botanic Gardens, Kew, Richmond, UK, Vol. 4, p 969, 1997.
Cagri-Mehmetoglu A, Sowemimo A, Maryna van de V. Evaluation of antibacterial activity and phenolic contents of four Nigerian medicinal plants. Int J Food Process Technol, 2017; 4:12–21. CrossRef
Carter S, Radcliffe-Smith A, 1988. Euphorbiaceae (part 2). In: Polhill RM (ed.). Flora of tropical East Africa, A.A. Balkema, Rotterdam, The Netherlands, pp 409–597.
Dounias E. Microdesmis puberula Hook. F. ex Planch. In: Schmeizer GH, Gurib-Fakim A (eds.). Plant resources of tropical Africa 11(1): medicinal plants 1, PROTA, Wageningen, The Netherlands, pp 380–5, 2008.
Egunyomi A, Moody J, Eletu O. Anti-sickling activies of two ethnomedicinal plant recipes used for the management of sickle cell anaemia in Ibadan, Nigeria. Afr J Biotechnol, 2009; 8:20–5.
Esonu BO, Azubuike JC, Emenalom OO, Etuk EB, Okoli IC, Ukwu H, Nneji CS. Effect of enzyme supplementation on the performance of broiler finisher fed Microdesmis puberula leaf meal. Int J Poult Sci, 2004; 3(2):112–4. CrossRef
Etuk IM, Daniel KS, Umoh UA. Ecology of undergrowth plant species in four selected natural forests in Akwa Ibom state, Nigeria. Afr J Environ Nat Sci Res, 2020; 3(4):12–34.
Flora of the World. 2015. Available via https://floraoftheworld.org/flora/17592186 091875 (Accessed 04 January 2023).
Gbadamosi IT, Oloyede AA. The mineral, proximate and phytochemical components of ten Nigerian medicinal plants used in the management of arthritis. Afr J Pharm Pharmacol, 2014; 8(23):638–43. CrossRef
Gnahore E, Kouadio KR, Grevin Amba AJ, Kone M, Bakayoko A. Ethnobotanical survey of plants used by the riparian population of Banco National Park (Abidjan, Ivory Coast). Asian J Ethnobiol, 2022; 5(2):121–9. CrossRef
Hartwell JL. Plants used against cancer. A survey. Lloydia, 1969; 32(3):247–96.
Herbpathy. Microdesmis puberula herb uses, benefits, cures, side effects, nutrients repertory. 2015. Available via https://herbpathy.com/Uses-and-Benefits-of-Microdesmis-Puberula-Cid6260 (Accessed 22 November 2022).
Honoré TI, Stéphane DK, Koffi N. Ethnopharmacological study of anti-diabetic plants sold on the markets of Abidjan, Côte d’Ivoire. J Phytopharmacol, 2020; 9(6):433–7. CrossRef
Idu M, Umweni AA, Odaro T, Ojelede L. Ethnobotanical plants used for oral healthcare among the Esan Tribe of Edo State, Nigeria. Ethnobot Leafl, 2009; 13:548–63.
Ihinmikaiye SO, Roberts EM, Ilesanmi OB, Simon CE. Ethnomedicinal survey of plant species used in managing erectile dysfunction (Ed) in Bayelsa state, Nigeria. J Biotechnol Biochem, 2021; 7(5):35–9.
Kayode J, Akinluyi SM. Documentation and conservation of Wild edible plants in Ado- Ekiti Region of Ekiti State, Nigeria. Can J Agric Crops, 2016; 1(2):43–9. CrossRef
Komlaga G, Agyare C, Dickson RA, Mensah ML, Annan K, Loiseau PM, Champy P. Medicinal plants and finished marketed herbal products used in the treatment of malaria in the Ashanti region, Ghana. J Ethnopharmacol, 2015; 172:333–46. CrossRef
Kpadehyea JT, Fernando ES, Tinio CE, Buot IE. Ethnobotany survey of the Wonegizi, Ziama Clan-Lofa County, Liberia. Electron J Biol, 2022; 11(4):165–74.
Kpadehyea J, Fernando E, Tinio C, Buot I. Ethnobotany survey of the Wonegizi, Ziama Clan-Lofa County, Liberia. Electron J Biol, 2015; 11:165–75.
Lawal IO, Rafiu BO, Ale JE, Majebi OE, Aremu AO. Ethnobotanical survey of local flora used for medicinal purposes among Indigenous people in five areas in Lagos State, Nigeria. Plants, 2022; 11(5):633. CrossRef
Lebbie AA, Turay M. Prevalence of snakebites and use of antivenom plants in Southern Sierra Leone. Sierra Leone J Biomed Res, 2017; 9(1):7–13.
Li X, Jiang J, Xia J, Jiang R. Effect of low androgen levels on the sulphur dioxide signalling pathway in rat penile corpus cavernosum. Andrologia, 2019; 51(1):e13167. CrossRef
Makinde SCO, Ojekale AB, Oshinaike TS, Awusinu TS. An ethnomedical and ethnobotanical survey of plants herbal therapy used for obesity, asthma, diabetes and fertility by the Badagry people of Lagos State, Nigeria. J Med Plant Stud, 2015; 3(5):1–6.
Malan DF, Neuba DFF. Traditional practices and medicinal plants use during pregnancy by Anyi-Ndenye women (Eastern Côte d’Ivoire). Afr J Reprod Health, 2011; 15(1):94.
Muanya C. Killing pains with local herbs. 2018. Available via https://guardian.ng/features/killing-pains-with-local-herbs/ (Accessed 19 November 2022).
Muanya CA, Odukoya OA. Lipid peroxidation as index of activity in aphrodisiac herbs. J Plant Sci, 2008; 3(1):92–8. CrossRef
Ncube NS, Afolayan AJ, Okoh AI. Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. Afr J Biotechnol, 2008; 7(12):1797–806. CrossRef
Neuwinger HD. African traditional medicine: a dictionary of plant use and applications. With supplement: search system for diseases. Medpharm, Stuttgart, Germany, p 599, 2000.
Obemebe OA. Stomata complex in some shrubs and trees. Glob J Biol Agric Health Sci, 2015; 4(2):164–72.
Odesanmi OS, Ojokuk SA, Apena A, Bikom OE, Lawal RA. Nutritional prospect of an aphrodisiac Microdermis keayana. J Med Plant Res, 2012; 6(7):1187–90. CrossRef
Ogunmefun OT. Phytochemicals—god’s endowment of curative power in plants. In: Asao T, Asaduzzaman M (eds.). Phytochemicals—source of antioxidants and role in disease prevention, Intechopen, 2018; doi: 10.5772/intechopen.77423. CrossRef
Ogunmefun OT, Gbile ZO. An ethnobotanical study of anti-rheumatic plants in South-Western states of Nigeria. Asian J Sci Technol, 2012; 4(11):63–6.
Okafor J, Ham R. Identification, utilization and conservation of medicinal plants in Southeastern Nigeria. Biodiversity Support Program, Washington, DC, 1999.
Okany CC, Ishola IO, Ashorobi RB. Evaluation of analgesic and antistress potential of methanolic stem wood extract of Microdesmis puberula Hook. f.ex. Planch (Pandaceae) in mice. Int J Appl Res Nat Prod, 2012; 5(3):30–6.
Okon JE, Ibanga IA, Esenowo GJ, Okon OG. Nutritional qualities and phytochemical constituents of two neglected Wild edible leafy vegetables in Akwa Ibom state, Nigeria. Biol Sci, 2017; 1:63–74.
Okon J, Ibanga I, Okon O. Nutritional qualities and phytochemical constituents of two neglected Wild edible leafy vegetables in Akwa Ibom state, Nigeria. Biol Sci, 2018; 1:67–75.
Ramandeep S, Sarabjeet S, Jeyabalan G, Ashraf A. An overview on traditional medicinal plants as aphrodisiac agent. J Pharmacogn Phytochem, 2012; 1(4):43–56.
Roumy V, Hennebelle T, Zamblé A, Yao JD, Sahpaz S, Bailleul F. Characterisation and identification of spermine and spermidine derivatives in Microdesmis keayana and Microdesmis puberula roots by electrospray ionisation tandem mass spectrometry and high-performance liquid chromatography/electrospray ionisation tandem mass spectrometry. Eur J Mass Spectrom, 2008; 14(2):111–5. CrossRef
Royal Botanical Garden Kew. Microdesmis keayana J.Léonard. 2022. Available via https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:352293-1 (Accessed 24 November 2022).
Salali GD, Chaudhary N, Thompson J, Olwen MG, van der Burgt XM, Dyble M, Page AE, Smith D, Lewis J, Mace R, Vinicius L, Migliano AB. Knowledge-sharing networks in hunter-gatherers and the evolution of cumulative culture. Curr Biol, 2016; 26:2516–21. CrossRef
Schmeizer GH, Gurib-Fakim A. Plants resources of tropical Africa medicinal plants. 11th edition, Prota Foundation, Wageningen, The Netherlands, pp 382–4, 2008.
Snow RW, Omumbo JA. Malaria. In: Jamison DT, Feachem RG, Makgoba MW, Bos ER, Baingana FK, Hofman KJ, Rogo KO (eds.). Disease and mortality in Sub-Saharan Africa. 2nd edition, The International Bank for Reconstruction and Development/the World Bank, Washington, DC, 2006.
Sofowora A. Medicinal plants and traditional medicine in Africa. John Wiley and Sons, Chichester, UK, p 274, 1982.
Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med, 2013; 10(5):210–29. CrossRef
Soladoye MO, Chukwuma EC, Olatunji MS, Roseline TF. Ethnobotanical survey of plants used in the traditional treatment of female infertility in southwestern Nigeria, Ethnobot Res Appl, 2014; 12:81–90.
Tjeck OP, Souza A, Mickala P, Lepengue AN, M’Batchi B. Bio-efficacy of medicinal plants used for the management of diabetes mellitus in Gabon: an ethnopharmacological approach. J Intercult Ethnopharmacol, 2017; 6(2):206–17. CrossRef
Umoh BI, Okon BI, James IO, Jacob ES. Effect of feeding varying levels of Microdesmis puberula and Alchornea cordifolia on the body size and carcass component of West African Dwarf Goats (WAD). Glob J Agric Sci, 2004; 3(1):45–51. CrossRef
Uwemedimo EU, Akaninyene UU, Emmanuel UD. Determination of nutrient, antinutrient compositions and median lethal dose of leaves of Microdesmis puberula grown in Nigeria. J Sci Res Rep, 2018; 17(4):1–10. CrossRef
Uzodimma DE. Medico-ethnobotanical inventory of Ogii, Okigwe ImoState, South Eastern Nigeria – I. Glob Adv Res J Med Plant (GARJMP), 2013; 2(2):30–44.
van Welzen PC. Pandaceae (formerly Euphorbiaceae s.l. subfam. Acalyphoideae tribe Galearieae). Flora Malesiana, 2011; 20:15–43.
Vonthron-Sénécheau C, Weniger B, Ouattara M, Bi FT, Kamenan A, Lobstein A, Brun R, Anton R. In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected Ivorian plants. J Ethnopharmacol, 2003; 87(2–3):221–5. CrossRef
Wahab OM. Ethnomedicinal antiepileptic plants used in parts of Oyo and Osun States, Nigeria. Botany Res Int, 2015; 8(4):77–81.
Wen J, Grenz A, Zhang Y, Dai Y, Kellems RE, Blackburn MR, Eltzschig HK, Xia Y. A2B adenosine receptor contributes to penile erection via PI3K/AKT signaling cascade-mediated eNOS activation. FASEB J, 2011; 25(8):2823–30. CrossRef
West African Plants. 2023. Available via http://www.westafricanplants.senckenberg.de/root/index.php?page_id=14&id=12101# (Accessed 04 January 2023).
World Health Organization (WHO). Malaria. 2022. [Online]. Available via https://www.who.int/news-room/fact-sheets/detail/malaria (Accessed 22 November 2022).
Zamblé A, Martin-Nizard F, Sahpaz S, Reynaert ML, Staels B, Bordet R, Duriez P, Gressier B, Bailleul F. Effects of Microdesmis keayana alkaloids on vascular parameters of erectile dysfunction. PTR, 2009; 23(6):892–5. CrossRef
Zamblé A, Sahpaz S, Brunet C, Bailleul F. Effects of Microdesmis keayana roots on sexual behavior of male rats. Phytomed Int J Phytother phytopharmacol, 2008; 15(8):625–9. CrossRef
Zamble A, Sahpaz S, Hennebelle T, Carato P, Bailleul, F. N1,N5,N10-Tris(4-hydroxycinnamoyl) spermidines from Microdesmis keayana roots. Chem Biodivers, 2006a; 3(9):982–9. CrossRef
Zamble A, Sahpaz S, Hennebelle T, Carato, Bailleul F. Two new quinoline and Tris(4-hydroxycinnamoyl) spermine derivatives from Microdesmis keayana roots. Chem Pharm Bull, 2007; 55(4):643–5. CrossRef
Zamblé A, Yao D, Martin-Nizard F, Sahpaz S, Offoumou M, Bordet R, Duriez P, Brunet C, Bailleul F. Vasoactivity and antioxidant properties of Microdesmis keayana roots. J Ethnopharmacolol, 2006b; 104(1–2), 26–269. CrossRef
Zirihi GN, Mambu L, Guédé-Guina F, Bodo B, Grellier P. In-vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. J Ethnopharmacol, 2005; 98(3):281–5. CrossRef